Abstract
Background and Aims
High-resolution microendoscopy (HRME) is a novel, low-cost “optical biopsy” technology that allows for subcellular imaging. The study aim was to evaluate the learning curve of HRME for the differentiation of neoplastic from non-neoplastic colorectal polyps.
Methods
In a prospective cohort fashion, a total of 162 polyps from 97 patients at a single tertiary care center were imaged by HRME and classified in real-time as neoplastic (adenomatous, cancer) or non-neoplastic (normal, hyperplastic, inflammatory). Histopathology was the gold standard for comparison. Diagnostic accuracy was examined at three intervals over time throughout the study; the initial interval included the first 40 polyps, the middle interval included the next 40 polyps examined, and the final interval included the last 82 polyps examined.
Results
Sensitivity increased significantly from the initial interval (50%) to the middle interval (94%, p = 0.02) and the last interval (97%, p = 0.01). Similarly, specificity was 69% for the initial interval but increased to 92% (p = 0.07) in the middle interval and 96% (p = 0.02) in the last interval. Overall accuracy was 63% for the initial interval and then improved to 93% (p = 0.003) in the middle interval and 96% (p = 0.0007) in the last interval.
Conclusions
In conclusion, this in-vivo study demonstrates that an endoscopist without prior colon HRME experience can achieve greater than 90% accuracy for identifying neoplastic colorectal polyps after 40 polyps imaged. HRME is a promising modality to complement white-light endoscopy in differentiating neoplastic from non-neoplastic colorectal polyps.
Keywords: Colorectal polyps, adenoma classification, microendoscopy
Introduction
Colonoscopy with polypectomy has resulted in a 76% reduction in the incidence of colorectal cancer and a significant reduction in colorectal cancer mortality 1, 2. However, as white light endoscopy cannot differentiate between neoplastic and non-neoplastic polyps, current standards foster an inefficient practice of removing virtually every polyp for histopathologic examination. This is despite the fact that significantly less than half of all resected polyps end up being adenomas , i.e. neoplastic polyps with malignant potential 3. Therefore, an unnecessarily high number of polyps are being removed for formal histopathologic analysis, leading to increased costs and risks to the patient.
By decreasing the costs of excess histological analyses, upwards of thirty million dollars could be saved annually 4, 5. Moreover, a selective biopsy approach would also decrease procedure time and reduce anesthesia cost. Cost is not the only drawback of the current system of excess biopsies. Polypectomy remains the most important risk factor for adverse events during colonoscopy with significant bleeding occurring in up to 5% of polypectomies 6, 7.
In order to move to a selective biopsy practice, a tool that increases the endoscopist's ability to differentiate between neoplastic and non-neoplastic polyps on a real-time basis would be very advantageous. Novel endoscopic imaging techniques, such as chromoendoscopy, narrow-band-imaging (NBI), and confocal laser endomicroscopy (CLE), have been developed in effort to improve the ability of white-light endoscopy at classifying neoplastic polyps from non-neoplastic polyps. While NBI produced mixed results, chromoendoscopy had moderate success especially with colorectal neoplasia detection in patients with ulcerative colitis 8-15. Recently, CLE has shown the most promise with studies demonstrating accuracy ranging from 81 to 94% 16. However, CLE is limited by high cost and need for intravenous contrast. In order for an imaging technology to be widely implemented, it must be affordable, quickly learned and easily applied.
High-resolution microendoscopy (HRME) is a low-cost “optical biopsy” technology consisting of a 1 millimeter diameter fiber-optic bundle that allows for subcellular imaging at 1000x magnification at 4 micrometer resolution. HRME has already been found to be effective in gastrointestinal pathology 8, 17, 18,19. In order for a complementary imaging tool to enhance the ability of white-light endoscopy in classifying polyps, the modality must not only be inexpensive but also one that can be learned quickly and interpreted accurately. While the HRME equipment is inexpensive at approximately $3,500 20, 21, it is unclear if an endoscopist's ability to classify colorectal neoplasia using HRME improves with experience. The purpose of this study was to evaluate the learning curve associated with use of the HRME for the discrimination of non-neoplastic (normal, hyperplastic, inflammatory) from neoplastic (adenomatous, cancer) colorectal polyps.
