Abstract
Better understanding of process controls over nitrous oxide (N2O) production in urine-impacted ‘hot spots’ and fertilizer bands is needed to improve mitigation strategies and emission models. Following amendment with bovine (Bos taurus) urine (Bu) or urea (Ur), we measured inorganic N, pH, N2O, and genes associated with nitrification in two soils (‘L’ and ‘W’) having similar texture, pH, C, and C/N ratio. Solution-phase ammonia (slNH3) was also calculated accounting for non-linear ammonium (NH4+) sorption capacities (ASC). Soil W displayed greater nitrification rates and nitrate (NO3−) levels than soil L, but was more resistant to nitrite (NO2−) accumulation and produced two to ten times less N2O than soil L. Genes associated with NO2− oxidation (nxrA) increased substantially in soil W but remained static in soil L. Soil NO2− was strongly correlated with N2O production, and cumulative (c-) slNH3 explained 87% of the variance in c-NO2−. Differences between soils were explained by greater slNH3 in soil L which inhibited NO2− oxidization leading to greater NO2− levels and N2O production. This is the first study to correlate the dynamics of soil slNH3, NO2−, N2O and nitrifier genes, and the first to show how ASC can regulate NO2− levels and N2O production.
Better understanding of biochemical process controls over soil N2O production is needed for improving N2O mitigation strategies and emissions models. Incorporation of improved process-related information into models will help improve emissions assessments at field, regional and global scales1,2. In cattle grazing systems, urine deposition events typically result in localized N loads equivalent to 1000 kg N ha−1,3, and can create ‘hot spots’ for elevated N2O emissions4. Similarly, N fertilizers applied in concentrated bands result in localized inorganic N concentrations ranging from several hundred to more than 2000 μg N g−1,5,6, and can result in substantially greater N2O emissions compared to uniformly applied fertilizer7,8,9. Thus, understanding the processes mediating N2O production within these concentrated zones is critical. Urine deposition and Ur application can cause elevated soil NO2− levels which in turn promote elevated N2O production9,10,11. Accumulation of NO2− is presumed to occur due to a sequence of chemical and microbial responses. Hydrolysis of Ur results in localized increases in both soil pH and NH4+ which together promote the formation of free NH312. Both groups of autotrophic nitrifying bacteria, i.e., the NH3-oxidizing bacteria (AOB) and the NO2− oxidizing bacteria (NOB), are sensitive to NH3 toxicity, but it is generally believed that NOB are more sensitive than AOB11,12,13,14; thus, soil NO2− accumulates in the presence of sufficiently high NH3 levels because NOB are unable to fully process the NO2− produced by AOB.
However, beyond this rather general understanding of soil NO2- dynamics, little is known about specific soil properties that regulate NO2− accumulation. While related processes have been well-studied in wastewater systems14, the presence of soil surface-solution interactions and other factors complicate our understanding in soils. For example, the partitioning of NH4+ between soil surfaces and solution could regulate solution-phase levels and therefore influence nitrifier activity and NH3 toxicity15. Simultaneous quantification of genes associated with activities of NOB, AOB and NH4+ oxidizing archaea in soil following amendment with N has been reported in a few studies16,17,18, but more data are needed to understand the role of nitrifier responses in regulating NO2− and N2O dynamics. Limited understanding of these and other factors limits our ability to predict NO2− dynamics for a particular soil, management practice, or climate condition, and our ability to predict N2O emissions resulting from NO2− transformations19,20.
In preliminary experiments, we observed that two soils collected from grazed fields in New Zealand, while having similar texture, pH, C content and C/N ratio, displayed substantially different N2O production rates when amended with Ur. Our general hypothesis was that differences in NO2− dynamics were responsible for the contrasting N2O production. In this study, we conducted a series of experiments designed to elucidate controls over NO2− and N2O production under conditions representative of concentrated BU patches or Ur bands, and to explain the differences in N2O production between these soils.
Results
Ammonium sorption
Ammonium sorption, determined in batch equilibrium experiments and modeled using Eq. 1, was significantly greater in soil W, which sorbed more NH4+ from solution compared to soil L (Fig. 1a). The modeled sorption parameters μ and K in soil W were four and two times greater, respectively, than in soil L (Fig. 1a).
Figure 1. Ammonium sorption capacity (ACS) and potential N2O production (pN2O).
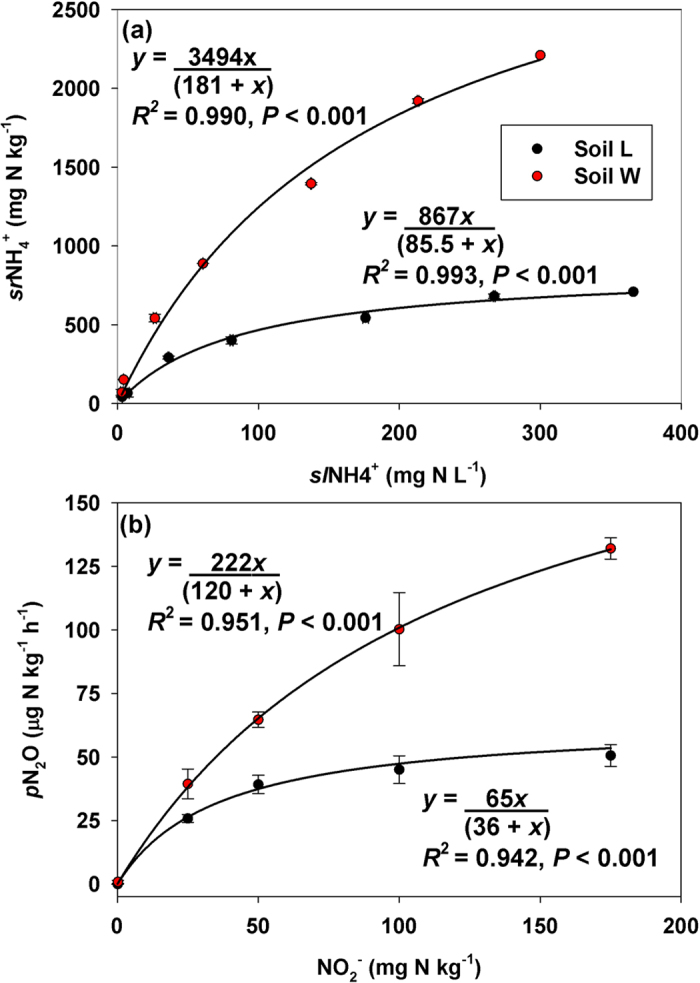
(a) ASC results showing equilibrium NH4+ concentrations in sorbed-phase (srNH4+) versus solution-phase (slNH4+) and (b) pN2O following addition of nitrite (NO2−) at 85% field capacity for soils L and W. Symbols are means with vertical standard error bars and lines are regression curves based on replicated data (in form of Eq. (1)). Horizontal error bars are displayed in (a) but are barely visible.
