Highlights
-
•
We examined individuals with screen-detected diabetes over five years.
-
•
Two cardio-protective agents were prescribed at diagnosis, 3 at one year and 4 at five years.
-
•
Increases in cardio-protective medication did not impact negatively on HRQoL.
Keywords: Diabetes, HRQoL, Medication
Abstract
Aims
Establishing a balance between the benefits and harms of treatment is important among individuals with screen-detected diabetes, for whom the burden of treatment might be higher than the burden of the disease. We described the association between cardio-protective medication and health-related quality of life (HRQoL) among individuals with screen-detected diabetes.
Methods
867 participants with screen-detected diabetes underwent clinical measurements at diagnosis, one and five years. General HRQoL (EQ5D) was measured at baseline, one- and five-years, and diabetes-specific HRQoL (ADDQoL-AWI) and health status (SF-36) at one and five years. Multivariable linear regression was used to quantify the association between change in HRQoL and change in cardio-protective medication.
Results
The median (IQR) number of prescribed cardio-protective agents was 2 (1 to 3) at diagnosis, 3 (2 to 4) at one year and 4 (3 to 5) at five years. Change in cardio-protective medication was not associated with change in HRQoL from diagnosis to one year. From one year to five years, change in cardio-protective agents was not associated with change in the SF-36 mental health score. One additional agent was associated with an increase in the SF-36 physical health score (2.1; 95%CI 0.4, 3.8) and an increase in the EQ-5D (0.05; 95%CI 0.02, 0.08). Conversely, one additional agent was associated with a decrease in the ADDQoL-AWI (−0.32; 95%CI −0.51, −0.13), compared to no change.
Conclusions
We found little evidence that increases in the number of cardio-protective medications impacted negatively on HRQoL among individuals with screen-detected diabetes over five years.
1. Introduction
Type 2 diabetes is associated with increased risk of morbidity and early mortality [1] and a reduced health related quality of life (HRQoL) [2]. Pharmacological management of individuals with established diabetes reduces cardiovascular risk [3]. However, treatment regimens may impact on a patient's illness experience and their HRQoL and interventions that improve cardiovascular risk factor levels do not necessarily improve HRQoL [4]. Establishing a balance between the benefits and harms of pharmacological treatment is particularly important among individuals with screen-detected diabetes, for whom the burden of treatment might be higher than the burden of the disease [5,6]. The advent of national screening programmes, such as the NHS Health Checks, means that more people with clinically asymptomatic diabetes will be diagnosed. There is limited research examining how the burden of treatment might affect HRQoL for individuals identified earlier in the diabetes disease trajectory.
Among patients with established diabetes, most research supports an inverse association between glycosylated haemoglobin (HbA1C) and diabetes-related QoL [7,8]. In a cohort of individuals with screen-detected diabetes, we recently showed that people whose HbA1C decreased from one to five years post-diagnosis were less likely to report a negative impact of diabetes on their HRQoL [9]. However, further research is needed to elucidate the relationship between cardio-protective medication and HRQoL. This information would help inform diabetes management strategies early in the diabetes disease trajectory.
Among 867 participants with screen-detected diabetes (the ADDITION-Cambridge trial cohort), we described the association between (i) change in cardio-protective medication from diagnosis to one year and change in general HRQoL (EQ-5D) and (ii) change in cardio-protective medication from one to five years and change in general (EQ-5D, SF-36) and diabetes-specific HRQoL (ADDQoL-AWI). Our secondary aim was to establish whether change in cardio-protective medication in the first year after diagnosis was associated with changes in HRQoL from one to five years.
2. Methods
We used data from the Cambridge centre of the ADDITION-Europe trial [10], a pragmatic cluster randomised controlled trial comparing intensive multifactorial treatment with routine care in a screen-detected diabetes population in primary care [11]. The study protocol has been published [10]. Individuals aged 40 to 69 years from 49 practices in Eastern England, not known to have diabetes, and with a diabetes risk score derived from practice records [12] corresponding to the top 25% of the population distribution were invited for stepwise screening. Exclusion criteria were pregnancy, lactation, an illness with a likely prognosis of less than one year or a psychiatric illness likely to limit study involvement or invalidate informed consent. 867 patients were found to have diabetes according to 1999 WHO diagnostic criteria [13] and agreed to take part in the treatment trial. The study was approved by the Eastern Multi-Centre Research Ethics Committee (ref: 02/5/54) [10] and all participants provided written informed consent.
2.1. Intervention
Individuals were treated according to the group to which their practice was allocated: routine care according to national guidelines [14] (n = 23) or intensive multifactorial treatment (n = 26). In the intensive treatment arm, GPs were encouraged through guidelines, educational meetings, and audits with feedback to introduce a stepwise target-led drug treatment regime to reduce hyperglycaemia, hypertension and hyperlipidaemia [10] based on the STENO-2 study [15]. The intervention also included funding for practices to facilitate more frequent contact, a recommendation to refer all participants to a dietician, and theory based diabetes education materials for participants.
