Abstract
Background:
This study aims at simplifying the practical patient management and offers some general indications for pharmacotherapeutic choice by the implementation of (Global Initiative for Chronic Lung Disease) guidelines. This study was designed to evaluate the clinical and economic consequences of salmeterol/fluticasone (SF), formoterol/budesonide (FB), and formoterol/fluticasone (FF) in severe and very severe chronic obstructive pulmonary disease (COPD) patients.
Objectives:
The aim was to find out the most cost-effective drug combination between the three combinations (SF/FB/FF) in COPD patients.
Materials and Methods:
A prospective observational comparative study (cost-effectiveness analysis), in which 90 severe (30 ≤ forced expiratory volume in 1 s [FEV1] <50% predicted) and very severe (FEV1 < 30% predicted) COPD patients (outpatients/inpatients) who are prescribed with any one of the following combinations (SF/FB/FF) were selected. In our study, we have divided 90 COPD patients into three groups (Group I, Group II, and Group III) each group consisting of 30 patients. Group I was prescribed with medication SF, Group II with medication FB, and Group III with medication FF. We used five different parameters such as spirometry test (mean FEV1 initial and final visit), number of symptom-free days (SFDs), number of moderate and severe exacerbations, Number of days of hospitalization and direct, indirect, and total cost to assess the cost-effectiveness of SF/FB/FF. Comparison of cost and effects was done during the period of 6 months of using SF/FB/FF.
Results:
The average FEV1 for Group I, Group II, and Group III subjects at initial visit was 33.47%, 33.73%, and 33.20% and was increased to 36.60%, 35.8%, and 33.4%, respectively. A 3% increment in FEV1 was reported for Group I subjects (SF) and was highly significant statistically (t = −8.833, P = 0.000) at 95% CI. For Group II subjects (FB), a 2% increment in FEV1 was reported and was highly significant statistically (t = −9.001, P = 0.000) at 95% CI. For Group III (FF) subjects 0.2% increment in FEV1. The overall mean total cost for Group I, Group II, and Group III subjects during the 6 months period was found to be Rs. 29,725/-, Rs. 32,602/- and Rs. 37,155/-. Incremental cost-effectiveness of FB versus SF was Rs. 37,781/- per avoided exacerbation and Rs. 661/-per SFD.
Conclusion:
This study highlights the favorable therapeutic performance of combined inhaled bronchodilators and corticosteroids (SF/FB/FF), thus suggesting that healthcare costs would be also affected positively. Results from our study showed that SF and FB were the most effective strategies in the treatment of COPD, with a slight clinical superiority of SF. The FF strategy was not much effective (i.e. associated with fewer outcomes and higher costs).
Keywords: Bronchodilators, cost-effectiveness, inhaled corticosteroids, severe and very severe chronic obstructive pulmonary disease, symptom-free day
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the world (WHO, 2000) and it is ranked among the first causes of disability in developed countries. COPD is a disease with substantial social costs. Exacerbation is the main cause of hospital admission in COPD patients. Exacerbation implies a substantial impairment of patient respiratory ability and quality of life and can even lead to death. It has been demonstrated that only smoking cessation can produce a relative slowdown in the chronic course of the disease while pharmaceutical therapies aim to improve patient's quality of life by reducing exacerbation frequency and severity.[1]
The Global Initiative for Chronic Lung Disease (GOLD) was established in 1997. Its goals are to increase awareness of COPD and decrease mortality and morbidity from the disease. The GOLD guidelines define COPD as a disease state characterized by airflow limitation that is not fully reversible, it is usually progressive and is associated with an abnormal inflammatory response of the lungs to inhaled noxious particles or gases.[2,3]
Global Initiative for Chronic Obstructive Lung Disease estimates suggests that COPD will raise from the fourth to the third most common cause of death world wide by 2020. The GOLD 2004 guideline classifies disease severity in four stages based on chronic symptoms, forced expiratory volume in 1 s (FEV1), and forced vital capacity (FVC).[4,5]
This pragmatic staging approach aims to simplify practical patient management and offer some general indications for pharmacotherapeutic choice. At the stage I, bronchodilators are generally prescribed on an as-needed basis for relief of persistent, or worsening, symptoms. The most commonly used bronchodilator drugs include β2-agonists, anticholinergics, and methylxanthines. At stage II, GOLD guidelines recommend the addition of pulmonary rehabilitation and regular treatment with one or more long-acting bronchodilators. Pulmonary rehabilitation aims at resolving a range of nonpulmonary problems including social isolation altered mood states (especially depression), muscle wasting and weight loss. The addition of regular treatment with inhaled glucocorticosteroids is appropriate for symptomatic COPD patients with an FEV1 < 50% predicted (stage III and stage IV). Combined inhaled glucocorticosteroids, and long-acting β2-agonists, are more effective than the individual components; according with GOLD judgments, combining drugs with different mechanisms and durations of action might increase the degree of bronchodilation with equivalent or fewer side-effects. Long-term oxygen therapy is generally added in stage IV, whereas other pharmacological treatments (such as antioxidants and mucolytic agents) are frequently used as adjuvant therapy.[6,7]