Nitrite addition experiments
Soil W produced more N2O after amendment with NO2− compared to soil L. The potential N2O production rate (pN2O) was well-described by models in the form of Eq. (1) with sorbed-phase NH4+ (srNH4+) replaced by potential N2O production rate (pN2O), and with solution phase NH4+ (slNH4+) replaced by NO2− concentration (Fig. 1b). The modeled μ and K parameters in soil W were each approximately three times greater than in soil L.
Microcosm experiments
Series 1 - Effect of BU addition rate at 85% of field capacity (FC)
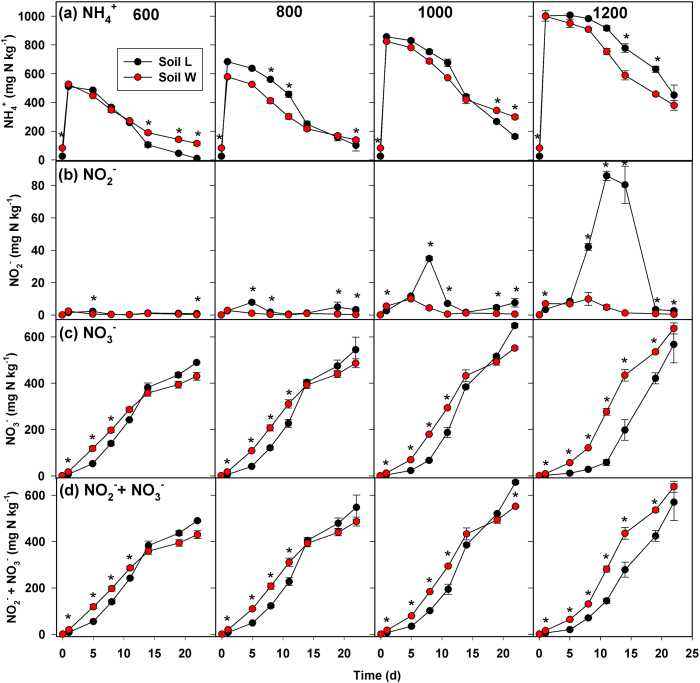
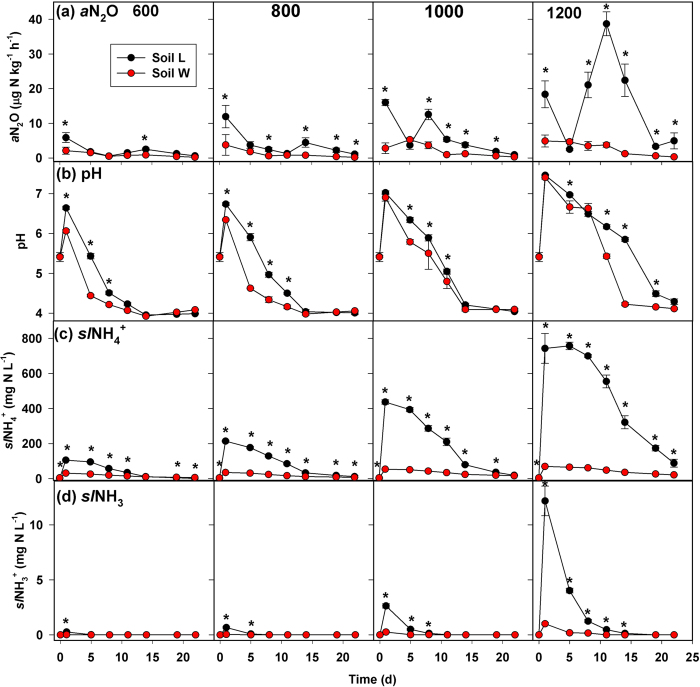
There were significant soil-by-BU addition rate-by-time interaction effects on all point-in-time (Figs 2, 3) and cumulative variables (Table 1). Most notably for point-in-time concentrations, soil L had greater NO2− and actual N2O production rate (aN2O) compared to W on at least two sampling dates at all N rates, and the frequency and magnitude of significant differences in NO2− and aN2O by soil increased with increasing BU addition (Figs 2b, 3a). Following BU addition, both soils showed a similar temporal pattern of increasing total extractable NH4+ (tNH4+) followed by decreasing tNH4+ (Fig. 2a). Differences in tNH4+ by soil were not observed until at least Day 8, and the timing and direction of significant differences varied by BU addition rate. Soil W had more nitrate (NO3−) and (NO2−+NO3−) present compared to soil L during at least the first 8 d, and the magnitude and duration of significant differences in NO3− by soil increased with increasing BU addition (Figs 2c–d). Soil L consistently produced more N2O on Day 1 compared to W. Soil pH, slNH4+ and slNH3 were frequently greater in soil L compared to W (Figs 3b–d). Most notably for cumulative indices (Table 1), soil L had consistently greater c-NO2−, c-slNH4+, c-NH3, and c-aN2O compared to W at all BU addition rates. During the first 5 d, soil W had greater NO2−+NO3− accumulation rate (NAR) compared to soil L at all BU addition rates, and both soils showed reduced NAR at N ≥ 1000 mg N kg−1 compared to N < 1000 mg N kg−1 (Table S1). During Days 5 through 11, significant differences in NAR by soil were only present at 1000 and 1200 mg N kg−1. During Days 11 through 19, soil L had greater NAR compared to W at all BU addition rates except 1200 mg N kg−1.
Figure 2. Results of Series 1 microcosm experiment.
Concentrations of extractable (a) NH4+, (b) NO2−, (c) NO3− and (d) NO2− + NO3− following addition of bovine urine at 600, 800, 1000 and 1200 mg N kg−1 at 85% field capacity. Asterisks indicate significant differences between soils at P < 0.05 for a given sampling date.
Figure 3. Results of Series 1 microcosm experiment (continued).
(a) Actual N2O production rate (aN2O), (b) pH, and theoretical concentrations of solution-phase (c) ammonium (slNH4+) and (d) ammonia (slNH3) following addition of bovine urine at 600, 800, 1000 and 1200 mg N kg−1 at 85% field capacity. Asterisks indicate significant differences between soils at P < 0.05 for a given sampling date.
