Abstract
Introduction
A consensus for which patients with thin melanomas (≤1 mm) should undergo sentinel lymph node biopsy (SLNB) is not established. We describe a large single institution experience with SLNB for thin melanomas to determine factors predictive of nodal metastases.
Methods
Retrospective review from 2005 to 2010 identified 271 patients with thin melanomas who underwent SLNB along with 13 additional cases not treated with SLNB who developed a nodal recurrence as first site of recurrence. Clinicopathologic characteristics were correlated with nodal status and outcome.
Results
Median age was 55 years and 53% of patients were male. Median Breslow thickness was 0.85 mm. Overall, a positive SLN was found in 22/271 cases (8.1%); 8.4% of melanomas ≥0.76 mm were SLN positive with 5% of T1a melanomas ≥0.76 mm and 13% of T1b melanomas ≥0.76 mm having SLN metastases. Only 2/33 highly selected patients with melanomas <0.76 mm (both T1b) had a positive SLN. Logistic regression analysis demonstrated that mitotic rate ≥1/mm2 significantly correlated with nodal disease (p<0.05) and ulceration correlated with SLN metastases (p<0.05). Median follow-up was 2.1 years. Overall survival did not differ between positive and negative SLN patients (p=0.53) but was worse for patients presenting with a nodal recurrence (p<0.01).
Conclusions
SLN metastases were seen in 8.4% of thin melanomas ≥0.76 mm, including 5% of T1a melanomas ≥0.76 mm. We believe these rates are sufficient to justify consideration of SLNB in these patients; while the indications for SLNB in melanomas <0.76 mm remain to be defined.
The incidence of melanoma is increasing with 70% of new cases diagnosed as thin melanomas (≤1 mm).[1–3] The prognosis for patients with thin melanoma is relatively good, with reported 10-year survival rates of 90% or greater in most series.[3, 4] However, a subset of patients with thin melanoma experience recurrent disease and melanoma-related mortality.[2–5] Recurrences at any site occur in close to 10% of thin melanomas with 3.7–5.2% of patients developing regional recurrences.[5–7] Notably, it is not unusual for recurrences to occur more than 10 years after excision of a thin primary.[2, 5–7]
The American Joint Committee on Cancer (AJCC) released the 7th edition staging system for melanoma in 2009.[4, 8, 9, 10] A notable change included the addition of mitotic rate (MR) as a predictor of survival for patients with thin melanoma.[4, 8, 9] However, MR as a predictor of nodal metastases was not addressed in the report. Sentinel lymph node biopsy (SLNB) has emerged as a powerful tool for assessing regional basins for occult nodal metastases.[11, 12] SLNB is recommended by the AJCC Melanoma Staging Committee for melanomas ≥1 mm in patients with no clinical evidence of metastasis.[4] However, the utility of SLNB for thin melanomas is debated.[13, 14] Arguments against SLNB include that thin melanomas have a low rate of nodal metastases, and the benefit of SLNB in this population is not established.[14] Others argue that there is a subset of patients with thin melanomas who will develop clinically-evident nodal disease and thus may benefit from SLNB.[14] Of note, Puleo et al. reported that a positive SLN was detected in 4.9% of patients with thin melanomas.[6]
Although no consensus exists regarding which subset of patients with thin melanomas are at greatest risk for nodal metastases, multiple predictive factors have been reported. However, none of these have been validated, and there is little agreement in the literature.[6, 14–30] At Moffitt Cancer Center, our philosophy has been that SLNB is potentially indicated if (a) the yield of a positive SLN is ≥5%; (b) the regional failure rate in SLN negative basins is very low relative to the yield of positive SLN; and (c) if nodal status has prognostic and therapeutic impact. This philosophy led us to routinely employ SLNB for otherwise healthy patients with melanomas ≥0.76 mm but only very selectively in patients with melanomas <0.76 mm. We reviewed our experience with SLNB for thin melanomas to evaluate tumor characteristics predictive of nodal metastases and assess the prognostic value of SLNB in patients with thin melanomas, in hopes of determining the validity of our criteria for selecting thin melanoma patients for SLNB.
Methods
After obtaining Institutional Review Board approval, a retrospective review was conducted involving patients referred to Moffitt Cancer Center between 2005 and 2010 with thin melanomas, defined as primary tumor thickness of ≤1 mm. Inclusion criteria were patients who had a SLNB or patients who did not have a SLNB at the time of primary excision but later developed a nodal recurrence (NR) as first site of recurrence. Demographic, clinical, primary tumor pathology, nodal status and outcome data were reviewed.
At Moffitt, SLNB is routinely performed for melanomas ≥0.76 mm and very selectively for thinner lesions based on findings of poor prognostic features (e.g. ulceration, extensive deep biopsy margin involvement, visible residual tumor present). MR ≥1/mm2 was not, absent other factors, considered sufficient to routinely recommend SLNB for melanomas <0.76 mm. SLNB was performed according to techniques previously described.[31, 32] Primary tumors were resected with a 1 cm margin. Patients with SLN metastases were offered completion lymph node dissection (CLND) but encouraged to enroll in the Multicenter Selective Lymphadenectomy Trial (MSLT) II, where participants are randomized to either immediate CLND or ultrasound surveillance of the affected nodal basin.
All cases were reviewed by a Moffitt dermatopathologist. Primary tumor characteristics were assessed, and evaluation of lymph nodes obtained through SLNB consisted of serial sectioning and review of hematoxylin and eosin-stained sections and S-100 and Melan-A immunohistochemistry. Evaluation of CLND specimens consisted of evaluation of lymph nodes <3 mm in diameter in their entirety, and a representative section through the largest diameter area of larger lymph nodes, review of H and E-stained sections, and S-100/Melan-A immunohistochemistry as needed. Clinicopathologic characteristics and outcome were correlated with nodal status.