Cost-effectiveness analysis (CEA) is a form of economic analysis that compares the relative costs and outcomes (effects) of two or more courses of action. CEA is distinct from cost-benefit analysis, which assigns a monetary value to the measure of effect. CEA is often used in the field of health services, where it may be inappropriate to monetize health effect. Typically the CEA is expressed in terms of a ratio where the denominator is a gain in health from a measure (years of life, premature births averted, and sight-years gained) and the numerator is the cost associated with the health gain. The most commonly used outcome measure is quality-adjusted life years (QALY).[8]
Examples include the number of people cured of the disease, the mmHg reduction in diastolic blood pressure and the number of symptoms free days (SFDs) experienced by a patient. The selection of the appropriate effect measure should be based on clinical judgment in the context of the intervention being considered.
CEA compares the relative difference of costs and consequences of different treatment strategies. In CEA, costs are measured in monetary terms and health consequences are measured in natural or physical units.
The incremental cost-effectiveness ratio (ICER) is an equation used commonly in health economics to provide a practical approach to decision-making regarding health interventions.[9] It is typically used in CEA. ICER is the ratio of the change in costs to incremental benefits of therapeutic intervention or treatment. The equation for ICER is:
ICER = (C1 – C2)/(E1 – E2)
where C1 and E1 are the cost and effect in the intervention or treatment group and where C2 and E2 are the cost and effect in the control care group. Costs are usually described in monetary units while benefits/effect in health status is measured in terms of QALYs gained or lost.
Incremental cost-effectiveness ratio provides a means of comparing projects or interventions across various disease states and treatments. As seen in the equation above, the ratio is created with the units of cost per benefits/effect unit. By using this ratio, comparisons can be made between treatment modalities to determine, which provides a more cost-effective therapy. ICER studies thus provide an opportunity to help contain health care costs without adverse health consequences. They also provide to policy makers information on where resources should be allocated when they are limited. As health care costs have continued to rise, many new-clinical trials are attempting to integrate ICER into results to provide more evidence of potential benefit.[10]
Current clinical guidelines recommend combined inhaled corticosteroids and bronchodilators as the mainstay of therapy for severe and very severe COPD patients.
Thus, the aim of the present study is to evaluate the clinical and economic consequences of the implementation of GOLD 2004 guidelines for severe and very severe COPD patients and to find out the most cost-effective drug combination among the three different drug combinations: Salmeterol/fluticasone (SF), formoterol/budesonide (FB), formoterol/fluticasone (FF) in COPD patients.
MATERIALS AND METHODS
We planned a CEA on the use of combined inhaled glucocorticosteroids and long-acting β2-agonists to evaluate the relative pharmacoeconomic performance of the three different drug combinations (SF; FF; and FB)
-
The CEA is the typical economic evaluation that should be performed when comparing two or more therapeutic alternatives whose clinical efficacy is not equivalent. In this analysis, both the costs and the health consequences of the alternatives are examined. The three therapeutic alternatives considered were:
- Salmeterol/fluticasone (SF): Use of combined SF 25/250 μg bid in GOLD stages III and IV patients in addition to the standard therapy already in use
- Formoterol/budesonide (FB): Use of combined FB 6/200 μg bid in GOLD stages III and IV patients
- Formoterol/fluticasone (FF): Use of combined FF 6/250 μg bid in GOLD stages III and IV patients
-
The direct comparison between two alternatives is obtained through the ICER. Comparing strategy 1 with strategy 2, the ICER value represents the relative increment of cost at which a relative unitary increment of benefit could be obtained. If we indicate the cost of the two alternatives by C1 and C2 and the benefits (for instance, life years saved, hospitalization avoided, etc.) by B1 and B2 this gives Eq. (1)
ICER = C1 − C2/B1 − B2 (1)
Plan of work
Ethical Committee approval was obtained from Institutional Review Board Committee of a teaching hospital
Informed consent was taken from patients
Literature review related to the study was done
Designing a data collection form
Spirometry (mainly FEV1)
To record and compare the average number of SFDs for each therapeutic alternative
To study and compare the number of moderate and severe exacerbation for each therapeutic alternative
Costs (direct, indirect, and total cost)
Review of patients
Report the data collected.
