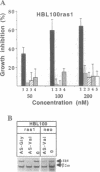
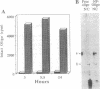
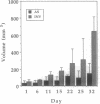
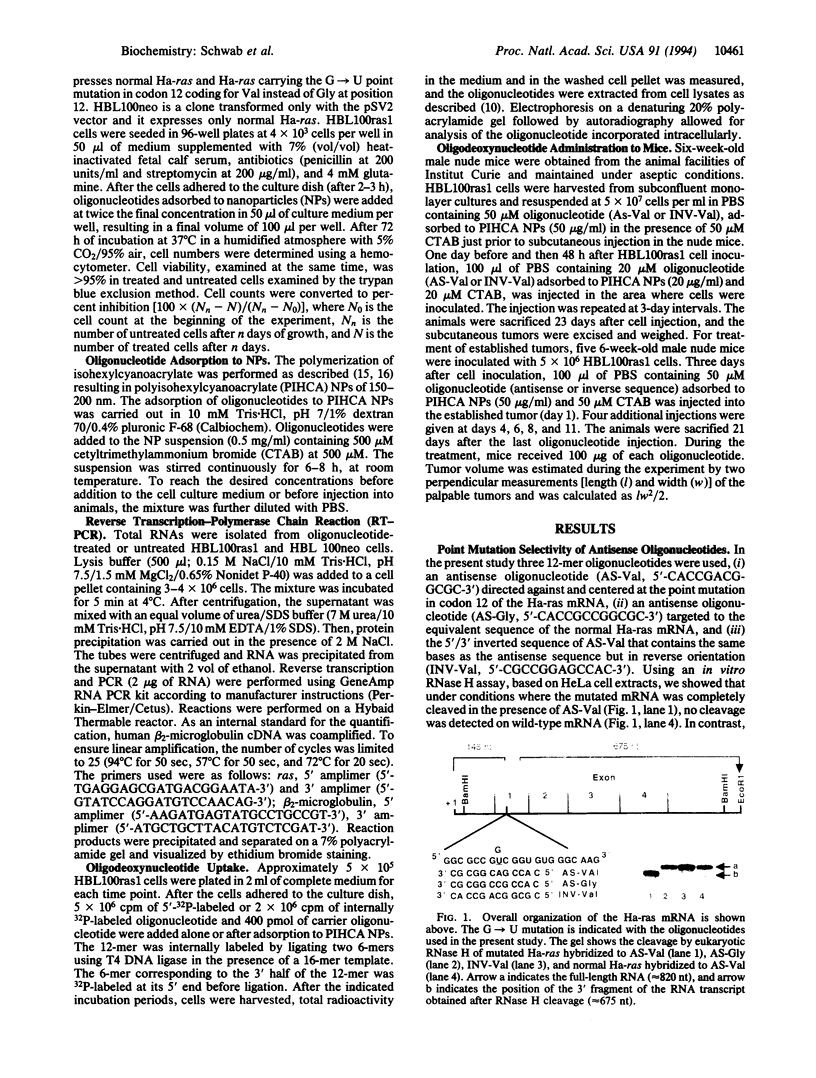
Abstract
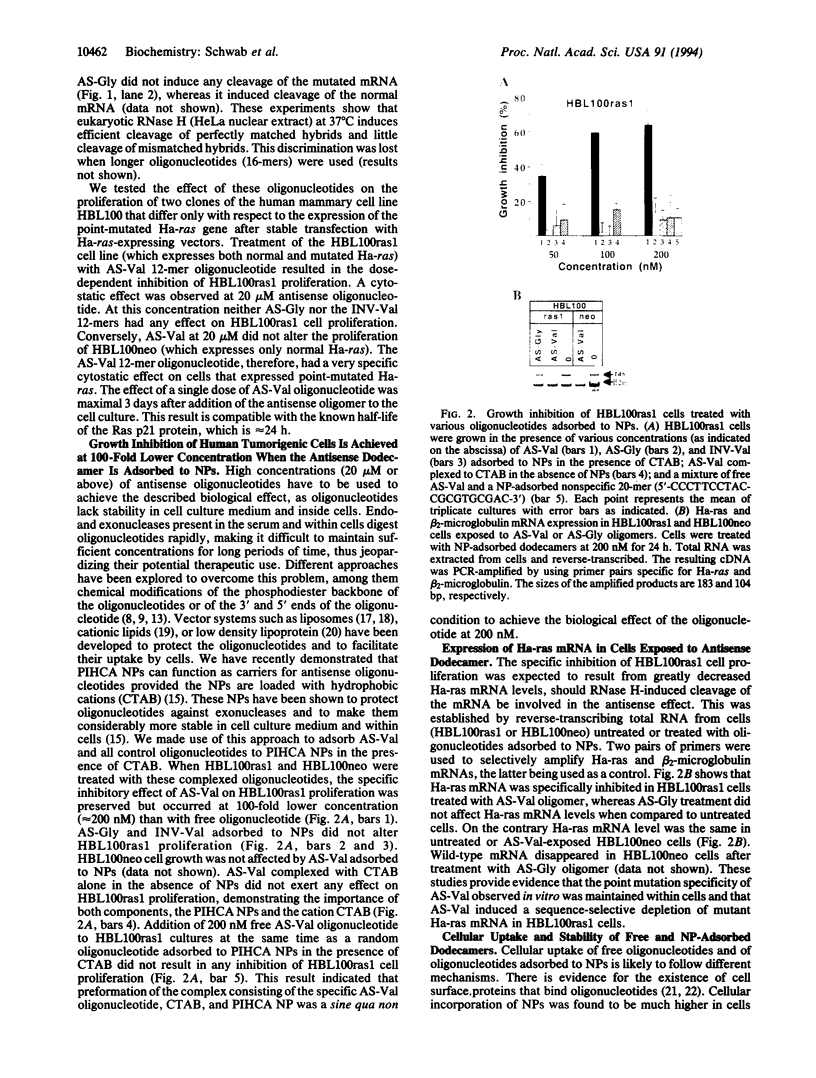
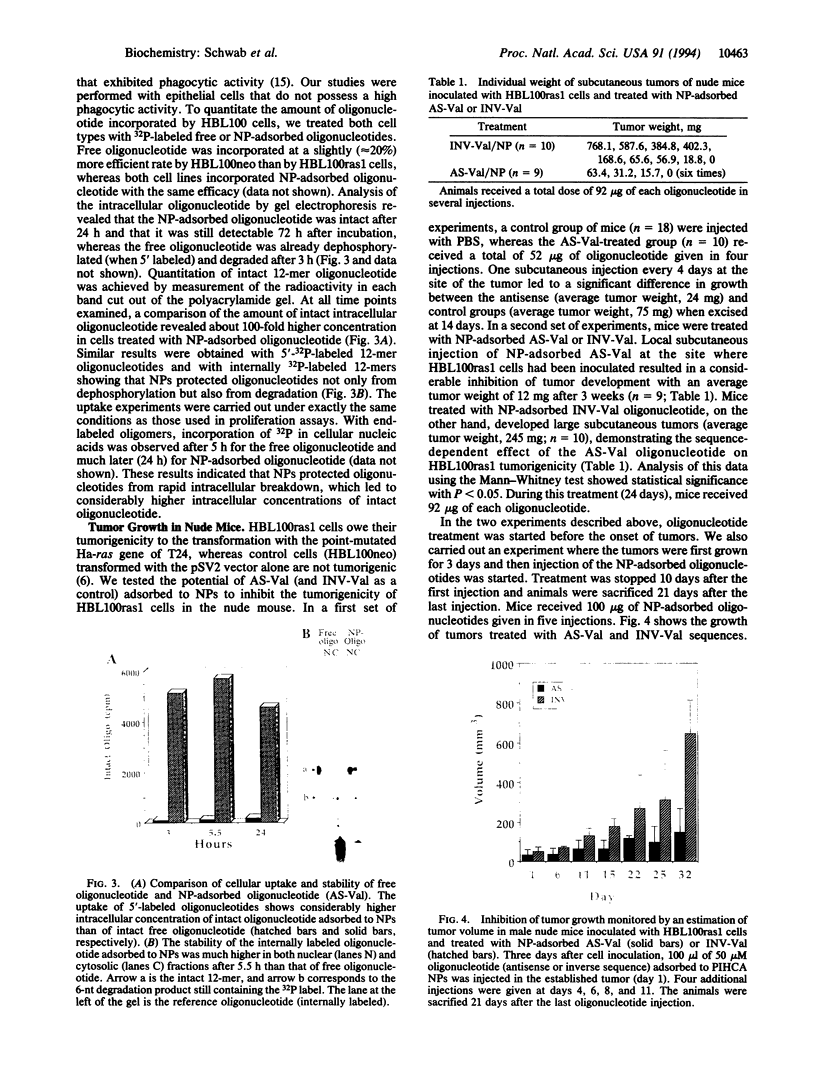
Ras oncogenes owe their transforming properties to single point mutations in the sequence coding for the active site of the p21 protein. These mutations lead to changes in cellular proliferation and induce tumorigenic properties. Point mutations represent a well-defined target for antisense oligonucleotides that can specifically suppress the translation of the targeted mutant mRNA. We show that the stability and cellular disponibility of antisense oligonucleotides can be markedly improved by adsorption to polyalkylcyanoacrylate nanoparticles. Nanoparticle-adsorbed antisense oligonucleotides directed to a point mutation (G-->U) in codon 12 of the Ha-ras mRNA selectively inhibited the proliferation of cells expressing the point-mutated Ha-ras gene at a concentration 100 times lower than free oligonucleotides. In addition they markedly inhibited Ha-ras-dependent tumor growth in nude mice after subcutaneous injection. These experiments show that inhibition of ras oncogenes by antisense oligonucleotides can block tumor development even though ras oncogenic activation might be an early event in tumor progression.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras oncogenes: their role in neoplasia. Eur J Clin Invest. 1990 Jun;20(3):225–235. doi: 10.1111/j.1365-2362.1990.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Burch R. M., Mahan L. C. Oligonucleotides antisense to the interleukin 1 receptor mRNA block the effects of interleukin 1 in cultured murine and human fibroblasts and in mice. J Clin Invest. 1991 Oct;88(4):1190–1196. doi: 10.1172/JCI115421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabretta B. Inhibition of protooncogene expression by antisense oligodeoxynucleotides: biological and therapeutic implications. Cancer Res. 1991 Sep 1;51(17):4505–4510. [PubMed] [Google Scholar]