Usual summary descriptive statistics, i.e., frequency and percentage for discrete variables and mean (standard deviation) and median (range), were provided for baseline demographics, disease characteristics, and outcome variables of interest. Both point estimates and their 95% confidence intervals (CIs) were reported, whenever appropriate. When exploring an association of a continuous variable with an outcome variable, e.g., nodal status, both its continuous version and a discrete version using an established cutpoint(s) or its median to divide patients into groups were considered. The Wilcoxon Rank-Sum test was used to compare age and Breslow thickness between patients of different outcomes, e.g., between patients with a positive and a negative SLN. Chi-square and Fisher’s exact tests were employed to study the association of nodal status with various categorical baseline demographics and disease characteristic variables. Simple logistic regression modeling was conducted to determine whether a demographic, disease or clinical variable was a significant predictor for nodal status. Multiple logistic regression with the stepwise variable selection technique was adopted to select the most significant risk factors from a set of pre-defined potential risk factors, with the level of entry set at 0.10 and the level of stay set at 0.05. Odds ratio and 95% CI were calculated for the odds of having a positive node and/or developing a regional recurrence. The Kaplan-Meier product-limit method was utilized to analyze overall survival (OS) and recurrence-free survival (RFS). The Log-rank test was used to test for differences in OS and RFS between any two groups of interest. A p-value of ≤0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.2 or higher.
Results
Patients
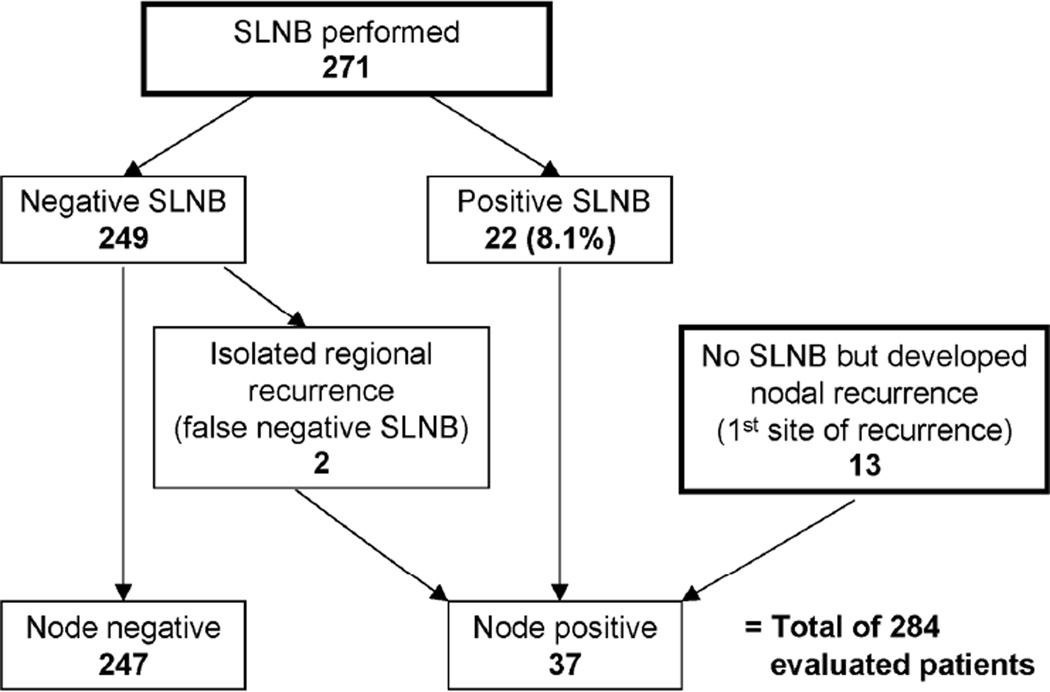
A total of 284 patients were included in the study (Figure 1). SLNB was performed in 271 patients while 13 patients did not have a SLNB but developed a NR as first site of recurrence. A positive SLN was seen in 22/271 patients (8.1%) undergoing SLNB. CLND was performed in 15 of the 22 positive SLN cases with no additional tumor-involved nodes found in any patient. Six patients with a positive SLN who did not undergo CLND were randomized to the observation arm of MSLT-II, while 1 patient was lost to follow-up.
Figure 1. Breakdown of patients.
Of the total 284 patients with thin melanomas who were included in the study, 271 patients had a sentinel lymph node biopsy (SLNB) while 13 patients did not have a SLNB at the time of primary excision but subsequently developed a nodal recurrence (NR) as first site of recurrence. All patients with evidence of nodal metastases were combined to form the node-positive group (37 patients) consisting of 22 positive SLNB patients, 13 NR patients and 2 false-negative SLNB patients.
SLNB was negative in 249 cases; however, two patients later developed regional recurrences (false-negative SLNB) leaving 247 patients who had no evidence of nodal disease at last follow-up and were designated as node-negative. To determine the association of primary tumor characteristics with nodal status, all patients with nodal metastases were analyzed. This node-positive group included 22 positive SLNB patients, 13 NR patients, and two false-negative SLNB patients for a total of 37 patients.
The overall median age was 55 years (range: 12 – 84 years) and the majority of patients in both the node-positive and node-negative groups were male (56.8% and 52.6%, respectively). Patient demographics and histopathologic characteristics are shown in Table 1.
