Abstract
Purpose
Allografts, xenografts, and alloplasts are commonly used in craniofacial medicine as alternatives to autogenous bone grafts; however, these materials lack important bone-inducing proteins. A method for enhancing the osteoinductive potential of these commercially available materials would provide a major clinical advance. In this study, a calcium-binding domain, polyglutamate, was added to an osteoinductive peptide derived from collagen type I, Asp-Gly-Glu-Ala (DGEA), to anchor the peptide onto four different materials: freeze-dried bone allograft (FDBA); anorganic bovine bone (ABB); β-tricalcium phosphate (β-TCP); and a calcium sulfate bone cement (CaSO4). The authors also examined whether peptide binding and retention could be tuned by altering the number of glutamate residues within the polyglutamate domain.
Materials and Methods
DGEA or DGEA modified with diglutamate (E2DGEA), tetraglutamate (E4DGEA), or heptaglutamate (E7DGEA) were evaluated for binding and release to the grafting materials. Peptides were conjugated with a fluorescein isothiocyanate (FITC) tag to allow monitoring by fluorescent microscopy or through measurements of solution fluorescence. In vivo retention was evaluated by implanting graft materials coated with FITC-peptides into rat subcutaneous pouches.
Results
Significantly more peptide was loaded onto the four graft materials as the number of glutamates increased, with E7DGEA exhibiting the greatest binding. There was also significantly greater retention of peptides with longer glutamate domains following a 3-day incubation with agitation. Importantly, E7DGEA peptides remained on the grafts after a 2-month implantation into skin pouches, a sufficient interval to influence bony healing.
Conclusion
Variable-length polyglutamate domains can be added to osteoinductive peptides to control the amount of peptide bound and rate of peptide released. The lack of methods for tunable coupling of biologics to commercial graft sources has been a major barrier toward developing materials that approach the clinical efficacy of autogenous bone. Modification of osteoinductive factors with polyglutamate domains constitutes a technically straightforward and cost-effective strategy for enhancing osteoinductivity of diverse graft products.
Keywords: bone graft, bone regeneration, osteoinductive peptide, peptide coupling, polyglutamate domain
Each year, more than 600,000 bone grafting procedures are performed in the United States, and 2.2 million worldwide.1,2 Bone grafts are widely used in medical procedures such as spinal fusion and in dental procedures such as sinus elevation, ridge augmentation, and periodontal regenerative therapy. Autogenous bone harvested from the patient is considered the gold standard material for grafting; however, harvesting bone can result in adverse events such as donor site pain and morbidity, and quantities of bone are limited.3–6 Because of these factors, clinicians often turn to commercial sources of graft materials.7 There are a variety of options available including cadaveric allografts with cortical and/or cancellous components, as well as xenograft and alloplast materials from bovine, porcine, coral, and synthetic origins. These materials offer several advantages, but their osteoinductive potential is less than that of autogenous bone. Xenograft and synthetic materials such as hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) lack organic components, whereas allograft bone may have lost osteoinductivity because of processing and sterilization procedures that degrade and denature critical regenerative proteins.8,9 A mechanism for coupling osteoinductive factors onto commercial grafting products would provide a major step toward the development of alternative grafting materials that approach the clinical efficacy of autogenous bone.
Most current methods for coupling osteoinductive factors to grafting materials have employed passive adsorption of molecules onto the material surface; however, this approach typically yields a rapid burst release.10–12 During a bolus release, most of the bioactive factor is disseminated away from the graft site, and its activity is diminished. There is a need to develop alternate approaches that achieve a more controlled and sustained delivery of these factors to enhance bone formation at the graft site. To address this issue, the authors and others have used selected calcium-binding amino acid motifs to anchor biologic modifiers to the surface of alloplastic materials.13–18 For example, polyglutamate domains have been utilized to attach bioactive peptides to synthetic forms of HA.14–16,18 Glutamate is a negatively charged amino acid that binds to the positively charged calcium ions within HA. The group conducting the present study has used a heptaglutamate (E7) domain to couple a range of bioactive peptides to HA, including DGEA (Asp-Gly-Glu-Ala, a collagen-derived peptide)15; RGD (Arg-Gly-Asp, an integrin-binding peptide)14; and FHRRIKA (Phe-His-Arg-Arg-Ile-Lys-Ala, a proteoglycanbinding peptide).19 In every case, better binding and retention of the peptide on HA was observed, suggesting that polyglutamate domains can serve as a generic tool for delivery of a wide array of bioactive molecules. Correspondingly, improved anchoring of the peptide resulted in enhanced activity, as evidenced by a comparison study of DGEA and E7-modified DGEA peptides.15 DGEA is a domain within collagen type I, the primary organic component of bone, which promotes osteoblastic differentiation of mesenchymal stem cells (MSCs).13,20,21 In prior studies, the authors reported that E7DGEA peptides, when coupled to HA biomaterials, stimulated greater in vitro osteoblastic differentiation of MSCs compared with DGEA-coated HA.15 Moreover, E7DGEA-coupled HA materials implanted into rat tibiae induced more new bone formation and bone-implant contact than DGEA-bound HA.15 These results confirmed that enhanced coupling of the osteoinductive peptide to the HA surface translated into a significant improvement in implant integration.
Synthetic peptides such as DGEA and RGD serve as surrogates for native adhesion proteins within the extracellular matrix, providing binding sites for cell surface receptors. Accordingly, tight anchoring of these peptides to the graft surface is important. However, for some types of regenerative molecules, release from the graft surface is required for maximal activity. Molecules that form chemotactic gradients to drive recruitment of MSCs or vascular cells represent one example. The authors previously reported that the number of glutamates within the polyglutamate domain can be manipulated to control the rate of peptide release from the surface of HA. Specifically, E7DGEA peptides exhibited a significantly slower rate of release than DGEA, and peptides modified with diglutamate (E2DGEA) or tetraglutamate (E4DGEA) displayed intermediate release kinetics.22 Given the technical simplicity of producing peptides with variable-length polyglutamate domains, this approach offers a feasible method to tune delivery of biomodifiers from graft carriers.
The purpose of the current study was to determine whether the polyglutamate-coupling approach could be employed to achieve controlled delivery of osteoinductive peptides from commercially available bone grafting materials including allografts, xenografts, and alloplasts. The binding, release, and in vivo retention of the polyglutamate-modified peptides to the graft surfaces were compared. The DGEA peptide was modified with variable-length polyglutamate domains (E2, E4, and E7), and binding and release of the peptides were measured for the following materials: (1) particulated cortical/cancellous mineralized freeze-dried bone allograft (FDBA), (2) particulated anorganic bovine bone (ABB), and (3) synthetic β-tricalcium phosphate particulate (β-TCP). Additionally, to test the versatility of this technology on a different type of material, calcium sulfate bone cement (CaSO4) was utilized as a fourth substrate. The authors found that for all four materials, the degree of peptide binding and rate of release were directly related to the length of the polyglutamate domain, with the greatest binding and retention observed with E7DGEA.