Methods
Patient Selection
The study was approved by the Icahn School of Medicine at Mount Sinai Institutional Review Board on November 24th, 2009 and all patients signed informed consent prior to enrolling. The clinical trial was registered on clinicaltrials.gov in June 2011 (NCT01384240). One hundred and fifty patients undergoing routine, scheduled screening or surveillance colonoscopies were prospectively enrolled at a single tertiary care center from July 2011 to December 2012. Of these, ninety-seven patients had visible polyps which were included in the final analysis. Patients were included if they were scheduled for a colonoscopy for screening or routine polyp surveillance, willing to sign informed consent and able to complete a telephone follow-up call post-procedure. Exclusion criteria were an unhealthy or unfit patient for standard colonoscopy or one who was unwilling to sign informed consent.
High Resolution Microendoscopy (HRME)
The description and technical specifications of the high-resolution microendoscope were previously described in detail by Muldoon et al and Pierce et al 20, 21. Briefly, the HRME unit comprises of a charge-coupled camera device, a flexible imaging probe, and a topical contrast agent. The imaging probe is a 1 millimeter-diameter fiber optic bundle that is passed through the biopsy channel or accessory port of any standard colonoscope. Once the mucosal surface has been sprayed by proflavine, a fluorescent agent, the tissue reflects fluorescence in response to the LED illumination. In essence, HRME functions as a battery-powered fluorescence microscope that like confocal endomicroscopy, allows visualization of the mucosa at a 1000x magnification. HRME provides a 720 micron field of view and 4.4 micron spatial resolution, both of which are less than endoscope-based confocal endomicroscopy (475 x 475 micron field of view, 7 micron spatial resolution) but similar to probe-based confocal endomicroscopy (240 micron field of view, 1 to 3.5 micron resolution).22 For HRME, the cost of the charge-coupled camera device is approximately $1500 and each probe costs approximately $300. The probe needs to be polished every 85-90 patients. CLE systems, in general, cost over $100,000.22 In this study, the HRME probe was repaired once..
Training in the HRME colon classification system
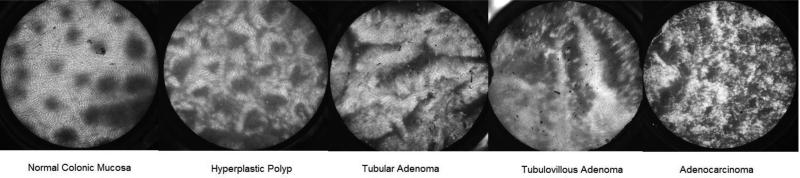
The HRME colon classification system was developed and described in detail by Chang et al 5. Briefly, polyp classification criteria were developed by two expert gastrointestinal pathologists based on glandular morphology, epithelial thickness and nuclear arrangement seen in corresponding HRME images and histology slides. Consensus HRME criteria were then established based on the WHO histopathologic criteria for each category of colorectal polyps: normal colonic mucosa, hyperplastic polyp, tubular adenoma, tubulovillous adenoma, and adenocarcinoma23. An HRME read of “nonneoplastic” was assigned to classification patterns for normal colonic mucosa, inflammatory or hyperplastic polyps. An HRME read of “neoplastic” included tubular adenoma, tubulovillous adenoma and adenocarcinoma. In the ex vivo, post-hoc study of this classification and training system, accuracy rates of 87% and 85% were achieved for the HRME characterization of polyps by novice and expert endoscopists respectively 5. Using this classification and training session model developed by Chang et al, the endoscopist in our study underwent a 5-minute scripted presentation of video images where the HRME image of each category of colorectal polyp was described and specific HRME features that would alert the endoscopist to identify that category were detailed. Representative HRME images of the differing morphological patterns of the various types of colorectal polyps are shown in Figure 1. Emphasis during the training session was placed on the HRME characteristics unique to the general categories of neoplastic and non-neoplastic polyps. Following the training session, the endoscopist took a 37-image test and received feedback on the incorrect answers.