Study site
The study was conducted in the outpatient and in-patient setup of Pulmonology Department of Princess Esra Hospital, a tertiary care teaching hospital in South India, during the period September 2013 to February 2014. It is a 1000-bedded teaching hospital situated in the heart of the city of Hyderabad, providing specialized health care services to all people.
Study design
A hospital-based prospective observational comparative study was conducted on 90 COPD patients. Data were collected from both case records and patients. Study period 6 months.
Sample size
A total of 90 patients who took treatment for COPD are selected according to inclusion and exclusion criteria for the study.
Study criteria
The following categories of patients admitted in RICU and MICU ward (inpatients) and also outpatients are enrolled into the study.
Inclusion criteria
Patients of both genders (male and female) above 18 years
Those patients who are prescribed with any one of the following drug combination: SF; FF; and FB
Patients who are willing to give their informed consent to participate in the study
Patients in RICU and MICU who are diagnosed with COPD.
Exclusion criteria
Patients who are not willing to participate in the study
Pregnant woman are excluded
Pediatrics patients are excluded
Patients without COPD.
Source of data
Patient's data relevant to the study will be obtained from the following sources:
Patient case record
Patient is counselling.
Expected outcomes
The 6 months average exacerbation number
Symptom-free days per patient
Percentage of FEV1 per patient.
Costs and cost perspective
Direct costs take into account hospitalizations, medical visits, laboratory investigations, pharmaceutical treatments (different from SF, FB or FF), oxygen therapy, lung ventilation, travelling cost, and rehabilitative therapy
Indirect costs account for lost productivity of the patient and first degree relatives
We have classified both direct and indirect costs in two parts; one caused directly by exacerbation and one independent of them
The pharmaceutical cost for the active treatment (SF, FB, or FF) should be added to direct exacerbation independent cost.
Assessment
We used five different parameters to assess the cost-effectiveness of SF/FB/FF:
Spirometry test (mean FEV1 initial and final visit)
Number of -SFDs
Number of moderate and severe exacerbations
Direct, indirect and total cost
Number of days of hospitalization.
Statistical analysis
Data were analyzed using Statistical Program for Social Science (SPSS) version: 13.0 (IBM Company). Descriptive statistics for improvement in lung functions are presented as mean and 95% confidence interval (CI). To assess the similarities between the groups at baseline analysis of variance (ANOVA) were used. General linear repeated measures using post-hoc Bonferroni method assessed significance between treatment groups during the study. The significant improvements in treatment groups were assessed by one-way ANOVA using post-hoc Bonferroni that compared means of FEV1, moderate and severe exacerbation, SFDs, costs (direct, indirect, and total cost) during individual follow-ups. Paired sample “t”- test was also used to assess the significant difference between different treatment groups. Statistical significance was fixed at P < 0.05.
RESULTS
A total of 90 COPD patients were assessed for cost-effectiveness of combined inhaled corticosteroids and bronchodilators (SF/FB/FF) with respect to spirometry (mainly FEV1), SFDs, number of moderate and severe exacerbation and costs (direct, indirect, and total cost) during the period of 6 months. These 90 COPD patients are divided into three groups (Group I, Group II, and Group III), each group consisting of 30 patients with an equal number of severe and very severe COPD patients. Group I subjects are those who are prescribed with medication SF, Group II subjects with medication FB, and Group III with medication FF.