Table 1. Cumulative indices for total extractable ammonium (c-tsNH4+), nitrite (c-NO2−), nitrate (c-NO3−), the sum of nitrite and nitrate (c- [NO2− + NO3 −]), actual N2O production rate (c-aN2O), acidity (c-H+) and solution-phase ammonium (c-slNH4+) and ammonia (c-slNH3) as affected by soil type and rate of bovine urine (BU) in Series 1 experiments.
| Soil | BU added (mg N kg−1) |
|||
|---|---|---|---|---|
| 600 | 800 | 1000 | 1200 | |
| c-tNH4+ (g N d kg−1) | ||||
| L | 5.48 D‡ | 8.78 C | 12.42 B | 18.05 A |
| W | 6.28 D | 7.21 C | 12.12 B | 15.61 A |
| P > |t| | <0.001§ | <0.001 | 0.216 | <0.001 |
| c-NO2− (mg N d kg−1) | ||||
| L | 23.95 D | 59.55 C | 209.37 B | 761.04 A |
| W | 15.43 B | 18.72 B | 72.64 A | 94.38 A |
| P > |t| | 0.005 | <0.001 | <0.001 | <0.001 |
| c-NO3− (g N d kg−1) | ||||
| L | 5.35 A | 5.53 A | 5.43 A | 3.64 B |
| W | 5.56 B | 6.03 AB | 6.22 AB | 6.25 A |
| P > |t| | 0.502 | 0.128 | 0.023 | <0.001 |
| c-(NO2− + NO3−) (g N d kg−1) | ||||
| L | 5.38 A | 5.59 A | 5.64 A | 4.40 B |
| W | 5.57 B | 6.05AB | 6.29 A | 6.35 A |
| P > |t| | 0.448 | 0.102 | 0.029 | <0.001 |
| c-H+ (mol H+ d kg−1) | ||||
| L | 0.315 A | 0.295 A | 0.256 B | 0.129 C |
| W | 0.267 A | 0.274 A | 0.243AB | 0.221 B |
| P > |t| | 0.010 | 0.217 | 0.433 | <0.001 |
| c-aN2O (mg N2O-N kg−1) | ||||
| L | 0.90 D | 1.72 C | 2.90 B | 8.08 A |
| W | 0.44 B | 0.52 B | 1.10 A | 1.29 A |
| P > |t| | 0.005 | <0.001 | <0.001 | <0.001 |
| c-slNH4+ (g N d kg−1) | ||||
| L | 0.94 D | 2.02 C | 4.45 B | 10.29 A |
| W | 0.35 C | 0.41 C | 0.74 B | 1.01 A |
| P > |t| | 0.020 | <0.001 | <0.001 | <0.001 |
| c-slNH3 (mg N d kg−1) | ||||
| L | 0.72 D | 1.99 C | 8.79 B | 50.26 A |
| W | 0.055 C | 0.11 C | 0.76 B | 3.70 A |
| P > |t| | <0.001 | <0.001 | <0.001 | <0.001 |
‡Within a row, means followed by the same letter are not significantly different at P ≤ 0.05.
§Significance of t test comparing the means from the two soils for a given rate of BU addition.
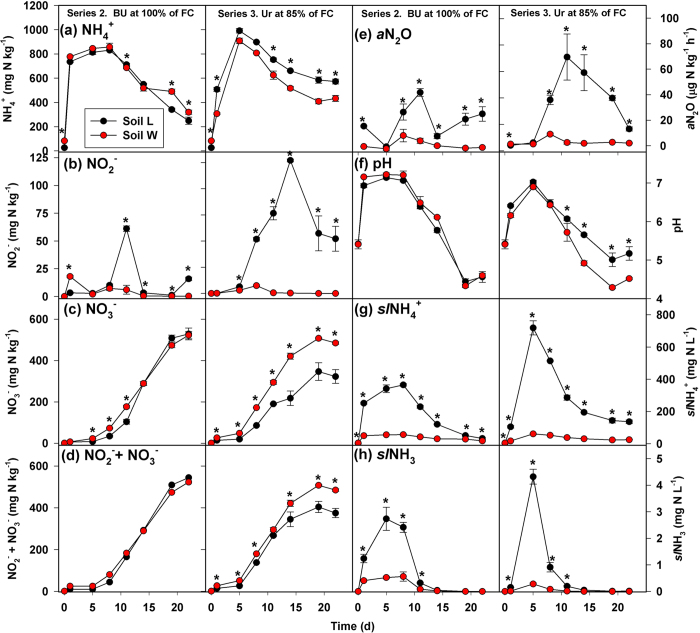
Series 2 - Effect of soil water content with 1000 mg N kg−1 of BU added
There were significant soil-by-water content-by-time interaction effects on point-in-time concentrations of tNH4+, NO2−, NO3−, aN2O, slNH4+, and slNH3. Differences by soil in the 100% of FC treatment were similar to differences by soil in the 85% of FC treatment with 1000 mg N kg−1, except for pH and NO2−+NO3− where no differences were observed (Fig. 4). For cumulative indices, there were significant soil-by-water content-by-time interaction effects on c-tNH4+, c-(NO2− + NO3−), c-slNH4+ and c-aN2O (Table S2). Differences in c-aN2O by soil were similar at 85% and 100% of FC, but both soils had greater c-aN2O at 100% compared to 85% of FC. Across both soils, c-NO2− and c-slNH3 were greater at 100% compared to 85% of FC, while c-NO3- and c-H+ were greater at 85% compared to 100% of FC (Table S3). Across both water content treatments, c-NO2− was greater in soil L compared to W.
Figure 4. Results of Series 2 and 3 microcosm experiments.
Concentrations of extractable (a) NH4+, (b) NO2−, (c) NO3− and (d) NO2− + NO3−, (e) actual N2O production rate (aN2O), (f) pH and theoretical concentrations of solution-phase (g) ammonium (slNH4+) and (h) ammonia (slNH3) in Series 2 (left-hand plates for each variable) and Series 3 (right-hand plates). Series 2 used bovine urine (BU) at 1000 mg N kg−1 with soils at 100% of field capacity (FC), and Series 3 used urea (Ur) at 1000 mg N kg−1 with soils at 85% of FC. Asterisks indicate significant differences between soils at P < 0.05 for a given sampling date.
Series 3- Effect of BU versus Ur added at 1000 ug N g−1 at 85% of FC
There were significant soil-by-N source-by-time interaction effects on all point-in-time variables, and differences by soil following Ur addition were generally consistent with differences following BU addition at 1000 mg N kg−1 (Fig. 4). However, differences by soil tended to be more consistent and/or to persist longer with Ur compared to BU. Also, tNH4+ and pH each took longer to reach their maximum values and did not decrease as rapidly with Ur compared to BU. Cumulative tNH4+, c-N2O, and c-slNH3 were greater with Ur, while c-H+ was lower with Ur, compared with BU, in both soils (Table S4). Cumulative NO2− was more than five times greater with Ur compared to BU for soil L.
Gene copies of bacterial ammonia monooxygenase (amoA-b) associated with AOB were greater in soil L compared to W, but increased over time in a similar manner in both soils until Day 14, after which the abundances did not change (Fig. 5). There was a significant soil-by-time interaction effect on gene copies of archaeal ammonia monooxygenase (amoA-a), but a difference by soil was observed only on the final sampling date (Fig. 5b). In both soils, amoA-a numbers did not change until after Day 14, corresponding to the cessation of any increases in amoA-b. Most notable was a significant soil-by-time interaction effect on gene copies of bacterial nitrite oxidoreductase (nxrA) associated with NOB (P < 0.001), which were initially lower in soil W than L, and subsequently increased in soil W by a factor of 60 while remaining static in soil L (Fig. 5c).