Figure 1.
Representative HRME Images
Performing the colonoscopy with HRME
Upon completion of the training in HRME colon classification, the endoscopist performed the colonoscopy with HRME. In this study, high-definition white-light endoscopy (Olympus SIFQ80) was first performed to detect colonic polyps. Upon detection of a colonic polyp by white-light endoscopy (WLE), the endoscopist would make a WLE diagnosis of non-neoplastic (normal, hyperplastic, or inflammatory) or neoplastic (adenoma or cancer). Once the WLE read was recorded, 1-4 ml of proflavine dye (0.01%) was sprayed onto the mucosal surface of the polyp via an endoscopic spray catheter. Proflavine is a fluorescent topical contrast agent that highlights cell nuclei with peak absorption and emission wavelengths of 445nm and 515nm, respectively. Originally developed and used as a topical antiseptic agent, proflavine was used in the study as an investigational drug under the FDA IND 102,217. Proflavine has been used in more than 500 patients in our studies worldwide (in the US, only commercial grade is available by Sigma). However, proflavine is used in confocal imaging in Asia and Australia without an IND. No adverse events or toxicity have been reported in human studies. After the proflavine was applied topically, the HRME probe was inserted through the biopsy port of the colonoscope and the fiber-optic tip was positioned directly onto the surface of the polyp. Once the probe was on the polyp, images appeared on the connected laptop at a rate of 12 frames per second. The average time required to apply the proflavine, position the probe, and make the optical diagnosis ranged from 50 to 80 seconds per polyp. Using the classification system developed by Chang et al, the endoscopist then made the in-vivo optical diagnosis of neoplastic or non-neoplastic 5. The imaged polyps were then removed by forceps or snare polypectomy and sent for formal histopathology analysis as per standard of care. A single expert gastrointestinal pathologist who was blinded to both the WLE and HRME diagnoses made the final diagnosis of non-neoplastic or neoplastic polyp, which served as the gold standard diagnosis for comparison.
Statistical Analysis
Log binomial regression models were used to estimate various measures of performance: sensitivity, specificity, positive predictive value, negative predictive value and accuracy. The models were implemented in SAS's Proc Genmod with a log link function, a binomial distribution and a repeated statement was used to account for the correlation among multiple biopsies on the same patient 24. Performance measures were then compared among the first 40, middle 40 and last 82 biopsies using these same regression models. The plot of HRME accuracy as a function of the chronological sequence of the biopsy was derived using a modified poisson regression model introduced by Zou. This model was employed using a log link function, a poisson distribution and a repeated statement was used to again account for the correlation among multiple biopsies on the same patient. The analyses were performed using PROC GENMOD in SAS v.9.2. (SAS Institute Inc. Cary, NC 2004). The sample size was calculated post hoc based on the fact that with n=162 and a postulated value for sensitivity or specificity of at least 80%, this sample size was guaranteed to estimate the performance characteristic with a precision (95% confidence interval) of +/− 6%. An interval of 40 polyps was chosen initially based on previously published CLE learning curve papers that have identified 30 to 50 polyps as the threshold to achieve moderate accuracy.16, 25 At the end of the second phase of 40 polyps, an interim analysis was done. Given the significant improvement in performance characteristics, the remainder of the trial (82 polyps) was performed without interruption to see if the increased diagnostic accuracy could be maintained.
Results
A total of 162 polyps from 97 patients were detected by WLE, imaged by HRME and evaluated histologically. Forty-one percent (n=67) of the polyps were neoplastic and fifty-three percent (n=86) were smaller than 10mm. Table 1 shows the breakdown of neoplastic versus non-neoplastic polyps among the sequential subgroups of biopsies taken.