Data were collected at two points one at the initial visit, that is, as soon as the patient diagnose with severe or very COPD and was prescribed with any one of the three combinations (SF/FB/FF) and the other after using the same medication for 6 months (that was prescribed at the initial visit). The test data obtained are enumerated in Table 1.
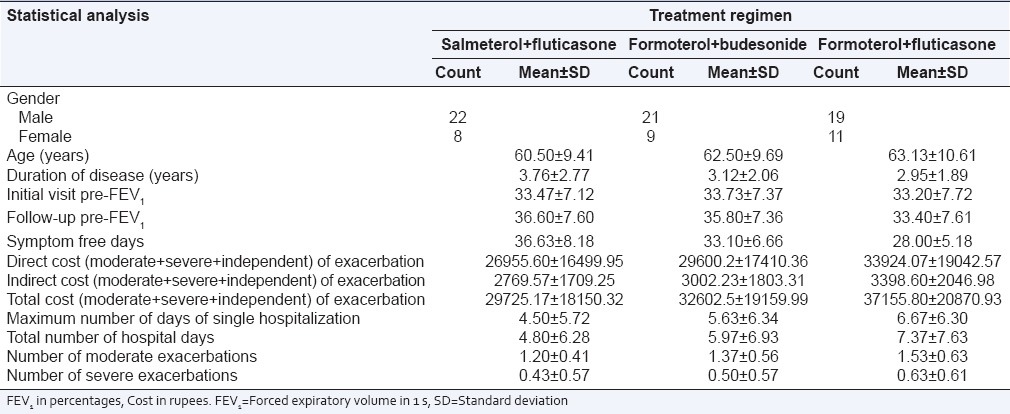
Table 1.
Mean±SD of patient demographic characteristics and other parameters
There were 171 episodes of exacerbation during the study period, of which 124 (72.5%) were classified as moderate, and 47 (27.5%) were severe. Of the 171 exacerbations, 128 (74.85%) used outpatient resources, 60 (35%) used ED resources and 47 (27.5%) were hospitalized as mentioned in Table 2.
Table 2.
Number of exacerbations visited to various departments

DISCUSSION
Attention to COPD is constantly increasing worldwide because its high prevalence, morbidity, and mortality represent a challenging problem for all healthcare systems. The burden of COPD, measured as its impact on patients’ symptoms and quality of life and the corresponding use of healthcare resources, is still a major aspect of the disease. For these reasons healthcare decision makers, before deciding which strategies should be preferred, need to improve their understanding of the concept of good value for money, in order to control the disease and reduce the huge costs required to meet patients’ needs.[11,12]
It is now well established that the main proportion of COPD costs depends on the clinically uncontrolled disease and its high exacerbation rate, frequently leading to the patients’ hospitalization.[13] Recommendations to treat COPD according to the most accepted guidelines have been disseminated over recent years even though COPD remains under-diagnosed and under-treated worldwide. Obviously, more severe degrees of COPD have received most attention both in terms of monitoring of clinical outcomes and in assessing the economic value of therapeutic interventions, although the effects of guideline recommendations have been investigated in terms of pharmacoeconomic convenience only.[14,15]
There is a good general consensus that combining medications of different pharmacological classes represents a much more convenient strategy in COPD, particularly for severe or very severe disease.[16] Additional effects have, in fact, been proven both in functional and in clinical terms under these conditions. In particular, health status, quality of life and exacerbations represent the most affected outcomes in severe (basal FEV1 < 50% predicted) and very severe COPD patients (basal FEV1 < 30% predicted) when treated with combined long-acting β2-agonists and inhaled corticosteroids over time. These data highlighted the favorable therapeutic performance of SF, FB, and FF thus suggesting that healthcare costs would be also affected positively.[17,18]
Current practice guidelines for the treatment of COPD recommend the use of combined inhaled corticosteroids and long-acting bronchodilators in severe and very severe patients (GOLD stages III and IV).[19]
The main scope of this study was to evaluate the clinical and economic consequences of implementation of GOLD guidelines for severe and very severe COPD patients.
A prospective observational comparative study (CEA) was conducted to assess the cost-effectiveness of combined inhaled corticosteroids and bronchodilators. We developed a CEA on three alternative therapeutic strategies (SF; FB; FF).