MATERIALS AND METHODS
Preparation of Peptides
DGEA, diglutamate DGEA (E2DGEA), tetraglutamate DGEA (E4DGEA), and heptaglutamate DGEA (E7DGEA) were synthesized and obtained from American Peptide Company. Fluorescein isothiocyanate (FITC) was added to the peptides to allow for quantification and visualization of peptide binding and release. The final peptides included a lysine (K) linker to attach the FITC: DGEA-K-FITC (907.9 g/mol), EE-DGEA-K-FITC (1,166.2 g/mol), EEEE-DGEA-K-FITC (1,424.4 g/mol), and EEEEEEE-DGEA-K-FITC (1,811.7 g/mol). The lyophilized peptides were reconstituted in deionized sterile water to a concentration of 1 mg/mL. They were aliquoted and stored at −20°C until use.
Bone Grafts
Four distinct commercially available bone graft materials were utilized: (1) cortical/cancellous FDBA (Miner-Oss [BioHorizons]; particle size: 0.60 to 1.25 mm), (2) ABB (BioOss [Geistlich]; particle size: 0.25 to 1.0 mm), (3) synthetic silicated β-TCP (IngeniOs [Zimmer]; particle size: 0.25 to 1 mm), and (4) calcium sulfate hemihydrate bone cement (CaSO4, Ace Surgical Supply).
Quantification of Peptide Binding and Release In Vitro
To measure binding and release of peptides, 1-mg, 5-mg, 10-mg, and 20-mg quantities of each bone graft were measured and placed in Eppendorf tubes. The particulated bone grafts were hydrated in Tris-buffered saline (TBS) for 10 minutes. The CaSO4 was reconstituted with the setting solution provided by the manufacturer by adding just enough solution for the material to set. While the grafts were hydrating, equimolar solutions (0.1 µmol/L) of FITC-tagged peptides were prepared. The fluorescence of the starting solution of each peptide was measured on a VersaFluor fluorometer (Bio-Rad) to verify similar fluorescence readings from each peptide solution. This number represented the baseline fluorescence. The TBS was aspirated from the grafts, and peptide solutions of DGEA-FITC, E2DGEA-FITC, E4DGEA-FITC, or E7DGEA-FITC were added. The samples were placed on a rotator and covered with aluminum foil to protect from the light for 30 minutes. This time point was chosen because pilot time course experiments (not shown) showed that maximal binding for each material was achieved at 30 minutes. At the end of the 30-minute incubation, the solution fluorescence was measured via fluorometer. This number represented the amount of peptide remaining in solution (unbound peptide). The unbound peptide measurement was subtracted from the baseline peptide fluorescence value and multiplied by 100 to give the percent of peptide bound.
To measure peptide release, the grafts were washed briefly in TBS after the 30-minute coating interval to remove unbound peptides, and 1 mL of fresh TBS was placed in each grafting material. The grafts were placed on a shaker with vigorous agitation for 3 days, and solution fluorescence was measured on the fluorometer. This value represents the amount of peptide released into solution. To determine the percent of peptide released, the amount of peptide released was divided by the amount of peptide initially bound.
Visualization of Peptide Binding and Release In Vitro
To visualize binding of the peptide, 50 mg of each bone graft was measured and placed into a 12-well plate. Grafts were hydrated, and equimolar solutions of the peptides were prepared as described previously. DGEAFITC or E7DGEA-FITC solutions (1 µmol/L) were added to each bone graft. Also, for each type of graft, TBS was added to serve as a no-peptide control to determine if any of the grafts had native fluorescence. The plate was covered in aluminum foil and placed on a shaker for 30 minutes. The peptide solution or TBS was aspirated, and the grafts were washed three times in TBS for 10 minutes on a shaker to remove any loosely bound peptides. A portion of the graft materials was removed and imaged using a Nikon fluorescent microscope.
Peptide Retention In Vivo
A rat subcutaneous pouch model was utilized to determine if the peptides were retained under physiologic conditions for a time period consistent with initial bony wound healing. Equimolar solutions (100 µmol/L) of DGEA-FITC or E7DGEA-FITC were prepared under sterile conditions as described previously. The peptides were coated onto the bone grafts for 30 minutes and washed three times in TBS for 10 minutes each to remove any unbound or loosely bound peptides. Approval from the University of Alabama at Birmingham Institutional Animal Care and Use Committee was obtained prior to animal studies. Male Sprague Dawley rats (325 to 350 g) were obtained from Harlan Laboratories. Rats were anesthetized with 1% to 3% isoflurane with an oxygen flow rate of 1 to 2 L/min. The backs of the rats were shaved, and 15 × 15–mm subcutaneous pouches were created by sharp and blunt dissection. The coated grafts were implanted into the pouches and the wounds closed with surgical staples. Four implants were placed into each rat. The staples were removed after 7 days. The rats were euthanized and the grafts explanted at 8 weeks. The grafts were imaged using a Nikon fluorescent microscope.
Statistical Analysis
Quantitative binding experiments were performed at least three independent times, with each experiment performed in duplicate or triplicate. For initial binding values, the percent of each peptide that bound to each graft material was compared at each quantity (1, 5, 10, and 20 mg) using a one-way analysis of variance (ANOVA). A one-way ANOVA test was also conducted for values collected on day 3 (% released). Values were considered significant with a P value of < .05. Three independent experiments were also performed for the imaging studies, with at least two individual samples evaluated per experiment.