Figure 5. Gene copy abundances in Series 3 microcosm experiment.

(a) amoA-b, (a) amoA-a, and (c) nxrA following addition of Ur at 1000 mg N kg−1 soil with soils at 85% of FC. Asterisks indicate significant differences between soils at P < 0.05. Normalized gene abundances are expressed relative to the number of copies of prokaryotic (bacteria + archaea) 16S rRNA genes in each sample47.
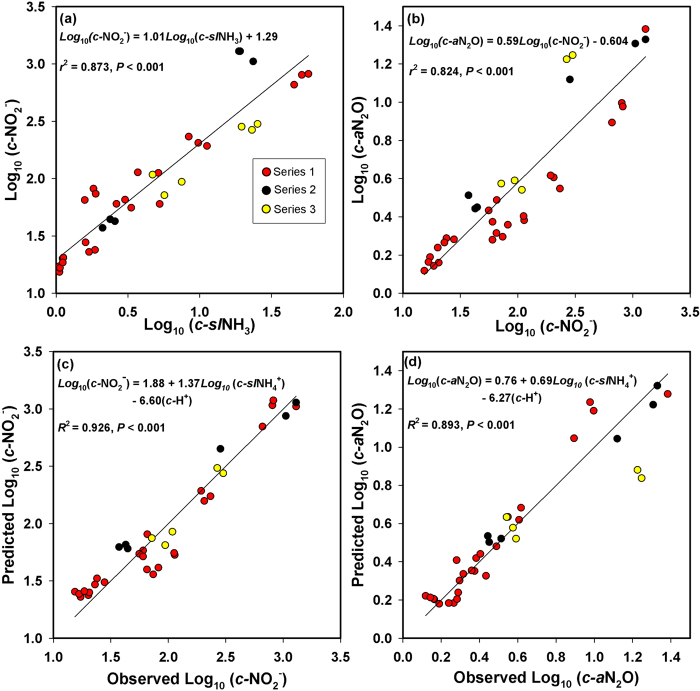
Correlation and regression analyses
Soil NO2− was positively correlated with tNH4+, slNH4+ and slNH3, and c-NO2− was positively correlated with c-tNH4+, c-slNH4+ and c-slNH3 (Table S5). Across all experiments (Series 1-3), c-NO2− was most strongly correlated with c-slNH3, which explained 87% of the total variance in c-NO2− (Fig. 6a). Soil NO2− was also positively correlated with aN2O, and c-NO2− was positively correlated with c-aN2O. Across all experiments, c-NO2− explained 82% of the total variance in c-aN2O (Fig. 6b). Soil NO3− and (NO2−+NO3−) tended to be negatively correlated with N2O (Table S5). Multiple regression models with c-slNH4+ and c-H+ as independent variables explained 93 and 89% of the variance in c-NO2− and c-aN2O, respectively (Figs 5c,d).
Figure 6. Regression results.
Single-factor regression models of (a) cumulative nitrite (c-NO2−) versus cumulative solution-phase ammonia (c-slNH3) and (b) cumulative actual N2O production (c-aN2O) versus c-NO2− with regression lines, and multiple regression models describing (c) c-NO2− and (d) c-aN2O as functions of cumulative solution-phase ammonium (c-slNH4+) and cumulative acidity (c-H+) with 1:1 lines, for all microcosm data (Series 1–3).
Discussion
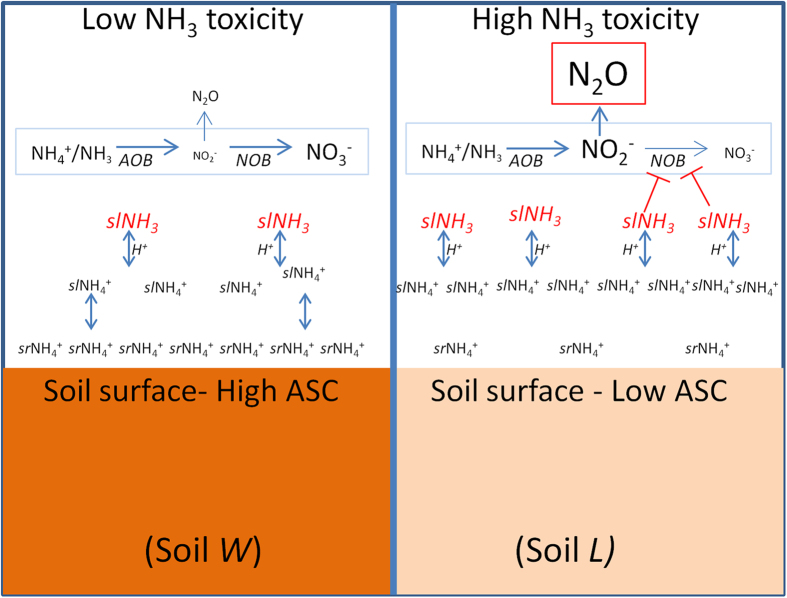
This is the first study to correlate the dynamics of slNH3, NO2−, N2O and nitrifier genes in incubating soil. The strong relationship (r2 = 0.87) between c-slNH3 and c-NO2− suggests that NH3 toxicity acting more strongly on NOB than AOB, and more strongly in soil L than in soil W, was responsible for the contrasting NO2− and N2O dynamics in the two soils11,12,13,14. The greater N2O production in soil L appeared to be driven by a greater NO2− accumulation which in turn resulted from greater slNH3 accumulation due to its lower ASC (Fig. 7). This explanation is further supported by the static nxrA gene copies in soil L in contrast to substantial increases in nxrA observed in soil W.
Figure 7. Conceptual schematic.
Decreased ammonium (NH4+) sorption capacity (ASC) results in increased ratio between solution-phase (sl) and sorbed-phase (sr) NH4+, which increases the potential formation of free ammonia (slNH3). When slNH3 differentially inhibits nitrite (NO2−) oxidizing bacteria (NOB) to a greater extent than NH3 ammonia-oxidizing bacteria (AOB), NO2− accumulates, leading to increased NO2−-driven N2O production in the low ASC soil.
Differences in ASC were related to differences in cation exchange capacity (CEC); i.e., the ratio (W:L) of Kd values in the two soils was 1.91 which was nearly identical to the ratio of their CEC values (1.93). Thus, while soil L and W had similar organic C and clay contents, differences in ASC were likely due to differences in chemical composition of soil organic matter and/or mineralogical composition of clay which control CEC21. It is not likely that a difference in the capacity of the two soils to fix N in clay minerals was an important factor; because NH4+ fixed by clay is not readily extracted by 2 M KCl nor is it readily available to microbes, such an effect would have been evident in differences by soil in tNH4+ that were not associated with differences in NAR and/or urea hydrolysis rates22.