During the 6-month study period, a total of 90 COPD patients among which 62 (68.9%) are males and 28 (31.1%) are females were assessed for cost-effectiveness of combined inhaled bronchodilators and corticosteroids [Figure 1]. The highest number of patients (n = 21) were in the age group of 66-70 years [Figure 2]. Among 90 patients enrolled for the study, 28 (31.1%) patients are employed, 25 (27.78%) patients are housewives, 7 (7.78%) patients are unemployed and 30 (33.33%) patients are retired [Table 3].
Figure 1.
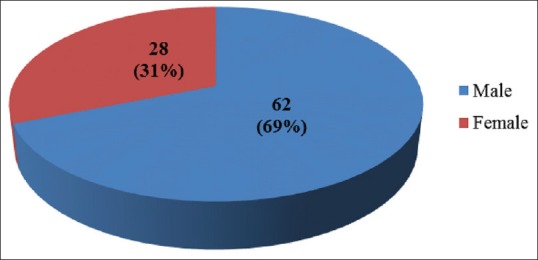
Gender distribution of patients
Figure 2.
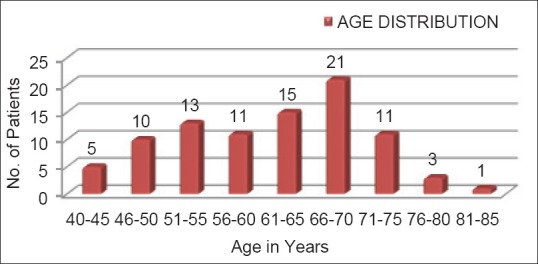
Age distribution of patients
Table 3.
Education distribution of patients studied
In our study of 90 COPD patients, it was observed that 30 (33.33%) patients are a current smoker, 17 (18.9%) patients are ex-smoker, 5 (5.55%) patients were addicted to alcohol, 10 (11.11%) patients were addicted to both tobacco and alcohol and 28 (31.11%) patients had no social addictions [Figure 3]. Among 90 participants, 23 (25.5%) subjects had positive family history of COPD and 67 (74.5%) subjects had no family history of COPD [Table 4].
Figure 3.
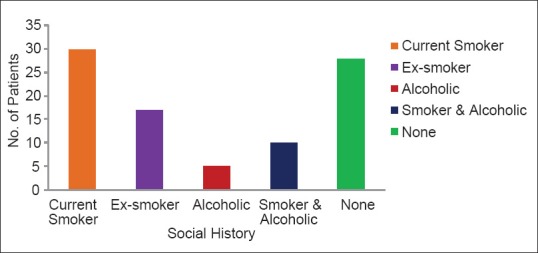
Social history of patients
Table 4.
Family history of COPD patients studied

In our study, we have divided 90 COPD patients into three groups (Group I, Group II, and Group III) each group consisting of 30 patients. Group I was prescribed with medication SF, Group II was prescribed with medication FB, and Group III was prescribed with medication FF. Costs and outcomes were noted for each group.
In our study, we used FEVI (initial visit and final visit), SFDs, number of moderate and severe exacerbation and costs (direct, indirect and total cost) to assess the effectiveness of SF, FB, and FF. We have used paired Student's t-test and ANOVA for analyzing the differences.
Mean age for Group I (SF) subjects was 60.5 years (standard deviation [SD] = 9.41), mean age for Group II (FB) subjects was 62.5 (SD = 9.69) and mean age for Group III (FF) subjects was 63.13 (SD = 10.61). Mean duration of disease for Group I subjects was 3.76 (SD = 2.77), mean duration of disease for Group II subjects was 3.12 (SD = 2.06), mean duration of disease for Group III subjects was 2.95 (SD = 1.89) [Table 1].
The average FEV1 for Group I, Group II, and Group III subjects at initial visit was 33.47%, 33.73% and 33.20% and was increased to 36.60%, 35.8%, and 33.4. A 3% increment in FEV1 was reported for Group I subjects (SF) and was highly significant statistically (t = −8.833, P = 0.000) at 95% CI. For Group II subjects (FB), a 2% increment in FEV1 was reported and was highly significant statistically (t = −9.001, P = 0.000) at 95% CI. For Group III subjects 0.2% increment in FEV1 was reported which was not significant statistically [Table 5, Figure 4].
Table 5.