RESULTS
Variable Length Polyglutamate Domains Facilitate Tunable Peptide Binding to Three Commercially Available Bone Graft Materials
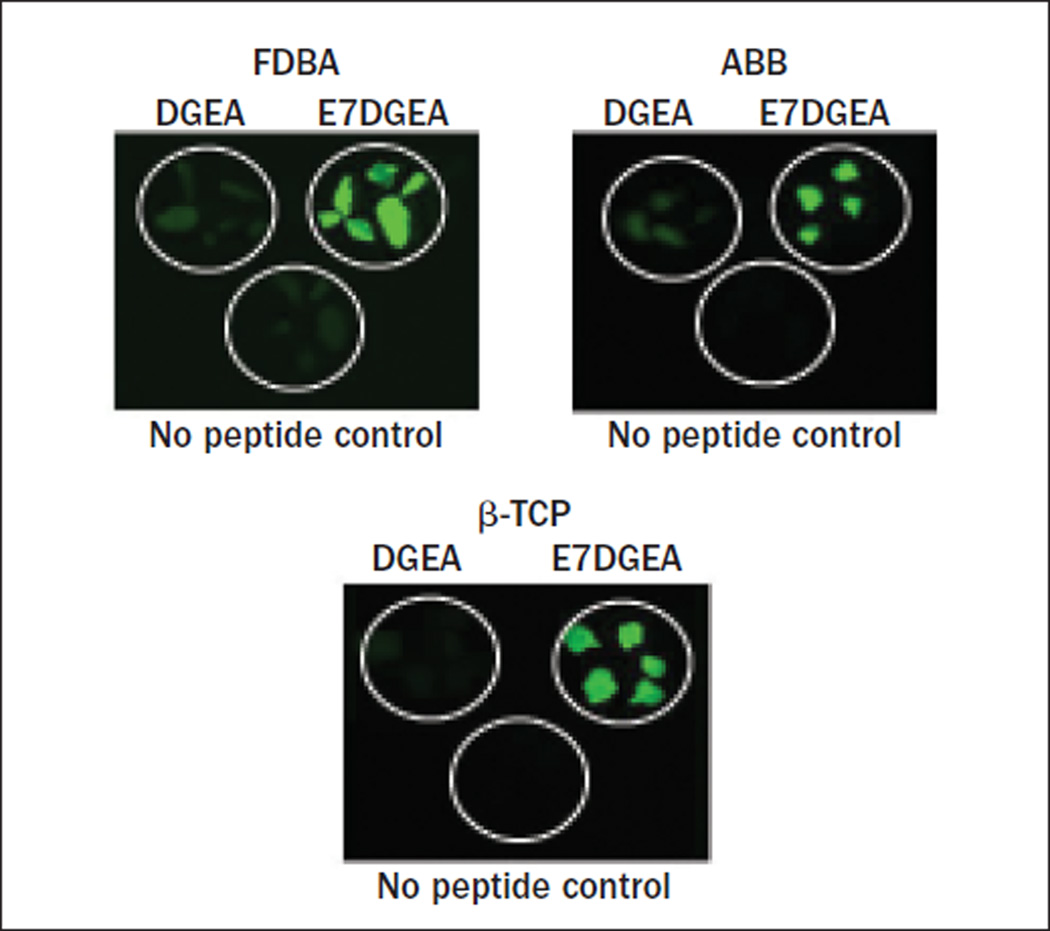
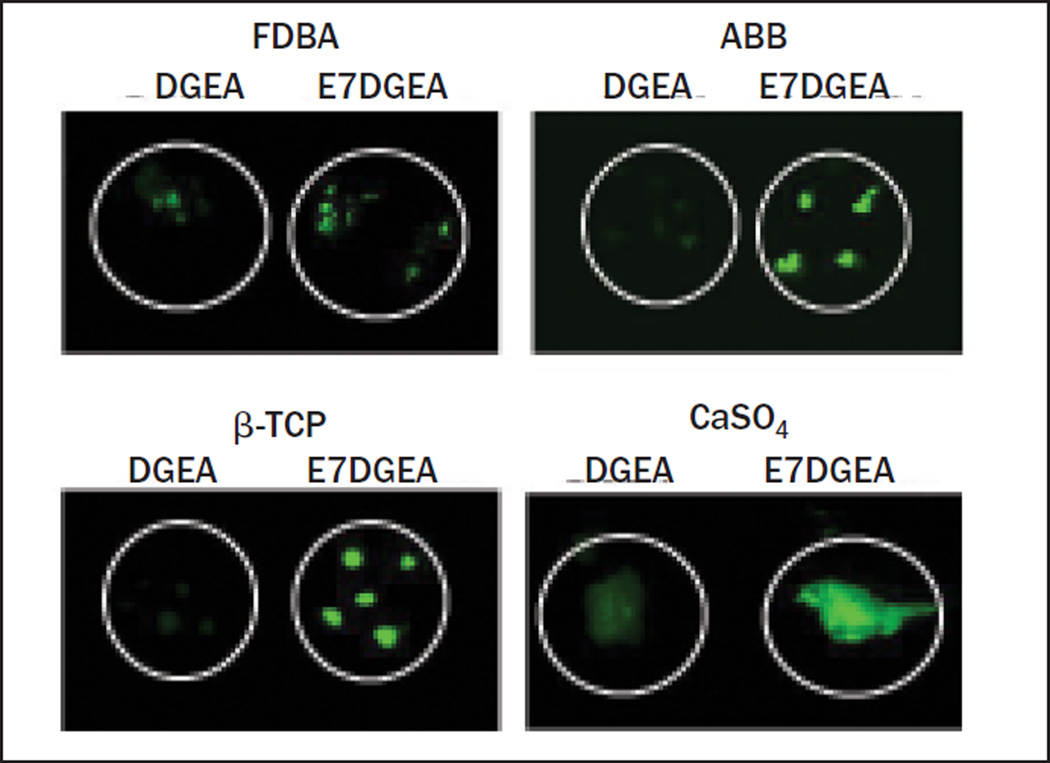
Direct visualization via fluorescent microscopy was performed to evaluate the binding of DGEA and E7DGEA to three distinct commercially available bone grafting materials: FDBA, ABB, and β-TCP. After coating for 30 minutes with FITC-tagged peptides, followed by several washing steps, the grafts were imaged. Greater binding of E7DGEA than DGEA to all three bone grafts was apparent, as shown in the fluorescent images (Fig 1).
Fig 1.
Fluorescent images showing that E7 directs greater binding and retention of DGEA. Grafts were coated with DGEA-FITC, E7DGEA-FITC, or left uncoated (TBS only) for 30 minutes. Grafts were washed and imaged.
