Abstract
Objectives
The metaplastic intestinal epithelium in Barrett esophagus (BE) occasionally contains Paneth cells; however, little is known regarding the prevalence and significance of Paneth cell metaplasia (PCM) in BE.
Methods
We evaluated 757 esophageal biopsy specimens with intestinal metaplasia (IM) for PCM. Outcome analysis was performed in 299 cases with complete clinical data using multinomial logistic regression.
Results
Thirty-one percent (234/757) of the IM cases showed PCM. Paneth cells are decreased when BE epithelium becomes increasingly dysplastic. Long-segment BE shows significantly more PCM than short-segment BE. On follow-up biopsies, patients without PCM (NPCM) are three times more likely to regress than patients with PCM, regardless of dysplasia, BE segment length, age, or sex. However, there is no significant difference in terms of progression to dysplasia/adenocarcinoma between the PCM and NPCM groups.
Conclusions
The presence of PCM is associated with less disease regression and is not associated with more disease progression.
Keywords: Paneth cell, Barrett esophagus, Dysplasia, Esophageal adenocarcinoma, GERD
INTRODUCTION
The incidence of esophageal adenocarcinoma has increased sixfold in the past 30 years in the Western world, faster than any other major malignancies. Evidence has shown that this increase is due to true growth of disease burden and unrelated to disease reclassification or overdiagnosis from increased usage of upper gastrointestinal (GI) endoscopy.1 Most esophageal adenocarcinomas develop from Barrett esophagus (BE), a form of “adaptive protection”2 against chronic insult from gastric acids and bile salts secondary to gastroesophageal reflux disease (GERD). On average, BE carries a 0.5% annum increased risk for malignancy, but only a small percentage of patients with BE will develop adenocarcinoma. Therefore, it is critical to identify the susceptible patient population so that diligent surveillance could be performed to detect malignant transformation at an early and possibly curable stage.
Despite intense studies of BE, our knowledge is still limited on how to accurately predict disease progression. Intriguingly, some patients with BE will rapidly develop adenocarcinoma after the first diagnosis of high-grade dysplasia (HGD), some patients will regress to lesser grades of dysplasia or even no intestinal metaplasia (IM), whereas others will live with high-grade dysplasia (HGD) for many years.2 Currently, histologic identification of dysplasia remains the gold standard for identifying patients with BE at an increased risk for progression to adenocarcinoma. Testing for other biomarkers, including mucin core polypeptide expression,3 DNA aneuploidy, and microRNA,4 may be helpful, but no single best biomarker is available thus far to provide a sensitivity and specificity adequate for optimal patient management.
Paneth cells are occasionally observed in esophagus with complete IM, first described by Schreiber et al.5 However, little is known regarding the prevalence and significance of Paneth cell metaplasia (PCM) in BE. We examined 757 esophageal biopsy specimens with IM to evaluate if there is a correlation between PCM and dysplasia/adenocarcinoma. Multinomial logistic regression analysis was performed on 299 cases with complete clinical information to assess if PCM and other covariates affect patient outcome.
MATERIALS AND METHODS
Study Group
The surgical pathology database at The Ohio State University Wexner Medical Center Pathology Department was searched for esophageal biopsy specimens with a diagnosis of IM. Biopsy specimens procured from the gastroesophageal junction were excluded, in an attempt to limit the study group to true BE cases and avoid cases with IM at the gastric cardia. In total, 950 consecutive cases were identified, and 757 cases were available for histologic review. Patients’ age, sex, and follow-up esophageal biopsy results were noted.
Among the 757 cases, we identified 299 cases (89 with PCM and 210 without PCM [NPCM]) that have complete clinical data for a more in-depth statistical analysis. All of the 299 cases have follow-up data for at least 1 year (mean [SD] follow-up time, 3.5 [1.6] years) and have information regarding length of the BE segment, duration of disease, and proton pump inhibitor (PPI) treatment history. Short-segment BE is defined as Barrett mucosa less than 3 cm, and long segment refers to Barrett mucosa equal to or more than 3 cm. The few cases of HGD on initial biopsy, which subsequently received endoscopic mucosal resection, radiofrequency ablation, or esophagectomy, were excluded from the outcome analysis, since the outcome of these patients will be different from the natural history of the disease. All cases with initial diagnosis of carcinoma were also excluded from the outcome analysis, since carcinoma is considered the end point of the study.
