Summary
Association of receptor activity-modifying proteins (RAMP1-3) with the G protein-coupled receptor (GPCR) calcitonin receptor-like receptor (CLR) enables selective recognition of the peptides calcitonin gene-related peptide (CGRP) and adrenomedullin (AM) that have diverse functions in the cardiovascular and lymphatic systems. How peptides selectively bind GPCR:RAMP complexes is unknown. We report crystal structures of CGRP analog-bound CLR:RAMP1 and AM-bound CLR:RAMP2 extracellular domain heterodimers at 2.5 and 1.8 Å resolutions, respectively. The peptides similarly occupy a shared binding site on CLR with conformations characterized by a β-turn structure near their C termini rather than the α-helical structure common to peptides that bind related GPCRs. The RAMPs augment the binding site with distinct contacts to the variable C-terminal peptide residues and elicit subtly different CLR conformations. The structures and accompanying pharmacology data reveal how a class of accessory membrane proteins modulate ligand binding of a GPCR and may inform drug development targeting CLR:RAMP complexes.
Graphical Abstract
Highlights
-
•
Crystal structures reveal how CGRP and AM peptides bind their heterodimeric receptors
-
•
CGRP and AM occupy a shared binding site on CLR with minimal contact to the RAMPs
-
•
Peptide binding modes were confirmed for intact receptors in cells by mutagenesis
-
•
Peptide selectivity arises from RAMP-specific contacts and subtle alteration of CLR
Booe et al. report two crystal structures that reveal how selectivity of the GPCR CLR for CGRP and AM peptides is modulated by RAMP proteins. The peptides similarly occupy a shared binding site on CLR. RAMPs augment the binding site with distinct contacts to the peptides and subtly alter CLR.
Introduction
G protein-coupled receptors (GPCRs) are a large family of cell surface receptors that regulate a multitude of biological processes in response to a diverse array of stimuli and they are important drug targets. The class B/Secretin family GPCRs in humans include 15 receptors that are activated by diverse neuropeptides, peptide paracrine factors, and peptide endocrine hormones (Hoare, 2005). These receptors are less well understood than the larger class A/Rhodopsin family, despite their physiological and clinical importance. Class B GPCRs comprise an extracellular domain (ECD) of about 120 amino acids in addition to the 7-transmembrane (7TM) domain in the membrane. The ECD has an N-terminal α-helix and a set of β sheets held together by three disulfide bonds (Archbold et al., 2011). Peptides bind class B GPCRs via a “two-domain” model whereby their C-terminal region binds the ECD and their N-terminal region binds and activates the 7TM domain. Crystal structures are available for class B GPCR ECDs with bound peptides related to PTH, CRF, GIP, and GLP-1 (Pal et al., 2010; Parthier et al., 2007; Pioszak et al., 2008, 2009; Pioszak and Xu, 2008; Runge et al., 2008; Underwood et al., 2010) and a consensus has emerged from these studies. The peptides bind as extended α helices to the same region of the receptor, in a groove between the N and C termini of the isolated ECDs. For PTH, GIP, and GLP-1 families, the peptides are closest to the N terminus; for the CRF-related peptides, they are displaced to be closer to the C terminus.
Although this model of binding is valid for several class B GPCRs, it cannot apply to all class B receptors. In particular, there are problems understanding the binding of members of the calcitonin (CT) family of peptides; calcitonin gene-related peptides alpha and beta (αCGRP, βCGRP), adrenomedullin (AM), adrenomedullin 2/intermedin (AM2), amylin (Amy), and CT (Hong et al., 2012; Poyner et al., 2002). These C terminally amidated peptides have a range of actions including neurogenic inflammation (CGRP), vasodilation/cardioprotection (CGRP, AM, and AM2), and regulation of blood and lymphatic vascular development (AM), nutrient intake and blood glucose (Amy), and bone turnover (CT). CGRP antagonists showed promise for the treatment of migraine and AM may be of value for the treatment of cardiovascular disorders (Durham and Vause, 2010; Karpinich et al., 2011). An Amy analog is used to treat insulin-dependent diabetes patients (Edelman et al., 2008) and CT has been long used to treat bone disorders (Purdue et al., 2002).
CGRP, AM, and AM2 binding to their cognate class B receptor, the calcitonin receptor-like receptor (CLR), is dependent on association of CLR with one of three accessory membrane proteins that determine ligand selectivity; receptor activity-modifying proteins (RAMPs) 1, 2, or 3 (Hong et al., 2012; McLatchie et al., 1998). RAMPs have an ECD of about 100 amino acids and a single TM segment (Parameswaran and Spielman, 2006). CLR:RAMP1 is a CGRP receptor, CLR:RAMP2 preferentially recognizes AM and is called the AM1 receptor, and CLR:RAMP3 binds both AM and AM2 with high affinities and is called the AM2 receptor. Amy by itself has a low affinity for the class B CT receptor (CTR); however, when CTR associates with any of the RAMPs, its affinity for Amy is markedly increased (Christopoulos et al., 1999; Poyner et al., 2002). CTR alone is the receptor for CT. Thus, the RAMPs profoundly alter the behavior of CLR and CTR. Although RAMPs are best characterized for their effects on CLR/CTR, they also interact with several other class B GPCRs and with certain class A/Rhodopsin and class C/Glutamate family GPCRs, making it particularly important to understand the molecular basis for RAMP actions (Bouschet et al., 2005; Lenhart et al., 2013; Wootten et al., 2010). RAMPs provide an excellent opportunity to explore how accessory membrane proteins can modulate GPCR pharmacology.
Crystal structures are available for ligand-free and small molecule antagonist-bound CLR:RAMP1 and ligand-free CLR:RAMP2 ECD complexes, but these provide little insight into how peptides bind or how RAMPs determine selectivity (Kusano et al., 2012; ter Haar et al., 2010). Extensive mutagenesis on the RAMPs (Qi and Hay, 2010) only provided clear evidence for the involvement of one RAMP residue (RAMP1 W84) in the binding of CGRP and two residues (RAMP2 F111 and E101) for AM binding (Moore et al., 2010; Watkins et al., 2014). It has not been possible to interpret these data mechanistically. A further complication is that it appears unlikely that CGRP and AM bind as extended helices as seen with other class B peptide ligands; there is evidence that only a small portion of these peptides form α helices and that at their C termini, there are one or more turn structures (Breeze et al., 1991; Carpenter et al., 2001; Pérez-Castells et al., 2012; Watkins et al., 2013b). Consequently, the mechanism of RAMP action and the mode of binding of CT family peptides remain unknown. Here, we describe high-resolution crystal structures of CGRP analog-bound CLR:RAMP1 and AM-bound CLR:RAMP2 ECD heterodimers that reveal bound peptide conformations starkly different from other class B GPCR peptide ligands, explain how RAMPs determine peptide selectivity, and provide molecular templates to guide drug development targeting CLR:RAMP complexes.
Results
Engineering CLR:RAMP ECD Complexes for Crystallization
We previously reported a tethered fusion protein approach to engineer the CLR:RAMP1 and CLR:RAMP2 ECD complexes for crystallization (Moad and Pioszak, 2013), inspired by previous successes using maltose binding protein (MBP) as a “crystallization module” for class B GPCR ECDs (Kumar et al., 2011; Pal et al., 2010; Pioszak et al., 2008, 2009, 2010; Pioszak and Xu, 2008). MBP-RAMP1 or MBP-RAMP2 ECD-CLR ECD fusion proteins in which the two ECDs were covalently tethered with a flexible (Gly-Ser)5 linker were designed to ensure complex stability and enforce 1:1 CLR:RAMP stoichiometry. The tethered RAMP1-CLR ECD fusion was a monomer, whereas the tethered RAMP2-CLR ECD fusion purified as a dimer, but the physiological relevance of oligomerization is unknown. Both proteins selectively bound their respective peptides but failed to yield crystals in the presence of peptides.