2.2. Measurement and outcomes
Trained staff assessed patients’ health at baseline, one year and five years and collected biochemical and anthropometric data according to standard operating procedures. Self-report questionnaires were used to collect information on socio-demographic information, lifestyle habits and medication use. Changes in biochemical measures and medication from baseline to five-year follow-up have been reported previously [11].
The EuroQol three level index score (EQ-5D) was administered at diagnosis, one and five years. The EQ-5D assesses health utility over five domains of health (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), each with three levels of functioning, which results in 243 health states with scores ranging from −0.594 to +1.00 (full health) [16]. The Short Form Health Survey (SF-36) measures health status and consists of 36 items over eight health domains; it can be summarised into physical (PCS) and mental health summary (MCS) scores that range from 0 to 100, with higher scores indicating better health [17]. The Diabetes-specific Audit of Diabetes Dependent Quality of Life (ADDQoL), measures an individual's perception of the impact of diabetes on various aspects of their QoL, and can be summarised as an average weighted index score (ADDQoL-AWI) that ranges from -9 (negative impact) to +3 (positive impact) [18]. The SF-36 and ADDQoL-AWI were collected at one and five years only. For the purposes of brevity, health status, diabetes-related QoL and HRQoL are treated as synonymous in the text.
Participants were encouraged to bring their repeat prescription summaries to each health assessment to aid with the completion of a health economics questionnaire [19], which asks for information on all prescribed medication. Self-reported medication was ATC coded [20] and grouped into 13 types of cardio-protective agent: aspirin; any statin; any other lipid lowering medication; any ACE inhibitor; any β-blocker; any calcium channel blocker; any diuretic; any other blood pressure lowering medication; any thiazolidinedione; any sulphonylurea; metformin; insulin; or any other glucose lowering medication. Cardio-protective medication count was defined as the total number of the 13 cardio-protective agents each participant reported taking at each time point: diagnosis, one and five years.
2.3. Statistical analysis
Individuals that died between diagnosis and one year (n = 8), and one year and five years (n = 47), were excluded from the analysis sample. Only cases with complete data were included. Descriptive characteristics were described at baseline, one year and five years using means, medians and proportions. Differences in characteristics between participants with and without complete data were examined using logistic regression.
To describe change in cardio-protective medication, data were collapsed into three groups: (i) no change or a reduction in the number of cardio-protective agents (0); (ii) an increase of one cardio-protective agent (1); and (iii) an increase of ≥2 cardio-protective agents (2). The baseline EQ-5D score was subtracted from one year to calculate the change in EQ-5D from diagnosis to one year. One-year HRQoL measures were subtracted from five-year measures to calculate change in HRQoL from one to five years. Multivariable linear regression was used to quantify the association between change in cardio-protective medication and change in EQ-5D from baseline to one year with standard errors adjusted for clustering by practice. A multilevel model accounting for individuals within practices was considered, but due to a lack of heterogeneity explained by practice in the primary analyses, it was rejected for a parsimonious model. All models were adjusted for age at diagnosis, gender, 2004 English Index of Multiple Deprivation (IMD) score [21], self-reported CVD at baseline, ethnicity, baseline value of the HRQoL measure, baseline HbA1C level, randomisation group and practice level clustering. In a second series of linear regression models, we examined the association between change in cardio-protective medication from one to five years and (i) change in EQ-5D; (ii) change in SF-36 (physical and mental score) and (iii) change in ADDQoL-AWI from one year to five years. We adjusted the model for the same factors outlined above, as well as self-reported CVD at one year.
In a secondary analysis, the association between change in cardio-protective medication in the first year after diagnosis and changes in HRQoL (EQ-5D, SF-36 and ADDQoL-AWI) from one to five years was assessed in a linear model analogous to the primary analysis.
Different versions of the ADDQoL were used (ADDQoL-18 and ADDQoL-19) at one and five years. The authors of the ADDQoL state that the measure remains robust if up to six items are removed [22]. We removed the following items from the summary score as they differed between questionnaires: ‘holidays/leisure activities’, ‘travel/journeys’, ‘society/people reaction’, ‘dependence’, ‘enjoyment of food’, and ‘closest personal relationship’. The Cronbach's alpha for the ADDQoL-AWI un-weighted items that were constant across both questionnaires at one- and five-year follow-up was 0.90 and 0.94, respectively. In addition, we included a sensitivity analysis using a Paretian model [23] of the complete ADDQoL questionnaires, which ignored the relative importance of change, instead focusing on the four possible directions of change. Four categories were derived; (A) increase in any ADDQoL domain, (B) no change across domains, (C) decrease in any domain, (D) mixed change, and regressed in a multinomial model that was analogous to the primary analysis.
Four additional sensitivity analyses were undertaken. Firstly, change in the number of medications was fitted as a continuous variable, rather than a categorical variable. Secondly, data points missing for ethnicity, IMD, change in agents, baseline of HRQoL measure and change in the HRQoL measure in the primary analysis were imputed 100 times using chained equations to account for missingness. Thirdly, change in energy intake (food frequency questionnaire derived kcal/day) or physical activity (EPAQ2 [24]) after diagnosis might have confounded the observations and were added to the model as covariates. Lastly, interactions between randomisation group and change in medication were explored and the main analysis was also repeated in only the routine care group.