Table 1.
Neoplasia Rate
| Polyps Imaged/Biopsied | Percentage of Neoplastic Polyps | p-value |
|---|---|---|
| Initial Interval: 1-40 | 35.0% | |
| Middle Interval: 41-80 | 40.0% | P = 0.69 (NS) |
| Final Interval: 81-162 | 45.1% | P = 0.34 (NS) |
Table 2 describes the performance characteristics of HRME in differentiating neoplastic and nonneoplastic polyps in the first 40 biopsies, the middle 40 biopsies, and then the last 82 biopsies. Sensitivity and specificity were 49.3% and 68.7%, respectively in the first 40 biopsies. Both measures improved to 93.6% and 91.5%, respectively, in biopsies 41 through 80. In the last 82 biopsies, HRME became even more sensitive (97.3%) and specific (95.5%). HRME's sensitivity and specificity significantly improved from the first interval of biopsies to the middle interval and from the first interval to the final interval. The improvement was maintained between the middle and final intervals. The PPV (positive predictive value) of the HRME was 88.2% in the middle set and 94.7% in the last set, both values being significantly greater than the PPV calculated for the first 40 biopsies (46.6%, p<0.04). Likewise, the NPV (negative predictive value) was only 71.9% for the first 40 biopsies but increased to 95.6% in the middle interval (p=0.0278) and 97.7% in the last 82 biopsies (p=0.0131).
Table 2.
Performance Characteristics
| Polyps Imaged/Biopsied | Initial Interval: 1-40 | Middle Interval: 41-80 | p-value | Final Interval: 81-162 | p-value* |
|---|---|---|---|---|---|
| Sensitivity | 49.3% | 93.6% | 0.0211 | 97.3% | 0.0126 |
| Specificity | 68.7% | 91.5% | 0.0668 | 95.5% | 0.0243 |
| Positive Predictive Value | 46.6% | 88.2% | 0.0367 | 94.7% | 0.0170 |
| Negative Predictive Value | 71.9% | 95.6% | 0.0278 | 97.7% | 0.0131 |
| Accuracy | 62.5% | 92.5% | 0.0032 | 96.3% | 0.0007 |
| Accuracy of Polyps < 10 mm (n = 86) | 44.4% | 90.5% | 0.0041 | 95.4% | 0.0009 |
p-value between characteristics of polyps 1-40 and polyps 81-162
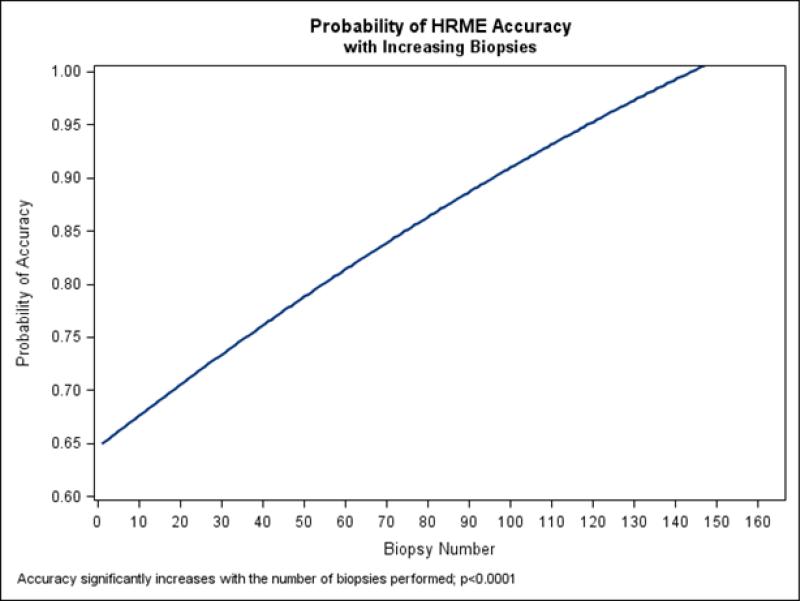
Figure 2 shows the accuracy probability of HRME compared to the gold standard of histopathology as a function of the chronological sequence of the HRME biopsy taken. The probability of HRME accuracy increased with each subsequent “optical” HRME biopsy taken. Accuracy was 62.5% in the first 40 biopsies and significantly increased to 92.5% in the middle set (p=0.0032). The endoscopist remained highly accurate in HRME at 96.3% in the last 82 biopsies.