- Chang E. H., Miller P. S., Cushman C., Devadas K., Pirollo K. F., Ts'o P. O., Yu Z. P. Antisense inhibition of ras p21 expression that is sensitive to a point mutation. Biochemistry. 1991 Aug 27;30(34):8283–8286. doi: 10.1021/bi00098a001. [DOI] [PubMed] [Google Scholar]
- Chavany C., Le Doan T., Couvreur P., Puisieux F., Hélène C. Polyalkylcyanoacrylate nanoparticles as polymeric carriers for antisense oligonucleotides. Pharm Res. 1992 Apr;9(4):441–449. doi: 10.1023/a:1015871809313. [DOI] [PubMed] [Google Scholar]
- Chiang M. Y., Chan H., Zounes M. A., Freier S. M., Lima W. F., Bennett C. F. Antisense oligonucleotides inhibit intercellular adhesion molecule 1 expression by two distinct mechanisms. J Biol Chem. 1991 Sep 25;266(27):18162–18171. [PubMed] [Google Scholar]
- Chiannilkulchai N., Ammoury N., Caillou B., Devissaguet J. P., Couvreur P. Hepatic tissue distribution of doxorubicin-loaded nanoparticles after i.v. administration in reticulosarcoma M 5076 metastasis-bearing mice. Cancer Chemother Pharmacol. 1990;26(2):122–126. doi: 10.1007/BF02897257. [DOI] [PubMed] [Google Scholar]
- Chiannilkulchai N., Driouich Z., Benoit J. P., Parodi A. L., Couvreur P. Doxorubicin-loaded nanoparticles: increased efficiency in murine hepatic metastases. Sel Cancer Ther. 1989;5(1):1–11. doi: 10.1089/sct.1989.5.1. [DOI] [PubMed] [Google Scholar]
- Higgins K. A., Perez J. R., Coleman T. A., Dorshkind K., McComas W. A., Sarmiento U. M., Rosen C. A., Narayanan R. Antisense inhibition of the p65 subunit of NF-kappa B blocks tumorigenicity and causes tumor regression. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9901–9905. doi: 10.1073/pnas.90.21.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C. Rational design of sequence-specific oncogene inhibitors based on antisense and antigene oligonucleotides. Eur J Cancer. 1991;27(11):1466–1471. doi: 10.1016/0277-5379(91)90033-a. [DOI] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Kashani-Sabet M., Funato T., Tone T., Jiao L., Wang W., Yoshida E., Kashfinn B. I., Shitara T., Wu A. M., Moreno J. G. Reversal of the malignant phenotype by an anti-ras ribozyme. Antisense Res Dev. 1992 Spring;2(1):3–15. doi: 10.1089/ard.1992.2.3. [DOI] [PubMed] [Google Scholar]