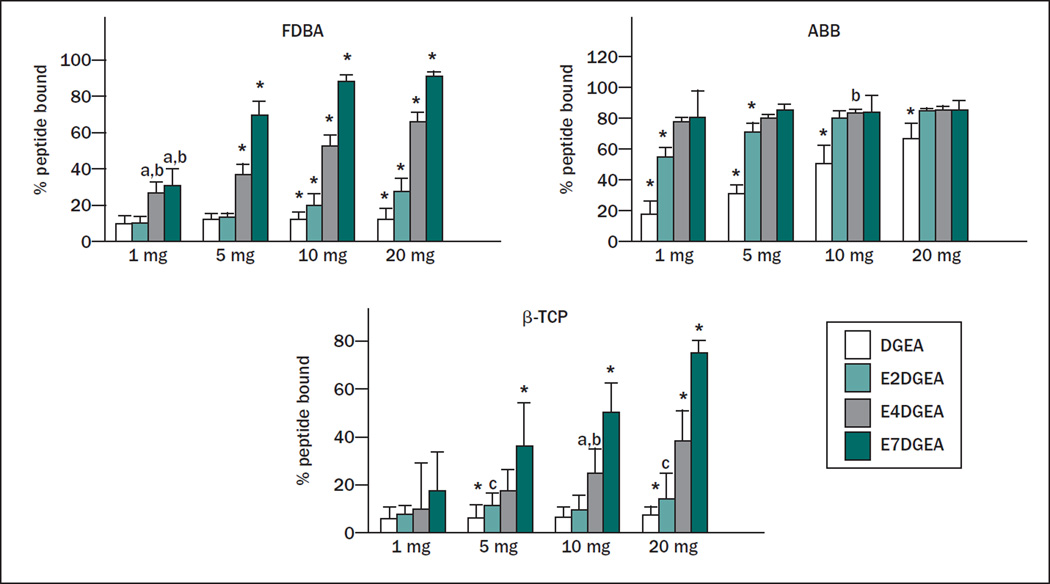
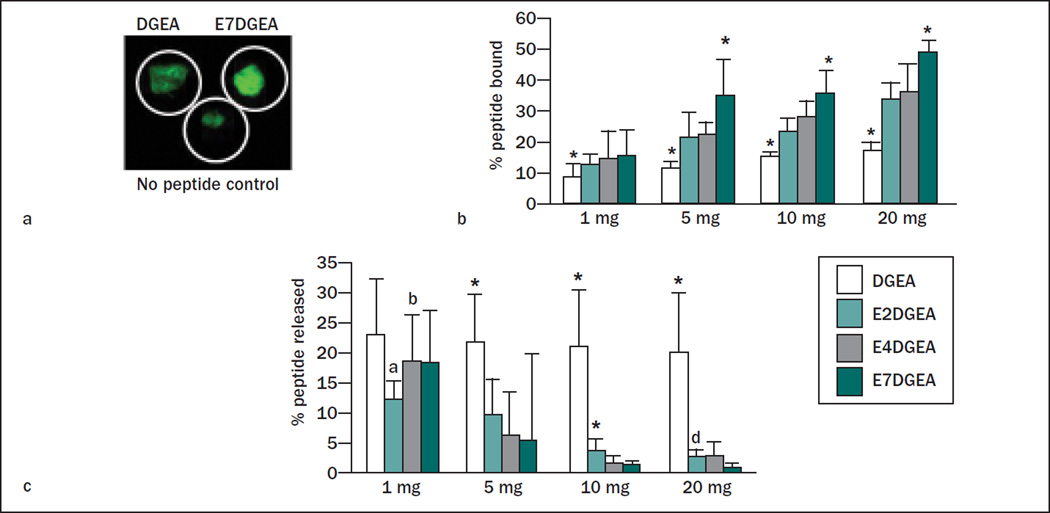
To quantify the amount of peptide loaded onto the grafts, solution depletion assays were performed. For these studies, the authors not only compared DGEA and E7DGEA, but also monitored the binding of DGEA modified with either two (E2) or four (E4) glutamate residues, with the expectation that the number of glutamate residues within the calcium-binding domain would directly influence binding affinity. Equimolar solutions of DGEA-FITC, E2DGEA-FITC, E4DGEA-FITC, and E7DGEA-FITC were prepared and the fluorescence measured to give initial solution fluorescence values. These solutions were then added to varying amounts of the bone grafting materials for 30 minutes, and residual fluorescence was measured and compared with the starting solution. As shown in Fig 2, there was a clear increase in peptide binding as the number of glutamates increased from two to seven, with E7 domains directing the greatest peptide loading to all three grafting materials. It was noted that the DGEA peptide (lacking a polyglutamate domain) exhibited higher binding to ABB relative to the other grafting materials. While the reason for this finding is currently unclear, it is possible that the high degree of porosity of ABB allowed some trapping of the peptide solution within the pores. Nonetheless, the collective data in Figs 1 and 2 show that the amount of peptide loaded can be tailored by altering the number of glutamates.
Fig 2.
Increasing length of glutamate domain confers greater peptide binding to three distinct bone grafts. Varying quantities of FDBA, ABB, or β-TCP (1 to 20 mg) were coated for 30 minutes with DGEA, E2DGEA, E4DGEA, or E7DGEA (all tagged with FITC). After the binding interval, the solution was collected and fluorescence measured via fluorometer. The percent of peptide bound was calculated by subtracting the residual fluorescence (representing unbound peptide) from the starting solution fluorescence (representing total amount of peptide added). Results show that increasing the length of the glutamate domain increases the amount of peptide bound to the three grafts tested. Significant difference (P < .05) between samples was denoted as follows: relative to DGEA = a, E2DGEA = b, E4DGEA = c, E7DGEA = d, and * to all.
Variable Length Polyglutamate Domains Mediate Controlled Release of Peptides from the Graft Surface
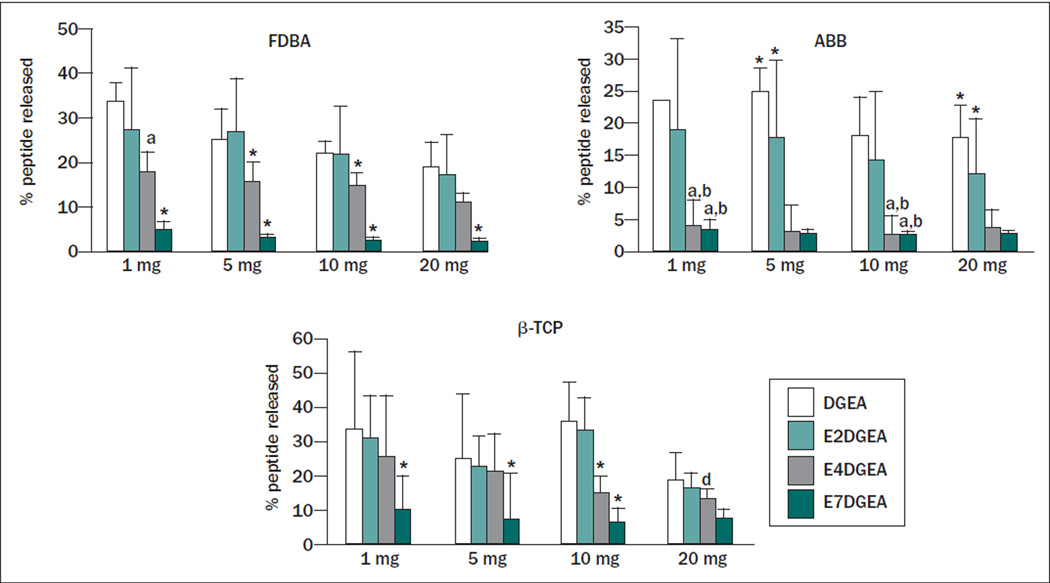
To determine if the length of the glutamate domain could be varied to tune release of DGEA, the three grafting materials, FDBA, ABB, and β-TCP, were coated with DGEA-FITC, E2DGEA-FITC, E4DGEA-FITC, or E7DGEA-FITC for 30 minutes, and the amount of peptide bound was measured as above. The samples were then placed into fresh TBS and agitated vigorously for 3 days. At the end of this interval, the amount of solution fluorescence, representing peptide that had been released back into solution from the graft surface, was measured. This number was divided by the amount of initial peptide bound to calculate the percentage of peptide released. In the case of all three bone grafts, a tapered release was observed as a function of the number of glutamates within the polyglutamate domain (Fig 3), with E7DGEA exhibiting the tightest binding to the graft surface.