The ADDITION-Cambridge trial was powered to detect a 20% relative effect of intensive treatment on modelled CVD risk, with 90% power at the 5% level of significance assuming 30% of participants were lost to follow up [10]. Statistical analyses were completed in Stata 13 and figures using R 3.0.2.
3. Results
Eight hundred and sixty seven patients agreed to participate in ADDITION-Cambridge and attended baseline measurement. Eight (0.9%) participants died before one year follow up, and 55 (6%) before five year follow up (Table 1). The median (IQR) value of the EQ-5D score at baseline for participants that were included in the analysis was (0.85; 0.73, 1). This was higher than the score for those who died and were excluded from the analysis (0.73; 0.62, 1). Participants who did not have complete data at five year follow-up reported lower levels of physical activity (at baseline) than those who attended. There were no other significant differences between those with complete data at five years and those with missing data for baseline age, sex, BMI, current smoker, self-reported previous CVD, health status (EQ5-D) or number of cardio-protective agents. The greatest amount of missing data at one and five years was for the SF-36 (18%, 151/860 and 19%, 151/805, respectively). Missing medication and HRQoL data at one and five years was not clustered in the same individuals, leading to an increased level of missing data in the complete case analysis models (Table 2).
Table 1.
Participant characteristics of ADDITION-Cambridge cohort at baseline, one and five years.
| Baseline |
One year |
Five Years |
||||
|---|---|---|---|---|---|---|
| N (%) | Value | N (%) | Value | N (%) | Value | |
| Median age at diagnosis in years (IQR) | 867 (100%) | 63 (56, 67) | – | – | – | – |
| % Male | 867 (100%) | 61% | – | – | – | – |
| Median IMD score* (IQR) | 750 (87%) | 11 (7, 18) | – | – | – | – |
| % White ethnicity | 859 (99%) | 96% | – | – | – | – |
| % Any lipid medication | 865 (100%) | 24% | 849 (99%) | 66% | 782 (96%) | 82% |
| % Any BP medication | 865 (100%) | 58% | 849 (99%) | 69% | 782 (96%) | 79% |
| % Any diabetes medication | 865 (100%) | 0.5% | 849 (99%) | 31% | 782 (96%) | 62% |
| % Aspirin medication | 865 (100%) | 20% | 849 (99%) | 35% | 782 (96%) | 44% |
| Median number of lipid medications (IQR) | 865 (100%) | 0 (0, 0) | 849 (99%) | 0 (1, 0) | 782 (96%) | 1 (1, 1) |
| Median number of BP medications (IQR) | 865 (100%) | 1 (0, 2) | 849 (99%) | 1 (0, 2) | 782 (96%) | 1.5 (1, 2) |
| Median number of diabetes medications (IQR) | 865 (100%) | 0 (0, 0) | 849 (99%) | 0 (0, 1) | 782 (96%) | 1 (0, 1) |
| HbA1C > 53 mmol mol−1 (7%) and not on any diabetes medication | 791 (91%) | 39% | 726 (85%) | 1% | 683 (84%) | 8% |
| Median HbA1C % (IQR) | 846 (98%) | 6.8 (6.3, 7.7) | 692 (81%) | 6.4 (6, 6.8) | 765 (88%) | 6.9 (6.4, 7.4) |
| Median HbA1C % (IQR) | 846 (98%) | 51 (45, 61) | 692 (81%) | 46 (42,51) | 765 (88%) | 52 (46, 57) |
| Median number reported cardio-protective medications (IQR) | 867 (100%) | 1 (0, 2) | 849 (99%) | 2 (1, 3) | 782 (96%) | 3 (2, 4) |
| Median EQ-5D index score (IQR) | 852 (98%) | 0.85 (0.73, 1) | 739 (86%) | 0.85 (0.73, 1) | 663 (82%) | 0.85 (0.73, 1) |
| Median SF-36 MCS (IQR) | – | – | 709 (83%) | 56 (48, 59) | 660 (81%) | 57 (51, 60) |
| Median SF-36 PCS (IQR) | – | – | 709 (83%) | 48 (39, 54) | 660 (81%) | 48 (36, 54) |
| Median ADDQoL-AWI (IQR) | – | – | 721 (84%) | −0.39 (−1, −0.06) | 669 (82%) | −0.37 (−0.11, −0.86) |
| % Had CVD event | – | – | – | – | 866 (100%) | 7% |
| % Alive | 867 (100%) | 100% | 866 (100%) | 99% | 866 (100%) | 94% |
− = Data unavailable; BP = blood-pressure; HBA1c = glycosylated haemoglobin; EQ-5D = European Quality of Life Questionnaire; MCS = Mental component score; PCS = Physical component score; ADDQoL-AWI = Audit of diabetes-dependent quality of life average weighted index; IQR = interquartile range.
Cambridgeshire county had a mean IMD score of 11.7 in 2004 (http://data.gov.uk/dataset/imd_2004).
Table 2.