We reasoned that tether flexibility and oligomerization of the AM1 receptor ECD complex hindered the crystallization efforts. We produced new constructs with a (Gly-Ser-Ala)3 tether designed to decrease flexibility and we identified a single amino acid substitution in the RAMP2 ECD, L106R, which prevented dimerization of the tethered RAMP2-CLR fusion protein (Figure S1A) by disrupting a putative oligomerization interface identified by examining crystal packing in the ligand-free CLR:RAMP2 ECD structure (Kusano et al., 2012). The monomeric RAMP2 L106R-tethered construct retained selectivity for AM over CGRP and bound AM(22-52)NH2 essentially identical to the wild-type tethered fusion in an AlphaScreen competition binding assay (IC50 ∼5–15 μM) (Figures S1B, S1C, and S1H). In a cell-based cAMP signaling assay the full-length AM1 receptor with RAMP2 [L106R] exhibited wild-type response to AM (Figure S1D; Table S4). High-quality crystals of MBP-RAMP2 ECD [L106R]-(GSA)3-CLR ECD grown in the presence of AM(25-52)NH2 were readily obtained (Figure S1E). Crystals of MBP-RAMP1 ECD-(GSA)3-CLR ECD grown in the presence of CGRP(20-37)NH2 diffracted poorly (data not shown); fortunately, high-quality crystals were obtained in the presence of a high-affinity CGRP analog CGRP(27-37)NH2 [D31, P34, F35] (Rist et al., 1998) (Figure S1F). In the competition assay, the CGRP receptor crystallization construct was selective for CGRP over AM and bound the CGRP analog with higher affinity (IC50 ∼0.46 μM) than CGRP(8-37)NH2 (IC50 ∼2 μM) (Figure S1G). The CGRP analog also bound the AM1 receptor crystallization construct with higher affinity than wild-type CGRP but was still lower affinity than AM (Figure S1H). The crystallized proteins thus exhibited peptide selectivity consistent with the intact receptors. The peptides in both crystal forms are antagonist fragments that lack the N-terminal 7TM domain-activating region (Figure S1I). The CGRP analog will hereafter be referred to as CGRPmut.
Structures of the CGRPmut-Bound CLR:RAMP1 and AM-Bound CLR:RAMP2 ECD Heterodimers
Diffraction data for the CGRPmut- and AM-bound receptor complexes were collected to resolutions of 2.5 and 1.8 Å, respectively (Table 1). The structures were solved by molecular replacement (MR) and refined to good Rwork and Rfree values (Table 1). Three copies of the tethered CGRP receptor fusion and one copy of the tethered AM1 receptor fusion were present in the asymmetric units. Molecule A (Mol A) of the CGRPmut-bound structure had the best electron density and lowest B-factors (Table 1); unless otherwise noted the figures use Mol A. The peptide-bound structures are shown in Figures 1A and 1B. The mFo-DFc electron density maps for the rebuilt MR models showed clear, unambiguous density for CGRPmut and AM (Figures S2A and S2C). MBP sits over the bound peptides, but it does not appear to alter their binding (Figures S2B and S2D). The final models include CGRPmut residues 27–37 and AM residues 35–52 (residues 25–34 were disordered) and the majority of the tethered fusion proteins other than the tethers and ∼20 residues at the C terminus, which were disordered (Figures S2B and S2D). The tethers appeared to be longer than necessary, making it unlikely that they altered RAMP-CLR interactions.
Table 1.
Data Collection and Refinement Statistics
| MBP-RAMP1-CLR:CGRP(27-37)NH2 [D31, P34, F35] | MBP-RAMP2-CLR:AM(25-52)NH2 | |
|---|---|---|
| Data collection | ||
| Space group | C2 | P212121 |
| Cell dimensions | ||
| a, b, c | 172.81 Å, 104.62 Å, 136.48 Å | 71.45 Å, 84.28 Å, 115.76 Å |
| α, β, γ | 90°, 122.43°, 90° | 90°, 90°, 90° |
| Resolution | 50.0–2.45 Å (2.49–2.45 Å)a | 50.0–1.76 Å (1.79–1.76 Å) |
| Rmerge | 0.058 (0.647) | 0.068 (0.966) |
| CC1/2 | (0.858) | (0.525) |
| I / σI | 24.30 (1.76) | 30.13 (1.05) |
| Completeness | 99.8% (98.6%) | 99.9% (98.2%) |
| Redundancy | 4.2 (3.9) | 7.2 (5.0) |
| Refinement | ||
| Resolution (Å) | 50.0-2.45 Å | 50.0-1.76 Å |
| No. reflections | 72,185 | 66,505 |
| Rwork / Rfree | 0.200/0.243 | 0.157/0.200 |
| Protein molecules/ASU | 3 | 1 |
| No. atoms (Mol 1/2/3) | ||
| MBP | 2,885/2,865/2,859 | 2,898 |
| RAMP1 or RAMP2 | 705/657/705 | 721 |
| CLR | 805/981/744 | 805 |
| CGRP or AM | 86/86/75 | 148 |
| Water | 70 | 394 |
| B-factors (Mol 1/2/3) | ||
| MBP | 71.62/94.58/96.57 | 37.14 |
| RAMP1 or RAMP2 | 72.26/101.20/96.41 | 30.93 |
| CLR | 61.60/67.61/88.84 | 35.75 |
| CGRP or AM | 72.24/101.71/81.79 | 44.55 |
| Water | 52.85 | 39.32 |
| RMS deviations | ||
| Bond lengths | 0.013 Å | 0.020 Å |
| Bond angles | 1.516° | 1.929° |
| Ramachandran Analysisb | ||
| Preferred regions | 95.72% | 96.8% |
| Allowed regions | 4.04% | 3.2% |
| Outliers | 0.24% | 0% |
Values in parentheses are for highest-resolution shell.
As defined in COOT.
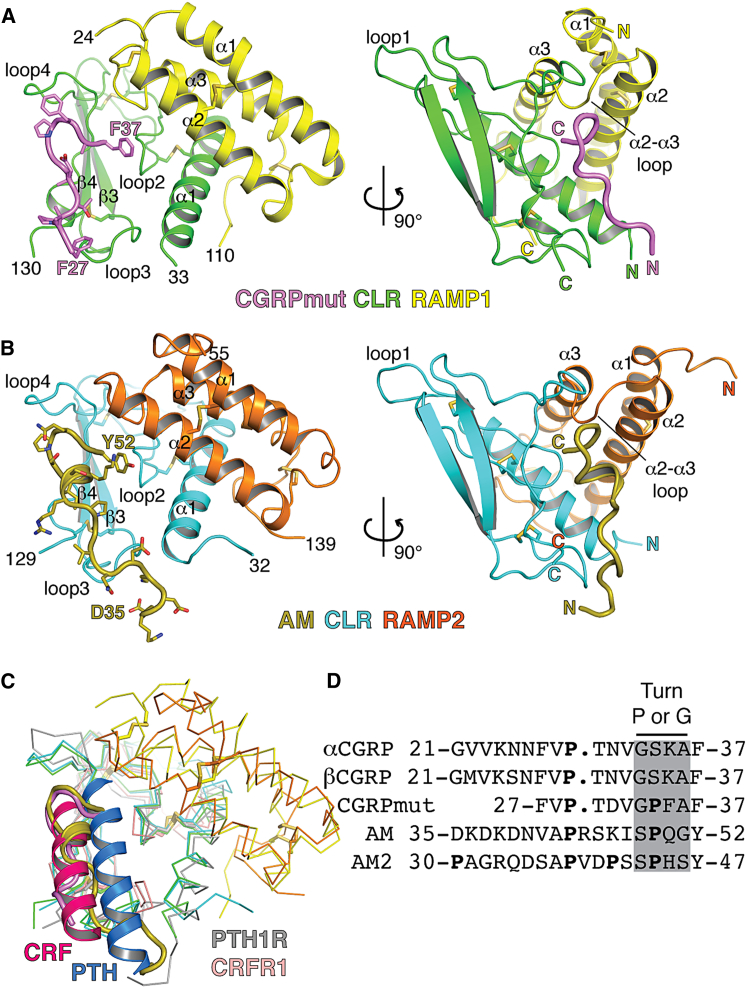
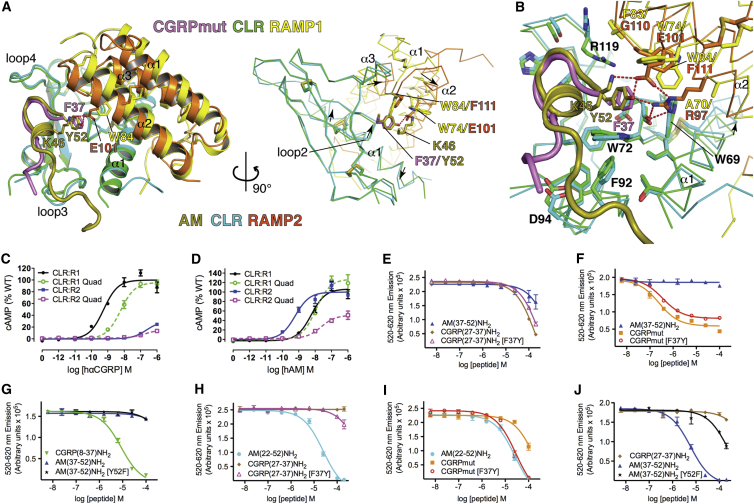
Figure 1.