Fig 3.
Increased length of glutamate domain confers greater retention to three distinct bone grafts after 3 days with vigorous agitation. Bone grafts were coated with DGEA, E2DGEA, E4DGEA, and E7DGEA (FITC-tagged) as described previously. Samples were then washed briefly in TBS, and resuspended in fresh TBS with agitation for 3 days. Peptide release was measured by monitoring the appearance of solution fluorescence. As the length of the glutamate domain increased, more peptide was retained on the graft surface despite agitation for 3 days. Significant difference (P < .05) between samples was denoted as follows: relative to DGEA = a, E2DGEA = b, E4DGEA = c, E7DGEA = d, and * to all.
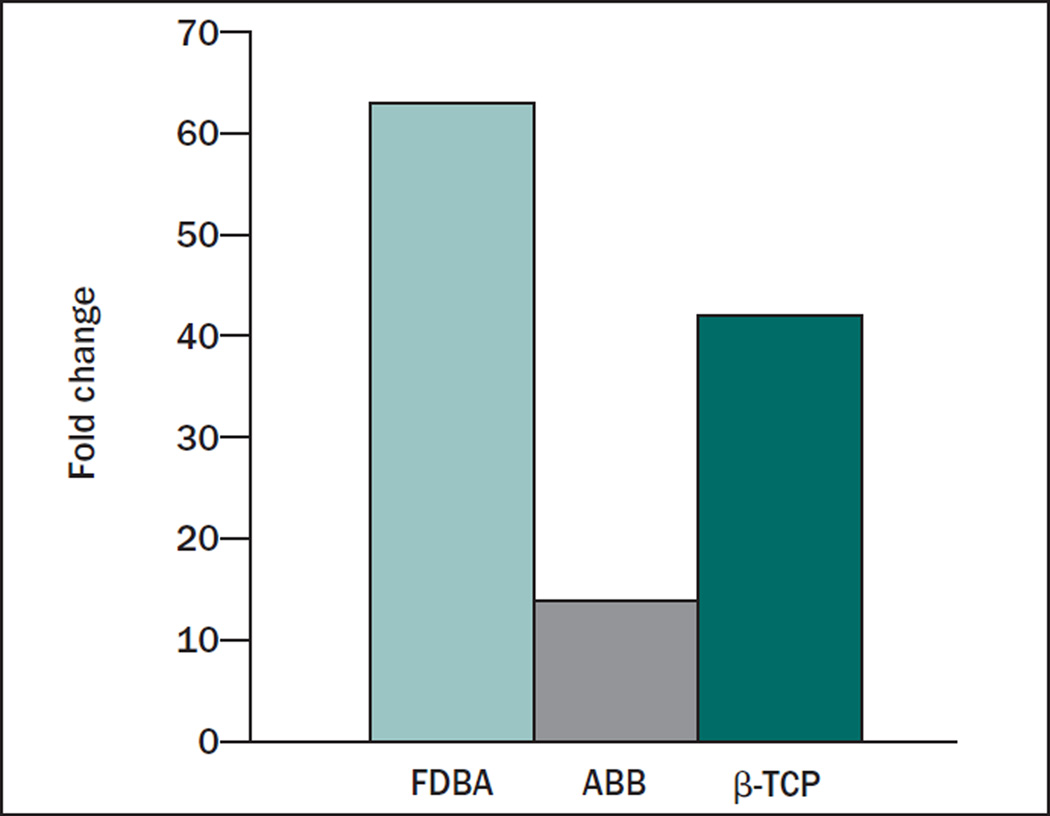
The values in Fig 3 were calculated as the percent of initially bound peptide that was released from the graft; however, the amount of peptide initially loaded onto the graft surface varied depending upon the length of the polyglutamate domain. The combined effects of differential peptide loading and release will dictate the absolute amount of peptide on the graft surface after an extended time interval. To estimate the combined influence of enhanced loading and retention on the capacity of E7 domains to increase peptide density on graft surfaces, the fold differences in initial loading were multiplied by the fold differences in peptide retention (Fig 4). As an example, for the 10-mg quantity of FDBA, there was 7 times greater initial loading, and 9 times greater retention, of E7DGEA relative to DGEA. This represents 63 times more E7DGEA than DGEA on the graft surface at 3 days. For ABB, there was 2 times more binding and 7 times more retention, which equates to 14 times more E7DGEA. For β-TCP, there was 7 times more binding and 6 times more retention, yielding 42 times more E7DGEA on the graft surface at 3 days.
Fig 4.
Fold enhancement in the amount of E7DGEA vs DGEA still bound at the end of 3 days. The combined effects of greater initial E7DGEA binding and retention after 3 days were calculated. As shown, 63-, 14-, and 42-fold more E7DGEA than DGEA was present on FDBA, ABB, and β-TCP, respectively.
Variable Length Polyglutamate Domains Can Be Used to Tailor Peptide Binding to, and Release from, Calcium-Containing Bone Cement
Figures 1 to 4 demonstrate tunable binding and release of polyglutamate-modified DGEA to three distinct bone grafting materials. To expand the range of materials for which this approach can be utilized, the authors examined the binding and release of DGEA with variable-length polyglutamate domains on CaSO4. DGEA-FITC and E7DGEA-FITC were coated onto the CaSO4 surface as described previously and imaged via fluorescent microscopy to visualize peptide loading. Figure 5a shows that E7 directed greater anchoring of DGEA to the cement surface. Of note, the CaSO4 has some native fluorescence, as can be seen in the nopeptide control. Solution depletion assays were also conducted as described previously. After 30 minutes, more DGEA was loaded onto the CaSO4 surface as the length of the glutamate domain was increased, with E7DGEA exhibiting the greatest amount of peptide bound (Fig 5b). Figure 5c shows that, despite vigorous agitation for 3 days, more peptide was retained on the CaSO4 surface as a function of the length of the polyglutamate domain. The fold change was also calculated as described in Fig 4, yielding a 38-fold increase in the amount of E7DGEA relative to DGEA after a 3-day interval. These results establish that polyglutamate domains can be used to anchor bioactive molecules to bone cements, and also show that the approach is effective for calcium-containing materials other than calcium phosphates.