Associations between change in number of cardio-protective agents and HRQoL in ADDITION-Cambridge cohort.
| Outcome measure | n (%) | Change in agents, relative to no change/decrease in agents |
|||
|---|---|---|---|---|---|
| One more agent |
More than one additional agent |
||||
| β (95%CI) | p-Value | β (95%CI) | p-Value | ||
| Complete case analysis (Primary) | |||||
| ΔEQ-5D, 0 to 1 year | 601 (70%) | −0.02 (−0.05, 0.01) | 0.210 | −0.02 (−0.05, 0.01) | 0.253 |
| ΔEQ-5D, 1 to 5 year | 513 (63%) | 0.02 (−0.02, 0.05) | 0.317 | 0.05 (0.02, 0.08) | 0.004 |
| ΔSF-36 MCS, 1 to 5 years | 488 (60%) | −0.5 (−2.2, 1.2) | 0.552 | −0.4 (−1.9, 1.0) | 0.536 |
| ΔSF-36 PCS, 1 to 5 years | 488 (60%) | 2.1 (0.3, 4.0) | 0.024 | 0.5 (−1.4, 2.3) | 0.632 |
| ΔADDQoL-AWI, 1 to 5 years | 510 (63%) | −0.11 (−0.36, 0.14) | 0.380 | −0.20 (−0.38, −0.02) | 0.030 |
| Imputed | |||||
| ΔEQ-5D, 0 to 1 year | 859 (100%) | −0.03 (−0.06, 0.05) | 0.102 | −0.02 (−0.06, 0.01) | 0.102 |
| ΔEQ-5D, 1 to 5 years | 811 (100%) | −0.01 (−0.05, 0.03) | 0.594 | 0.06 (0.02, 0.10) | 0.007 |
| ΔSF-36 MCS, 1 to 5 years | 811 (100%) | −0.1 (−1.5, 1.3) | 0.862 | −0.5 (−2.0, 1.1) | 0.541 |
| ΔSF-36 PCS, 1 to 5 years | 811 (100%) | 2.1 (0.4, 3.8) | 0.019 | 0.2 (−1.6, 1.9) | 0.832 |
| ΔADDQoL-AWI, 1 to 5 years | 811 (100%) | −0.20 (−0.44, 0.05) | 0.116 | −0.32 (−0.51,−0.13) | 0.002 |
| Including ΔPA and ΔEnergy | |||||
| ΔEQ-5D, 0 to 1 year | 593 (69%) | −0.02 (−0.05,0.02) | 0.277 | −0.02 (−0.05, 0.01) | 0.232 |
| Routine care arm only | |||||
| ΔEQ-5D, 0 to 1 years | 301 (73%) | −0.05 (−0.10,0.00) | 0.073 | 0.00 (−0.05, 0.05) | 0.976 |
| ΔEQ-5D, 1 to 5 years | 252 (66%) | −0.02 (−0.04,0.08) | 0.458 | 0.03 (−0.02, 0.08) | 0.458 |
| ΔSF-36 MCS, 1 to 5 years | 242 (64%) | 0.5 (−1.6, 2.6) | 0.636 | −0.2 (−2.2, 1.8) | 0.825 |
| ΔSF-36 PCS, 1 to 5 years | 242 (64%) | 0.8 (−3.0, 4.7) | 0.759 | −0.2 (−3.4, 3.1) | 0.909 |
| ΔADDQoL-AWI, 1 to 5 years | 245 (64%) | −0.18 (−0.50, 0.15) | 0.275 | −0.26 (−0.49, −0.03) | 0.028 |
β coefficients (95% confidence interval) from a linear regression model adjusted for age at diagnosis, gender, 2004 IMD, self-reported CVD at baseline, ethnicity, baseline value of the HRQoL measure, randomisation group and practice level clustering.
Δ = Change; BP = blood-pressure; EQ-5D = European Quality of Life questionnaire MCS = Mental component score; PCS = Physical component score; ADDQoL-AWI = Audit of diabetes-dependent quality of life average weighted index.
3.1. Change from baseline to one year
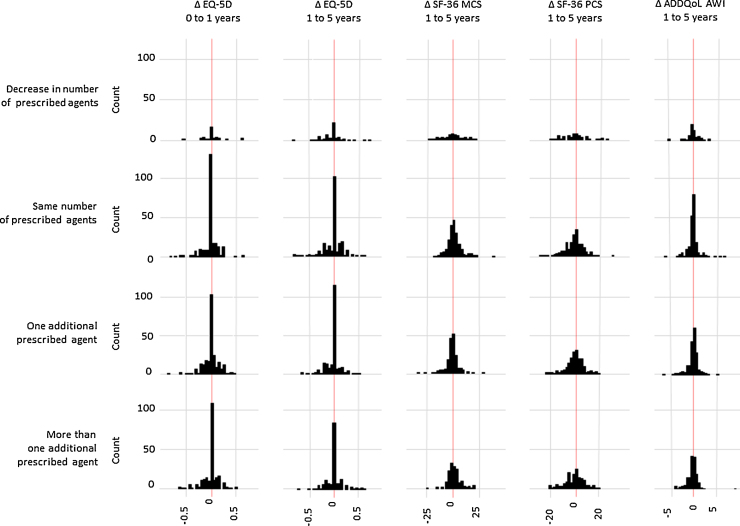
Four individuals (0.5%) reported being prescribed a glucose-lowering agent before diagnosis (Table 1) (three metformin, one a sulphonylurea). 24% of participants were taking a lipid-lowering agent, 58% a blood pressure-lowering agent and 19% aspirin at baseline. From diagnosis to one year there was an increase in the median number of prescribed agents, from 2 (IQR 1, 3) to 3 (2, 4). At one year follow-up, 251 (34%) individuals reported the same or a reduced number of prescribed cardio-protective agents, 185 (25%) one additional agent and 295 (40%) two or more agents. From baseline to one year, median EQ-5D scores remained constant at 0.85 (IQR 0.73, 1) and a large proportion of individuals (45%, 327/729) reported no change in health utility (Fig. 1). There was no evidence for an association between change in the number of cardio-protective medications and change in the EQ-5D score from baseline to one year (Table 2).