Peptide-Bound CGRP and AM1 Receptor ECD Heterodimer Structures
(A and B) CGRPmut- and AM-bound complexes in cartoon representation with disulfide bonds as sticks and secondary structure elements labeled. Peptide, CLR, and RAMP terminal residues are numbered. Peptide side chains are shown as sticks in the left images but are omitted in the right images. MBP is not shown. The color scheme is consistent throughout the figures with carbon atoms varied in color to distinguish the proteins/peptides and oxygen, nitrogen, and sulfur atoms in CPK colors.
(C) Superpositions of the peptide-bound complexes with the PTH:PTH1R ECD (PDB: 3C4M) and CRF:CRFR1 ECD (PDB: 3EHU) structures. The receptors are shown as Cα traces and the peptides as cartoons.
(D) Amino acid sequences of the C-terminal regions of the human CGRP, CGRPmut, AM, and AM2 peptides. The turn structure region is highlighted in gray and Pro residues are in bold. See also Figures S1 and S2.
CGRPmut and AM occupy similar positions near CLR loops 2, 3, and 4; only their C termini are in proximity to the RAMPs (Figures 1A and 1B). Strikingly, CGRPmut adopts a receptor-bound conformation devoid of secondary structure. Receptor-bound AM lacks secondary structure other than one α-helical turn. Shared turn structures near the peptide C termini similarly position the C-terminal residues adjacent to α2 and the α2-α3 loop of the RAMPs. CGRPmut and AM occupy the same face of the CLR ECD as observed for other class B GPCRs with their positions more similar to that of CRF than PTH (Figure 1C). Helix-breaking Pro residues are prevalent in the CGRP, AM, and AM2 sequences and the four residue segment prior to the C-terminal residue contains turn-favoring Pro or Gly residues, consistent with the observed peptide conformations (Figure 1D). The structures are consistent with our knowledge of the architectures of the intact receptor complexes. The ECDs are oriented such that their C termini could continue toward the membrane with a similar number of residues between the termini visible in the structures and the predicted start of the TM segments (∼17 residues for CLR and ∼8 for the RAMPs). The peptides are oriented such that their N termini containing the receptor-activating regions would be directed toward the 7TM domain.
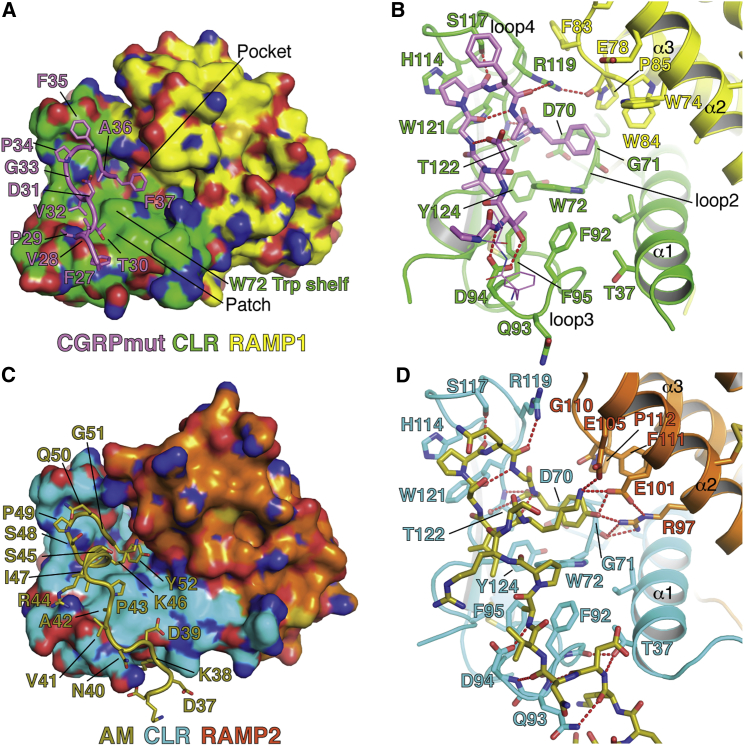
CGRPmut and AM primarily contact CLR, but key RAMP contacts are also formed (Tables S1 and S2). Two key features of the peptide-binding sites are a hydrophobic patch extending from the base of CLR loop 4 to loop 3 and a pocket extending from the base of CLR loop 4 to loop 2 and the RAMPs (Figure 2). The CLR W72 bulge, previously called the “Trp shelf” (ter Haar et al., 2010), demarcates patch and pocket. The patch comprises the Trp shelf, F92, F95, and Y124. The pockets comprise the Trp shelf, D70, G71, W121, T122, Y124, and RAMP1 W84 and P85 in the CGRP receptor (Figures 2A and 2B) or RAMP2 R97, E101, E105, and P112 in the AM1 receptor (Figures 2C and 2D). CGRPmut G33-A36 and AM S48-G51 form type II β-turns that contact CLR loop 4 in part via hydrogen bonds between the turn main chain and CLR S117, R119, and W121 side chains (Figures 2B and 2D). The β-turns enable the peptide C termini to occupy their respective pockets where their amide groups hydrogen bond with the CLR T122 main chain and their C-terminal residues pack against the Trp shelf, CLR G71, and RAMP1 W84 from the α2-α3 loop, which makes hydrophobic contact with CGRPmut F37 (Figures 2A and 2B), or RAMP2 R97, E101, and E105 from α2, which hydrogen bond with AM Y52 and K46 (Figures 2C and 2D). Prior to the β-turns CGRPmut V32 and AM I47 similarly contact the patch, but moving backward thence the peptides diverge in their interactions. CGRPmut T30 forms main chain- and side chain-mediated hydrogen bonds with CLR D94 on loop 3 and contacts the patch via its side chain methyl group (Figures 2A and 2B). The single helical turn in AM enables K46 to contact the Trp shelf and pack against AM Y52 and AM P43 and A42 contact the patch (Figures 2C and 2D). AM K38-A42 form a series of main chain-mediated hydrogen bonds with the main chain of CLR loop 3 and the side chains of D94 in loop 3 and T37 on α1 (Figure 2D). For the CGRPmut-bound structure, 94% of the solvent accessible surface area (ASA) of the ECD complex buried at the interface with the peptide is from CLR (478 Å2) and only 6% is from RAMP1 (29 Å2). More ASA is buried at the interface with AM, but the majority is still from CLR, 90% (781 Å2), whereas RAMP2 contributes only 10% (85 Å2).
Figure 2.
CGRPmut and AM Interactions with Their Receptor ECD Complexes
(A and C) The peptide-bound structures viewed with the receptor complexes in molecular surface representation and the peptides as cartoons with side chains as sticks.
(B and D) Detailed views with the receptors in cartoon representation and selected receptor residues and the peptides as sticks. Red dashes are hydrogen bonds. Peptide residues are labeled in (A) and (C) and receptor residues are labeled in (B) and (D). In (B), CGRPmut residues F27 and V28 are shown as lines for visualization of the CGRPmut T30-CLR D94 interaction. See also Tables S1 and S2.
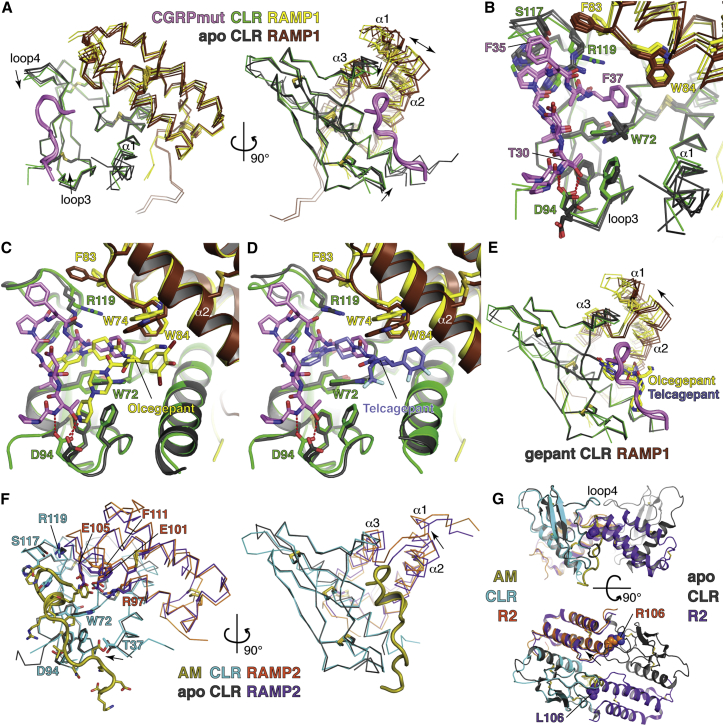
Comparisons to Ligand-free and Small Molecule Antagonist-Bound Structures
Superpositions of CGRPmut-bound and ligand-free CLR:RAMP1 complexes (ter Haar et al., 2010) revealed clamp-like movement of CLR loops 3 and 4 upon CGRPmut binding, presumably mediated by the CGRPmut T30-CLR D94 interaction and β-turn contacts with CLR loop 4 including the CGRPmut F35-CLR S117 interaction (Figures 3A and 3B). CLR R119 shifts to accommodate the peptide and RAMP1 F83 rotates away from CLR loop 4 (Figure 3B). The RAMP1 position relative to CLR varies in the structures (Figure 3A), but the positions of the α2-α3 loop and W84, which contacts CGRPmut F37, remain relatively similar (Figures 3A and 3B).