Table 1.
Patient demographics and primary tumor characteristics stratified by nodal status (n=284)
| All patients | Node positive | Node negative | p- value† |
|
|---|---|---|---|---|
| n | 284 | 37 | 247 | |
| Age (years) | NS | |||
| Median (range) | 55 (12 – 84) | 55 (32 – 84) | 55 (12 – 82) | |
| Mean (STD) | 55.5 (14.3) | 55.1 (13.3) | 54.5 (14.4) | |
| Gender (n, %) | NS | |||
| Male | 151 (53.2) | 21 (56.8) | 130 (52.6) | |
| Female | 133 (46.8) | 16 (43.2) | 117 (47.4) | |
| Histologic type (n, %) | -- | |||
| Superficial spreading | 210 (73.9) | 24 (64.9) | 186 (75.3) | |
| Nodular | 26 (9.2) | 2 (5.4) | 24 (9.7) | |
| Acral lentiginous | 11 (3.9) | 2 (5.4) | 9 (3.6) | |
| Lentigo Maligna | 9 (3.2) | 2 (5.4) | 7 (2.8) | |
| Other | 6 (2.1) | 1 (2.7) | 5 (2.0) | |
| Not specified | 22 (7.7) | 6 (16.2) | 16 (6.5) | |
| Location (n, %) | NS | |||
| Head and Neck | 48 (16.9) | 9 (24.3) | 39 (15.8) | |
| Trunk | 105 (37.0) | 13 (35.1) | 92 (37.2) | |
| Extremities | 131 (46.1) | 15 (40.5) | 116 (47.0) | |
| Thickness (mm) | NS | |||
| Median (range) | 0.85 (0.3 – 1) | 0.85 (0.32 – 1) | 0.85 (0.3 – 1) | |
| Mean (STD) | 0.84 (0.13) | 0.81 (0.17) | 0.84 (0.13) | |
| Ulceration (n, %)$ | 17 (6.3) | 5 (14.3) | 12 (5.1) | 0.06a |
| Mitotic rate (n, %)$ | <0.05b | |||
| ≥1 mm2 | 116 (43.1) | 21 (63.6) | 95 (40.3) | |
| <1 mm2 | 153 (56.9) | 12 (36.4) | 141 (59.7) | |
| Clarks level (n, %)$ | NS | |||
| IV/V | 172 (61.0) | 20 (55.6) | 152 (61.8) | |
| ≤III | 110 (39.0) | 16 (44.4) | 94 (38.2) | |
| Regression (n, %)$ | 27 (10.0) | 3 (8.8) | 24 (10.1) | NS |
| VGP (n, %)$ | 167 (64.5) | 20 (66.7) | 147 (64.2) | NS |
| TIL (n, %)$ | NS | |||
| Brisk | 48 (31.6) | 10 (45.5) | 38 (29.2) | |
| Non-brisk | 60 (39.5) | 8 (36.4) | 52 (40) | |
| Absent | 44 (28.9) | 4 (18.2) | 40 (30.8) | |
For comparing between node positives and node negatives based on Wilcoxon, Chi-square or Fisher’s exact test.
Significant on chi-square and univariable logistic analysis for node positive vs. node negative (p<0.05) but not significant on multiple logistic regression analysis (p=0.06).
Significant on multiple logistic regression analysis for node positive vs. node negative.
Data unavailable or missing for ulceration in 13 cases, for mitotic rate in 15 cases, for Clarks level in 2 cases, for regression in 13 cases, for VGP in 25 cases and for TIL in 132 cases.
SLNB: sentinel lymph node biopsy, VGP: vertical growth phase, TIL: tumor infiltrating lymphocytes, NS: not significant.
Primary tumor characteristics correlated with nodal status
Primary tumor characteristics were correlated with nodal status (Table 1). Overall median Breslow thickness was 0.85 mm (range: 0.3–1 mm), and there was no significant difference between node-positive and node-negative patients. MR was ≥1/mm2 in 21 node-positive patients (63.6%) and 95 node-negative patients (40.3%). Ulceration was seen in 14.3% of node-positive and 5.1% of node-negative patients. Univariate analysis demonstrated that ulceration (OR=3.11, 95% CI: 1.02–9.45; p=0.05) and MR ≥1/mm2 (OR=2.60, 95% CI: 1.22–5.53; p=0.01) were statistically significant predictors of nodal metastasis. Multiple regression analysis showed that MR ≥1/mm2 was the only significant predictor for nodal metastasis (OR=2.45, 95% CI: 1.14–5.25; p=0.02) while ulceration trended towards significance (OR=3.09, 95% CI: 0.98–9.77; p=0.06). Clark level IV/V, regression, vertical growth phase and brisk, non-brisk and absent tumor infiltrating lymphocytes (TIL) did not differ significantly between the two groups.
Recurrence-Free Survival
First site of recurrences are shown in Table 2. Overall, 3.3% of SLNB patients experienced recurrences, with 13.6% of positive SLNB patients but only 2.4% of negative SLNB patients developing a recurrence. Local recurrences were rare and seen in only 0.7% (2/271) of all SLNB patients. Two patients (0.8%) in the negative SLNB group developed regional recurrences, for a negative predictive value of 99.2% and a false-negative rate of 8.3% (2 positive nodes missed out of 24 total). One of the false-negative SLNB patients had an ulcerated 0.97 mm melanoma while the second had a 0.87 mm melanoma with MR 1/mm2. Distant recurrences as first site of recurrence were seen in 1.5% (4/271) of SLNB patients, occurring in 9.1% (2/22) of positive but only 0.8% (2/249) of negative SLNB patients. Of note, no recurrences have occurred as of the last follow-up in the 6 positive SLN patients being observed on MSLT-II.