Fig. 1.
Distribution of change in quality of life measures by change in cardio-protective agents in ADDITION-Cambridge cohort. Δ = Change; SF-36 MCS = SF36 mental health summary score; SF-36 PCS = SF36 physical health summary score; ADDQoL-AWI = Audit of diabetes-dependent quality of life average weighted index.
3.2. Change from one to five years
From one to five years after diagnosis, use of any anti-hypertensive agent increased from 69% to 79%; larger increases were seen in the reporting of any lipid-lowering agents (66% to 82%) and any glucose-lowering agents (31% to 62%). Aspirin use increased from 35% at one year, to 44% at five years. At one and five years, a median total of 3 (IQR 2, 4) and 4 (IQR 3, 5) cardio-protective agents were reported, respectively. Over the same time period, 219 (36%) individuals reported no increase in cardio-protective medication, 192 (32%) one more agent and 193 (32%) two or more additional cardio-protective agents. At one year, the median ADDQoL-AWI score was −0.39 (IQR −1, −0.06), suggesting that the majority of individuals reported a negative impact of diabetes on their HRQoL. Consistent with the baseline to one year results, change in EQ-5D, SF-36 and ADDQoL-AWI measures between one and five years were distributed evenly around no change (Fig. 1). There was no association between increases in cardio-protective medication and change in the SF-36 MCS score (Table 2). Increasing cardio-protective medication was associated with an increase in the SF36-PCS score, but the association was only statistically significant for an increase of one agent (2.1; 95%CI 0.3, 4.0). Conversely, while an increase in one, or more than one, agents was associated with an increase in the EQ-5D index score, the relationship was only statistically significant for one or more additional agents (0.05; 95%CI 0.02, 0.08). An association in the opposite direction was observed between change in cardio-protective medication and the ADDQoL-AWI score: more than one additional agent was associated with a statistically significant decrease in the ADDQoL-AWI score (−0.20; 95%CI −0.38, −0.02) (Table 2).
3.3. Secondary analyses
We found no associations between change in medication in the first year after diagnosis, and subsequent change in EQ-5D, SF-36 PCS and MCS, or ADDQoL-AWI from one to five years in models that were adjusted for potential confounders and HRQoL at one year.
3.4. Sensitivity analyses
When modelling cardio-protective medication as a continuous variable, similar statistically non-significant associations were identified, replicating findings from the main analysis. Similarly, coefficients from models based on imputed data replicated findings from the complete case analysis. There was no evidence of an association between change in the ADDQoL-AWI and cardio-protective medication in a multinomial analysis of no change against an increase, decrease or mixed change across ADDQoL domain scores. Changes in physical activity and energy intake in the year after diagnosis did not influence the associations between change in HRQoL and change in cardio-protective medication. Models analogous to the primary analysis run in the routine care arm of ADDITION-Cambridge suggested that treatment arms could be merged. Likewise, no interactions between the randomisation group and change in agents were detected.
4. Discussion
We found little evidence that increases in the number of cardio-protective medications impacted negatively on HRQoL among individuals with screen-detected diabetes over five years. The few significant associations that we did observe were linked to clinically negligible changes in HRQoL measures.
For the EQ-5D, the smallest change associated with a clinically meaningful improvement in health status amongst individuals with diabetes is between 0.058 and 0.158 [25], while in the general population a change in the EQ-5D of >0.07 can indicate a potential clinically relevant change [26]. This suggests that the increase in EQ-5D associated with change in medication in our analysis, while statistically significant, is not likely to be clinically meaningful. More complex is an apparent decrease in diabetes-specific QoL associated with more than one additional agent (−0.20; 95%CI −0.38, −0.02). In an Australian population of 14,439 people with diabetes the mean difference in ADDQoL between those with and without complications was 0.69 [27]. It remains unclear whether a decrease of up to 0.38 in the ADDQoL, which ranges from −9 to +3, is clinically relevant.