Fig 5.
Polyglutamate domains are effective in anchoring peptides to CaSO4 bone cement. (a) E7DGEA-FITC exhibited greater binding than DGEA-FITC to CaSO4 bone cement, as evidenced by fluorescent microscopy. Note that CaSO4 has inherent fluorescence, as can be seen in the no-peptide control. (b) Solution depletion assays show that increasing the length of the glutamate domain increases the amount of peptide bound to CaSO4. (c) CaSO4 samples were coated with peptides as before, and then placed in fresh TBS with vigorous agitation for 3 days. As the length of the glutamate domain increased, more peptide was retained on the CaSO4 surface. (b and c) Significant difference (P < .05) between samples was denoted as follows: relative to DGEA = a, E2DGEA = b, E4DGEA = c, E7DGEA = d, and * to all.
Addition of a Polyglutamate Domain Increases Peptide Retention In Vivo
To determine if E7DGEA could be retained on the bone graft surface under physiologic conditions, the three bone grafts, as well as the bone cement, were coated with DGEA-FITC or E7DGEA-FITC as described previously. The modified grafts were then placed into rat subcutaneous pouches. After 8 weeks, the grafts were explanted and imaged using a fluorescent microscope. As shown in Fig 6, E7DGEA peptides were retained on all four materials for at least 8 weeks in vivo.
Fig 6.
E7 directs greater retention of DGEA in vivo. Grafts were coated with DGEA-FITC or E7DGEA-FITC and placed into rat subcutaneous pouches for 8 weeks. Grafts were retrieved, washed, and imaged. Of note, the FDBA group resorbed at a quicker rate than the other grafts, and minimal particles were found upon retrieval.
DISCUSSION
Due to the limitations associated with autogenous bone grafting, extensive research has been devoted to developing substitute grafting materials that can produce comparable clinical outcomes. The lack of effective and commercially feasible methods for coupling osteoinductive molecules onto substitute graft materials represents a major barrier to this goal. A commonly used approach for delivering osteoinductive molecules on grafting materials involves passive adsorption onto the graft surface. However, passively adsorbed molecules are typically released very rapidly, which limits therapeutic potential and can, in some instances, cause adverse side effects. As an example, dissemination of the highly osteoinductive protein, bone morphogenic protein-2 (BMP-2), away from the graft site has been linked to inflammation and heterotopic calcification.23 Additionally, supraphysiologic doses of BMP-2 are needed because of inadequate coupling to carriers.23
To circumvent the problems associated with passive adsorption, a number of methods have been investigated to anchor bioactive factors to bone grafting materials. For synthetic materials such as HA, covalent attachment of molecules has been achieved through first silanizing the HA surface, followed by various chemical coupling approaches.24–26 However, there are several potential disadvantages of covalent coupling: (1) silanization of the material surface may mask the natural osteoconductive properties of calcium phosphate moieties; (2) chemical immobilization may alter the conformation and/or activity of the target biologic; and (3) well-controlled orientation of the anchored biologic is difficult to achieve. Furthermore, covalent immobilization would require modification of the graft surface prior to commercialization. As an alternative, biologics conjugated with domains that bind to the graft through high-affinity ionic, or other noncovalent, interactions can be utilized with products already in the clinic. Most noncovalent coupling strategies have centered on the use of negatively charged domains that bind calcium. These include bisphosphonates,27–30 polyglutamate or polyaspartate sequences,14–16,18,31,32 and domains that incorporate noncanonical amino acids such as γ-carboxyglutamate or phosphoserine.33–35 These domains are all effective in tethering molecules to calcium-containing substrates. However, the use of bisphosphonate as an anchoring domain introduces a nonneeded pharmacologic agent into the graft site, and some clinicians may be wary of this approach given the link between bisphosphonates and osteonecrosis of the jaw.36,37 For domains incorporating noncanonical amino acids, chemical synthesis may be more complex than that required for standard polyglutamate or polyaspartate sequences.
The concept underlying the use of polyglutamate/ polyasparate domains is derived from the mechanism employed by native bone-localized proteins to bind bone matrix. Acidic amino acid motifs are present within several bone matrix proteins and are responsible for protein binding to the calcium within bone HA.38,39 Verification that polyglutamate serves as an HA-binding domain was provided by early work from Kuboki’s group. In this study, osteonectin was adsorbed onto synthetic HA and then digested.40 After digestion, it was found that the glutamate-rich regions of the protein remained bound to the HA surface. Subsequently, polyglutamate domains have been exploited to couple many different bioactive peptides to HA-containing biomaterials. As an example, Fujisawa et al examined the binding of an E7-RGD peptide to synthetic HA.18 It was found that the dissociation constant for E7-RGD was 500 times greater than that of bone sialoprotein, a protein that contains both polyglutamate and RGD sequences. Another important property of polyglutamate domains is that they are very selective for calcium-containing materials, evidenced by the negligible binding of the E7 domain to titanium, stainless steel, and polycaprolactone.15 Further supporting specificity, E7-modified peptides overlaid onto tissue sections harvested from neonatal mice bound tightly to bone, but not to soft tissue.31
Polyglutamate domains offer additional advantages compared with other coupling approaches. The polyglutamate domain is synthesized as a contiguous sequence with the bioactive part of the peptide using a commercial peptide synthesizer, thus avoiding the need for complex chemical coupling strategies. Large quantities of highly pure peptides can be produced in an efficient and relatively inexpensive manner. Moreover, the binding of polyglutamate-modified peptides to substrate occurs rapidly, with near maximal binding observed within 30 to 60 minutes.22,31 These features suggest that polyglutamate-modified peptides could be produced as a lyophilized product for coating onto off-the-shelf bone grafting products immediately before surgical placement.