Table 2.
Recurrences and deaths
| SLNB performed |
Positive SLNB |
Negative SLNB |
Nodal recurrence |
|
|---|---|---|---|---|
| n | 271 | 22 | 249 | 13 |
| Recurrence (n, %) | 9 (3.3) | 3 (13.6) | 6 (2.4) | 13 (100) |
| First site of recurrence (n, %) | ||||
| Local | 2 (0.7) | 1 (4.5) | 1 (0.4) | 0 |
| In-transit | 1 (0.4) | 0 | 1 (0.4) | 0 |
| Regional | 2 (0.7) | 0 | 2 (0.8) | 13 (100) |
| Distant | 4 (1.5) | 2 (9.1) | 2 (0.8) | 0 |
| Total deaths (n, %) | 4 (1.5) | 1 (4.5) | 3 (1.2) | 4 (30.8) |
| Melanoma related | 1 (0.4) | 1(4.5) | 0 | 3 (23.1) |
| Other causes | 2 (0.7) | 0 | 2 (0.8) | 0 |
| Unknown | 1 (0.4) | 0 | 1 (0.4) | 1 (7.7) |
SLNB: sentinel lymph node biopsy.
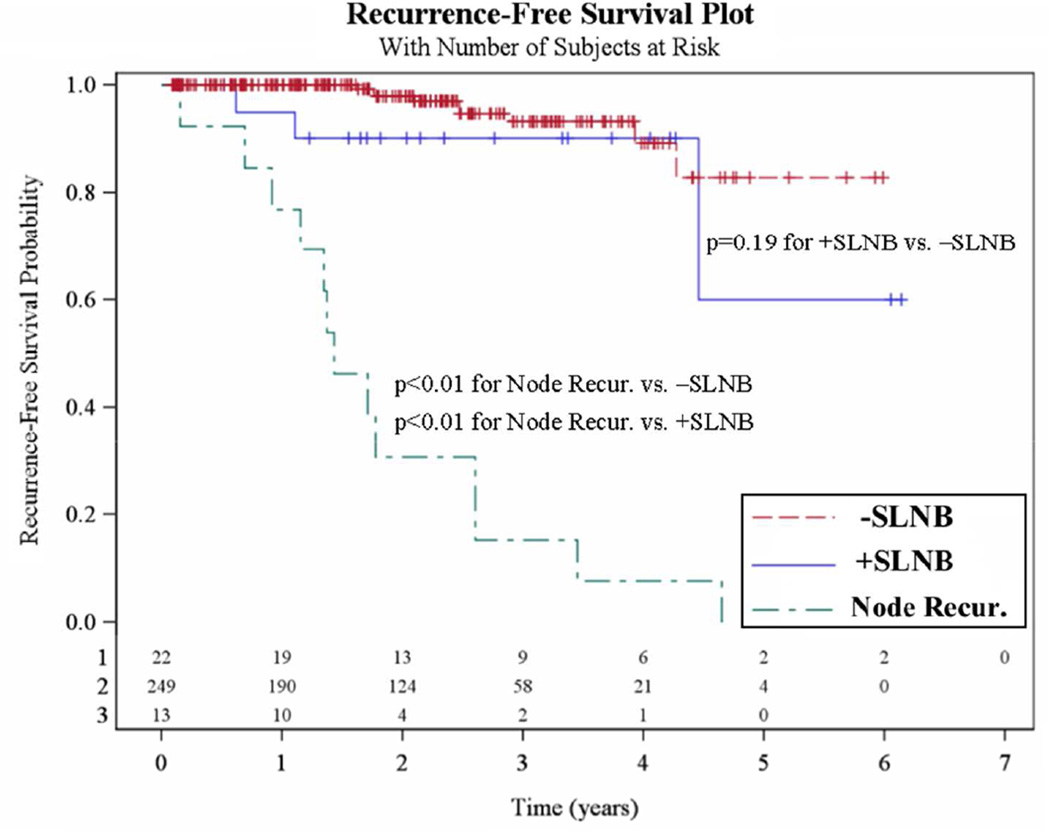
RFS curves for the negative SLNB, positive SLNB and NR groups are shown in Figure 2. RFS was significantly worse for the NR group compared with the negative and positive SLNB groups (both p<0.01). A trend towards worse RFS was seen for positive SLNB patients when compared with negative SLNB patients (p=0.19).
Figure 2. Recurrence-free survival (RFS).
The 249 patients who had a negative sentinel lymph node biopsy (SLNB) are compared with both the 22 positive SLNB patients and the 13 nodal recurrence (NR) patients (Node Recur).