As ADDITION-Cambridge is a novel cohort of individuals with screen-detected diabetes, few direct comparisons with published literature are possible. Shortly after diagnosis, 43% of individuals with screen-detected diabetes from the Hoorn Study were prescribed anti-hypertensive medication, 17% lipid lowering medication and 24% oral diabetes medication [28]. Among middle aged populations with established diabetes, the average number of prescribed cardio-protective medications is between four and five [5,29]. Despite a significant treatment burden, many individuals with established diabetes remained untreated for CVD risk factors such as blood pressure and cholesterol [29]. In ADDITION-Cambridge, individuals reported a median of two (IQR 3, 4) cardio-protective medication at diagnosis and four (IQR 3, 5) by five year follow-up. This is likely due to the population being diagnosed earlier in the disease trajectory. However, there was still evidence of under-treatment in our cohort [30].
While populations with diabetes tend to have a lower HRQoL than the general population [31,32], individuals with screen-detected diabetes have better HRQoL than those with clinically diagnosed diabetes at diagnosis [28]. There is limited literature with which to compare our findings on change in HRQoL among individuals with screen-detected diabetes as most published research has been conducted in populations with long-standing diabetes. Seppälä et al, in a Finnish population, found that SF-36 assessed HRQoL was lower in the 91 individuals with undiagnosed diabetes than in those with normal glucose tolerance [32]. Grandy et al. [33] demonstrated a small decrease in mean EQ-5D index score (−0.031 SD 0.158) over a five year time period in people with an average diabetes duration of nine years (SD 7.8) [33].
In terms of the association between medication and HRQoL, Wexler et al reported an inverse association between HRQoL and longer diabetes duration, prescription of more than 7 medications, older age and being female [2]. Trial evidence on the relationship between intensifying treatment and HRQoL is generally under-reported [34]. The UKPDS trial, which enrolled recently diagnosed individuals more than a decade before addition, found no difference between individuals with a conventional or intensified treatment protocol [35]. The ACCORD trial, which included individuals with established diabetes and early CVD, concluded that there was no HRQoL benefit from very intensive (HbA1C < 42 mmol mol−1 [6%]) over moderate glycaemic control (HbA1C 53–63 mmol mol−1 [7.0–7.9%]) [7]. In a trial analysis of the ADDITION-Europe cohort, in which relatively small differences in treatment intensity were achieved, there were no differences between EQ-5D or SF-36 scores for individuals in the routine care and intensive treatment groups [11]. In our observational analysis, we found no consistent association between an increase in medication and reduced HRQoL. While this suggests that increasing the number of prescribed cardio-protective medications does not impact negatively on quality of life among individuals with screen-detected diabetes, more research in populations with diabetes detected early in the disease trajectory is needed to confirm this finding.
4.1. Strengths and limitations
ADDITION-Cambridge is a large cohort of individuals with screen-detected diabetes and long-term follow-up. Standardised measurements and high response rates at diagnosis, one year and five years allowed the examination of changes in treatment burden and HRQoL measures. In addition to disease specific and general HRQoL measures after diagnosis, a unique strength of this study is the measurement of general HRQoL before a screen diagnosis of diabetes. Participants were encouraged to bring repeat prescription summaries, and we collected self-report medication data using an adaption of a validated questionnaire [19]. We computed the total number of cardio-protective agents to describe treatment burden, a method which applies equal weight to each agent. We did not examine the potential differing effect of individual drugs on HRQoL. Nor did we conduct pill counts or account for differing doses of prescribed treatments. In the sensitivity analysis, cardio-protective medication was explored as a continuous variable and results did not differ; this suggests that collapsing medication change into an ordered categorical variable did not obscure a small change. The use of fewer questions from the original ADDQoL questionnaire might have affected the instrument's sensitivity. However, the Cronbach's alpha indicated high reliability in the shortened ADDQoL-AWI version at both time points (0.90 and 0.94). Our analysis was conducted in the first five years after detection by screening. This population was younger and closer to ideal health than cohorts with established diabetes. The association between treatment intensity and HRQoL could change as duration of diabetes and age increases.
Only a general HRQoL measure (the EQ-5D) was measured before individuals were diagnosed with diabetes. At baseline, our population had a mean EQ-5D index score of 0.81 (SD 0.21; median 0.85; IQR 0.73, 1). The average value for a general British population aged 55–64 is 0.80 (SD 0.26) [36]. This suggests individuals with screen detected diabetes have a comparable HRQoL to the general public, which potentially limits the ability of the EQ-5D to detect small changes in HRQoL when many individuals may remain at ‘ideal health (score of 1)’. However, the EQ-5D has demonstrated an ability to distinguish between populations with and without different complications of diabetes [37]. The difference in our estimates for the EQ-5D, and SF-36 PCS, compared to the ADDQoL-AWI and SF36 MCS, provide weak evidence that the association between cardio-protective medication and mental HRQoL differs from physical HRQoL. This finding is surprising as qualitative interviews suggest that the initial process of being screened and labelled with the condition of early detected diabetes is more often seen as a positive “wake up call” than a negative experience [38]. Further research is needed to establish if there is a clinically or economically relevant association.
We compared concurrent changes in cardio-protective medication and HRQoL between two time points, which were one and four years apart. This may hide short term changes in the prescription of medications and HRQoL within these time points. Understanding such changes would inform the temporality of the association, but would require a much finer resolution of prescription patterns and HRQoL over the five year period.