Our calculations of slNH3 concentrations are theoretical approximations. Because soil pH is by its nature operationally defined, any subsequent calculations are also operationally defined; e.g., a different range of absolute NH3 values would have resulted if a different pH solvent were used23, although slNH3 levels based on a single solvent provide a basis for relative comparison. Our methods assumed that the NH4+-sorption equilibria in the isotherm experiments also described the solid-liquid NH4+ partitioning in the microcosm experiments. Nonetheless, it is interesting that the maximum slNH3 concentrations observed in soil L (approximately 0.5 to 10 mg N L−1) were within the range observed to inhibit NOB and cause NO2− accumulation in nitrifying wastewater systems14. Apart from any theoretical slNH3 calculations, the multiple regression model (Fig. 6c) is further suggestive of NH3 toxicity; that is, increased slNH4+ combined with reduced acidity (which together are the ‘raw ingredients’ for NH3 formation) explained 93% of the variance in c-NO2−.
The slNH3 levels reached maximum values the day after N addition, but NO2− did not reach maxima until at least Day 5 and generally remained elevated for longer than slNH3. These results are reflected in stronger correlations between c-NH3 and c-NO2− compared with correlations between point-in-time concentrations. This result could have been due to residual inhibitory effects of slNH3 on NOB that persisted even after slNH3 had subsided. It also is possible that once NO2− started to accumulate, NO2− itself (or its protonated form [HNO2]) became a source of toxicity to NOB. Kinetic models that account for NH3 and NO2−/HNO2 as separate sources of toxicity affecting AOB and NOB have been developed for wastewater systems14.
Previous studies have found a significant correlation between NO2− and N2O dynamics9,11,24. The NO2− molecule is an immediate precursor substrate for N2O produced via nitrifier-denitrification19 and chemo-denitrification20,25, and there is evidence that N2O can be produced via reaction of NO2− with hydroxylamine (NH2OH)26. Previous studies have also found significant differences among soils in pN2O following amendment with NO2−,20,27. The current results show that pN2O is not necessarily a reliable indicator of actual N2O production (aN2O) following amendment of soil with BU or Ur; i.e., although soil W had greater pN2O after being artificially amended with NO2−, soil L had greater aN2O because it accumulated more NO2− biologically than soil W following amendment with BU or Ur.
These experiments were not designed to precisely distinguish among all potential pathways of N2O production, e.g. nitrification, nitrifier-denitrification, heterotrophic denitrification, or chemodenitrification19,20,28,29. The experiments were designed to maintain aerobic conditions, and therefore the influence of denitrification of NO3− as an N2O source was expected to be minimal. This was supported by the lack of positive correlation between aN2O and soil NO3− levels; in fact, NO3− was negatively correlated with aN2O in many cases. A previous study20 found that headspace O2 levels <5% were required for N2O production to proceed in NO3−-amended soil; in contrast, NO2−-amended soil readily produced N2O at ambient O2 and displayed increasing N2O per unit NO2− as O2 decreased below 20%. Similar findings have been shown in culture studies examining nitrifier-denitrification30. Thus, in the current study, greater N2O production at greater water content was likely due to N2O derived from nitrifier-denitrification. At 100% of FC, nitrification proceeded more slowly than at 85% of FC, possibly due decreased O2 availability to support NH4+ oxidation. Because tNH4+ and pH did not decrease as quickly (owing to slower nitrification-induced H+ production), this resulted in greater slNH3, which in turn could have caused the greater NO2− at 100% of FC compared to 85% of FC. Thus, greater availability of NO2−, as well as increased potential for nitrifier-denitrification to produce N2O with reduced O2, likely enhanced N2O at 100% of FC.
Both soils responded differently to Ur compared to BU. With BU, it took 1 d for tNH4+ and pH to reach their maximum values compared to 5 d with Ur; this was likely due to compounds such as hippuric acid present in BU which accelerate Ur hydrolysis31. Soil pH also remained elevated for a longer period with Ur (again, indicating slower nitrification-induced H+ production), which resulted in a doubling of c-slNH3 compared to BU. In soil L, this resulted in a 4- to 5-fold increase in NO2− and a 7-fold increase in c-N2O. These results highlight the interactions involving several processes and substrates following BU and Ur addition that can regulate N2O production.
On the first day following addition of BU to soil L (in Series 1 and 2), N2O production was elevated without a corresponding increase in soil NO2− or NO3−, and then declined on Day 3. This result was not observed with Ur, nor was it observed with soil W. Further research would be needed to explain this result, but it is possible that any NO2− produced during the initial onset of nitrification was consumed in the N2O-producing reactions and therefore was not measurable. Urine addition could have stimulated ‘co-denitrification’ reactions32, or alternatively, N2O may have been produced from reactions involving NH2OH produced during AOB activity28.
While NH3 is the main substrate utilized by AOB33, at higher levels, NH3 can itself inhibit AOB activity. Decreasing NAR with increasing slNH3 was observed in a grassland soil amended with BU and attributed to NH3 toxicity effects on AOB34. Similar results were found for NAR in Series 1 during Days 0–5. The qPCR data from Series 3 indicate that any NH3 toxicity effects on AOB were not large enough to inhibit amoA-b genes from increasing during Days 0–14. The amoA data also are consistent with results in New Zealand grasslands soils35, where amoA-b genes increased following N addition but amoA-a genes did not. Here, we found an increase in amoA-a numbers occurred only after amoA-b gene copies stopped increasing. This could indicate that amoA-b growth was inhibited after Day 14 by increasing acidity36 or NO2−/HNO214 which might have favored amoA-a activity.
These results have implications for increased fundamental understanding as well as pointing in new directions for modeling and mitigating N2O emissions. The current experiments were aimed at studying processes occurring in localized zones receiving concentrated N inputs representative of potential N2O production hot spots. Under field conditions, these processes may be further modified by water infiltration or evaporation, temperature dynamics and other factors. Nonetheless, this study shows that soils having largely similar physical and chemical properties but differing in ASC can display dramatically different potentials for NH3 toxicity, NO2− accumulation and N2O production. A previous study showed effects of altering CEC on nitrification dynamics15, but effects on NO2− or N2O dynamics have not been considered. Further research comparing soils differing in ASC, and examining the effects of altering soil CEC, should be performed under both lab and field conditions to further investigate these variables as modeling parameters and potential N2O mitigation factors.