The current study provides an advance by showing that polyglutamate domains are effective in coupling an osteoinductive peptide, DGEA, to multiple types of grafting materials including allograft (FDBA), xenograft (ABB), alloplast (β-TCP), and a bone cement (CaSO4). All of these materials are currently used in craniofacial and/ or orthopedic medicine, and each has distinct benefits and limitations. Allograft materials retain some osteoinductive proteins; however, this material can potentially introduce pathogens or elicit an immune response. Xenograft products, which are more widely used in parts of Europe than allograft bone, have been treated to remove organic components, which eliminates the risk of pathogen transfer and immunogenicity, but correspondingly reduces osteoinductivity. Alloplast materials are abundant and can be produced synthetically at low cost; however, these also lack osteoinductivity. Another point to consider is that these various materials have different resorption rates and mechanical properties, which can influence the choice of grafting material depending upon the specific clinical objective. For example, some clinical situations warrant the use of allograft, which resorbs at a faster rate than a xenograft like ABB, for which residual graft particles can be found for years after implantation.41,42 A technically feasible and cost-effective mechanism to functionalize all of these diverse materials with osteoinductive molecules would have widespread clinical applicability. Another important finding of this study is that the concentration of peptide bound to the graft surface, as well as the rate of peptide release, can be controlled by varying the number of glutamate residues, offering a simple method for tuning the delivery of bioactive molecules. While matrix-derived mimetic peptides such as DGEA require tight anchoring for optimal effect, shorter polyglutamate domains (E2, E4), or mixtures of variable-length glutamate domains, may be better suited for factors that function as chemotactic gradients, such as angiogenic factors. For instance, controlled release, with appropriate temporal kinetics, is important for the angiogenesis-inducing activities of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF).43,44 Recently, biomimetic peptides derived from VEGF have been developed45; these peptides constitute promising candidates for modification with variable-length polyglutamate domains to enable gradient release. Additionally, variable-length polyglutamate domains may be useful for delivering two distinct peptides with differing optimal temporal kinetics. Finally, the authors recently reported that polyglutamate domains are effective in anchoring cargo-bearing nanoparticles to calcium-containing graft materials, which opens new avenues for drug delivery and imaging.46
CONCLUSION
Results herein demonstrate that osteoinductive peptides such as DGEA can be synthesized with a polyglutamate domain to increase peptide binding and retention on the surface of four distinct bone grafting materials including allograft, xenograft, alloplast, and a bone cement. Peptides with a polyglutamate domain were retained on the graft surface for at least 2 months in vivo, highlighting the potential for a sustained effect on bone healing. Furthermore, the authors found that the amount of peptide bound, and rate of peptide released, can be controlled by varying the number of glutamates within the polyglutamate domain. These collective findings suggest that variable-length polyglutamate domains enable tunable delivery of bioactive peptides to enhance the regenerative capacity of off-the-shelf grafting materials used in many craniofacial and orthopedic procedures.
ACKNOWLEDGMENTS
This research was supported by a grant from the Osseointegration Foundation (SLB and MSR). Dr Bain was supported by the Dental Academic Research Training Program T32 NIDCR DE017607 and NIH/NIDCR postdoctoral fellowship 1F32DE022997-01.
Footnotes
The authors reported no conflicts of interest related to this study.
REFERENCES
- 1.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: An update. Injury. 2005;36(suppl 3):S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Marino JT, Ziran BH. Use of solid and cancellous autologous bone graft for fractures and nonunions. Orthop Clin North Am. 2010;41:15–26. doi: 10.1016/j.ocl.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Brighton CT, Shaman P, Heppenstall RB, Esterhai JL, Jr, Pollack SR, Friedenberg ZB. Tibial nonunion treated with direct current, capacitive coupling, or bone graft. Clin Orthop Relat Res. 1995:223–234. [PubMed] [Google Scholar]
- 5.Fernyhough JC, Schimandle JJ, Weigel MC, Edwards CC, Levine AM. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine. 1992;17:1474–1480. doi: 10.1097/00007632-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;339:76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Bostrom MP, Seigerman DA. The clinical use of allografts, demineralized bone matrices, synthetic bone graft substitutes and osteoinductive growth factors: A survey study. HSS J. 2005;1:9–18. doi: 10.1007/s11420-005-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker W, Urist MR, Tucker LM, Becker BE, Ochsenbein C. Human demineralized freeze-dried bone: Inadequate induced bone formation in athymic mice. A preliminary report. J Periodontol. 1995;66:822–828. doi: 10.1902/jop.1995.66.9.822. [DOI] [PubMed] [Google Scholar]
- 9.Shigeyama Y, D’Errico JA, Stone R, Somerman MJ. Commercially-prepared allograft material has biological activity in vitro. J Periodontol. 1995;66:478–487. doi: 10.1902/jop.1995.66.6.478. [DOI] [PubMed] [Google Scholar]
- 10.Romagnoli C, D’Asta F, Brandi ML. Drug delivery using composite scaffolds in the context of bone tissue engineering. Clin Cases Miner Bone Metab. 2013;10:155–161. [PMC free article] [PubMed] [Google Scholar]
- 11.Holland TA, Mikos AG. Biodegradable polymeric scaffolds. Improvements in bone tissue engineering through controlled drug delivery. Adv Biochem Eng Biotechnol. 2006;102:161–185. doi: 10.1007/b137205. [DOI] [PubMed] [Google Scholar]
- 12.Bose S, Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012;8:1401–1421. doi: 10.1016/j.actbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert M, Giachelli CM, Stayton PS. Biomimetic peptides that engage specific integrin-dependent signaling pathways and bind to calcium phosphate surfaces. J Biomed Mater Res A. 2003;67:69–77. doi: 10.1002/jbm.a.10053. [DOI] [PubMed] [Google Scholar]
- 14.Sawyer AA, Weeks DM, Kelpke SS, McCracken MS, Bellis SL. The effect of the addition of a polyglutamate motif to RGD on peptide tethering to hydroxyapatite and the promotion of mesenchymal stem cell adhesion. Biomaterials. 2005;26:7046–7056. doi: 10.1016/j.biomaterials.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Culpepper BK, Phipps MC, Bonvallet PP, Bellis SL. Enhancement of peptide coupling to hydroxyapatite and implant osseointegration through collagen mimetic peptide modified with a polyglutamate domain. Biomaterials. 2010;31:9586–9594. doi: 10.1016/j.biomaterials.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh D, Yoneda S, Kuroda S, et al. Enhancement of osteogenesis on hydroxyapatite surface coated with synthetic peptide (EEEEEEE-PRGDT) in vitro. J Biomed Mater Res. 2002;62:292–298. doi: 10.1002/jbm.10338. [DOI] [PubMed] [Google Scholar]