Histologic Review
H&E slides were available in all cases. All six levels in each biopsy specimen were examined for the presence of PCM, since the distribution of Paneth cells may be sparse, with only a few cells present on one or two levels. Paneth cells were identified by their distinct eosinophilic cytoplasmic granules on H&E stain using conventional light microscopy. Cases with IM containing PCM were further evaluated by two pathologists (W.C. and M.M.Y.) with an interest in GI pathology for dysplasia, location of PCM, associated inflammation, pancreatic acinar metaplasia, and multilayered epithelium.
Statistical Methods
Patients’ age between PCM and NPCM groups was compared using the Student t test. Comparison study between PCM and NPCM groups, as well as PCM frequencies between different groups of dysplastic BE, was performed using the Fisher exact test. A P value of less than .05 was considered statistically significant. Multinomial logistic regression was employed to study association of the presence of PCM with disease outcome (progression/same/regression). In this study, regression was defined as a finding of a lower degree of dysplasia, or no dysplasia, in biopsy specimens from a patient with prior samples that showed dysplasia. In other words, regression was called if the patient’s follow-up biopsy specimens demonstrated a lower degree of dysplasia in the sequence of HGD, low-grade dysplasia (LGD), indefinite for dysplasia (IFD), negative for dysplasia (NFD), and no IM. Vice versa, progression in our study refers to greater degree of dysplasia in the above sequence. Patients’ age, sex, BE segment length, and dysplasia were adjusted in the model. No significant interaction was found between covariates. Odds ratios were reported.
RESULTS
Demographic Features
Patients’ age ranged from 24 to 88 years (mean [SD], 61.5 [12.9] years) in the PCM group and 22 to 92 years (59.5 [12.8] years) in the NPCM group. The difference was statistically significant (P = .0413). There was no significant difference in sex ratio between PCM and NPCM groups (male-to-female ratio of 1.8:1 and 2.4:1, respectively, P = .2592).
Prevalence of PCM in BE
Paneth cells were identified in 31% (234 of 757) of the esophageal biopsy specimens on routine H&E-stained slides. The prevalence of PCM in nondysplastic, dysplastic, and malignant BE was compared. Significantly more frequent PCM was found in cases of IM with NFD (196/626 [31%]) than that of IM with esophageal adenocarcinoma (EAC) (1/21 [5%], P = .0069). Significantly more frequent PCM was also present in the IFD and LGD groups (32/86 [37%]) than in the HGD and EAC groups (6/45 [13%], P = .0045) (Table 1).
Table 1.
Prevalence of Paneth Cell Metaplasia in 757 Barrett Esophagus Cases
| Group | No. (%) of Patients
|
||||
|---|---|---|---|---|---|
| NFD (n=626) | IFD (n=48) | LGD (n=38) | HGD (n=24) | EAC (n=21) | |
| PCM | 196 (31) | 18 (38) | 14 (37) | 5(21) | 1 (5) |
| NPCM | 430 (69) | 30 (62) | 24 (63) | 19 (79) | 20 (95) |
| P valuea | .4214 | .4764 | .3695 | .0069 | |
EAC, esophageal adenocarcinoma; HGD, high-grade dysplasia; IFD, indefinite for dysplasia; LGD, low-grade dysplasia; NFD, negative for dysplasia; NPCM, no Paneth cell metaplasia; PCM, Paneth cell metaplasia.
P value of each group compared with NFD group. P value of group (IFD + LGD) compared with (HGD + EAC) was .0045.
In the 299 cases with complete clinical data, PCM was more frequently found in patients with long-segment BE (42/115 [37%]) than in patients with short-segment BE (44/184 [24%], P = .0253). There was no significant difference in the prevalence of PCM between patients who had PPI therapy before biopsy (74/259 [29%]) and patients who did not (7/40 [18%], P = .1813).