Figure 3.
Comparisons of the CGRPmut- and AM-Bound Structures to Ligand-free and Small Molecule Antagonist-Bound Structures
(A) CGRPmut-bound complexes from Mol A and Mol C were aligned with four independent ligand-free CLR:RAMP1 complexes (PDB: 3N7P) based on the CLR positions. Mol B of the CGRPmut-bound structure was omitted because crystal packing altered its conformation. Receptors are shown as Cα traces. Arrows denote the directions of loop movements upon CGRPmut binding. The double-headed arrow highlights variability in the RAMP1 position relative to CLR.
(B) Detailed view of differences between the CGRPmut-bound and ligand-free states of the CLR:RAMP1 ECD complex. CGRPmut F27 and V28 are omitted for clarity.
(C and D) Superposition of CGRPmut-bound and olcegepant-bound (PDB: 3N7S) (C) or telcagepant-bound (PDB: 3N7R) (D) structures aligned based on the CLR positions. The peptide and small molecules are shown as sticks.
(E) Superpositions of the CGRPmut-bound structures with two independent olcegepant-bound complexes and a single telcagepant-bound complex based on the CLR positions. The arrow indicates the direction of movement of RAMP1 from the small molecule antagonist-bound to CGRPmut-bound states.
(F) Superposition of the AM-bound and ligand-free (PDB: 3AQF) CLR:RAMP2 ECD structures aligned based on the CLR positions. Receptors are shown as Cα traces and selected receptor and peptide residues as sticks. Arrows indicate directions of movement from ligand-free to AM-bound states.
(G) Putative dimer of ligand-free CLR:RAMP2 ECD heterodimers with the AM-bound CLR:RAMP2 ECD [L106R] heterodimer superimposed based on the CLR positions. The receptors and peptide are in cartoon representation and residues L/R106 are in space-filling representation. The arrow indicates the shift of RAMP2 in the AM-bound structure as compared to the ligand-free state. The top image is oriented similar to that in (F), right image.
The CGRPmut-bound structure explains antagonism by the CGRP receptor-selective small molecule drugs olcegepant and telcagepant. Superposition of the CGRPmut-bound and drug-bound structures (ter Haar et al., 2010) indicated that olcegepant and telcagepant block key interactions of the CGRP C-terminal amide and F37 with the receptor pocket by hydrogen bonding with the CLR T122 main chain at the base of the pocket and packing of their piperidyl moieties against the Trp shelf and G71 (Figures 3C and 3D). Olcegepant also hydrogen bonds with CLR D94, thereby blocking the CGRP T30-CLR D94 interaction (Figure 3C). The position of RAMP1 relative to CLR in the peptide-bound versus drug-bound structures varies such that the drugs appear to favor RAMP1 α2 shifting closer to the small molecule binding sites (Figure 3E), presumably due to drug-RAMP1 interactions including packing against W74 (Figures 3C and 3D).
Superposition of the AM-bound and ligand-free CLR:RAMP2 complexes (Kusano et al., 2012) revealed minor CLR conformational differences involving the N-terminal region of α1 moving toward AM in the AM-bound state presumably due to AM-CLR T37 contacts (Figure 3F). CLR loops 3 and 4 do not move as in the CGRPmut-bound structure and there are no significant side-chain conformational differences at the peptide-binding site. The position of RAMP2 relative to CLR varies in the two structures (Figure 3F), but the ligand-free RAMP2 position is probably constrained by formation of the dimer of heterodimers in which the C-terminal end of RAMP2 α2 occupies the peptide-binding site of CLR from the opposing heterodimer (Figure 3G). Dimerization of the CLR:RAMP2 ECD heterodimer thus occludes the AM-binding site, which suggested that the RAMP2 L106R substitution (at the end of RAMP2 α2) was key to obtaining AM-bound crystals.
Validation of the Structures for Intact CGRP and AM1 Receptors in Cells
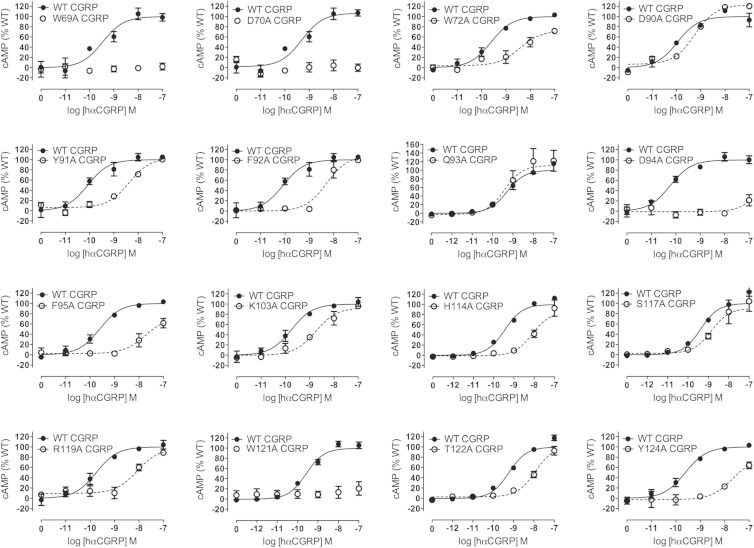
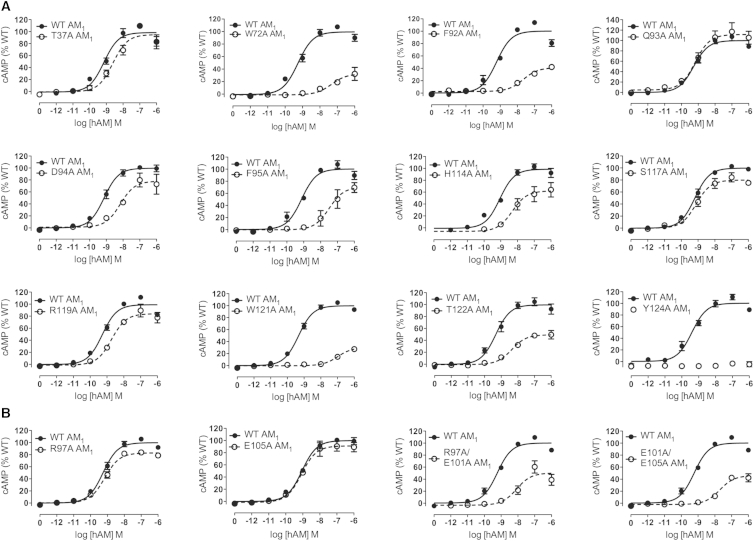
To validate the structures, we constructed several Ala substitution mutants in the CLR and RAMP2 ECDs, and they were analyzed for their effects on peptide-stimulated cAMP formation in COS-7 cells. For the CGRP receptor, Ala substitution of CLR W69, D70, K103, or Y91 significantly reduced CGRP potency, likely due to the structural roles of these residues (Figure 4, Table S3). Ala substitution of CLR W72, F92, D94, F95, H114, R119, W121, T122, or Y124, which are contacted by CGRPmut in the structure, reduced potency of the full-length CGRP >20-fold with the CLR D94A mutant being strikingly defective.
Figure 4.
Validation of the CGRPmut-Bound ECD Heterodimer Structure for the Intact CGRP Receptor Transiently Expressed in COS-7 Cells
Concentration-response curves for each of the CLR alanine substitution mutants tested with hαCGRP in cAMP signaling assays. Data are represented as mean ± SEM. See also Table S3.