Methods
Soils
Soil ‘L’ was collected from a research field at Lincoln University, New Zealand (43.648 S; 172.454 E) that had been under pasture (Lolium perenne L.) for at least 5 yr. Soil ‘W’ was collected from a farmer’s field east of Waikari, New Zealand (42.964 S; 172.629 E) planted to alfalfa (Medicago sativa L.) and rotationally grazed by sheep (Ovis aries). Soils were collected from the upper 0.10 m and allowed to dry at 25 °C, and then ground and sieved (2 mm). Soils L and W were both classified as silt loams and had similar clay content (116 and 130 g kg−1, respectively), organic C (26, 33 g kg−1), C/N ratio (11.2, 10.6), and pH in H2O (6.3, 6.1), but differing CEC (14 and 27 cmolc kg−1) (Table S6).
Ammonium sorption capacity and solution-phase concentrations
Ammonium sorption isotherms were obtained using a batch equilibrium method37. Solutions (15 mL) containing NH4+ as NH4Cl (5, 10, 50, 100, 200, 300 and 400 mg NH4+-N L−1) were added to 50-mL polyethylene tubes containing 0.75 g soil. Three replicate tubes of each NH4+ concentration were equilibrated on a reciprocating shaker for 18 h at 100 rpm. Mixtures were filtered (Whatman 42) and the filtrate analyzed for NH4+ 38 with a flow-injection analyzer (FIA) (Lachat QuikChem 8500 or Alpkem FS3000). The amount of srNH4+ was calculated from the difference in slNH4+ at the beginning and end of equilibration and accounting for the initial 2 M KCl-extractable NH4+ content of the soil. The resulting data were not consistent (R2 < 0.6) with commonly used models39 but were well-described (R2 ≥ 0.99) by models of the form
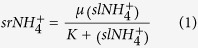 |
where μ (mg N kg−1) is the maximum sorption capacity and K (mg N L−1) is the slNH4+ concentration at which srNH4+ equals one-half of μ. Eq. (1) is similar in form to the Langmuir model and has a ‘linear portion’ for the case where slNH4+<< K, such that srNH4+=(slNH4+) where Kd = μ/K is the slope with units (L kg−1). Eq. (1) was used to calculate theoretical concentrations of slNH4+ and slNH3 by expressing the total extractable ammonium (tNH4+, mg N kg−1) under equilibrium conditions as follows:
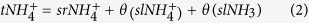 |
where θ is the soil water content (L kg−1). Eq. (1) was used to replace srNH4+ in Eq. (2), and slNH3 in Eq. (2) was replaced by:
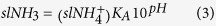 |
where KA is the acid dissociation constant (9.245) for the NH4+-NH3 acid-base pair at 25 °C40. This resulted in the quadratic equation:
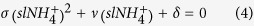 |
where σ, ν and δ are constants containing the parameters μ, K, θ, KA, tNH4+ and 10pH. For each sampling event, measured values of tNH4+ and pH were substituted into the constant terms, Eq. (4) was solved for slNH4+ using the quadratic formula, and slNH3 was calculated using Eq. (3) Example calculations are provided as Supplementary Information (SI). The above procedure was compared to another method where θ(slNH3) was omitted from Eq. (2), and the results agreed within <1%. Thus, assumptions regarding whether slNH3 was captured in the tNH4+ analysis affected the results to a negligible extent.
Nitrite-addition experiments
The potential for each soil to produce N2O when amended with NO2− was determined20. Soil was amended with solutions containing KNO2 to achieve concentrations of 0, 25, 50, 100 and 175 mg NO2−-N kg−1 at a water content equivalent to 85% of FC. Solutions were added to 10.0 g of air-dried soil in ‘wide-mouth’ 250 mL glass jars (69 mm diameter by 65 mm) and homogenized with a spatula. Jars were sealed with septum-equipped caps and incubated for 1 h at 25 °C with sampling of the headspace at 0, 30 and 60 min. Gas samples were immediately transferred to evacuated glass vials which were analyzed for N2O with a gas chromatograph (8610, SRI Instruments, CA) equipped with an electron capture detector and interfaced to an autosampler (Gilson 222XL, Middleton, WI)41. The rate of increase in headspace N2O concentration, headspace volume and soil mass were used to calculate pN2O20.
Microcosm experiments
Three series of microcosm experiments were conducted. Series 1 used each soil amended with four rates of BU equivalent to 600, 800, 1000 and 1200 mg N kg−1 at a water content equivalent to 85% of FC. Series 2 used each soil amended with BU at 1000 mg N kg−1 at 100% of FC. Series 3 used each soil amended with Ur at 1000 mg N kg−1 at 85% of FC. At the start of the experiment, 21 replicate 250-mL glass jars of each treatment were established by adding solutions by pipette to 10.0 g of dry soil and homogenizing with a spatula such that the wetted soil occupied a thin layer (~3 mm) in the bottom of the jar. Three replicate jars of each treatment were sacrificed for destructive analysis after 1, 5, 8, 11, 14, 19 and 22 d. An additional three jars containing soil amended with deionized H2O were used to represent ‘Day 0’. Jars were incubated in the dark at 25 °C. On each sampling day, three randomly selected jars of each treatment were opened for 5 min to equilibrate the jar headspace with lab air and then sealed with caps, equipped with rubber septa, for 1 h. The jar headspace was sampled at 0, 30 and 60 min by syringe and aN2O was determined using methods described above. Nitrous oxide measured in the microcosm experiments is referred to as ‘actual’ N2O production rate (aN2O) to distinguish from pN2O. Immediately following gas sampling, approximately one-half of the soil mass in each jar was gravimetrically transferred to a polyethylene tube and extracted in 40 mL of 2 M KCl for 1 h. The extracts were filtered and stored at 4 °C until determination of tNH4+,38. Subsamples of the extract also were used to determine soil pH. Soil remaining in each jar was amended with 40 mL of a separate 2 M KCl solution and extracted for 10 min and then filtered for determination of NO2− and the sum of NO2−+NO3−. The pH of the 2M KCl used for NO2− and NO2−+NO3− extraction was adjusted so that, during extraction, the pH of the soil-solution mixture was ≥ 8.542. The NO2− analysis was performed within 3 h with a spectrophotometer (Shimadzu UV mini–1240)38. The NO2−+NO3− analysis was conducted within 24 h using a FIA preceded by Cd-reduction of NO3− to NO2−, and NO3− was determined by difference38.
The microcosms were designed to maintain aerobic conditions with minimal need for aeration due to the high ratio of headspace volume to soil mass and the high ratio of jar diameter to volume. The jars were opened for 10 min on Days 1, 8 and 15. This procedure minimized evaporative moisture losses while maintaining headspace O2 above 18% as determined by gas chromatographic analysis with a thermal conductivity detector. Opening of the jars on Day 1 also allowed release of CO2 produced during hydrolysis of Ur. Field capacities (0.35 and 0.45 kg H2O kg−1 for soil L and W, respectively) were determined by incremental water addition until free water was observed. Bovine urine was collected from the Lincoln University dairy farm where cows were grazing perennial ryegrass (Lolium perenne L.)/white clover (Trifolium repens L.). Urine was kept frozen until the day prior to setting up the experiment, at which time the urine was thawed and analyzed for total N content. Appropriate volumes of BU and H2O were added to soil in each jar to achieve target N concentrations and water contents. Because these grazed soils are commonly dry at the surface for days at a time prior to receiving urine deposition, we did not add water or pre-incubate the soils prior to amendment.