Overall Survival
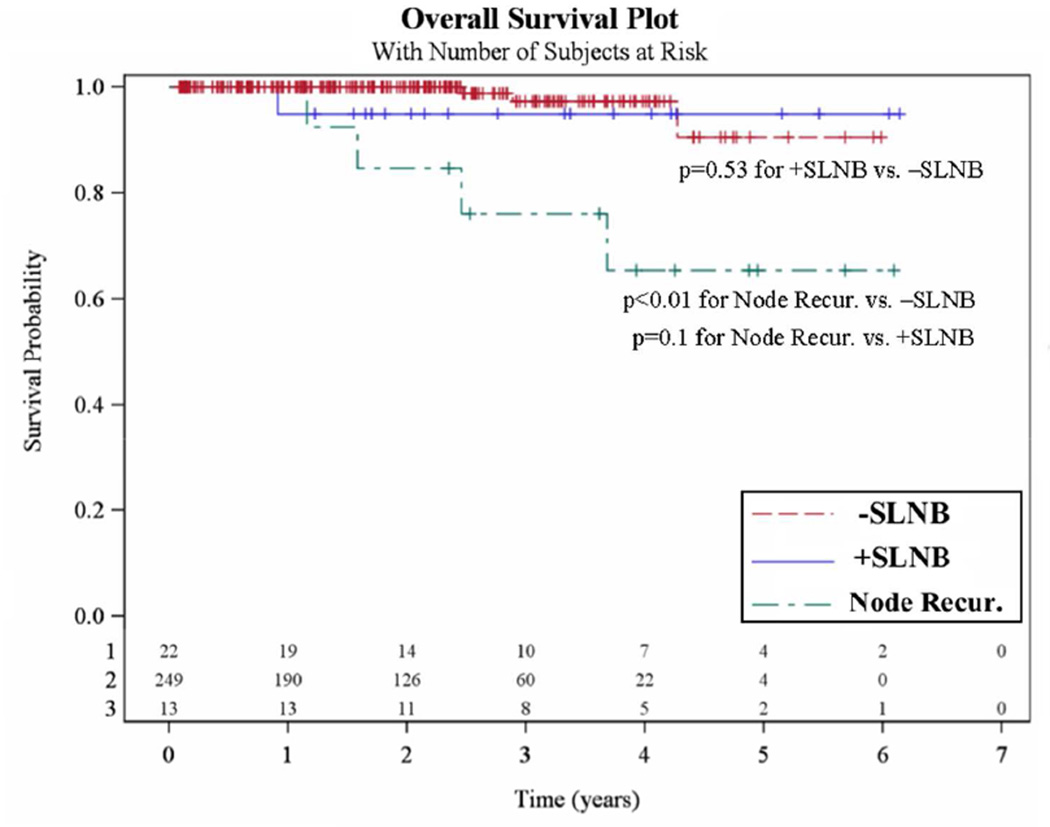
In total, there were 8 deaths after a median follow-up of 2.1 years. Four deaths occurred in the SLNB cohort (1.5%): one positive SLNB patient (4.5%) died of metastatic melanoma while 3 negative SLNB patients (1.2%) died of pancreatic carcinoma, renal cell carcinoma, and an unknown cause, respectively. Four deaths were seen in the NR group, with 3 of these deaths being melanoma-related and 1 death with an unknown cause. Overall survival data is shown in Table 2.
OS curves for the negative SLNB, positive SLNB and NR groups are shown in Figure 3. OS was significantly worse for the NR group compared with the negative SLNB group (p<0.01) but only a trend was seen when compared with the positive SLNB group (p=0.10). There was no significant difference in OS between positive and negative SLNB patients (p=0.53).
Figure 3. Overall survival (OS).
The 249 patients who had a negative sentinel lymph node biopsy (SLNB) are compared with both the 22 positive SLNB patients and the 13 nodal recurrence (NR) patients (Node Recur).
Predictors of sentinel lymph node metastases
Clinicopathologic characteristics were evaluated and correlated with SLN metastasis (Table 3). A total of 238 melanomas (88%) were ≥0.76 mm while only 33 (12%) were <0.76 mm. Twenty (91%) of 22 positive SLNB cases were in melanomas ≥0.76 mm. A positive SLN was seen in 8.4% of melanomas ≥0.76 mm and in 6.1% of melanomas <0.76 mm; this difference was not statistically significant.
Table 3.
Predictors of sentinel lymph node metastases (n=271)
| SLNB performed |
Rate of positive SLNB |
p-value† | |
|---|---|---|---|
| n (%) | 271 (100) | 22 (8.1) | |
| Age group (n, %) | NS | ||
| <40 years | 43 (15.9) | 4 (9.3) | |
| 40 – 70 years | 195 (72.0) | 16 (8.2) | |
| >70 years | 33 (12.2) | 2 (6.1) | |
| Thickness (n, %) | NS | ||
| ≥0.76 mm | 238 (87.8) | 20 (8.4) | |
| <0.76 mm | 33 (12.2) | 2 (6.1) | |
| Ulceration (n, %)$ | <0.05a | ||
| Yes | 17 (6.6) | 4 (23.5) | |
| No | 241 (93.4) | 16 (6.6) | |
| Mitotic rate (n, %)$ | <0.05b | ||
| ≥1/mm2 | 109 (42.3) | 13 (11.9) | |
| <1/mm2 | 149 (57.8) | 7 (4.7) | |
| Clarks level (n, %)$ | NS | ||
| IV/V | 167 (61.9) | 15 (9.0) | |
| ≤III | 103 (38.2) | 7 (6.8) | |
| Regression (n, %)$ | NS | ||
| Yes | 26 (10.0) | 2 (7.7) | |
| No | 233 (90.0) | 18 (7.7) | |
| VGP (n, %)$ | NS | ||
| Yes | 162 (64.5) | 13 (8.0) | |
| No | 89 (35.5) | 7 (7.9) | |
| TIL (n, %)$ | NS | ||
| Brisk | 42 (29.4) | 4 (9.5) | |
| Non-brisk | 57 (39.9) | 5 (8.8) | |
| Absent | 44 (30.8) | 3 (6.8) | |
| +Deep biopsy margin (n, %)$ | NS | ||
| Yes | 109 (40.8) | 9 (8.3) | |
| No | 158 (59.2) | 13 (8.2) | |
| T stage stratified by thickness | <0.05c | ||
| Stage T1a (n, %) | 148 (54.6) | 6 (4.1) | |
| ≥0.76 mm | 126 (46.5) | 6 (4.8) | |
| <0.76 mm | 22 (8.1) | 0 (0) | |
| Stage T1b (n, %) | 123 (45.4) | 16 (13.0) | |
| ≥0.76 mm | 112 (41.3) | 14 (12.5) | |
| <0.76 mm | 11 (4.1) | 2 (18.2) | |
For testing the association between node metastases and each of the covariates based on Chi-square or Fisher’s exact test.