For the AM1 receptor, Ala substitution of CLR W72, F92, F95, W121, or Y124, which are contacted by AM in the structure, resulted in >40-fold decreases in AM potency (Figure 5A, Table S4). CLR D94, H114, R119, or T122 mutants were less deleterious with 4- to 9-fold decreases in AM potency. Mutation of RAMP2 E101 yielded 26-fold reduced AM potency (Table S4; Watkins et al., 2014). Surprisingly, Ala substitution of RAMP2 R97 or E105 did not affect AM signaling potency despite their contacts with AM (Figure 5B). RAMP2 R97A/E101A and E101A/E105A double mutants had defects similar to the E101A single mutant. These data emphasize that RAMP2 E101 provides the crucial contacts to AM. The Ala substitutions did not significantly alter receptor cell surface expression levels other than reduced AM1 receptor expression with CLR Y124A (Tables S3 and S4).
Figure 5.
Validation of the AM-Bound ECD Heterodimer Structure for the Intact AM1 Receptor Transiently Expressed in COS-7 Cells
Concentration-response curves for each of the CLR (A) or RAMP2 (B) alanine substitution mutants tested with hAM in cAMP signaling assays. Data are represented as mean ± SEM. See also Table S4.
Peptide Selectivity Determinants
RAMPs may confer selectivity by providing distinct contacts to the peptides, altering CLR conformation, or a combination of the two. Superposition of the CGRPmut- and AM-bound structures indicated that the RAMPs augment the peptide-binding site pocket with distinct residues from their α2-α3 loop and α2 (Figure 6A). RAMP1 W84 in the α2-α3 loop makes hydrophobic contact with the CGRPmut F37 phenyl ring. This contact would be lost in RAMP2, which has the smaller F111 at the equivalent position. RAMP2 E101 on α2 hydrogen bonds with AM K46 and Y52. The equivalent RAMP1 W74 cannot make these contacts. Two other peptide-proximal RAMP positions differ: RAMP1 F83/RAMP2 G110 on the α2-α3 loop and RAMP1 A70/RAMP2 R97 on α2 (Figure 6B). The F83/G110 position is close to CLR loop 4 and the R119 side chain that has different conformations in the two structures. RAMP2 R97, which participates in the hydrogen bond network near AM Y52, would sterically clash with a Trp at position 111. The small A70 in RAMP1 avoids a clash with W84.
Figure 6.
Peptide Selectivity Determinants for CLR:RAMP1/2 Complexes
(A) Superposition of the CGRPmut- and AM-bound ECD heterodimers aligned based on the CLR positions. The receptors and peptides are in cartoon representation with selected residues as sticks in the left image. In the right image, the receptors are Cα traces and the peptide cartoons were omitted for clarity. Arrows indicate directions of movement of CLR α1 and loop 2 and RAMP α2 and α3 from the CGRPmut/RAMP1-bound state to the AM/RAMP2-bound state. Red dashes are hydrogen bonds.
(B) Detailed view of the aligned CGRPmut- and AM-bound complexes with selected residues as sticks and the receptors as Cα traces. The arrow highlights the shift of the C-terminal region of CLR α1 from the CGRPmut/RAMP1- to AM/RAMP2-bound states.
(C and D) Concentration-response curves for the CGRP and AM1 receptors with the RAMP1 (A70R, W74E, F83G, W84F) and RAMP2 (R97A, E101W, G110F, F111W) quadruple “swap” mutants tested with hαCGRP and hAM in cAMP assays in COS-7 cells. The cell surface expression was: RAMP1 quad 116.4 ± 5.25 (n = 4) % WT, RAMP2 quad 73.7 ± 9.52 (n = 4) % WT p < 0.01 by one-way ANOVA followed by Dunnett’s test for the RAMP2 quad mutant.
(E–J) Competition AlphaScreen assays with purified receptor ECD heterodimer proteins and the indicated competitor “swap” peptides. (E)–(G) are for the MBP-RAMP1-(GSA)3-CLR-H6 protein with biotin-CGRP (100 nM each) and (H)–(J) are for the MBP-RAMP2[L106R]-(GSA)3-CLR-H6 protein with biotin-AM (100 nM each). The binding data are representative of at least three independent experiments each performed in duplicate. The error bars represent the SEM of the experiment. Determinable pIC50 values were as follows: (F), CGRPmut 6.79 ± 0.10 and CGRPmut [F37Y] 6.24 ± 0.09; (G), CGRP(8-37) 5.01 ± 0.04; (H), AM(22-52) 4.92 ± 0.16; (I), AM(22-52) 4.84 ± 0.09 and CGRPmut [F37Y] 4.79 ± 0.07; (J), AM(37-52) 5.00 ± 0.11.
The positions of RAMP1 and RAMP2 relative to CLR differ in the two structures and the RAMPs elicit subtly different CLR conformations (Figure 6A). Equivalent RAMP1/2 Cα positions at the end of α2 differ by ∼3 to 4.5 Å such that RAMP2 α2 is closer to the peptide-binding site than RAMP1 α2. A similar 3 to 4.5 Å displacement of the RAMP1/2 α3 helices is accompanied by shifts in the position of the C-terminal end of CLR α1 (Figures 6A and 6B). The RAMPs and CLR α1 appear to move somewhat as a unit relative to the remainder of CLR, which is also evident in the comparisons of the peptide-bound structures to the ligand-free and small molecule antagonist-bound structures (Figures 3A, 3E, and 3F). The subtly different CLR loop 2 positions in the structures may reflect RAMP-dependent differences at the interface with CLR α1 propagated to loop 2 via CLR W69 (Figures 6A and 6B).
To explore the contribution of RAMP binding site augmentation to selectivity, we constructed RAMP “swap” mutants in which the four variable residue positions near the peptide C termini were reciprocally exchanged between RAMP1 and RAMP2 (A70/R97, W74/E101, F83/G110, and W84/F111) and we tested their response to CGRP and AM in the cAMP assay (Figures 6C and 6D). The clearest effect was one of a modest decrease in cognate ligand potency. Accordingly, CGRP potency decreased ∼10-fold at the CGRP receptor that included the RAMP1 mutant with RAMP2 residues and AM potency decreased ∼50-fold in the AM1 receptor with the RAMP2 mutant that contained RAMP1 residues. Thus, swapping these RAMP residues was insufficient to exchange pharmacological profiles, but the differing RAMP1/2 positions probably complicated the experiment.
We turned to peptide swap experiments to test whether reciprocal exchanges of the C-terminal residues of minimal ECD complex-binding CGRP and AM peptides (Moad and Pioszak, 2013) could exchange their receptor selectivity. In the competition AlphaScreen assay, CGRP(27-37)NH2 [F37Y] retained the ability to bind the CGRP receptor ECD complex (Figure 6E) and did not gain AM-like affinity for the AM1 receptor ECD complex (Figure 6H). CGRPmut [F37Y] retained CGRP receptor ECD complex binding (Figure 6F) and gained the ability to bind the AM1 receptor ECD complex as strongly as AM (Figure 6I). AM(37-52)NH2 [Y52F] exhibited significantly diminished binding to the AM1 receptor ECD complex (Figure 6J) but did not gain increased affinity for the CGRP receptor ECD complex (Figure 6G). These results suggested that the RAMP2 E101-AM Y52 hydrogen bond is a key contributor to AM1 receptor selectivity, whereas Phe as the peptide C-terminal residue is insufficient to confer CGRP receptor selectivity.
Discussion
RAMPs are an important class of accessory membrane proteins that modulate GPCR pharmacology. The CGRPmut-bound CLR:RAMP1 ECD and AM-bound CLR:RAMP2 ECD structures presented here expand our understanding of the mechanisms by which peptides can bind to class B GPCRs and increase our understanding of how RAMPs enable peptide selectivity. The engineered tethered ECD fusion proteins used for crystallization exhibited the same peptide selectivity rank order as the intact receptors, which indicated that they are valid reagents for studying selective peptide binding. The purified proteins bound their respective peptides with apparent affinities in the low μM range (Figures S1C, S1G, and S1H), which are lower than the affinities of the agonist peptides for intact receptors but typical for truncated peptides at class B GPCR ECDs (Pal et al., 2010; Parthier et al., 2007; Pioszak and Xu, 2008).
The oligomeric states of CLR:RAMP complexes has been a source of debate with evidence for 1:1, 2:1, and 2:2 CLR:RAMP stoichiometries (Héroux et al., 2007; Hill and Pioszak, 2013; Kusano et al., 2012; Moad and Pioszak, 2013; Watkins et al., 2013a). Dimerization of the purified CLR:RAMP2 ECD heterodimer to form a 2:2 complex may be an artifact because the RAMP2 L106R mutation prevented oligomerization yet did not affect AM1 receptor function (Figure S1). Occlusion of the AM-binding site by dimerization explains our inability to measure AM binding to the tethered RAMP2-CLR ECD fusion protein in an assay using μM receptor concentration, whereas AM binding was readily measured in an assay using nM receptor concentration where the dimeric species was likely not significantly present (Moad and Pioszak, 2013). Héroux et al. (2007) provided evidence for a homo-oligomer of CLR with a single RAMP1 as the functional CGRP receptor in cells. We cannot rule out a role for higher-order oligomerization in the function of the intact receptors, but the structures indicate that the 1:1 heterodimers are sufficient to bind peptides.