Quantitative polymerase chain reaction (qPCR)
For Series 3, additional soil sub-samples were collected for DNA isolation and quantification of nitrifier gene abundances. On Days 0, 5, 8, 11, 14, 19, 22, 25 and 28, sub-samples (0.25 g) were extracted using a PowerLyzer PowerSoil DNA isolation Kit (MoBio, Carlsbad, CA) in accordance with manufacturer recommendations except for the final washing step which was performed twice rather than once. Abundances of 16S ribosomal RNA (16S rRNA), amoA-b, amoA-a and nxrA were determined using appropriate primers43,44,45,46. Abundances of amoA-b, amoA-a and nxrA were normalized to recovered 16S rRNA abundances47. Additional details are provided as SI.
Data analysis
Concentrations of all N species and production of N2O are expressed on a dry weight soil basis. Chemical concentrations and pH determined at individual times were used to calculate cumulative ‘exposure’ indices using trapezoidal integration of concentration versus time data7,9,48. We use a ‘c-’ prefix to distinguish cumulative variables (e.g. c-NO2−) from point-in-time concentrations (e.g. NO2−). For cumulative acidity (c-H+), pH was first converted to theoretical hydrogen ion concentration using H+ = 10−pH prior to integration. Integration of aN2O versus time also was performed, but in this case the resulting variable (c-aN2O) represents cumulative N2O production. The NAR was calculated over different time intervals from the difference in NO2−+NO3− concentration divided by elapsed time. Three sets of data from the microcosm experiments were analyzed independently. The first set included all data from Series 1, the second set included data from Series 2 plus the 1000 mg N kg−1 treatment from Series 1 (to examine water content effects) and the third set included data from Series 3 plus the 1000 mg N kg−1 treatment from Series 1 (to examine N source effects). Each set was analyzed as a completely randomized design at P ≤ 0.05 using the MIXED procedure of SAS [Version 9.2, SAS Institute, Cary, NC] with time as a repeated measurement. Additional details are provided as SI.
Additional Information
How to cite this article: Venterea, R. T. et al. Ammonium sorption and ammonia inhibition of nitrite-oxidizing bacteria explain contrasting soil N2O production. Sci. Rep. 5, 12153; doi: 10.1038/srep12153 (2015).
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the assistance of S. Mitchell, P. Wang and M. Sadowsky in establishing the PCR methods, and S. Khan, S. Mitchell, K. Sidla and M. Dolan in assisting with laboratory analysis. This work was supported by a Global Research Alliance Senior Scientist (GRASS) fellowship to R. Venterea and T. Clough from the Livestock Emissions and Abatement Research Network.
Footnotes
Author Contributions R.V. and T.C. designed and conducted the experiments. J.C. performed the statistical analyses. F.B-S. conducted the qPCR analyses. R.V. wrote the main manuscript. All authors reviewed and contributed to writing specific sections of the manuscript.
References
- Del Grosso S. J. et al. Global scale DAYCENT model analysis of greenhouse gas emissions and mitigation strategies for cropped soils. Global Planet. Change. 67, 44–50 (2009). [Google Scholar]
- Vogeler I., Giltrap D. & Cichota R. Comparison of APSIM and DNDC simulations of nitrogen transformations and N2O emissions. Sci. Total Environ. 465, 147–155 (2013). [DOI] [PubMed] [Google Scholar]
- Haynes R. J. & Williams P. H. Nutrient cycling and soil fertility in the grazed pasture ecosystem. Adv. Agron. 49, 119–199 (1993). [Google Scholar]
- Flessa H., Dorsch P., Beese F., Konig H. & Bouwman A. F. Influence of cattle wastes on nitrous oxide and methane fluxes in pasture land. J. Environ. Qual. 6, 1366–1370 (1996). [Google Scholar]
- Wetselaar R., Passioura J. B. & Singh B. R. Consequences of banding nitrogen fertilizers in soil. i. Effects on nitrification. Plant Soil. 36, 159–175 (1972). [Google Scholar]
- Singh Yadvinder & Beauchamp E. G. Nitrogen transformations near urea in soil: effects of nitrification inhibition, nitrifier activity and liming. Fert. Res. 18, 201–212 (1989). [Google Scholar]
- Engel R., Liang D. L., Wallander R. & Bembenek A. Influence of urea fertilizer placement on nitrous oxide production from a silt loam soil. J. Environ. Qual. 39, 115–125 (2010). [DOI] [PubMed] [Google Scholar]
- Halvorson A. D. & Del Grosso S. J. Nitrogen placement and source effects on nitrous oxide emissions and yields of irrigated corn. J. Environ. Qual. 42, 312–322 (2013). [DOI] [PubMed] [Google Scholar]
- Maharjan B. & Venterea R. T. Nitrite intensity explains N management effects on N2O emissions in maize. Soil Biol. Biochem. 66, 229–238 (2013). [Google Scholar]
- Khan S., Clough T. J., Goh K. M. & Sherlock R. R. Nitric and nitrous oxide fluxes following bovine urine deposition to summer-grazed pasture. New Zealand J. Agri. Res. 57, 136–147 (2014). [Google Scholar]
- Ma L., Shan J. & Yan X. Nitrite behavior accounts for the nitrous oxide peaks following fertilization in a fluvo-aquic soil. Biol. Fert. Soils. 10.1007/s00374-015-1001-8 (2015). [DOI] [Google Scholar]
- Van Cleemput O. & Samatar A. H. Nitrite in soils: accumulation and role in the formation of gaseous N compounds. Fert. Res. 45, 81–89 (1996). [Google Scholar]
- Anthonisen A. C., Loehr R. C., Prakasam T. B. S. & Srinath E. G. Inhibition of nitrification by ammonia and nitrous acid. J. Water Poll. Control Fed. 48, 835–852 (1976). [PubMed] [Google Scholar]
- Park S. & Bae W. Modeling kinetics of ammonium oxidation and nitrite oxidation under simultaneous inhibition by free ammonia and free nitrous acid. Process Biochem. 44, 631–640 (2009). [Google Scholar]
- Jiang X., Ma Y., Yuan J., Wright A. L. & Li H. Soil particle surface electrochemical property effects on abundance of ammonia-oxidizing bacteria and ammonia-oxidizing archaea, NH4+ activity, and net nitrification in an acid soil. Soil Biol. Biochem. 43, 2215–2221 (2011). [Google Scholar]
- Wang B., Zhao J., Guo Z., Ma J., Xu H. & Jia Z. Differential contributions of ammonia oxidizers and nitrite oxidizers to nitrification in four paddy soils. ISME J. 10.1038/ismej.2014.194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke X., Angel R., Lu Y. & Conrad R. Niche differentiation of ammonia oxidizers and nitrite oxidizers in rice paddy soil. Environ. Microbiol. 15, 2275–2292 (2013). [DOI] [PubMed] [Google Scholar]