- 17.Murphy MB, Hartgerink JD, Goepferich A, Mikos AG. Synthesis and in vitro hydroxyapatite binding of peptides conjugated to calcium-binding moieties. Biomacromolecules. 2007;8:2237–2243. doi: 10.1021/bm070121s. [DOI] [PubMed] [Google Scholar]
- 18.Fujisawa R, Mizuno M, Nodasaka Y, Kuboki Y. Attachment of osteoblastic cells to hydroxyapatite crystals by a synthetic peptide (Glu7-Pro-Arg-Gly-Asp-Thr) containing two functional sequences of bone sialoprotein. Matrix Biol. 1997;16:21–28. doi: 10.1016/s0945-053x(97)90113-x. [DOI] [PubMed] [Google Scholar]
- 19.Sawyer AA, Hennessy KM, Bellis SL. The effect of adsorbed serum proteins, RGD and proteoglycan-binding peptides on the adhesion of mesenchymal stem cells to hydroxyapatite. Biomaterials. 2007;28:383–392. doi: 10.1016/j.biomaterials.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JM, Kushwaha M, Tambralli A, Bellis SL, Camata RP, Jun HW. Osteogenic differentiation of human mesenchymal stem cells directed by extracellular matrix-mimicking ligands in a biomimetic self-assembled peptide amphiphile nanomatrix. Biomacromolecules. 2009;10:2935–2944. doi: 10.1021/bm9007452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennessy KM, Pollot BE, Clem WC, et al. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials. 2009;30:1898–1909. doi: 10.1016/j.biomaterials.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Culpepper BK, Webb WM, Bonvallet PP, Bellis SL. Tunable delivery of bioactive peptides from hydroxyapatite biomaterials and allograft bone using variable-length polyglutamate domains. J Biomed Mater Res A. 2014;102:1008–1016. doi: 10.1002/jbm.a.34766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in BMP delivery. Biotechnol Lett. 2009;31:1817–1824. doi: 10.1007/s10529-009-0099-x. [DOI] [PubMed] [Google Scholar]
- 24.Durrieu MC, Pallu S, Guillemot F, et al. Grafting RGD containing peptides onto hydroxyapatite to promote osteoblastic cells adhesion. J Mater Sci Mater Med. 2004;15:779–786. doi: 10.1023/b:jmsm.0000032818.09569.d9. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Cheng K, Weng W, Yang C. Immobilization of RGD peptide on HA coating through a chemical bonding approach. J Mater Sci Mater Med. 2009;20:2349–2352. doi: 10.1007/s10856-009-3794-1. [DOI] [PubMed] [Google Scholar]
- 26.Nelson M, Balasundaram G, Webster TJ. Increased osteoblast adhesion on nanoparticulate crystalline hydroxyapatite functionalized with KRSR. Int J Nanomedicine. 2006;1:339–349. [PMC free article] [PubMed] [Google Scholar]
- 27.Uludag H, Gao T, Wohl GR, Kantoci D, Zernicke RF. Bone affinity of a bisphosphonate-conjugated protein in vivo. Biotechnol Prog. 2000;16:1115–1118. doi: 10.1021/bp000066y. [DOI] [PubMed] [Google Scholar]
- 28.Schuessele A, Mayr H, Tessmar J, Goepferich A. Enhanced bone morphogenetic protein-2 performance on hydroxyapatite ceramic surfaces. J Biomed Mater Res A. 2009;90:959–971. doi: 10.1002/jbm.a.31745. [DOI] [PubMed] [Google Scholar]
- 29.Gittens SA, Bagnall K, Matyas JR, Lobenberg R, Uludag H. Imparting bone mineral affinity to osteogenic proteins through heparin-bisphosphonate conjugates. J Control Release. 2004;98:255–268. doi: 10.1016/j.jconrel.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Uludag H, Kousinioris N, Gao T, Kantoci D. Bisphosphonate conjugation to proteins as a means to impart bone affinity. Biotechnol Prog. 2000;16:258–267. doi: 10.1021/bp990154m. [DOI] [PubMed] [Google Scholar]
- 31.Culpepper BK, Bonvallet PP, Reddy MS, Ponnazhagan S, Bellis SL. Polyglutamate directed coupling of bioactive peptides for the delivery of osteoinductive signals on allograft bone. Biomaterials. 2013;34:1506–1513. doi: 10.1016/j.biomaterials.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokogawa K, Miya K, Sekido T, et al. Selective delivery of estradiol to bone by aspartic acid oligopeptide and its effects on ovariectomized mice. Endocrinology. 2001;142:1228–1233. doi: 10.1210/endo.142.3.8024. [DOI] [PubMed] [Google Scholar]
- 33.Sakuragi M, Kitajima T, Nagamune T, Ito Y. Recombinant hBMP4 incorporated with non-canonical amino acid for binding to hydroxyapatite. Biotechnol Lett. 2011;33:1885–1890. doi: 10.1007/s10529-011-0637-1. [DOI] [PubMed] [Google Scholar]
- 34.Lee JS, Murphy WL. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta Biomater. 2010;6:21–28. doi: 10.1016/j.actbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brounts SH, Lee JS, Weinberg S, Lan Levengood SK, Smith EL, Murphy WL. High affinity binding of an engineered, modular peptide to bone tissue. Mol Pharm. 2013;10:2086–2090. doi: 10.1021/mp300662r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Coskun Benlidayi I, Guzel R. Oral bisphosphonate related osteonecrosis of the jaw: A challenging adverse effect ISRN. Rheumatol. 2013 May 16; doi: 10.1155/2013/215034. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oldberg A, Franzen A, Heinegard D. The primary structure of a cellbinding bone sialoprotein. J Biol Chem. 1988;263:19430–19432. [PubMed] [Google Scholar]
- 39.Hoang QQ, Sicheri F, Howard AJ, Yang DS. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003;425:977–980. doi: 10.1038/nature02079. [DOI] [PubMed] [Google Scholar]
- 40.Fujisawa R, Wada Y, Nodasaka Y, Kuboki Y. Acidic amino acid-rich sequences as binding sites of osteonectin to hydroxyapatite crystals. Biochim Biophys Acta. 1996;1292:53–60. doi: 10.1016/0167-4838(95)00190-5. [DOI] [PubMed] [Google Scholar]
- 41.Traini T, Valentini P, Iezzi G, Piattelli A. A histologic and histomorphometric evaluation of anorganic bovine bone retrieved 9 years after a sinus augmentation procedure. J Periodontol. 2007;78:955–961. doi: 10.1902/jop.2007.060308. [DOI] [PubMed] [Google Scholar]
- 42.Piattelli M, Favero GA, Scarano A, Orsini G, Piattelli A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: A histologic long-term report of 20 cases in humans. Int J Oral Maxillofac Implant. 1999;14:835–840. [PubMed] [Google Scholar]
- 43.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24:258–264. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 44.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 45.D’Andrea LD, Iaccarino G, Fattorusso R, et al. Targeting angiogenesis: Structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proc Natl Acad Sci. 2005;102:14215–14220. doi: 10.1073/pnas.0505047102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Culpepper BK, Morris DS, Prevelige PE, Bellis SL. Engineering nanocages with polyglutamate domains for coupling to hydroxyapatite biomaterials and allograft bone. Biomaterials. 2013;34:2455–2462. doi: 10.1016/j.biomaterials.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]