5. Conclusion
We found little evidence that increases in cardio-protective medication had an adverse impact on HRQoL in people with screen detected diabetes. There was no association between change in cardio-protective medication and the EQ-5D from diagnosis to one year. The few observations we observed from one to four years were small, in different directions, and the changes in HRQoL were clinically negligible. Targeted management of CVD risk factors in diabetes improves cardiovascular health [3]. Our results suggest that clinicians should not be concerned that increasing the number of cardio-protective medications will impact negatively on quality of life among individuals with screen-detected diabetes.
Conflicts of interest statement
SJG received an honorarium and reimbursement of travel expenses from Eli Lilly associated with membership of an independent data monitoring committee for a randomised trial of a medication to lower glucose. The remaining authors declare that they have no conflicts of interest.
Funding sources
ADDITION-Cambridge was supported by the Wellcome Trust (grant reference No G061895) the Medical Research Council (grant reference no: G0001164), National Health Service R&D support funding (including the Primary Care Research and Diabetes Research Networks), and the National Institute for Health Research (08/116/300). We received an unrestricted grant from University of Aarhus, Denmark, to support the ADDITION-Cambridge trial. Bio-Rad provided equipment to undertake capillary glucose screening by HbA1c in general practice. The Primary Care Research Unit is supported by NIHR Research funds. SJG receives support from the Department of Health NIHR Programme Grant funding scheme (RP-PG-0606-1259). This article presents independent research funded by the NIHR under the Programme Grants for Applied Research programme (RP-PG-0606-1259]. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
References
- 1.Zimmet P., Alberti K.G.M.M., Shaw J.E. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Wexler D.J., Grant R.W., Wittenberg E. Correlates of health-related quality of life in type 2 diabetes. Diabetologia. 2006;49:1489–1497. doi: 10.1007/s00125-006-0249-9. [DOI] [PubMed] [Google Scholar]
- 3.Holman R.R., Paul S.K., Bethel M.A. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 4.Huang E.S., Brown S.E.S., Ewigman B.G. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30:2478–2483. doi: 10.2337/dc07-0499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant R.W., Devita N.G., Singer D.E., Meigs J.B. Polypharmacy and medication adherence in patients with type 2 diabetes. Diabetes Care. 2003;26:1408–1412. doi: 10.2337/diacare.26.5.1408. [DOI] [PubMed] [Google Scholar]
- 6.Murphy E., Kinmonth A.L. No symptoms, no problem? Patients’ understandings of non-insulin dependent diabetes. Fam Pract. 1995;12:184–192. doi: 10.1093/fampra/12.2.184. [DOI] [PubMed] [Google Scholar]
- 7.Anderson R.T., Narayan K.M.V., Feeney P. Effect of intensive glycemic lowering on health-related quality of life in type 2 diabetes: ACCORD trial. Diabetes Care. 2011;34:807–812. doi: 10.2337/dc10-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X., Norris S.L., Chowdhury F.M. The effects of interventions on health-related quality of life among persons with diabetes: a systematic review. Med Care. 2007;45:820–834. doi: 10.1097/MLR.0b013e3180618b55. [DOI] [PubMed] [Google Scholar]
- 9.Kuznetsov L., Griffin S.J., Davies M.J. Diabetes-specific quality of life but not health status is independently associated with glycaemic control among patients with type 2 diabetes: a cross-sectional analysis of the ADDITION-Europe trial cohort. Diabetes Res Clin Pract. 2014;104:281–287. doi: 10.1016/j.diabres.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Echouffo-Tcheugui J.B., Simmons R.K., Williams K.M. The ADDITION-Cambridge trial protocol: a cluster—randomised controlled trial of screening for type 2 diabetes and intensive treatment for screen-detected patients. BMC Public Health. 2009;9:136. doi: 10.1186/1471-2458-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Donk M., Griffin S.J., Stellato R.K. Effect of early intensive multifactorial therapy compared with routine care on self-reported health status, general well-being, diabetes-specific quality of life and treatment satisfaction in screen-detected type 2 diabetes mellitus patients (ADDITION-Eur) Diabetologia. 2013 doi: 10.1007/s00125-013-3011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin S.J., Little P.S., Hales C.N. Diabetes risk score: towards earlier detection of type 2 diabetes in general practice. Diabetes Metab Res Rev. 2000;16:164–171. doi: 10.1002/1520-7560(200005/06)16:3<164::aid-dmrr103>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Richard J.-L., Sultan a, Daures J.-P. Diagnosis of diabetes mellitus and intermediate glucose abnormalities in obese patients based on ADA (1997) and WHO (1985) criteria. Diabet Med. 2002;19:292–299. doi: 10.1046/j.1464-5491.2002.00647.x. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Clinical Excellence . National Institute for Clinical Excellence; London: 2002. Management of type 2 diabetes: management of blood pressure and blood lipids. [Google Scholar]