- Ollivier J. et al. Effects of repeated application of sulfadiazine-contaminated pig manure on the abundance and diversity of ammonia and nitrite oxidizers in the root-rhizosphere complex of pasture plants under field conditions. Front. Microbiol. 4, 22 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrage N., Velthof G. L., van Beusichem M. L. & Oenema O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem. 33, 1723–1732 (2001). [Google Scholar]
- Venterea R. T. Nitrite-driven nitrous oxide production under aerobic soil conditions: Kinetics and biochemical controls. Global Change Biol. 13, 1798–1809 (2007). [Google Scholar]
- McBride M. in Environmental chemistry of soils, Ch. 3, 63–120 (Oxford University Press, 1994). [Google Scholar]
- Kissel D. E., Cabrerra M. L. & Paramasivam S. Ammonium, ammonia, and urea reactions in Soils in Nitrogen in agricultural systems, (eds Schepers J. S. & Raun W. R.) Ch. 4, 101–155 (American Society of Agronomy, 2008). [Google Scholar]
- Sumner M. Measurement of soil pH: Problems and solutions. Commun. Soil Sci. Plan. 25, 859–879 (1994). [Google Scholar]
- Venterea R. T. & Rolston D. Nitric and nitrous oxide emissions following fertilizer application to agricultural soil: Biotic and abiotic mechanisms and kinetics. J. Geophys. Res-Atm. 105, 15117–15129 (2000). [Google Scholar]
- Stevenson F., Harrison R., Wetselaar R. & Leeper R. Nitrosation of soil organic matter: II. Nature of gases produced by reaction of nitrite with lignins, humic substances and phenolic constituents under neutral and slightly acidic conditions. Soil Sci. Soc. Amer. Proc. 34, 430–435 (1970). [Google Scholar]
- Minami K. & Fukushi S. Emission of nitrous oxide from a well-aerated Andosol treated with nitrite and hydroxylamine. Soil Sci. Plant Nutr. 32, 233–237 (1986). [Google Scholar]
- Venterea R. T. & Rolston D. E. Mechanisms and kinetics of nitric and nitrous oxide production during nitrification in agricultural soil. Global Change Biol. 6, 303–316 (2000). [Google Scholar]
- Huang T. et al. Ammonia-oxidation as an engine to generate nitrous oxide in an intensively managed calcareous Fluvo-aquic soil. Sci. Reports. 4, 3950. 10.1038/srep03950 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Burger M., Doaneb T. A. & Howarth W. R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. P. Natl. Acad. Sci. USA 110, 6328–6333 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goreau T. J. et al. Production of NO2− and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl. Environ. Microbiol. 40, 526–32 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead D. C., Lockyer D. R. & Raistrick N. Volatilization of ammonia from urea applied to soil: Influence of hippuric acid and other constituents of livestock urine. Soil Biol. Biochem. 21, 803–808 (1989). [Google Scholar]
- Spott O., Russow R. & Stange C. F. Formation of hybrid N2O and hybrid N2 due to codenitrification: First review of a barely considered process of microbially mediated N-nitrosation. Soil Biol Biochem. 43, 1995–2011 (2011). [Google Scholar]
- Suzuki I., Dular U. & Kwok S. C. 1974. Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J. Bacteriol. 120, 556–558 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan R. M. & Barraclough D. Some chemical and physical factors affecting the rate and dynamics of nitrification in urine-affected soil. Plant Soil. 143, 11–18 (1992). [Google Scholar]
- Di H. J., Cameron K. C., Shen J. P., Winefield C. S., O’Callaghan M. S. B. & He J. Z. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geoscience. 2, 621–624 (2009). [Google Scholar]
- Gubry-Rangin C., Nicol G. W. & Prosser J. I. Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol. Ecol. 74, 566–574 (2010). [DOI] [PubMed] [Google Scholar]
- Zhao W. et al. Adsorption and desorption characteristics of ammonium in eight loams irrigated with reclaimed wastewater from intensive hogpen. Environ. Earth Sci. 69, 41–49 (2013). [Google Scholar]
- Mulvaney R. L. Nitrogen-Inorganic forms in Methods of soil analysis Part 3-Chemical Methods, (ed. Sparks D. L.) Ch. 38, 1123–1184 (American Society of Agronomy, 1996).
- Vogeler I., Cichota R., Snow V. O., Dutton T. & Daly B. Pedotransfer functions for estimating ammonium adsorption in soils. Soil Sci. Soc. Am. J. 75, 324–331 (2011). [Google Scholar]
- Bates R. G. & Pinching G. D. Acidic dissociation constant of ammonium ion at 0° to 500o C and the base strength of ammonia. Res. Paper RP1982. J. Res. Natl. Bur. Standards. U.S. Dep. Commerce, Natl. Bureau Standards. 42, 419–430 (1949). [Google Scholar]
- Clough T. J., Kelliher F. M., Wang Y. P. & Sherlock R. R. Diffusion of 15N-labelled N2O into soil columns: a promising method to examine fate of N2O in subsoils. Soil Biol. Biochem. 38, 1462–1468 (2006). [Google Scholar]
- Stevens R. & Laughlin R. Nitrite transformations during soil extraction with potassium chloride. Soil Sci. Soc. Am. J. 59, 933–938 (1995). [Google Scholar]
- Caporaso J. G. et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. A., Roberts K. J., Beman J. M., Santoro A. E. & Oakley B. B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. P. Natl. Acad. Sci. USA 102, 14683–14688 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotthauwe J. H., Witzel K. P. & Liesack W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 63, 4704–4712 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz S., Poly F., Le Roux X. & Degrange V. Development and application of a PCR-denaturing gradient gel electrophoresis tool to study the diversity of Nitrobacter-like nxrA sequences in soil. FEMS Microbiol. Ecol. 63, 261 (2008). [DOI] [PubMed] [Google Scholar]
- Caffrey J. M., Bano N., Kalanetra K. & Hollibaugh J. T. Ammonia oxidation and ammonia-oxidizing bacteria and archaea from estuaries with differing histories of hypoxia. ISME J. 1, 660–662 (2007). [DOI] [PubMed] [Google Scholar]
- Burton D. L., Li X. & Grant C. A. Influence of fertilizer nitrogen source and management practice on N2O emissions from two Black Chernozemic soils. Can. J. Soil Sci. 88, 219–227 (2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.