Mean FEV1 (initial and final visit) with different treatment groups

Figure 4.
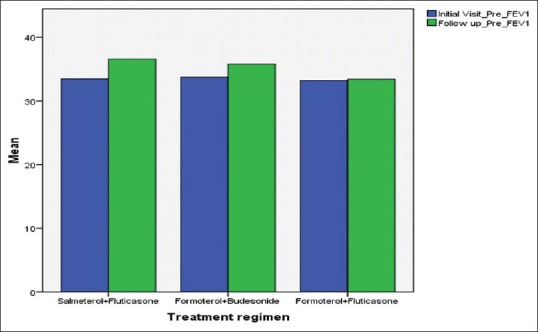
Initial visit forced expiratory volume in 1 s (FEV1) and final visit FEV1 with different treatment groups
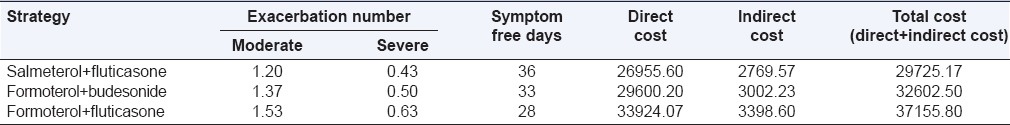
In our study, we observed that the average number of moderate exacerbation per patient of Group I, Group II, and Group III subjects during the 6 months period was 1.2 (±0.41), 1.37 (±0.56), 1.53 (±0.63). Average number of severe exacerbation per patient of Group I, Group II, and Group III subjects during the 6 months period was 0.43 (±0.57), 0.50 (±0.57), and 0.63 (±0.61) respectively [Table 6]. Our study builds on the results of other economic analysis investigating the burden of COPD and COPD exacerbations. Miravitlles et al.[20] have reported an average cost of exacerbation at $159. Costs were based on the cohorts of 2414 patients over a 1 month time horizon.
Table 6.
Costs (rupees) and outcomes at the end of 6 months (average values per patient)
Andersson et al.[21] reported costs of moderate and severe exacerbation at SEK 2111 and SEK 21,852, respectively. The small study cohort (n = 61) was based on a larger epidemiological cohort. The cost driver in the severe category was hospitalization cost, which accounted for more than 90% of the overall mean cost. However, the average length of hospital stay (6.6 days) was similar to that of present study.
The average total number of hospital days per patient of Group I, Group II, and Group III subjects was 4.8 (±6.28), 5.97 (±6.93), and 7.37 (±7.63), respectively.
The SFDs per patient using SF, FB and FF strategy was 36, 33 and 28 during the 6 months period [Figure 5].
Figure 5.
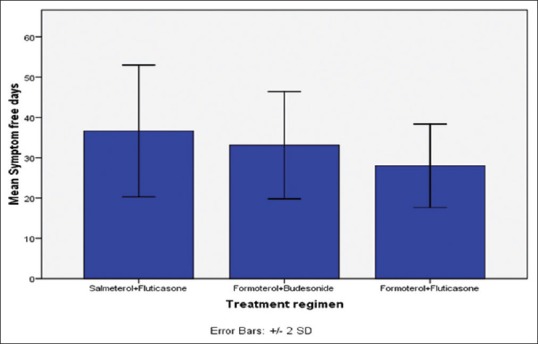
Symptom free days in 6 months with different treatment groups
The cost of moderate exacerbation per patient using SF strategy was Rs. 1895/-, FB strategy was Rs. 2200/-, and with FF strategy was Rs. 2576/-. The cost of severe exacerbation per patient using SF strategy was Rs. 13,286/-, FB strategy was Rs. 15,718/-, and with FF strategy was Rs. 19,883/-.
The overall mean direct cost for Group I, Group II, and Group III subjects during the 6 months period was found to be Rs. 26,955/-, Rs. 29,600/-, and Rs. 33,924/-. The overall mean total cost for Group I, Group II, and Group III subjects during the 6 months period was found to be Rs. 29,725/-, Rs. 32,602/-, and Rs. 37,155/- [Table 6, Figure 6]. Incremental cost-effectiveness of FB versus SF was Rs. 37,781/- per avoided exacerbation and Rs. 661/- per SFD [Table 7].
Figure 6.
