Abstract
The clinical research industry today is undergoing a major facelift. Companies are continuously looking to adopt and implement effective and innovative ways to accelerate drug launches in the market. Companies today are more open and do not view patients as mere “subjects” who generate data, – but as informed collaborators whose participation is “core” to the overall success of trials leading to the emergence of the concept of “patient-centric trials.” This paper is intended to highlight the current trends and new opportunities that can be seen in industry -indicative of crucial role patients today play in their own health care using technology, social media and self education.
Keywords: Data transparency patient communities, direct to patient, electronic health records, internet, patient-centric trials, patient education, patient engagement, social media
INTRODUCTION
In an era of increased complexity and escalating costs of clinical research, a focus on personalized medicine and patient empowerment, drug development is undergoing a metamorphosis. Typically, when a clinical trial is conducted, clinicians and patients collaborate with the sponsor for determining the safety and effectiveness of a molecule under experimental treatment. Trials are usually designed keeping in mind the feasibility and ease with which the sponsor can conduct the study. This can lead to a large number of costly and complex trials being conducted, without addressing the patient's convenience or needs. As per an IMS Health Study forecast, global spending on medicines is targeted to increase 30% to $1.3 trillion by 2018.[1] The spend has grown, but patient's needs are not being met. Thus, patient-centric drug development is now becoming the model that the industry is following. Today, patients are aware, technology-driven, and informed-driving the change in mindset and way clinical trials are being approached and conducted.
DIGITAL PATIENT – PATIENT AT THE CENTER OF THE NETWORK ENVIRONMENT
A number of factors can influence a patient's decision to participate in a clinical trial including financial, social, philanthropic or altruistic.[2] Prior to enrolling in trials, patients today invest time in learning about the disease of interest, the drug mechanism for cure, locations where trials are being conducted and results from similar trials to name a few, through the internet. This has enhanced the quality of discussions occurring between the physician and patient. Benefits of engaging these “digital patients” have been realized. Contract research organizations (CROs) are offering a ready repository of patients who have enrolled in different trials to pharma companies keen on conducting trials-driving cost and effort saving. For example, quintiles have more than three million patients under their “Digital Patient Unit” program which also helps to use real world patients for the faster testing of inclusion/exclusion criteria and allows sponsors to prescreen the subjects and refer them, if needed, to clinical trial sites.[3]
INVOLVING PATIENTS FROM THE INITIAL STAGES IN DRUG DEVELOPMENT
Pharma are using technology to run contests and competitions to gather ideas and feedback on trial designs, informed consent forms (ICFs) and protocols. Open discussions on draft protocols specially focused on endpoints and visited structure are encouraged, and updates are made based on the responses received. Some of the areas where patients are being actively engaged are listed below.
Seeking patient inputs in informed consent forms
The draft guidelines on the informed consent released by the Food and Drug Administration (FDA) in July 2014 could be used as a ready reference for building effective ICFs. While building ICFs, an approach, which is behavioral but science-based helps uncover patient insights. The usage of simple language easily deciphered by the participating subject will establish expectations regarding foreseen and unforeseen risks. ICFs that are short and precise will hold the attention. Clearly defining study objectives, end points and providing a detailed summary would retain the interest of the patient. It could also help in understanding the enrollment risks and also highlight factors that could be adjusted in the protocol for minimizing those risks.[3] Having the ICF reviewed by a layman could also be considered as it would shed light on content that is too scientific in nature. Understanding the section of the protocol that led to distress while review (e.g., surgical procedure related requirement) could help one make amendments, if needed, in earlier stages of protocol development. Seeking clarification from patients on their understanding of the protocol could provide insights on building ICFs that would be better accepted by a larger patient population.[4]
Building patient friendly protocols and grooming study staff
While designing the study protocols, time should be invested in understanding the lifestyle of the patient population. Real world patients providing their inputs to building study protocols and study designs, which are more real and closer to life experiences, cost-effective and patient-centric is needed. Designing patient friendly visit schedules that are more flexible is important. In the case, patients are dependent on caregivers, considering the schedule of a caregiver would be beneficial. For better retention, care should be taken to provide facilities and an environment conducive to patients, especially during prolonged on-site visits, or visits that require patients to be in a fasted state. By being sensitive to a patient's comfort and needs, a better retention rate could be achieved.[5]
Companies should provide appropriate trainings to the study staff and groom them to handle sensitive situations, especially when invasive procedures are followed.