The CGRPmut and AM peptides adopted receptor-bound conformations different from typical α-helical class B GPCR peptide ligands. CGRPmut and AM are characterized by a shared turn structure that positions their C-terminal residue to occupy the pocket near the RAMP. Previous studies indicated the presence of turns in the C-terminal region of CGRP (and an absence of α-helix in this area), but how this region interacted with the receptor was unclear (Carpenter et al., 2001; Watkins et al., 2013b). Prior to the turns, the peptides diverge in their structure and interactions with CLR, but they contact the same area of CLR with little or no peptide secondary structure. NMR structures of CGRP and AM suggested that α-helix is restricted to residues 8-18/22-34 of these peptides (Boulanger et al., 1995; Breeze et al., 1991; Pérez-Castells et al., 2012; Watkins et al., 2013a) and indeed the C-terminal regions of both contain helix-breaking Pro residues (Figure 1D). Accordingly, the lack of substantial helical content in the bound peptide fragments is consistent with what is known about the structures of the full-length peptides. A turn structure and paucity of α-helicity may be a general feature of the receptor ECD-binding portions of CT family peptides.
The CGRPmut and AM binding modes are consistent with peptide mutagenesis studies. CGRP T30, V32, and F37 and the C-terminal amide were important for binding purified CLR:RAMP1 ECD (Moad and Pioszak, 2013) and intact CGRP receptor (Carpenter et al., 2001; Rist et al., 1998; Watkins et al., 2013b). Modified CGRP peptides as short as 30–37 maintained the ability to bind the receptor (Carpenter et al., 2001), consistent with this region providing most of the contacts. Increased affinity of CGRPmut over that of CGRP can be explained by their differences in the turn region (Figure 1D). P34 favors β-turn formation better than S34 and F35 provides better hydrophobic contact to CLR loop 4 than K35. AM P43, K46, I47, G51, Y52, and the C-terminal amide were most critical for binding purified CLR:RAMP2 ECD and intact AM1 receptor, and truncation beyond residue 38 diminished binding even though the K38-V41 side chains were not important (Moad and Pioszak, 2013; Watkins et al., 2013a).
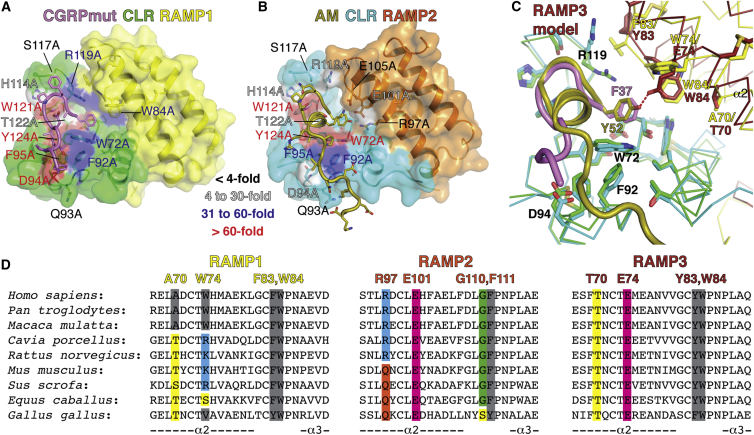
Mapping the receptor mutagenesis data (Figures 4 and 5) onto the surface of the receptor structures (Figures 7A and 7B) suggests that CGRP binds in a similar manner to CGRPmut and that the structures are good models for full-length CGRP and AM binding to intact receptors. Mutation of CLR residues that form the shared binding site diminished CGRP and AM potencies, and the effects of some of the mutations were similar for both peptides (e.g., F92). Noteworthy divergent effects of several mutations support the differences in the structures. CLR D94 was far more important for CGRP action than AM, consistent with the crucial role of D94 in contacting CGRP T30 and its less important role in contacting the AM main chain. CLR R119A diminished the potency of CGRP much more than that of AM, which may reflect an important role for the different R119 conformations observed in the two structures. CLR W72A was more deleterious for AM action than CGRP, which is consistent with the greater number of AM contacts to CLR W72 as compared to CGRP.
Figure 7.
Summary of Peptide Recognition and Selectivity Determinants for CLR:RAMP1-3 Complexes
(A and B) Structures of the CGRPmut- and AM-bound CLR:RAMP1/2 ECD heterodimers with the surface of receptor residues colored according to their effect on CGRP (A) or AM (B) signaling potency when mutated to alanine. Color coding signifies the extent of reduced signaling potency at intact receptor complexes in cells compared to wild-type as indicated by the inset legend. RAMP1 W84A data are from Moore et al. (2010) (34-fold reduced potency).
(C) Model for RAMP3 binding site augmentation. A homology model of the AM-bound CLR:RAMP3 ECD complex (Supplemental Experimental Procedures) was superimposed with the CGRPmut-bound CLR:RAMP1 and AM-bound CLR:RAMP2 ECD structures based on the CLR positions. Only the RAMP3 subunit from the homology model is shown and RAMP2 is omitted. The receptors are shown as Cα traces and selected residues as sticks.
(D) Amino acid sequence alignments for RAMP1-3 from the indicated species showing the α2 and α2-α3 loop regions that augment the peptide-binding site.
RAMP1 W84 and RAMP2 E101 were previously identified as key residues for CGRP and AM function, respectively (Moore et al., 2010; Watkins et al., 2014). These data are explained by how these residues augment the binding site pocket (Figures 7A and 7B). Apparently, packing of the CGRP F37 and AM Y52 phenyl rings against the CLR Trp shelf and G71 is insufficient for strong binding. RAMP1 W84 or RAMP2 E101 is required to complete the pocket to enable strong “anchoring” of the peptide C termini. RAMP2 R97 and E105 also augment the pocket, but the R97A and E105A mutants did not diminish AM potency. These data along with the peptide swap data indicate that the RAMP2 E101-Y52 hydrogen bond is the crucial AM anchoring contact. Ionic interactions of AM K46 and RAMP2 E101/E105 do not appear to be significant. The main role of AM K46 thus appears to be intramolecular packing against Y52 and contacting the Trp shelf.
Distinct RAMP binding site augmentation clearly contributes to peptide selectivity (Figure 6). RAMP2 E101 favors AM binding because it can hydrogen bond with Y52 and RAMP2 F111 discourages CGRP binding because it is too small to contact the F37 phenyl ring. Indeed, the F37Y swap in CGRPmut conferred strong affinity for the AM1 receptor ECD complex and the Y52F swap in AM(37-52)NH2 significantly diminished its binding. The lack of Glu at RAMP1 position 74 would disfavor strong AM binding. RAMP1 W84 enables strong CGRP binding by contacting F37, but this contact alone is apparently insufficient for selectivity because AM(37-52)NH2 [Y52F] did not gain affinity for the CGRP receptor ECD complex.
Modeling the AM-bound AM2 receptor ECD complex (Supplemental Experimental Procedures) suggests that RAMP3 augments the binding site as a RAMP1-2 hybrid (Figure 7C). RAMP3 E74, which is equivalent to RAMP2 E101, would favor AM binding by hydrogen bonding with Y52. RAMP3 W84, which is equivalent to RAMP1 W84, could contact the AM Y52 and CGRP F37 phenyl rings, thereby explaining diminished potency of both peptides at the AM2 receptor with RAMP3 W84A and why CGRP is more active at the AM2 receptor than the AM1 receptor (Watkins et al., 2014). The key RAMP residues proposed as selectivity determinants are conserved across species: W84 and a lack of Glu at position 74 in RAMP1, E101 and F or Y at position 111 in RAMP2, and E74 and W84 in RAMP3 (Figure 7D). A small amino acid is conserved at position 70 in RAMP1/3, which would avoid steric clash with W84. Notably, the lack of conservation of RAMP2 R97 and E105 is consistent with the mutagenesis data that indicated that these residues are not critical for AM signaling.
Previous RAMP single swap mutant studies supported the importance of Glu at position 74/101 as a determinant for AM selectivity. RAMP1 W74E had no effect on CGRP potency but increased AM potency at the CGRP receptor (Qi et al., 2008, 2011). RAMP3 E74W decreased AM potency at the AM2 receptor, while having a negligible effect on CGRP potency (Hay et al., 2006; Qi et al., 2008, 2011). More extensive quadruple swap mutants in this study failed to exchange the pharmacological profiles, but these experiments are complicated by the different RAMP positions relative to CLR and variable RAMP effects on CLR conformation.