Figure 2.
HRME Accuracy with Increasing Polyps Imaged/Biopsied
Discussion
This real-time, in-vivo study demonstrates that an endoscopist can achieve accuracy greater than 90% after a brief training session and 40 “optical” biopsies performed. Moreover, once the endoscopist moves past this brief learning period, the accuracy remains at an excellent level.
While this is the first such study of the HRME modality, other trials have assessed the learning curve of confocal laser endomicroscopy (CLE) in colorectal neoplasia. Buchner et al evaluated probe-based CLE (pCLE) during the differentiation of 76 colorectal polyps in 54 patients 25. All colonoscopies were performed by a single endoscopist but 11 separate observers were subsequently trained post-hoc and tested on the pCLE images. For all 11 observers, accuracy was 63% for the first 20 polyps, increased to 79% for the third 20 polyps and then 86% for the last 16 polyps. Kuiper et al conducted a similar study of the endoscope-based CLE (eCLE) system 16. This was also a post-hoc analysis using 3 observers for 90 polyps detected by a single endoscopist in 47 patients. Results showed that after a brief training set, all 3 observers achieved greater than 90% accuracy with no change in accuracy over the subsequent subsets of 30 polyps interpreted.
In 2011, the American Society of Gastrointestinal Endoscopy (ASGE) issued a statement that in order for a new technology to guide the decision of not resecting diminutive and suspected non-neoplastic polyps, it must demonstrate a greater than 90% negative predictive value for adenomatous histology 26. In our study, HRME reached a negative predictive value of 95.7% after the first 40 polyps and then maintained a value of 97.7% for the last 82 polyps. Given these findings, the HRME modality could qualify as an excellent complementary tool for endoscopists during the decision of leaving diminutive, non-neoplastic appearing polyps alone without resection.
Compared to CLE studies, our study has several notable strengths. While both CLE studies demonstrated that the probe and endoscope-based systems can be learned quickly after brief training sets, they were not in-vivo real-time assessments. Rather, the learning curve was evaluated through a sequential training and test set with multiple observers. In the case of Kuiper et al, the best possible CLE images were pre-selected, adding an additional layer of bias to the study. Our study was a true real-time assessment of the HRME's ability to complement white-light endoscopy in differentiating between neoplastic and non-neoplastic colorectal polyps. In spite of the fact that images in our study were obtained in-vivo and therefore not quality controlled, the HRME was able to achieve, by the final 82 lesions, greater sensitivity, specificity and accuracy compared to either CLE systems. In the pCLE system, accuracy only reached 80% after the first 60 lesions while the HRME reached 90% after 40 biopsies. Similarly, sensitivity ranged from 71% to 86% for the first 66 lesions in the pCLE modality while HRME again was greater than 90% after 40 biopsies. Specificity reached a maximum of 67% for the pCLE compared to 96% for the HRME. Finally, a further limitation of the pCLE study was that the proportion of neoplastic lesions was not the same across each group of 10 or 20 polyps. In our study, the percentage of neoplastic polyps did not change significantly from subset to subset.