Morphologic Features of PCM in BE
Paneth cells were most frequently seen at the base and rarely at the neck of the metaplastic glands (Image 1A). They were distributed singly, in small clusters, or circumferentially (Image 1B) within the crypt. Background mild to moderate chronic inflammation was seen in most cases. Occasional acute inflammation was also present. Morphologically, Paneth cells in the metaplastic intestinal epithelium exhibited small, basally oriented, ovoid nuclei, with cytoplasmic eosinophilic, refractile granules concentrated toward the apical side of the cell. No Paneth cell dysplasia was observed. Degranulation of the Paneth cells, evidenced by the presence of eosinophilic granules in the crypt lumen near the Paneth cells (Image 1C), was occasionally present. Less frequently, scattered Paneth cells were seen within the dysplastic glands in IM (Image ID). Interestingly, Paneth cells were also rarely seen in nonmetaplastic mucinous glands (Image IE) or hybrid mucinous/metaplastic glands (Image IF). Multilayered epithelium and pancreatic acinar metaplasia were occasionally seen.
Image 1.
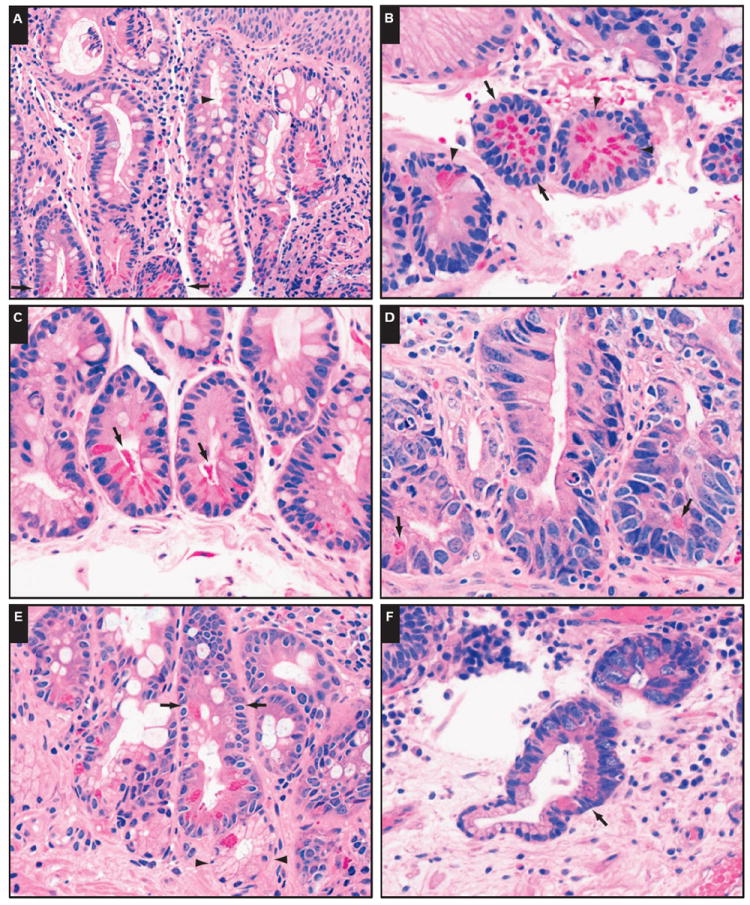
Paneth cell metaplasia (PCM) in distal esophageal biopsy specimens with intestinal metaplasia. A, PCM is seen primarily at the base (arrows) and rarely at the neck (arrowhead) of metaplastic crypts (H&E, ×200). B, Paneth cells can be distributed circumferentially (arrows) or scattered (arrowheads) (H&E, ×400). C, Degranulation of Paneth cells (arrows) is occasionally seen (H&E, ×400). D, PCM in dysplastic crypts (arrows) (H&E, × 400). E, PCM in metaplastic glands (arrows) and nonmetaplastic mucinous glands (arrowheads) (H&E, ×400). F, PCM in a hybrid mucinous/metaplastic gland (arrow) (H&E, ×400).
Distribution of Paneth Cells in BE With LGD
In eight (57%) of 14 BE cases with LGD, Paneth cells were present in both dysplastic and nondysplastic glands, with the number of Paneth cells in the nondysplastic glands outnumbering that in the dysplastic glands. In five (36%) of 14 cases, Paneth cells were present only in nondysplastic glands adjacent to the dysplastic glands, and in one (7%) of 14 cases, Paneth cells were present only in dysplastic glands. Nine (64%) of 14 cases also showed focal acute inflammation in addition to the chronic inflammation seen in all BE cases.