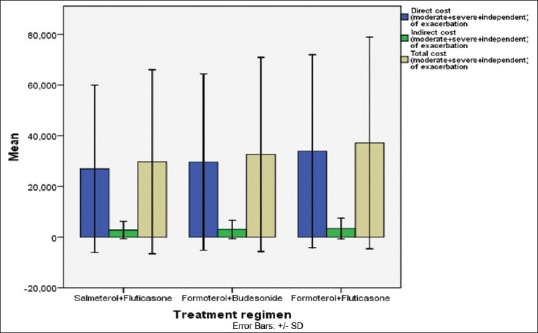
Direct cost, indirect cost, and total cost (moderate + severe + independent of exacerbation) with different treatment groups
Table 7.
ICER calculation with respect to the less expensive strategy (salmeterol+fluticasone)

However, other studies, viz., Dal Negro et al. reveals that the average exacerbation number per patient at the end of a life-long simulation for patients using SF strategy was 9.09, FB was 9.66 and salmeterol alone was 10.07. The average SFDs per patient was 257 (SF), 220 (FB) and 55 (salmeterol). The average direct cost at the end of a life-long simulation for patients using SF strategy was € 34,037, FB strategy was € 33,944 and salmeterol was € 33,369, respectively. Incremental cost-effectiveness of SF versus salmeterol was € 679.5 per avoided exacerbation and € 3.3 per SFD.
The same conclusion was drawn in recent studies (Gagnon et al., 2005; Lofdahl et al., 2005; Spencer et al., 2005), based on similar approach, developed for the US, Canadian, and Swedish healthcare systems, respectively.
Limitations of the study
The study was carried out for a short term period of 6 months. A long-term study with a larger group of patients can be carried out in the RICU department as the treatment requires longer duration and more number of follow-ups. Among 90 patients enrolled for the study, 2% of the patients have not utilized SFDs form.
CONCLUSION
There is a good general consensus that combining medications of different pharmacological classes represents a much more convenient strategy in COPD, particularly for severe or very severe disease. Additional effects have in fact been proven both in functional and in clinical terms under these conditions. In particular, health status, quality of life, and exacerbations represent the most affected outcomes in more severe COPD (basal FEV1 < 50% predicted) when treated with combined long-acting β2-agonists and inhaled corticosteroids over time. This study highlighted the favorable therapeutic performance of combined inhaled bronchodilators and corticosteroids (SF/FB/FF), thus suggesting that healthcare costs would be also affected positively. Results from our study showed that the recommended use of combined inhaled corticosteroids and long-acting bronchodilators for severe and very severe COPD patient treatment, compared with current practice, had the potential to improve clinical outcomes, and consequently patients’ quality of life, without increasing healthcare costs.
Based on five different parameters used in our study to assess the cost-effectiveness of combined inhaled bronchodilators and corticosteroids, we found that SF and FB were the most effective strategies with a slight clinical superiority of SF. The FF strategy was not much effective (i.e. associated with fewer outcomes and higher costs).
This conclusion seems relevant not only from the patient's perspective (such as improvement of clinical conditions), but also from a societal perspective. This study confirm that it is possible to improve substantially the health status of severe and very severe COPD patients without further increasing social costs.