There are multiple potential limitations to our study. While the single endoscopist who performed the colonoscopies and made the in-vivo HRME interpretations was not previously trained in the HRME technology at the time of study initiation, she was an advanced endoscopist and thus perhaps, not representative of the general gastroenterologist in practice. Nevertheless, most gastroenterologists performing complementary modalities such as chromoendoscopy or CLE, tend to also be advanced endoscopists. Furthermore, while the CLE studies used multiple observers, our study had only one endoscopist and therefore, we could not assess inter-observer agreement. Finally, while the CLE studies selected quality-controlled images for training and testing purposes, we did not assess the quality of our HRME images for this study. However, we believe that this fact further enhances our study as a true invivo assessment modality. Despite the potential for suboptimal images, the HRME produced excellent performance characteristics compared to its quality-controlled predecessors.
Both CLE and HRME provide 1000x magnification views and therefore, in any given image, only evaluate a portion of the polyp and thus could theoretically lead to sampling error of lesions that may have both neoplastic and non-neoplastic components. However, the HRME takes images at an approximate rate of 12 per second and therefore, inevitably, multiple images from slightly different locations along the polyp are captured. This significantly reduces sampling error. Sessile serrated adenomas/polyps (SSA/Ps) are most likely to have predominant benign features with subtle neoplastic features and therefore, at high risk for such a sampling error. However, all 3 SSA/Ps that were identified histologically in this study were diagnosed correctly as neoplastic lesions by HRME. Given the small sample size, it is not appropriate to draw any conclusions about the diagnostic capability of HRME in terms of SSA/Ps but it is encouraging that they were identified as neoplastic as opposed to non-neoplastic lesions. SSA/Ps presented with more glandular disarray and increased stroma to crypt ratio compared to hyperplastic polyps but did not have the elongated nuclei seen typically in tubular adenomas.
In conclusion, HRME is a promising modality to complement white-light endoscopy in the identification of non-neoplastic versus neoplastic colorectal polyps. As an inexpensive tool compared to the more costly CLE systems, it has the potential to truly allow the implementation of “resect and discard” approaches during colorectal cancer screening. Our study shows that HRME can be learned quickly with accuracy greater than 90% obtained just after a brief training session and 40 imaged polyps. Moreover, once competency is achieved, it can be maintained. A prospective study with multiple endoscopists to enhance the generalizability of these results and assess the inter-observer variability is being planned.
Acknowledgments
This work was supported by the NIH/NCI grant “Optical Systems for In Vivo Molecular Imaging of Cancer” (CA 103830-07). N. Parikh, D. Perl, M. Lee, S. Chang, A. Polydorides, E. Moshier, J. Godbold, E. Zhou, J. Mitcham, and S. Anandasabapathy have no conflicts of interest to report. Rebecca Richards-Kortum serves as an unpaid scientific advisor to Remicalm LLC, holds patents related to optical diagnostic technologies that have been licensed to Remicalm LLC, and holds minority ownership in Remicalm LLC.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/jgh.12937
References
- 1.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. The New England journal of medicine. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 2.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond SJ, Enestvedt BK, Jiang Z, et al. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointest Endosc. 2011;74:135–40. doi: 10.1016/j.gie.2011.03.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. 2010;8:865–9. 9 e1–3. doi: 10.1016/j.cgh.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Chang SS, Shukla R, Polydorides AD, et al. High resolution microendoscopy for classification of colorectal polyps. Endoscopy. 2013;45:553–9. doi: 10.1055/s-0032-1326502. [DOI] [PubMed] [Google Scholar]