Follow-up of Patients With BE With and Without PCM
We analyzed the outcome data on the 299 patients with more than 1 year of follow-up. In the group of patients with BE with PCM, 67 (69%) of 97 cases remained the same, 11 (11%) of 97 progressed to higher grades of dysplasia or carcinoma, and 19 (20%) of 97 regressed to lesser grades of dysplasia, no dysplasia, or even no IM (Table 2). In contrast, the NPCM group showed significantly more cases regressing to lesser grades of dysplasia, no dysplasia, or no IM (63/202 [31%], P = .0383). There is no significant difference between PCM and NPCM groups for cases that remained the same (P = .0987) or progressed (P = .6899).
Table 2.
Outcome Analysis of 299 Barrett Esophagus Cases With and Without Paneth Cell Metaplasia
| Group | No. (%) of Patients
|
||
|---|---|---|---|
| Remain Same | Progress to Higher Grade of Dysplasia | Regress to Lesser Grade of Dysplasia | |
| PCM (n=97) | 67 (69) | 11 (11) | 19 (20) |
| NPCM (n=202) | 119 (59) | 20 (10) | 63 (31) |
| P valuea | 0.987 | .6899 | .0383 |
NPCM, no Paneth cell metaplasia; PCM, Paneth cell metaplasia.
When comparing between PCM and NPCM groups.
Multinomial logistic regression study showed that NPCM patients were three times more likely to regress than patients with PCM, regardless of other covariates, including dysplasia, BE segment length, age, or sex. However, there is no statistical significance in terms of progression between PCM and NPCM groups (Table 3).
Table 3.
Odds Ratio Estimates Evaluating Multiple Variates Affecting Outcome in 299 Barrett Esophagus Cases
| Effect | Outcome Category | Point Estimate | 95% Wald Confidence Limits |
|---|---|---|---|
| PCM no vs yes | Progression | 2.026 | 0.748-5.490 |
| PCM no vs yes | Regression | 3.230 | 1.466-7.115 |
| BE SS vs LS | Progression | 0.426 | 0.170-1.069 |
| BE SS vs LS | Regression | 4.028 | 1.895-8.560 |
| LGD/HGD yes vs no | Progression | 7.759 | 1.306-46.083 |
| LGD/HGD yes vs no | Regression | 27.626 | 5.554-137.411 |
| Age (10 y older) | Progression | 2.328 | 1.470-3.684 |
| Age (10 y older) | Regression | 0.752 | 0.566-0.999 |
| Male vs female | Progression | 10.394 | 2.096-51.554 |
| Male vs female | Regression | 0.537 | 0.280-1.028 |
BE, Barrett esophagus; HGD, high-grade dysplasia; LGD, low-grade dysplasia; LS, long segment; NPCM, no Paneth cell metaplasia; PCM, Paneth cell metaplasia; SS, short segment.
In addition, in our cohort, regardless of PCM or not, those patients with short-segment BE were four times more likely to regress than those with long-segment BE (Table 3). Patients with BE positive for dysplasia (LGD or HGD) were 7.8 times more likely to progress than patients with BE NFD. Male BE patients were 10 times more likely to progress than female patients. Patients who were 10 years older were two times more likely to progress than patients who were 10 years younger. Neither duration of disease nor history of PPI therapy showed any association with disease outcome.
DISCUSSION
Paneth Cells
After half a century since its first discovery, the Paneth cell is still a mysterious cell type with its function less defined than other cell types in the intestinal epithelium. Recent studies gained exciting insight into Paneth cells’ role in the innate immunity and the homeostasis of intestinal mucosa. Dysfunction of Paneth cells leads to disruption of the intestinal mucosal barrier, dysbiosis, and impaired regeneration, which contribute to the pathogenesis of intestinal inflammatory and infectious diseases,6-8 such as ileal Crohn disease9 and neonatal necrotizing enteritis.10
Paneth cells are normally present in the small intestine, mainly distributed at the base of the crypts of Lieberkühn. Paneth cells also reside sparsely in the proximal colon and appendix but are usually absent in the rest of the luminal GI tract. However, PCM has been reported in a variety of organs/tissues that are subjected to chronic inflammation, including those within the GI tract, such as the esophagus and stomach, as well as those outside the GI tract, such as the urinary epithelium and gallbladder.11
Histologically, Paneth cells are pyramidal-shaped secretory epithelial cells that show prominent eosinophilic cytoplasmic granules on H&E stain. Early ultra-structural and cytochemical studies have shown that these granules contain heavy metals, lysozymes, and immunoglobulin IgA and IgG.11 Recent studies identified additional granule contents, including antimicrobials secreted by Paneth cells, such as human α-defensin-5 (HD5), human α-defensin-6, and secretory phospholipase A2.12 The differentiation and function of Paneth cells are regulated by the APC/β-catenin/Tcf pathway.13-16
Prevalence of Paneth Cells in BE
The presence of Paneth cells in BE has been described in a few earlier works with small patient cohorts, but the prevalence and significance of PCM in BE are unclear. We found that 31% of the distal esophageal biopsy specimens with IM showed PCM, in concordance with the previously reported frequencies (31% to ~50%).5,17,18 There is no significant difference in sex ratios between the PCM and NPCM groups.