Significant on multiple logistic regression analysis for positive vs. negative SLNB.
Significant on univariable analysis for positive vs. negative SLNB (p<0.05) but not significant on multiple logistic regression analysis (p=0.06).
Significant on univariable analysis for positive vs. negative SLNB.
Data unavailable or missing for ulceration in 13 cases, for mitotic rate in 13 cases, for Clarks level in 1 case, for regression in 12 cases, for VGP in 20 cases, for TIL in 128 cases and for deep biopsy margin in 4 cases.
SLNB: sentinel lymph node biopsy, VGP: vertical growth phase, TIL: tumor infiltrating lymphocytes, NS: not significant.
Primary tumor ulceration was seen in 17 patients (6.6%) who had a SLNB, with a positive SLN in 4/17 (23.5%). In contrast, only 16 of 241 non-ulcerated melanomas (6.6%) had a positive SLN. A positive SLN was seen in 11.9% of cases with a MR ≥1/mm2 versus only 4.7% of cases with a MR <1/mm2. Nine percent of Clark level IV/V melanomas had a positive SLNB versus 6.8% of Clark level ≤III melanomas. The positive SLNB rate in tumors with and without regression was 7.7%. The positive SLNB rates were similar in vertical (8.0%) and radial (7.9%) growth phase melanomas and in melanomas with (8.3%) and without (8.2%) a positive deep biopsy margin. A positive SLN was seen in 9.5% of cases with brisk TIL, 8.8% of cases with non-brisk TIL and in 6.8% of cases with absent TIL. Both ulceration (OR=5.72, 95% CI: 1.82–17.91; p=0.03) and MR ≥1/mm2 (OR=2.58, 95% CI: 1.04–6.39; p=0.04) were correlated with SLN metastasis on univariate analysis. However, on multiple logistic regression only ulceration was a significant predictor of SLN metastases (OR=3.93, 95% CI: 1.11–13.91; p=0.03) while MR ≥1/mm2 trended towards significance (OR=2.50, 95% CI: 0.95–6.60; p=0.06).
Melanomas were also stratified by T stage, with 148 (54.6%) tumors classified as T1a and 123 (45.4%) tumor classified as T1b. A positive SLNB was seen in only 4.1% of T1a but in 13% of T1b melanomas (p<0.05). T stage was further stratified by thickness, with 126 T1a melanomas ≥0.76 mm and 22 T1a melanomas <0.76 mm. A positive SLNB was seen in 4.8% of T1a melanomas ≥0.76 mm. None of the T1a melanomas <0.76 mm had a positive SLNB. Among T1b tumors, there were 112 melanomas ≥0.76 mm and 11 melanomas <0.76 mm. A positive SLNB was seen in 12.5% of T1b melanomas ≥0.76 mm. Two of 11 T1b melanomas <0.76 mm (18.2%) had SLN metastases; both of these cases had mitotically active tumors with one case having a 0.6 mm non-ulcerated melanoma with a MR of 3/mm2 and the second having a 0.7 mm non-ulcerated melanoma with a MR of 1/mm2.
Discussion
No consensus exists as to which patients with thin melanomas are at risk for nodal metastases with numerous studies evaluating predictive risk factors for nodal disease in thin melanomas, including Breslow thickness, Clark level, ulceration, MR, regression, growth phase and TIL.[6, 7, 15–30] Several studies have found that a higher MR is associated with nodal metastases in thin melanomas.[7, 15, 16, 17] Karakousis et al. reported that MR >0/mm2 significantly predicted regional nodal disease while Kesmodel et al. showed that MR >0/mm2 independently predicted SLN metastases.[7, 15] Our results are consistent with Karakousis et al. in that MR ≥1/mm2 significantly predicted nodal status when all node-positive cases were considered, but differ from Kesmodel et al. since MR only trended towards significance as a predictor of a positive SLN in our study. We also utilized a slightly different MR threshold, defining MR by the current AJCC definition of ≥1/mm2.
Ulceration was a significant predictor of SLN metastases in our study. Yonick et al. also showed in their study of SLNB in 147 thin melanoma patients that ulceration was an independent predictor of SLN disease (p=0.047). In contrast, the majority of studies have not shown ulceration to be a significant predictor of SLN metastases in thin melanomas.[15, 16, 18, 19, 21, 23, 24, 25] However, since ulceration is uncommon in thin melanomas (4–9%), ulcerated cases may not have occurred with sufficient frequency to be adequately assessed in these studies.[14] Alternatively, local trauma may have resulted in misclassification of some low-risk tumors as ulcerated, further diluting the limited statistical power to see a difference, especially in multi-institution studies without central pathology review.[33]
Breslow thickness ≥0.76 mm is associated with a 4.9–12.8% rate of SLN metastases while only 0–2.3% of melanomas <0.76 mm are associated with SLN disease.[7, 15, 16, 18, 23, 29] Our results are similar to these previously published studies since the risk for SLN metastases was proportional to tumor thickness, with 6.1% of melanomas <0.76 mm and 8.4% of melanomas ≥0.76 mm having a positive SLN. Furthermore, the vast majority (91%) of patients with a positive SLN in the current study had melanomas ≥0.76 mm. When we analyzed T stage as stratified by thickness, SLN metastases were seen in approximately 5% of T1a melanomas ≥0.76 mm and 13% of T1b melanomas ≥0.76 mm. SLNB does not appear to be indicated for T1a melanomas <0.76 mm since none of our cases had SLN metastases. SLN metastases were seen in T1b tumors <0.76 mm. However, in this study the <0.76 mm group represents highly selected patients (only 33 cases out of many hundreds of very thin melanomas seen during that time interval with only 11 T1b) and still we only found 2 node-positive cases.