PROMOTING OPEN COMMUNICATION CHANNELS BETWEEN BIOPHARMA, PHYSICIANS, PATIENTS, AND MEDIA
The traditional communication mechanisms where Biopharma companies were at the center and messages were directed toward physicians, who in turn shared the messages with patients, have changed. There is a need to adopt newer tools and technologies that drive two-way communication. Promoting and accelerating direct to consumer advertising where companies still control the delivery of the messages, but the loop in media for a greater effect. Companies are actively using social listening techniques to review what patients are discussing online regarding disease state and issues to drive better patient participation and relationships.[6] Researchers are skeptical regarding the type and extent of clinical information that is being shared and discussed by patients via online chats, such as protocol details, their experience of participating in trials, adverse event reactions etc., that can introduce bias for future trials.[7]
Understanding the importance social media and its role in the dissemination of information, FDA has released draft guidance for industry on social media usage in June 2014. It provides recommendations for presenting benefit and risk information for FDA regulated prescription drugs or devices using social media such as Twitter, Yahoo, and Google.[8] Medicine's New Zealand also released its updated code of practice in June 2014 where it has included its guidance on social media usage by the pharmaceuticals. This was done to separate social media from other type of advertising and clearly outline pathway for pharma companies keen to use social media channels – an indication of increasing global acceptance of social media usage.[9]
ENHANCING THE FOCUS ON PATIENT EDUCATION, ENGAGEMENT AND RETENTION USING TECHNOLOGY
To improve clinical outcomes, increase patient satisfaction and incur profit revenue, engaging patients in their own healthcare is critical. The US government is encouraging the use of Electronic Health Records (EHRs) via HealthIT.gov and promoting incentives to doctors who use EHRs meaningfully to reduce medical errors and improve the quality of care. CROs and independent service providers are designing educative websites that can be accessed via mobiles or the internet for educating patients on diseases.
A few examples of available websites and tools below
Agency for Health Research and Quality maintained by US Department of Health and Human Services
ClinicalResearch.com
WELVU – Mobile First, an iPad- and iPhone-based educative tool providing medical illustration, quality scores, and health outcomes to engage patients
Krames patient education from StayWell
ExitCare OnScreen™ video solutions for patient education.[10,11,12,13]
The retention of patients in a trial is the key to the success of the overall project. Acurian, a service provider for recruitment and retention services uses platforms such as Facebook and Myspace for patient referrals and retention strategies.[14] The easier it is to be compliant to study schedule, the better is the retention till the end. Dose compliance tracking tools like MediGuard™ enable reminders to be set up for dose intake.
USE OF CROWDSOURCING TECHNIQUES FOR ASSESSING THE PULSE OF THE PUBLIC
Crowdsourcing has been used since long as a powerful tool to engage the masses in other industries. Wikipedia is one classic example. To maintain 23 million articles rich in content, the company uses crowd participation where the site is maintained by a community of passionate 80,000 users, who in turn are incentivized via a gamified award mechanism.[15]
Companies are holding contests where relevant stakeholders, including medical communities, patient communities, and researchers are looped in to provide responses to survey questions via tools like “protocol builder.” The FDAs approval of the first completely crowd-sourced protocol for multiple sclerosis by Transparency Life Science's reconfirms the potential that regulatory agencies see in the application of crowdsourcing. Online patient communities such as Mediguard.org and Clinical research.com are instrumental in changing face of healthcare, clinical trials and outcomes. They are beneficial to patients as connections can be developed between people with similar conditions, sharing clinical trials information and advice on management of diseases. The communities also aid in patient recruitment by prescreening potential participants online. Digital observational research where data are directly collected from patients and compared to data results in healthcare records (with reduced physician involvement) can be especially helpful for postmarketing surveillance of products consumed for extended periods.[16]
Researchers, however, still feel concerned while opening complex clinical problems to a large number of strangers. Issues related to the misuse of intellectual property, the lack of surety in receiving solutions-raises questions on the effectiveness of crowdsourcing. However, companies can leverage on the potential of crowdsourcing and actively engage patients via several methods. Survey questions could be targeted to seek public views on inclusion-exclusion criteria, visit schema, alternative endpoints, etc. Inputs on the protocol design could also be requested from the physicians specialized in areas other than the study indication, e.g., Seeking inputs from an endocrinologist for the study indication diabetes could shed some light on the addition of some biochemical tests for specific analyte tracking crucial to diabetes. Also asking direct questions in the survey related to what kind of results patients expect to see at the end can help derive the primary and secondary endpoint for the trials.[17]
One of the best-known examples where the application of crowdsourcing in research yielded great results was “Foldit.” Here, a group of online gamers decoded the molecular structure of a monomeric retroviral protease, a long-standing scientific problem, by a protein folding game.[18]
Pharma giants such as Eli Lily, AstraZeneca and Cleveland Clinic, have partnered with companies like innocentive. The company hosts challenges and supports the crowdsourcing of innovation solutions across the globe.