We noted that Paneth cells are decreased in advanced neoplasia (see Table 1), which seems to parallel what has been described for goblet cells. The decreased incidence of specialized intestinal cells in the metaplastic epithelium, such as Paneth cells and goblet cells, may be explained by the fast clonal expansion of the neoplastic epithelial cells that take over the regenerative efforts of the benign meta plastic epithelium.
Role of Paneth Cells in BE
It is unclear why PCM is present in some cases of BE but not in others. This could be the result of sampling error, different stage of the disease, or different responses of the metaplastic epithelium to the environment, based on the severity of mucosal injury from GERD and whether there is concurrent bacterial infection, among others.
As a member of the innate immunity, Paneth cells help to maintain homeostasis of the small intestinal mucosa where bacterial load is kept at a lower level than the large intestine,6 whereas gastric acid and bile salt levels are relatively high. In animal models and in vitro studies of BE, acid and/or bile could induce columnar metaplasia of the distal-esophageal-squamous mucosa.2 It is tempting to speculate that the columnar mucosa can inheritably handle acid/bile insults and associated inflammation better than squamous mucosa, hence the IM in BE.
A mild to moderate mucosal chronic inflammation is a universal finding in BE with and without PCM. In addition, focal acute inflammation and/or increased chronic inflammation are frequently observed in cases with morphologic features of increased Paneth cell activity, such as circumferential distribution of Paneth cells within the crypt and luminal degranulation. Notably, the highest PCM incidence occurs in BE with IFD (38%) and LGD (37%), in which cases focal acute inflammation is frequent. Furthermore, long-segment BE cases show more frequent PCM than do short-segment cases, which may suggest PCM as a mucosal response to more severe/prolonged acid/bile salt insults. Interestingly, in patients with active celiac disease, increased HD5 and lysozyme levels are seen in metaplastic Paneth cells in the upper two-thirds of the jejunal intestinal crypts and are thought to be induced by high interferon-γ production by intraepithelial lymphocytes.19
PCM as a Histologic Marker for Less Disease Regression in BE
To our knowledge, our study represents the first to evaluate PCM in BE in the context of dysplasia and patient outcome. We found that older patient age, male sex, and dysplasia are associated with more disease progression. However, PCM is not associated with disease progression. Interestingly, PCM is associated with three times less disease regression than NPCM. The observation of increased PCM in patients with long-segment BE, PCM’s association with more frequent acute inflammation, and patients with PCM showing less disease regression prompt us to hypothesize that the presence of PCM is a histologic marker for more severe/prolonged mucosal injuries and therefore decreased disease regression.
Intriguingly, a recent in vitro study by Nomura et al20 demonstrated that when cultured squamous cells are incubated with synthetic HD5 (an antimicrobial secreted by Paneth cells), the expression of E-cadherin is reduced. Therefore, Nomura et al hypothesized that in BE, HD5 secreted by the metaplastic Paneth cells could potentially accelerate initiation and progression of BE due to decreased cell-cell interaction. However, it remains to be proven whether this holds true in vivo. In fact, our study appears to argue against Paneth cells being the “culprit” for causing BE and disease progression. First, most BE cases with PCM remained NFD or IFD, similar to the NPCM group. Second, there are fewer Paneth cells in dysplastic BE cases than in nondysplastic BE cases. Third, the metaplastic Paneth cells in BE do not exhibit overt cytologic dysplastic features. And last, the finding that more Paneth cells are distributed in nondysplastic glands and the associated active inflammatory activity may suggest that PCM in BE is more likely to be related to inflammatory injury rather than dysplasia. PCM probably reflects the host’s attempt to repair/adapt to the local insult, and it is the local insult that ought to be blamed for promoting carcinogenesis.