- 15.Gaede P., Vedel P., Larsen N. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 16.Kind P., Dolan P., Gudex C., Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316:736–741. doi: 10.1136/bmj.316.7133.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware J.E., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 18.Bradley C., Todd C., Gorton T. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res. 1999;8:79–91. doi: 10.1023/a:1026485130100. [DOI] [PubMed] [Google Scholar]
- 19.Knapp M., Beecham J. Reduced list costings: examination of an informed short cut in mental health research. Health Econ. 1993;2:313–322. doi: 10.1002/hec.4730020404. [DOI] [PubMed] [Google Scholar]
- 20.WHO . WHO; 2013. Guidelines for ATC classification and DDD assignment. 2013. [Google Scholar]
- 21.Office of the Deputy Prime Minister (UK) Office of the Deputy Prime Minister (UK); 2004. The English indices of deprivation 2004: summary. [Google Scholar]
- 22.Bradley C. Procedures for use of the ADDQoL. 1998. The audit of diabetes-dependent quality of life (ADDQoL) pp. 1–15. [Google Scholar]
- 23.Devlin N.J., Parkin D., Browne J. Patient-reported outcome measures in the NHS: new methods for analysing and reporting EQ-5D data. Health Econ. 2010;19:886–905. doi: 10.1002/hec.1608. [DOI] [PubMed] [Google Scholar]
- 24.Wareham N.J., Rennie K.L. The assessment of physical activity in individuals and populations: why try to be more precise about how physical activity is assessed? Int J Obes Relat Metab Disord. 1998;22(Suppl 2):S30–S38. [PubMed] [Google Scholar]
- 25.Mulhern B., Meadows K. Investigating the minimally important difference of the Diabetes Health Profile (DHP-18) and the EQ-5D and SF-6D in a UK diabetes mellitus population. Health (Irvine, Calif) 2013;05:1045–1054. [Google Scholar]
- 26.Crosby R.D., Kolotkin R.L., Williams G.R. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56:395–407. doi: 10.1016/s0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 27.Ostini R., Dower J., Donald M. The Audit of Diabetes-Dependent Quality of Life 19 (ADDQoL): feasibility, reliability and validity in a population-based sample of Australian adults. Qual Life Res. 2012;21:1471–1477. doi: 10.1007/s11136-011-0043-0. [DOI] [PubMed] [Google Scholar]
- 28.Adriaanse M.C., Dekker J.M., Spijkerman A.M.W. Health-related quality of life in the first year following diagnosis of Type 2 diabetes: newly diagnosed patients in general practice compared with screening-detected patients. The Hoorn Screening Study. Diabet Med. 2004;21:1075–1081. doi: 10.1111/j.1464-5491.2004.01277.x. [DOI] [PubMed] [Google Scholar]
- 29.Voorham J., Haaijer-Ruskamp F.M., Stolk R.P. Influence of elevated cardiometabolic risk factor levels on treatment changes in type 2 diabetes. Diabetes Care. 2008;31:501–503. doi: 10.2337/dc07-1043. [DOI] [PubMed] [Google Scholar]
- 30.Black J.A., Sharp S.J., Wareham N.J. Change in cardiovascular risk factors following early diagnosis of type 2 diabetes: a cohort analysis of a cluster-randomised trial. Br J Gen Pract. 2014;64:e208–e216. doi: 10.3399/bjgp14X677833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brod M., Hammer M., Christensen T. Understanding and assessing the impact of treatment in diabetes: the treatment-related impact measures for diabetes and devices (TRIM-Diabetes and TRIM-Diabetes Device) Health Qual Life Outcomes. 2009;7:83. doi: 10.1186/1477-7525-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seppälä T., Saxen U., Kautiainen H. Impaired glucose metabolism and health related quality of life. Prim Care Diabetes. 2013;7:223–227. doi: 10.1016/j.pcd.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Grandy S., Fox K.M. Change in health status (EQ-5D) over 5 years among individuals with and without type 2 diabetes mellitus in the SHIELD longitudinal study. Health Qual Life Outcomes. 2012;10:99. doi: 10.1186/1477-7525-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magwood G.S., Zapka J., Jenkins C. A review of systematic reviews evaluating diabetes interventions: focus on quality of life and disparities. Diabetes Educ. 2008;34:242–265. doi: 10.1177/0145721708316551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.UK Prospective Diabetes Study Group Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). U.K. Prospective Diabetes Study Group. Diabetes Care. 1999;22:1125–1136. doi: 10.2337/diacare.22.7.1125. [DOI] [PubMed] [Google Scholar]
- 36.Kind P., Hardman G., Macran S. 1999. UK population norms for the EQ-5D. [Google Scholar]
- 37.Janssen M.F., Lubetkin E.I., Sekhobo J.P., Pickard aS. The use of the EQ-5D preference-based health status measure in adults with Type 2 diabetes mellitus. Diabet Med. 2011;28:395–413. doi: 10.1111/j.1464-5491.2010.03136.x. [DOI] [PubMed] [Google Scholar]
- 38.Eborall H., Davies R., Kinmonth A.-L. Patients’ experiences of screening for type 2 diabetes: prospective qualitative study embedded in the ADDITION (Cambridge) randomised controlled trial. BMJ. 2007;335:490. doi: 10.1136/bmj.39308.392176.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]