DRIVING THE DATA TRANSPARENCY PRINCIPLE FOR BUILDING TRUST AND CONFIDENCE
The need for data transparency in clinical trials has been long discussed since it helps tackle multiple issues of concern. Having data transparency can help building trust and confidence in patients participating in trials. It also helps researchers prevent unnecessary duplication of trials, primarily when results of the similar trials have indicated that the product may be unsafe. The US Department of Health and Human services rolled out the notice of proposed rulemaking by detailing the process to be followed to ensure requirements established by FDA Amendments Act to strengthen public access to clinical trial data[19] clearly demarcating cases where some companies would be apprehensive to share patient data citing patient confidentiality. To ensure data transparency, pharma companies can take simple, but effective measures like publishing the copy of the clinical trial protocol on safe sites like www.clinicaltrials.gov and www.clinicaltrialsregister.eu, making the results of the trials available irrespective of the outcome (positive or negative), publishing the results of the trials in peer-reviewed journals or company websites, ensuring that the results are available in languages well understood by the participants, etc.[20]
ROLE OF TECHNOLOGY IN DRIVING PATIENT-CENTRICITY
The paradigm shift that we see in the way clinical research is evolving is attributed to a very large extent to technology and its widespread access to public.
HealthPatch MD, a wearable biosensor partnered between Vital Connect and Medidata helps in efficient remote patient monitoring. Its sensors and advanced algorithm provide continuous measurement of electrocardiogram grading, respiratory rate, skin temperature, heart rate, physical activity, etc. The combined technology (Medidata Clinical cloud and biosensor device) enables near real-time review of patient's health metrics[21]
Iodine, a health information website developed a new web-based application for cold and flu season. The app helps consumers to review >300 options related to medication for cold and flu and compare medications that can help cure their own symptom[22]
Reg4all or Registries for All developed by Genetic Alliance with support from Sanofi works on the principle of matchmaking between patients and clinical trials. The tool provides privacy and flexibility to patients to decide, which groups can have a view/access to their data thus empowering the patients
Treato, a data mining company, monitors conversations of patients on Facebook, Twitter, and patient forums and helps pharma companies make sense of data that is being published by millions of patients across the globe. The company captures near real time comments from social media using a combination of natural language processing algorithms, patient language dictionaries, and big data analytics. These conversations help pharma gain insights into patients’ lives and focus their efforts in the correct areas of drug development[23]
Patients like me have strong inbuilt mechanism where data are pulled from clinicaltrials.gov each night and it is matched with the list of 2,50,000 registered patients across globe helping the patients get better and updated results pertaining to the trials that might be conducted in their vicinity. The site also provides a forum when open communication and information exchange occurs between patients and feedback to improve the trial design can also be shared [Figure 1].[24]
Figure 1.