Indeed, recent studies using mouse models demonstrated that Paneth cells play a crucial role within the intestinal stem cell niche, which allows orderly regeneration of intestinal surface epithelia on a continuous basis and aids in recovery following substantial insult.21,22 Study of the microbiome in BE showed increased gram-negative bacteria, which elicit increased inflammation and reflux through the action of lipopolysaccharides in the bacterial outer membrane.23 Therefore, we hypothesize that Paneth cells in BE may signal a more severe mucosal damage from reflux and/or bacteria, which is associated with less disease regression.
Other Features of PCM in BE
Interestingly, PCM is occasionally seen in mucinous nonmetaplastic glands or hybrid glands. Hybrid glands are metaplastic intestinal glands confined to the superficial aspect of mucinous glands.24 Given the current thought that BE originates from pluripotent stem cells locally residing in the esophageal squamous epithelium or gland duct epithelium, it is not surprising to see PCM in mucinous glands or hybrid glands since they probably arise from the same type of stem cells. PCM in mucinous or hybrid glands is likely to also represent a form of “adaptive protection” by the mucinous glands exposed to the same reflux injuries. It is interesting to note that mucous cells in Brunner’s glands also express lysozyme,12 suggesting a shared role of mucous cells in mucosal protection as Paneth cells. In supporting this notion, mucous gland metaplasia is also observed in the distal esophagus in baboons from lifelong gastroesophageal reflux caused by habitual chewing of regurgitated food.25
As expected, multilayered epithelium is occasionally observed in BE cases with PCM. Multilayered epithelium is thought to be the early transitional form of columnar metaplasia in patients with GERD.26,27 Pancreatic acinar metaplasia is also observed in some BE with PCM cases but does not seem to have any association with PCM or disease severity.
Despite a four-quadrant biopsy protocol and the usage of jumbo biopsy forceps, evaluation of the Barrett mucosa is subject to inherent sampling error, thus limiting the assessment of PCM in these patients. Future studies with more extensive sampling in autopsy patients may be helpful to confirm the prevalence of the PCM in patients with BE.
In conclusion, 31% of our cohort of esophageal biopsy specimens with IM showed PCM. Follow-up multinomial logistic regression analysis showed that PCM is associated with less disease regression, regardless of patient age, sex, segment length, or dysplasia. The presence of Paneth cells in BE could potentially be used as a histologic marker to identify patients with more severe mucosal injury with decreased chance of disease regression.
References
- 1.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 2.Appelman HD, Umar A, Orlando RC, et al. Barrett’s esophagus: natural history. Ann N Y Acad Sci. 2011;1232:292–308. doi: 10.1111/j.1749-6632.2011.06057.x. [DOI] [PubMed] [Google Scholar]
- 3.Glickman JN, Blount PL, Sanchez CA, et al. Mucin core polypeptide expression in the progression of neoplasia in Barrett’s esophagus. Hum Pathol. 2006;37:1304–1315. doi: 10.1016/j.humpath.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Wu X, Ajani JA, Gu J, et al. MicroRNA expression signatures during malignant progression from Barrett’s esophagus to esophageal adenocarcinoma. Cancer Prev Res (Phila) 2013;6:196–205. doi: 10.1158/1940-6207.CAPR-12-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreiber DS, Apstein M, Hermos JA. Paneth cells in Barrett’s esophagus. Gastroenterology. 1978;74:1302–1304. [PubMed] [Google Scholar]
- 6.Wehkamp J, Stange EF. Paneth cells and the innate immune response. Curr Opin Gastroenterol. 2006;22:644–650. doi: 10.1097/01.mog.0000245541.95408.86. [DOI] [PubMed] [Google Scholar]