ACKNOWLEDGMENTS
We would like to express our profound gratitude to Dr. S.A. Azeez, the honorable Principal of Deccan School of Pharmacy, Hyderabad and Mr. Syed Amir Ali, Dr. Arshad Hussain Mohd, Assistant Professor, Department of Pharmacy Practice, Deccan School of Pharmacy and also Dr. Mohammed Aleemuddin Naveed, Associate Professor, Department of Pulmonology, Princess Esra Hospital, Anas Rasheed and Dr. Mohammed Abdul Hannan Hazari, Associate Professor, Department of Physiology, Deccan College of Medical Sciences for providing necessary facilities, valuable guidance, and continuous encouragement.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hoogendoorn M, Feenstra TL, Hoogenveen RT, Rutten-van Mölken MP. Long-term effectiveness and cost-effectiveness of smoking cessation interventions in patients with COPD. Thorax. 2010;65:711–8. doi: 10.1136/thx.2009.131631. [DOI] [PubMed] [Google Scholar]
- 2.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 3.Pride NB. Pulmonary physiology. In: Barnes PJ, Drazen JM, Rennard S, Thomson NC, editors. Asthma and COPD: Basic Mechanisms and Clinical Management. San Diego Calif USA: Academic Press; 2002. pp. 43–56. [Google Scholar]
- 4.De Marco R, Accordini S, Cerveri I. An international survey of chronic obstructive pulmonary disease in young adults according to GOLD stages. Thorax. 2004;59:120–5. doi: 10.1136/thorax.2003.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonelli-Incalzi R, Imperiale C, Bellia V, Catalano F, Scichilone N, Pistelli R, et al. Do GOLD stages of COPD severity really correspond to differences in health status? Eur Respir J. 2003;22:444–9. doi: 10.1183/09031936.03.00101203. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Inhaled corticosteroids vs placebo for preventing COPD exacerbations:A systematic review and metaregression of randomized controlled trials. Chest. 2010;137:318–25. doi: 10.1378/chest.09-1305. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro SD, Reilly JJ, Jr, Rennard SI. Chronic bronchitis and emphysema. In: Mason RJ, Broaddus VC, Martin TR, editors. Murray and Nadel's Textbook of Respiratory Medicine. 5th ed. ch. 39. Philadelphia, PA: Saunders Elsevier; 2010. [Google Scholar]
- 8.Bleichrodt H, Quiggin J. Life-cycle preferences over consumption and health: When is cost-effectiveness analysis equivalent to cost-benefit analysis? J Health Econ. 1999;18:681–708. doi: 10.1016/s0167-6296(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 9.Tengs TO, Adams ME, Pliskin JS, Safran DG, Siegel JE, Weinstein MC, et al. Five-hundred life-saving interventions and their cost-effectiveness. Risk Anal. 1995;15:369–90. doi: 10.1111/j.1539-6924.1995.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 10.Bell CM, Urbach DR, Ray JG, Bayoumi A, Rosen AB, Greenberg D, et al. Bias in published cost effectiveness studies: Systematic review. BMJ. 2006;332:699–703. doi: 10.1136/bmj.38737.607558.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41:582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 12.Barr JT, Schumacher GE, Freeman S, LeMoine M, Bakst AW, Jones PW. American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin Ther. 2000;22:1121–45. doi: 10.1016/S0149-2918(00)80089-2. [DOI] [PubMed] [Google Scholar]
- 13.Hilleman DE, Dewan N, Malesker M, Friedman M. Pharmacoeconomic evaluation of COPD. Chest. 2000;118:1276–85. doi: 10.1378/chest.118.5.1278. [DOI] [PubMed] [Google Scholar]
- 14.Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941–7. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 15.David M, Halpin G. Health economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:227–33. doi: 10.1513/pats.200507-072SF. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117:398S–401. doi: 10.1378/chest.117.5_suppl_2.398s. [DOI] [PubMed] [Google Scholar]
- 17.Osman IM, Godden DJ, Friend JA, Legge JS, Douglas JG. Quality of life and hospital re-admission in patients with chronic obstructive pulmonary disease. Thorax. 1997;52:67–71. doi: 10.1136/thx.52.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rutten-van Mölken MP, Goossens LM. Cost effectiveness of pharmacological maintenance treatment for chronic obstructive pulmonary disease: A review of the evidence and methodological issues. Pharmacoeconomics. 2012;30:271–302. doi: 10.2165/11589270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Friedman M, Serby CW, Menjoge SS, Wilson JD, Hilleman DE, Witek TJ., Jr Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest. 1999;115:635–41. doi: 10.1378/chest.115.3.635. [DOI] [PubMed] [Google Scholar]
- 20.Miravitlles M, Murio C, Guerrero T, Gisbert R. DAFNE Study Group. Decisiones sobre Antibioticoterapia y Farmacoeconomía en la EPOC. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest. 2002;121:1449–55. doi: 10.1378/chest.121.5.1449. [DOI] [PubMed] [Google Scholar]
- 21.Andersson F, Borg S, Jansson SA, Jonsson AC, Ericsson A, Prutz C, et al. The costs of exacerbations in chronic obstructive pulmonary disease (COPD) Respir Med. 2002;96:700–708. doi: 10.1053/rmed.2002.1334. [DOI] [PubMed] [Google Scholar]