- Kitajima I., Shinohara T., Bilakovics J., Brown D. A., Xu X., Nerenberg M. Ablation of transplanted HTLV-I Tax-transformed tumors in mice by antisense inhibition of NF-kappa B. Science. 1992 Dec 11;258(5089):1792–1795. doi: 10.1126/science.1299224. [DOI] [PubMed] [Google Scholar]
- Lebeau J., Le Chalony C., Prosperi M. T., Goubin G. Constitutive overexpression of a 89 kDa heat shock protein gene in the HBL100 human mammary cell line converted to a tumorigenic phenotype by the EJ/T24 Harvey-ras oncogene. Oncogene. 1991 Jul;6(7):1125–1132. [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke S. L., Stein C., Zhang X., Avigan M., Cohen J., Neckers L. M. Delivery of c-myc antisense phosphorothioate oligodeoxynucleotides to hematopoietic cells in culture by liposome fusion: specific reduction in c-myc protein expression correlates with inhibition of cell growth and DNA synthesis. Curr Top Microbiol Immunol. 1988;141:282–289. doi: 10.1007/978-3-642-74006-0_38. [DOI] [PubMed] [Google Scholar]
- Milhaud P. G., Machy P., Lebleu B., Leserman L. Antibody targeted liposomes containing poly(rI).poly(rC) exert a specific antiviral and toxic effect on cells primed with interferons alpha/beta or gamma. Biochim Biophys Acta. 1989 Dec 11;987(1):15–20. doi: 10.1016/0005-2736(89)90449-5. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T., Steinberg S. M., Oie H. K., Mulshine J. L., Phelps R., Viallet J., Pass H., Minna J. D., Gazdar A. F. ras gene mutations in non-small cell lung cancers are associated with shortened survival irrespective of treatment intent. Cancer Res. 1991 Sep 15;51(18):4999–5002. [PubMed] [Google Scholar]
- Miyamoto H., Harada M., Isobe H., Akita H. D., Haneda H., Yamaguchi E., Kuzumaki N., Kawakami Y. Prognostic value of nuclear DNA content and expression of the ras oncogene product in lung cancer. Cancer Res. 1991 Dec 1;51(23 Pt 1):6346–6350. [PubMed] [Google Scholar]