Considering the low false-negative and complication rates of SLNB, we believe that using a 5% risk of nodal metastasis as a threshold for performing SLNB is reasonable.[11, 34] Using a 5% risk threshold, our results suggest that melanoma patients whose tumors are ≥0.76 mm thick should be considered for SLNB, since SLN metastases were seen in 8.4% of all thin melanomas ≥0.76 mm and specifically in 5% of T1a melanomas ≥0.76 mm and 13% of T1b melanomas ≥0.76 mm. However, further study is needed to determine which patients with melanomas <0.76 mm, if any, should be offered SLNB.
A key part of the decision-making process in offering a staging test like SLNB, however, is its ability to predict relapse and survival. In our study, negative SLNB patients had the best RFS and OS while patients with a nodal relapse fared worst. However, we were unable to demonstrate that a positive SLN had an impact on overall survival and only saw a trend towards decreased RFS for positive SLNB patients. The median follow-up in our study was relatively short, at just over 2 years, and we saw a number of deaths from causes other than melanoma. Wright et al. demonstrated that for patients with thin melanoma, melanoma-specific survival differences between positive and negative SLNB patients were not seen until after approximately 5 years.[19] We will need to follow our thin melanoma patients and allow our data to mature further before any definitive conclusions about survival can be made. It is worth noting that distant metastasis as a first site of relapse was much more common in our positive SLNB patients, adding credence to the possibility that melanoma-specific survival differences may emerge with extended follow-up.
In summary, thin melanomas have a significant risk for SLN metastases (8.1% in our series). MR and ulceration both influence nodal status, but how best to use them in selecting patients for SLNB remains unclear. Using a 5% risk for nodal metastases as a threshold, SLNB should be considered for all melanomas ≥0.76 mm since SLN metastases were seen in 8.4% of all melanomas ≥0.76 mm. Importantly, our population was not preselected for ulceration, mitotic rate, growth phase or other “high risk” factors. For surgeons (or patients) who would utilize a 10% threshold before recommending SLNB, our data would suggest choosing T1b melanomas ≥0.76 mm (13% node-positive in our series). The benefits of detecting a positive SLN in patients with thin melanomas include treatment of regional nodal basins at an occult stage, which may improve OS and decrease morbidity when compared with patients who develop a macroscopic NR.[26] Furthermore, detection of a positive SLN also allows for evaluation for systemic therapy or inclusion in clinical trials at an earlier stage.
Acknowledgments
Disclosures:
VKS: Consultant/Advisory Board: Merck, Navidea (Neoprobe)
JSZ: Consultant/Scientific Advisory Board: Delcath Systems, Inc., Consultant/Advisory Board: IGEA
JLM: Consultant: Glaxo Smith Kline, Consultant: Durect Corporation
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Gimotty PA, Guerry D, Ming ME, et al. Thin primary cutaneous malignant melanoma: a prognostic tree for 10-year metastasis is more accurate than American Joint Committee on Cancer staging. J Clin Oncol. 2004;22(18):3668–3676. doi: 10.1200/JCO.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 3.McKinnon JG, Yu XQ, McCarthy WH, Thompson JF. Prognosis for patients with thin cutaneous melanoma: long-term survival data from New South Wales Central Cancer Registry and the Sydney Melanoma Unit. Cancer. 2003;98(6):1223–1231. doi: 10.1002/cncr.11624. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalady MF, White RR, Johnson JL, Tyler DS, Seigler HF. Thin melanomas: predictive lethal characteristics from a 30-year clinical experience. Ann Surg. 2003;238(4):528–535. doi: 10.1097/01.sla.0000090446.63327.40. discussion 535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puleo CA, Messina JL, Riker AI, et al. Sentinel node biopsy for thin melanomas: which patients should be considered? Cancer Control. 2005;12(4):230–235. doi: 10.1177/107327480501200404. [DOI] [PubMed] [Google Scholar]
- 7.Karakousis GC, Gimotty PA, Botbyl JD, et al. Predictors of regional nodal disease in patients with thin melanomas. Ann Surg Oncol. 2006;13(4):533–541. doi: 10.1245/ASO.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Thompson JF, Soong SJ, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol. 2011;29(16):2199–2205. doi: 10.1200/JCO.2010.31.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20(1):1–17. doi: 10.1016/j.soc.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–2459. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355(13):1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 12.Phan GQ, Messina JL, Sondak VK, Zager JS. Sentinel lymph node biopsy for melanoma: indications and rationale. Cancer Control. 2009;16(3):234–239. doi: 10.1177/107327480901600305. [DOI] [PubMed] [Google Scholar]