The failure of the CGRP and AM peptide C-terminal residue swaps to exchange their receptor preferences (Figure 6) strongly suggests that RAMP binding site augmentation alone is insufficient to account for selectivity. Thus, the subtle differences in CLR conformation in the two structures may also be important for selectivity. RAMP-induced changes in CLR R119 side-chain conformation and/or subtle shifting of loop 2 may sufficiently alter the pocket to favor one peptide over the other. Future studies will be required to determine to what extent such allostery contributes to selectivity. Peptide selectivity determinants may also exist in portions of the receptors that were not addressed in this study.
In summary, the structures presented here provide the first structural views of any accessory membrane protein modulating GPCR ligand binding and may provide a basis for understanding modulation of other GPCRs by accessory proteins. Our data indicate that RAMPs determine peptide selectivity of CLR through a combination of binding site augmentation and alteration of CLR conformation. It is striking that relatively minor differences in RAMP-specific peptide contacts and subtle RAMP-induced changes in CLR conformation lead to such profoundly different pharmacological profiles. Of practical value, the structures may inform rational drug design targeting CLR:RAMP complexes with clinical relevance for migraine headache and cardiovascular disorders. Lastly, the MBP-tethered ECD fusion protein approach to crystallization should facilitate structural studies of other CT family peptides bound to their respective receptor ECD complexes, which will enable a more complete understanding of how RAMPs modulate both CLR and CTR.
Experimental Procedures
Protein Production and Characterization and Peptides
Plasmid construction, mutagenesis, protein expression, purification, and the AlphaScreen peptide-binding assay were as previously described with minor modifications to the AlphaScreen assay (Hill and Pioszak, 2013; Moad and Pioszak, 2013). Synthetic peptides were from RS Synthesis, Bachem, or were synthesized in-house. Details are in Supplemental Experimental Procedures.
Crystallization, Structure Solution, and Homology Modeling
The tethered MBP-RAMP1 ECD-CLR ECD and MBP-RAMP2 ECD [L106R]-CLR ECD proteins were complexed with CGRP(27-37)NH2 [D31, P34, F35] or AM(25-52)NH2 and crystallized with a reservoir solution of 22% PEG3350, 8% Tacsimate (pH 6.0) for the CGRP receptor complex or 19% PEG3350, 0.1 M Tris-HCl (pH 8.3), 225 mM sodium acetate, and 20% ethylene glycol for the AM1 receptor complex. Diffraction data collected at the APS synchrotron were processed with HKL2000 (Otwinowski and Minor, 1997) and the CCP4 suite (Winn et al., 2011). The structures were solved by molecular replacement with Phaser (McCoy et al., 2007), rebuilt with COOT (Emsley et al., 2010), and refined with REFMAC5 (Murshudov et al., 1997). Details and homology modeling are in Supplemental Experimental Procedures.
Cell-Based Assays
Transfection of COS-7 cells, cAMP assay, ELISA for cell surface expression, and data analysis were as previously described (Barwell et al., 2010; Watkins et al., 2014).
Author Contributions
J.M.B. produced and characterized proteins, performed crystallization, collected diffraction data, and constructed CLR and RAMP mutants; C.S.W., M.A.J., and D.L.H. designed, performed, and analyzed cell-based assays for the CGRP and AM1 receptors; J.B., G.K., J.S., R.M.B., and D.R.P. designed and constructed CLR mutants and performed and analyzed cell-based assays for the CGRP receptor; J.S. performed RAMP3 homology modeling; M.L.W. performed peptide binding experiments; P.W.H. and M.A.B. synthesized CGRP analog; A.A.P. designed and managed the structural project, solved/refined the structures, and wrote the manuscript with D.R.P. and D.L.H.
Acknowledgments
This research was supported by NIH grant R01GM104251 (A.A.P.), Wellcome Trust grant 091496 (D.R.P.), and grants from the Marsden Fund and Health Research Council of New Zealand (D.L.H.). Use of the Leica microscope for crystal imaging was supported by an Institutional Developmental Award from the National Institute of General Medical Sciences of the NIH under grant number P20GM103640. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817). We thank Zdzislaw Wawrzak and Spencer Anderson for assistance with remote data collection at APS sector 21 and Amanda Roehrkasse for pilot studies with the tethered RAMP1-(GSA)3-CLR construct.
Published: May 14, 2015
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, two figures, and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2015.04.018.
Accession Numbers
The accession numbers for the coordinates and structure factors reported in this paper for the AM:CLR:RAMP2 and CGRPmut:CLR:RAMP1 complexes, respectively are PDB: 4RWF, 4RWG.
Supplemental Information
References
- Archbold J.K., Flanagan J.U., Watkins H.A., Gingell J.J., Hay D.L. Structural insights into RAMP modification of secretin family G protein-coupled receptors: implications for drug development. Trends Pharmacol. Sci. 2011;32:591–600. doi: 10.1016/j.tips.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Barwell J., Miller P.S., Donnelly D., Poyner D.R. Mapping interaction sites within the N-terminus of the calcitonin gene-related peptide receptor; the role of residues 23-60 of the calcitonin receptor-like receptor. Peptides. 2010;31:170–176. doi: 10.1016/j.peptides.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger Y., Khiat A., Chen Y., Senécal L., Tu Y., St-Pierre S., Fournier A. Structure of human calcitonin gene-related peptide (hCGRP) and of its antagonist hCGRP 8-37 as determined by NMR and molecular modeling. Pept. Res. 1995;8:206–213. [PubMed] [Google Scholar]
- Bouschet T., Martin S., Henley J.M. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J. Cell Sci. 2005;118:4709–4720. doi: 10.1242/jcs.02598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeze A.L., Harvey T.S., Bazzo R., Campbell I.D. Solution structure of human calcitonin gene-related peptide by 1H NMR and distance geometry with restrained molecular dynamics. Biochemistry. 1991;30:575–582. doi: 10.1021/bi00216a036. [DOI] [PubMed] [Google Scholar]
- Carpenter K.A., Schmidt R., von Mentzer B., Haglund U., Roberts E., Walpole C. Turn structures in CGRP C-terminal analogues promote stable arrangements of key residue side chains. Biochemistry. 2001;40:8317–8325. doi: 10.1021/bi0102860. [DOI] [PubMed] [Google Scholar]
- Christopoulos G., Perry K.J., Morfis M., Tilakaratne N., Gao Y., Fraser N.J., Main M.J., Foord S.M., Sexton P.M. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- Durham P.L., Vause C.V. Calcitonin gene-related peptide (CGRP) receptor antagonists in the treatment of migraine. CNS Drugs. 2010;24:539–548. doi: 10.2165/11534920-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman S., Maier H., Wilhelm K. Pramlintide in the treatment of diabetes mellitus. BioDrugs. 2008;22:375–386. doi: 10.2165/0063030-200822060-00004. [DOI] [PubMed] [Google Scholar]
- Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D.L., Christopoulos G., Christopoulos A., Sexton P.M. Determinants of 1-piperidinecarboxamide, N-[2-[[5-amino-l-[[4-(4-pyridinyl)-l-piperazinyl]carbonyl]pentyl]amino]-1-[(3,5-dibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl) (BIBN4096BS) affinity for calcitonin gene-related peptide and amylin receptors—the role of receptor activity modifying protein 1. Mol. Pharmacol. 2006;70:1984–1991. doi: 10.1124/mol.106.027953. [DOI] [PubMed] [Google Scholar]
- Héroux M., Hogue M., Lemieux S., Bouvier M. Functional calcitonin gene-related peptide receptors are formed by the asymmetric assembly of a calcitonin receptor-like receptor homo-oligomer and a monomer of receptor activity-modifying protein-1. J. Biol. Chem. 2007;282:31610–31620. doi: 10.1074/jbc.M701790200. [DOI] [PubMed] [Google Scholar]
- Hill H.E., Pioszak A.A. Bacterial expression and purification of a heterodimeric adrenomedullin receptor extracellular domain complex using DsbC-assisted disulfide shuffling. Protein Expr. Purif. 2013;88:107–113. doi: 10.1016/j.pep.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare S.R. Mechanisms of peptide and nonpeptide ligand binding to Class B G-protein-coupled receptors. Drug Discov. Today. 2005;10:417–427. doi: 10.1016/S1359-6446(05)03370-2. [DOI] [PubMed] [Google Scholar]