- Monia B. P., Johnston J. F., Ecker D. J., Zounes M. A., Lima W. F., Freier S. M. Selective inhibition of mutant Ha-ras mRNA expression by antisense oligonucleotides. J Biol Chem. 1992 Oct 5;267(28):19954–19962. [PubMed] [Google Scholar]
- Neckers L., Whitesell L., Rosolen A., Geselowitz D. A. Antisense inhibition of oncogene expression. Crit Rev Oncog. 1992;3(1-2):175–231. [PubMed] [Google Scholar]
- Ratajczak M. Z., Kant J. A., Luger S. M., Hijiya N., Zhang J., Zon G., Gewirtz A. M. In vivo treatment of human leukemia in a scid mouse model with c-myb antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11823–11827. doi: 10.1073/pnas.89.24.11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saison-Behmoaras T., Tocqué B., Rey I., Chassignol M., Thuong N. T., Hélène C. Short modified antisense oligonucleotides directed against Ha-ras point mutation induce selective cleavage of the mRNA and inhibit T24 cells proliferation. EMBO J. 1991 May;10(5):1111–1118. doi: 10.1002/j.1460-2075.1991.tb08051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasawa S., Furuse M., Yokoyama N., Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science. 1993 Apr 2;260(5104):85–88. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Whitesell L., Rosolen A., Neckers L. M. In vivo modulation of N-myc expression by continuous perfusion with an antisense oligonucleotide. Antisense Res Dev. 1991 Winter;1(4):343–350. doi: 10.1089/ard.1991.1.343. [DOI] [PubMed] [Google Scholar]
- Yakubov L. A., Deeva E. A., Zarytova V. F., Ivanova E. M., Ryte A. S., Yurchenko L. V., Vlassov V. V. Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc Natl Acad Sci U S A. 1989 Sep;86(17):6454–6458. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smidt P. C., Le Doan T., de Falco S., van Berkel T. J. Association of antisense oligonucleotides with lipoproteins prolongs the plasma half-life and modifies the tissue distribution. Nucleic Acids Res. 1991 Sep 11;19(17):4695–4700. doi: 10.1093/nar/19.17.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]