- 13.Thompson JF, Shaw HM. Is sentinel lymph node biopsy appropriate in patients with thin melanomas: too early to tell? Ann Surg Oncol. 2006;13(3):279–281. doi: 10.1245/ASO.2006.08.927. [DOI] [PubMed] [Google Scholar]
- 14.Andtbacka RHI, Gershenwald JE. Role of sentinel lymph node biopsy in patients with thin melanoma. J Natl Compr Canc Netw. 2009;7:308–317. doi: 10.6004/jnccn.2009.0023. [DOI] [PubMed] [Google Scholar]
- 15.Kesmodel SB, Karakousis GC, Botbyl JD, et al. Mitotic rate as a predictor of sentinel lymph node positivity in patients with thin melanomas. Ann Surg Oncol. 2005;12(6):449–458. doi: 10.1245/ASO.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Ranieri JM, Wagner JD, Wenck S, Johnson CS, Coleman JJ., 3rd The prognostic importance of sentinel lymph node biopsy in thin melanoma. Ann Surg Oncol. 2006;13(7):927–932. doi: 10.1245/ASO.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Sondak VK, Taylor JM, Sabel MS, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity: lessons learned from the generation of a probabilistic model. Ann Surg Oncol. 2004;11(3):247–258. doi: 10.1245/aso.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 18.Wong SL, Brady MS, Busam KJ, Coit DG. Results of sentinel lymph node biopsy in patients with thin melanoma. Ann Surg Oncol. 2006;13(3):302–309. doi: 10.1245/ASO.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Wright BE, Scheri RP, Ye X, Faries MB, Turner RR, Essner R, Morton DL. Importance of sentinel lymph node biopsy in patients with thin melanoma. Arch Surg. 2008;143(9):892–899. doi: 10.1001/archsurg.143.9.892. discussion 899–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris KT, Busam KJ, Bero S, Patel A, Brady MS. Primary cutaneous melanoma with regression does not require a lower threshold for sentinel lymph node biopsy. Ann Surg Oncol. 2008;15(1):316–322. doi: 10.1245/s10434-007-9675-2. [DOI] [PubMed] [Google Scholar]
- 21.Olah J, Gyulai R, Korom I, Varga E, Dobozy A. Tumour regression predicts higher risk of sentinel node involvement in thin cutaneous melanomas. Br J Dermatol. 2003;149(3):662–663. doi: 10.1046/j.1365-2133.2003.05502.x. [DOI] [PubMed] [Google Scholar]
- 22.Warycha MA, Zakrzewski J, Ni Q, et al. Meta-analysis of sentinel lymph node positivity in thin melanoma (<or=1 mm) Cancer. 2009;115(4):869–879. doi: 10.1002/cncr.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murali R, Haydu LE, Quinn MJ, et al. Sentinel lymph node biopsy in patients with thin primary cutaneous melanoma. Ann Surg. 2012;255(1):128–133. doi: 10.1097/SLA.0b013e3182306c72. [DOI] [PubMed] [Google Scholar]
- 24.Stitzenberg KB, Groben PA, Stern SL, Thomas NE, Hensing TA, Sansbury LB, Ollila DW. Indications for lymphatic mapping and sentinel lymphadenectomy in patients with thin melanoma (Breslow thickness < or =1.0 mm) Ann Surg Oncol. 2004;11(10):900–906. doi: 10.1245/ASO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Yonick DV, Ballo RM, Kahn E, et al. Predictors of positive sentinel lymph node in thin melanoma. Am J Surg. 2011;201(3):324–327. doi: 10.1016/j.amjsurg.2010.09.011. discussion 327-8. [DOI] [PubMed] [Google Scholar]
- 26.Faries MB, Wanek LA, Elashoff D, Wright BE, Morton DL. Predictors of occult nodal metastasis in patients with thin melanoma. Arch Surg. 2010;145(2):137–142. doi: 10.1001/archsurg.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaquerano J, Kraybill WG, Driscoll DL, Cheney R, Kane JM., 3rd American Joint Committee on Cancer clinical stage as a selection criterion for sentinel lymph node biopsy in thin melanoma. Ann Surg Oncol. 2006;13(2):198–204. doi: 10.1245/ASO.2006.03.092. [DOI] [PubMed] [Google Scholar]
- 28.Hershko DD, Robb BW, Lowy AM, Ahmad SA, Ramadas GH, Soldano DA, Sussman JJ. Sentinel lymph node biopsy in thin melanoma patients. J Surg Oncol. 2006;93(4):279–285. doi: 10.1002/jso.20415. [DOI] [PubMed] [Google Scholar]
- 29.Vermeeren L, Van der Ent F, Sastrowijoto P, Hulsewe K. Sentinel lymph node biopsy in patients with thin melanoma: occurrence of nodal metastases and its prognostic value. Eur J Dermatol. 2010;20(1):30–34. doi: 10.1684/ejd.2010.0837. [DOI] [PubMed] [Google Scholar]
- 30.Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25(7):869–875. doi: 10.1200/JCO.2006.08.9755. [DOI] [PubMed] [Google Scholar]
- 31.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 32.Ross MI, Reintgen D, Balch CM. Selective lymphadenectomy: emerging role for lymphatic mapping and sentinel node biopsy in the management of early stage melanoma. Semin Surg Oncol. 1993;9(3):219–223. [PubMed] [Google Scholar]
- 33.White RL, Jr, Ayers GD, Stell VH, et al. Factors predictive of the status of sentinel lymph nodes in melanoma patients from a large multicenter database. Ann Surg Oncol. 2011;18(13):3593–3600. doi: 10.1245/s10434-011-1826-9. Epub 2011 Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wrightson WR, Wong SL, Edwards MJ, et al. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10(6):676–680. doi: 10.1245/aso.2003.10.001. [DOI] [PubMed] [Google Scholar]