- 7.Roth S, Franken P, Sacchetti A, et al. Paneth cells in intestinal homeostasis and tissue injury. PLoS One. 2012;7:e38965. doi: 10.1371/journal.pone.0038965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salzman NH, Bevins CL. Dysbiosis—a consequence of Paneth cell dysfunction. Semin Immunol. 2013;25:334–341. doi: 10.1016/j.smim.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Wehkamp J, Salzman NH, Porter E, et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci U S A. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherman MP, Bennett SH, Hwang FF, et al. Paneth cells and antibacterial host defense in neonatal small intestine. Infect Immun. 2005;73:6143–6146. doi: 10.1128/IAI.73.9.6143-6146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandow MJ, Whitehead R. The Paneth cell. Gut. 1979;20:420–431. doi: 10.1136/gut.20.5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wehkamp J, Chu H, Shen B, et al. Paneth cell antimicrobial peptides: topographical distribution and quantification in human gastrointestinal tissues. FEBS Lett. 2006;580:5344–5350. doi: 10.1016/j.febslet.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 13.van Es JH, Jay P, Gregorieff A, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 14.Andreu P, Peignon G, Slomianny C, et al. A genetic study of the role of the Wnt/beta-catenin signalling in Paneth cell differentiation. Dev Biol. 2008;324:288–296. doi: 10.1016/j.ydbio.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Joo M, Shahsafaei A, Odze RD. Paneth cell differentiation in colonic epithelial neoplasms: evidence for the role of the Apc/beta-catenin/Tcf pathway. Hum Pathol. 2009;40:872–880. doi: 10.1016/j.humpath.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Pai RK, Rybicki LA, Goldblum JR, et al. Paneth cells in colonic adenomas: association with male sex and adenoma burden. Am J Surg Pathol. 2013;37:98–103. doi: 10.1097/PAS.0b013e318267b02e. [DOI] [PubMed] [Google Scholar]
- 17.Thompson JJ, Zinsser KR, Enterline HT. Barrett’s metaplasia and adenocarcinoma of the esophagus and gastroesophageal junction. Hum Pathol. 1983;14:42–61. doi: 10.1016/s0046-8177(83)80045-8. [DOI] [PubMed] [Google Scholar]
- 18.Takubo K, Nixon JM, Jass JR. Ducts of esophageal glands proper and Paneth cells in Barrett’s esophagus: frequency in biopsy specimens. Pathology. 1995;27:315–317. doi: 10.1080/00313029500169213. [DOI] [PubMed] [Google Scholar]
- 19.Forsberg G, Fahlgren A, Hörstedt P, et al. Presence of bacteria and innate immunity of intestinal epithelium in childhood celiac disease. Am J Gastroenterol. 2004;99:894–904. doi: 10.1111/j.1572-0241.2004.04157.x. [DOI] [PubMed] [Google Scholar]
- 20.Nomura Y, Tanabe H, Moriichi K, et al. Reduction of E-cadherin by human defensin-5 in esophageal squamous cells. Biochem Biophys Res Commun. 2013;439:71–77. doi: 10.1016/j.bbrc.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 21.McElroy SJ, Underwood MA, Sherman MP. Paneth cells and necrotizing enterocolitis: a novel hypothesis for disease pathogenesis. Neonatology. 2013;103:10–20. doi: 10.1159/000342340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parry L, Young M, El Marjou F, et al. Evidence for a crucial role of Paneth cells in mediating the intestinal response to injury. Stem Cells. 2013;31:776–785. doi: 10.1002/stem.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Francois F, Pei Z. Molecular pathways: pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin Cancer Res. 2012;18:2138–2144. doi: 10.1158/1078-0432.CCR-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava A, Odze RD, Lauwers GY, et al. Morphologic features are useful in distinguishing Barrett esophagus from carditis with intestinal metaplasia. Am J Surg Pathol. 2007;31:1733–1741. doi: 10.1097/PAS.0b013e318078ce91. [DOI] [PubMed] [Google Scholar]
- 25.Rubio CA, Owston M, Orrego A, et al. Mucous gland metaplasia in the esophagus and gastric mucosa in baboons. Anticancer Res. 2011;31:2187–2190. [PMC free article] [PubMed] [Google Scholar]
- 26.Shields HM, Rosenberg SJ, Zwas FR, et al. Prospective evaluation of multilayered epithelium in Barrett’s esophagus. Am J Gastroenterol. 2001;96:3268–3273. doi: 10.1111/j.1572-0241.2001.05324.x. [DOI] [PubMed] [Google Scholar]
- 27.Glickman JN, Spechler SJ, Souza RF, et al. Multilayered epithelium in mucosal biopsy specimens from the gastroesophageal junction region is a histologic marker of gastroesophageal reflux disease. Am J Surg Pathol. 2009;33:818–825. doi: 10.1097/PAS.0b013e3181984697. [DOI] [PubMed] [Google Scholar]