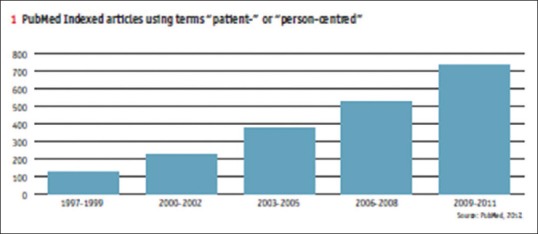
The graph depicts a six-fold increase in the last 12 years related to a number of searches related to the term “patient-centered” in PubMed. It is indicative of the curiosity and awareness that exists in the industry for this concept. Reference for this is “Reinventing Biopharma: Strategies for an evolving marketplace, The Patient Led R and D strategy, An Economist Intelligence Unit report Sponsored by Quintiles.” Available from: http://www.quintiles.com/~/media/library/white%20papers/reinventing-biopharma-strategies-for-an-evolving-marketplace-the-patient-led-rampd-strategy.pdf
CONCLUSION
The above graph depicts a six-fold increase in the last 12 years related to a number of searches related to the term “patient-centered” in PubMed.[25] It is indicative of the curiosity and awareness that exists in the industry for this concept.
Thus, we see that as the focus on healthcare and its access to patient's increases, it calls for the clinical research industry to adapt. It needs to consider technology advancement such as technology driving patient education, application of crowdsourcing to drive patient's contributions towards innovation, activities like ICF and protocol build based on patient's inputs, the usage of social media for data transparency as an integral part of the patient-centric move. The future seems to be bright where pharma and patients have the potential to work symbiotically that could result in better research results, improved health care and profits.
ACKNOWLEDGMENT
I would like to acknowledge insights and guidance offered by Dr. Nimita Limaye that has helped me while drafting this article.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Spike in Growth in 2014-15 Driven by Innovation Surge and Few Patent Expiries, IMS Health News. [Last accessed on 2014 Dec 22]. Available from: http://www.imshealth.com/portal/site/imshealth/menuitem.c76283e8bf81e98f53c753c71ad8c22a/?vgnextoid=8269f454d4ac9410VgnVCM10000076192ca2RCRDandvgnextchannel = 5ec1e590cb4dc310VgnVCM100000a48d2ca2RCRDandvgnextfmt=default .
- 2.Shore E. A Framework for Patient-Centered Clinical Trials. [Last accessed on 2014 Nov 18]. Available from: http://www.christenseninstitute.org/patient-centered-clinical-trials/
- 3.Conman D, Jeffrey A. Spaeder. Harnessing the Power of the Digital Patient. [Last accessed on 2014 Nov 19]. Available from: http://www.quintiles.com/library/white.papers/harnessing-the-power-of-the-digital-patient-white-paper .
- 4.Gossen R. Re-imagining Clinical Trial Info in a Patient-Centric Way. [Last accessed on 2014 Nov 19]. Available from: http://www.rebarinteractive.com/clinical-trial-visualization-redesign-challenge/
- 5.Capsey L, Butlin R. In the Patients Shoes from Protocol to Publication-How to Achieve a Patient-Centric Approach to Clinical Trial Design. [Last accessed on 2014 Nov 20]. Available from: http://www.beaufortcro.com/wp-content/uploads/2012/04/Monitor-In_the_Patients_Shoes.pdf .
- 6.Rose AW. Behavioral Science and “Social Listening”: The DNA of Clinical Trial Recruitment. [Last accessed on 2014 Dec 24]. Available from: http://www.clinicalleader.com/doc/behavioral-science-social-listening-the-dna-of-clinical-trial-recruitment-0001 .
- 7.Wechsle J. Social Media Raises Special Concerns for Clinical Trial Sponsors in Applied Clinical Trials. [Last accessed on 2014 Dec 24]. Available from: http://www.appliedclinicaltrialsonline.com/node/246735 .
- 8.Abrams T. FDA Issues Draft Guidance's for Industry on Social Media and Internet Communications about Medical Products: Designed with Patients in Mind. [Last accessed on 2014 Dec 24]. Available from: http://www.blogs.fda.gov/fdavoice/index.php/2014/06/fda-issues-draft-guidances-for-industry-on-social-media-andinternet-communications-about-medical-products-designed-with-patients-in-mind/#sthash.T2SwWund.dpuf .