- 6.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–906. 906 e1. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS. Delayed postpolypectomy bleeding. J Korean Soc Coloproctol. 2011;27:3. doi: 10.3393/jksc.2011.27.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trecca A, Gaj F, Di Lorenzo GP, et al. Improved detection of colorectal neoplasms with selective use of chromoendoscopy in 2005 consecutive patients. Tech Coloproctol. 2006. 10:339–44. doi: 10.1007/s10151-006-0304-z. [DOI] [PubMed] [Google Scholar]
- 9.Kiesslich R, von Bergh M, Hahn M, Hermann G, Jung M. Chromoendoscopy with indigocarmine improves the detection of adenomatous and nonadenomatous lesions in the colon. Endoscopy. 2001;33:1001–6. doi: 10.1055/s-2001-18932. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Kim JW, Cho YK, et al. Detection of colorectal adenomas by routine chromoendoscopy with indigocarmine. Am J Gastroenterol. 2003;98:1284–8. doi: 10.1111/j.1572-0241.2003.07473.x. [DOI] [PubMed] [Google Scholar]
- 11.Pasha SF, Leighton JA, Das A, et al. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012;107:363–70. doi: 10.1038/ajg.2011.436. quiz 71. [DOI] [PubMed] [Google Scholar]
- 12.Kaltenbach T, Friedland S, Soetikno R. A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut. 2008;57:1406–12. doi: 10.1136/gut.2007.137984. [DOI] [PubMed] [Google Scholar]
- 13.Rastogi A, Early DS, Gupta N, et al. Randomized, controlled trial of standard-definition white-light, high-definition white-light, and narrow-band imaging colonoscopy for the detection of colon polyps and prediction of polyp histology. Gastrointest Endosc. 2011;74:593–602. doi: 10.1016/j.gie.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 14.Kiesslich R, Goetz M, Lammersdorf K, et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874–82. doi: 10.1053/j.gastro.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 15.Marion JF, Waye JD, Present DH, et al. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103:2342–9. doi: 10.1111/j.1572-0241.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuiper T, Kiesslich R, Ponsioen C, Fockens P, Dekker E. The learning curve, accuracy, and interobserver agreement of endoscope-based confocal laser endomicroscopy for the differentiation of colorectal lesions. Gastrointest Endosc. 2012;75:1211–7. doi: 10.1016/j.gie.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Vila PM, Kingsley MJ, Polydorides AD, et al. Accuracy and interrater reliability for the diagnosis of Barrett's neoplasia among users of a novel, portable high-resolution microendoscope. Dis Esophagus. 2013 doi: 10.1111/dote.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regunathan R, Woo J, Pierce MC, et al. Feasibility and preliminary accuracy of high-resolution imaging of the liver and pancreas using FNA compatible microendoscopy (with video). Gastrointest Endosc. 2012;76:293–300. doi: 10.1016/j.gie.2012.04.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MH VP, Polydorides AD, Mitcham J, Moshier E, Godbold JH, Richards-Kortum R, Anandasabapathy S. Diagnostic accuracy of a novel low cost microendoscope for the detection of Barrett's neoplasia: a prospective, single-center trial. Gastroenterology. 2012;142:330–5. [Google Scholar]
- 20.Muldoon TJ, Anandasabapathy S, Maru D, Richards-Kortum R. High-resolution imaging in Barrett's esophagus: a novel, low-cost endoscopic microscope. Gastrointest Endosc. 2008;68:737–44. doi: 10.1016/j.gie.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce M, Yu D, Richards-Kortum R. High-resolution fiber-optic microendoscopy for in situ cellular imaging. J Vis Exp. 2011 doi: 10.3791/2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Committee AT, Chauhan SS, Abu Dayyeh BK, et al. Confocal laser endomicroscopy. Gastrointest Endosc. 2014;80:928–38. doi: 10.1016/j.gie.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton SR BF, Boffetta P. Carcinoma of the colon and rectum. In: Bosman FTCF, Hruban RH, editors. WHO Classification of Tumours of the Digestive System. IARC Press; 2010. pp. 134–46. [Google Scholar]
- 24.Diggle PJLKY, Zeger SL. Analysis of longitudinal data. Oxford University Press; New York: 1994. [Google Scholar]
- 25.Buchner AM, Gomez V, Heckman MG, et al. The learning curve of in vivo probe-based confocal laser endomicroscopy for prediction of colorectal neoplasia. Gastrointest Endosc. 2011;73:556–60. doi: 10.1016/j.gie.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Rex DK, Kahi C, O'Brien M, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419–22. doi: 10.1016/j.gie.2011.01.023. [DOI] [PubMed] [Google Scholar]