- Hong Y., Hay D.L., Quirion R., Poyner D.R. The pharmacology of adrenomedullin 2/intermedin. Br. J. Pharmacol. 2012;166:110–120. doi: 10.1111/j.1476-5381.2011.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinich N.O., Hoopes S.L., Kechele D.O., Lenhart P.M., Caron K.M. Adrenomedullin function in vascular endothelial cells: insights from genetic mouse models. Curr. Hypertens. Rep. 2011;7:228–239. doi: 10.2174/157340211799304761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Pioszak A., Zhang C., Swaminathan K., Xu H.E. Crystal structure of the PAC1R extracellular domain unifies a consensus fold for hormone recognition by class B G-protein coupled receptors. PLoS ONE. 2011;6:e19682. doi: 10.1371/journal.pone.0019682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano S., Kukimoto-Niino M., Hino N., Ohsawa N., Okuda K., Sakamoto K., Shirouzu M., Shindo T., Yokoyama S. Structural basis for extracellular interactions between calcitonin receptor-like receptor and receptor activity-modifying protein 2 for adrenomedullin-specific binding. Protein Sci. 2012;21:199–210. doi: 10.1002/pro.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart P.M., Broselid S., Barrick C.J., Leeb-Lundberg L.M., Caron K.M. G-protein-coupled receptor 30 interacts with receptor activity-modifying protein 3 and confers sex-dependent cardioprotection. J. Mol. Endocrinol. 2013;51:191–202. doi: 10.1530/JME-13-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J. Phaser crystallographic software. J. Appl. Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie L.M., Fraser N.J., Main M.J., Wise A., Brown J., Thompson N., Solari R., Lee M.G., Foord S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Moad H.E., Pioszak A.A. Selective CGRP and adrenomedullin peptide binding by tethered RAMP-calcitonin receptor-like receptor extracellular domain fusion proteins. Protein Sci. 2013;22:1775–1785. doi: 10.1002/pro.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E.L., Gingell J.J., Kane S.A., Hay D.L., Salvatore C.A. Mapping the CGRP receptor ligand binding domain: tryptophan-84 of RAMP1 is critical for agonist and antagonist binding. Biochem. Biophys. Res. Commun. 2010;394:141–145. doi: 10.1016/j.bbrc.2010.02.131. [DOI] [PubMed] [Google Scholar]
- Murshudov G.N., Vagin A.A., Dodson E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z., Minor W. Processing of X-ray diffraction data collected in oscillation mode. In: Carter C.W.J., Sweet R.M., editors. Methods in Enzymology. Academic Press; New York: 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- Pal K., Swaminathan K., Xu H.E., Pioszak A.A. Structural basis for hormone recognition by the Human CRFR2alpha G protein-coupled receptor. J. Biol. Chem. 2010;285:40351–40361. doi: 10.1074/jbc.M110.186072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N., Spielman W.S. RAMPs: The past, present and future. Trends Biochem. Sci. 2006;31:631–638. doi: 10.1016/j.tibs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Parthier C., Kleinschmidt M., Neumann P., Rudolph R., Manhart S., Schlenzig D., Fanghänel J., Rahfeld J.U., Demuth H.U., Stubbs M.T. Crystal structure of the incretin-bound extracellular domain of a G protein-coupled receptor. Proc. Natl. Acad. Sci. USA. 2007;104:13942–13947. doi: 10.1073/pnas.0706404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Castells J., Martín-Santamaría S., Nieto L., Ramos A., Martínez A., Pascual-Teresa Bd., Jiménez-Barbero J. Structure of micelle-bound adrenomedullin: a first step toward the analysis of its interactions with receptors and small molecules. Biopolymers. 2012;97:45–53. doi: 10.1002/bip.21700. [DOI] [PubMed] [Google Scholar]
- Pioszak A.A., Xu H.E. Molecular recognition of parathyroid hormone by its G protein-coupled receptor. Proc. Natl. Acad. Sci. USA. 2008;105:5034–5039. doi: 10.1073/pnas.0801027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioszak A.A., Parker N.R., Suino-Powell K., Xu H.E. Molecular recognition of corticotropin-releasing factor by its G-protein-coupled receptor CRFR1. J. Biol. Chem. 2008;283:32900–32912. doi: 10.1074/jbc.M805749200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioszak A.A., Parker N.R., Gardella T.J., Xu H.E. Structural basis for parathyroid hormone-related protein binding to the parathyroid hormone receptor and design of conformation-selective peptides. J. Biol. Chem. 2009;284:28382–28391. doi: 10.1074/jbc.M109.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioszak A.A., Harikumar K.G., Parker N.R., Miller L.J., Xu H.E. Dimeric arrangement of the parathyroid hormone receptor and a structural mechanism for ligand-induced dissociation. J. Biol. Chem. 2010;285:12435–12444. doi: 10.1074/jbc.M109.093138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyner D.R., Sexton P.M., Marshall I., Smith D.M., Quirion R., Born W., Muff R., Fischer J.A., Foord S.M. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Purdue B.W., Tilakaratne N., Sexton P.M. Molecular pharmacology of the calcitonin receptor. Receptors Channels. 2002;8:243–255. [PubMed] [Google Scholar]
- Qi T., Hay D.L. Structure-function relationships of the N-terminus of receptor activity-modifying proteins. Br. J. Pharmacol. 2010;159:1059–1068. doi: 10.1111/j.1476-5381.2009.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T., Christopoulos G., Bailey R.J., Christopoulos A., Sexton P.M., Hay D.L. Identification of N-terminal receptor activity-modifying protein residues important for calcitonin gene-related peptide, adrenomedullin, and amylin receptor function. Mol. Pharmacol. 2008;74:1059–1071. doi: 10.1124/mol.108.047142. [DOI] [PubMed] [Google Scholar]
- Qi T., Ly K., Poyner D.R., Christopoulos G., Sexton P.M., Hay D.L. Structure-function analysis of amino acid 74 of human RAMP1 and RAMP3 and its role in peptide interactions with adrenomedullin and calcitonin gene-related peptide receptors. Peptides. 2011;32:1060–1067. doi: 10.1016/j.peptides.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Rist B., Entzeroth M., Beck-Sickinger A.G. From micromolar to nanomolar affinity: a systematic approach to identify the binding site of CGRP at the human calcitonin gene-related peptide 1 receptor. J. Med. Chem. 1998;41:117–123. doi: 10.1021/jm970533r. [DOI] [PubMed] [Google Scholar]
- Runge S., Thøgersen H., Madsen K., Lau J., Rudolph R. Crystal structure of the ligand-bound glucagon-like peptide-1 receptor extracellular domain. J. Biol. Chem. 2008;283:11340–11347. doi: 10.1074/jbc.M708740200. [DOI] [PubMed] [Google Scholar]
- ter Haar E., Koth C.M., Abdul-Manan N., Swenson L., Coll J.T., Lippke J.A., Lepre C.A., Garcia-Guzman M., Moore J.M. Crystal structure of the ectodomain complex of the CGRP receptor, a class-B GPCR, reveals the site of drug antagonism. Structure. 2010;18:1083–1093. doi: 10.1016/j.str.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Underwood C.R., Garibay P., Knudsen L.B., Hastrup S., Peters G.H., Rudolph R., Reedtz-Runge S. Crystal structure of glucagon-like peptide-1 in complex with the extracellular domain of the glucagon-like peptide-1 receptor. J. Biol. Chem. 2010;285:723–730. doi: 10.1074/jbc.M109.033829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins H.A., Au M., Bobby R., Archbold J.K., Abdul-Manan N., Moore J.M., Middleditch M.J., Williams G.M., Brimble M.A., Dingley A.J., Hay D.L. Identification of key residues involved in adrenomedullin binding to the AM1 receptor. Br. J. Pharmacol. 2013;169:143–155. doi: 10.1111/bph.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins H.A., Rathbone D.L., Barwell J., Hay D.L., Poyner D.R. Structure-activity relationships for α-calcitonin gene-related peptide. Br. J. Pharmacol. 2013;170:1308–1322. doi: 10.1111/bph.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins H.A., Walker C.S., Ly K.N., Bailey R.J., Barwell J., Poyner D.R., Hay D.L. Receptor activity-modifying protein-dependent effects of mutations in the calcitonin receptor-like receptor: implications for adrenomedullin and calcitonin gene-related peptide pharmacology. Br. J. Pharmacol. 2014;171:772–788. doi: 10.1111/bph.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn M.D., Ballard C.C., Cowtan K.D., Dodson E.J., Emsley P., Evans P.R., Keegan R.M., Krissinel E.B., Leslie A.G., McCoy A. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootten D.L., Simms J., Hay D.L., Christopoulos A., Sexton P.M. Receptor activity modifying proteins and their potential as drug targets. Prog. Mol. Biol. Transl. Sci. 2010;91:53–79. doi: 10.1016/S1877-1173(10)91003-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.