- 9.Medicines New Zealand Incorporated Code of Practice. 16th ed. [Last accessed on 2015 Feb 03]. pp. 22–3. Available from: http://www.rgpn.org.nz/Network/media/documents/pdfs/Medicines-New-Zealand-Draft-Revision-Code-of-Practice-Ed-16.pdf .
- 10.Agency for Healthcare Research and Quality, Education and Training for Health professionals. [Last accessed on 2014 Dec 25]. Available from: http://www.ahrq.gov/
- 11.WELVU, Solutions for Mobile. [Last accessed on 2014 Dec 29]. Available from: http://www.welvu.com .
- 12.STAYWELL, Products and Solutions. [Last accessed on 2014 Dec 29]. Available from: http://www.staywell.com/patient-education/krames-on-demand/
- 13.ELSEVIER, Exit care Onscreen. [Last accessed on 2014 Dec 29]. Available from: http://www.exitcare.com/solutions/video-solutions/exitcare-onscreen/
- 14.Weinberg T. How social Media has become a tool for clinical research, Techipedia. [Last accessed on 2014 Dec 25]. Available from: http://www.techipedia.com/2014/social-media-clinical-research/
- 15.Takher G. The Power of Crowd Curation: We Investigate Wikipedia. [Last accessed on 2014 Dec 22]. Available from: http://portal.lillycoi.com/2012/10/17/the-power-of-crowd-curation-we-investigate-wikipedia/
- 16.Kremidas J. Online Patient Communities: Accelerating Clinical Trials and improving patient outcomes. [Last accessed on 2015 Feb 03]. Available from: http://www.clinicalleader.com/doc/online-patient-communities-accelerating-clinical-0001 .
- 17.Carlson RH. Crowdsourcing clinical trial protocols. Oncol Times. 2014;36:31–3. [Google Scholar]
- 18.Khatib F, DiMaio F. Foldit Contenders Group, Foldit Void Crushers Group, Cooper S, Kazmierczyk M, et al. Crystal structure of a monomeric retroviral protease solved by protein folding game players. Nat Struct Mol Biol. 2011;18:1175–7. doi: 10.1038/nsmb.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HHS and NIH take steps to enhance transparency of clinical trial results. [Last accessed on 2014 Dec 02]. Available from: http://www.nih.gov/news/health/nov2014/od-19.htm .
- 20.LEO Pharma's Position on Public Access to Clinical Trials Information. [Last accessed on 2014 Nov 25]. Available from: http://www.leo-pharma.com/Home/Research-and-Development/Clinical-trial-disclosure/LEO-Pharmas-position-on-transparency.aspx .
- 21.Vital Connect, Medidata partner on continuous vital sign monitoring. [Last accessed on 2014 Dec 26]. Available from: http://www.centerwatch.com/news_online/article/7300/vital-connect -medidata -partner -on -continuous -vital -sign-monitoring#sthash-KHRIHtMm.dpuf .
- 22.Iodine launches cold and flu app to help consumers find best OTC options for their symptoms. [Last accessed on 2014 Dec 26]. Available from: http://www. centerwatch.com /news-online/article/7352/iodine-launches-cold-and-fluapp-to-help-consumers-find-best-otc-options-for-theirsymptoms#sthash.xncXmNgP.dpuf .
- 23.Wiggington C. Technology Trends that will Transform Clinical Trials. [Last accessed on 2014 Dec 29]. Available from: http://www.datatrak.com/wp -content/uploads/2014/07/Technology -Trends -Transforming -Clinical-Trials.pdf .
- 24.Baum S. Patient -centric clinical trial recruitment tools want to eliminate [medical] language barrier – Simple Language usage advocated. [Last accessed on 2014 Nov 17]. Available from: http://www.medcitynews.com/2014/01/patient -centric -clinical -trial -recruitment -tools-want -eliminate -medical -language -barrier/
- 25.Reinventing Biopharma: Strategies for an evolving marketplace, The Patient Led R and D strategy, An Economist Intelligence Unit report Sponsored by Quintiles. [Last accessed on 2014 Dec 26]. Available from: http://www.quintiles.com/~/media/library/white%20papers/reinventing -biopharma -strategies-for -an -evolving -marketplace -the -patient -led-rampd-strategy.pdf .


