Abstract
Background
Cells deploy quality control mechanisms to remove damaged or misfolded proteins. Recently, we have reported that a mutation (R43W) in the Frank-ter Haar syndrome protein Tks4 resulted in aberrant intracellular localization.
Results
Here we demonstrate that the accumulation of Tks4R43W depends on the intact microtubule network. Detergent-insoluble Tks4 mutant colocalizes with the centrosome and its aggregate is encaged by the intermediate filament protein vimentin. Both the microtubule inhibitor nocodazole and the histone deacetylase inhibitor Trichostatin A inhibit markedly the aggresome formation in cells expressing Tks4R43W. Finally, pretreatment of cells with the proteasome inhibitor MG132 markedly increases the level of aggresomes formed by Tks4R43W. Furthermore, two additional mutant Tks4 proteins (Tks41–48 or Tks41–341) have been investigated. Whereas the shorter Tks4 mutant, Tks41–48, shows no expression at all, the longer Tks4 truncation mutant accumulates in the nuclei of the cells.
Conclusions
Our results suggest that misfolded Frank-ter Haar syndrome protein Tks4R43W is transported via the microtubule system to the aggresomes. Lack of expression of Tks41–48 or aberrant intracellular expressions of Tks4R43W and Tks41–341 strongly suggest that these mutations result in dysfunctional proteins which are not capable of operating properly, leading to the development of FTHS.
Keywords: Tks4, Aggresome, Frank-ter Haar Syndrome, Microtubules, Misfolded protein
Background
Cells deploy quality control mechanisms to remove damaged or misfolded proteins. These mechanisms include up-regulated chaperones to facilitate protein refolding, ubiquitin-dependent degradation of misfolded/damaged proteins, and formation of detergent insoluble aggresomes at the juxtanuclear region [1–4]. Aggresome formation is a vital mechanism to eliminate misfolded proteins, nevertheless, it is not clear if it is an independent process or it is activated only when the degradative capacity of the ubiquitin-proteasome system is overwhelmed [5]. In this system, misfolded and aggregated proteins are selectively recognized, ubiquitinated, and delivered via HDAC6 (histone deacetylase 6)-dependent, microtubule-based transport toward the microtubule-organizing center (MTOC) [6]. Aggresome formation not only protects cells from proteotoxicity but also facilitates the clearance of damaged proteins by autophagy [6]. The accumulation of protein aggregates is commonly linked with various human diseases referred to as protein conformation disorders [7].
Frank-ter Haar syndrome (FTHS) is a rare disorder associated with cardiovascular, skeletal and craniofacial anomalies including macrocornea with or without glaucoma, brachycephaly, large anterior fontanels, hypertelorism, anteverted nostrils, thoracolumbar kyphosis, and short hands [8, 9]. Protruding simple ears and prominent coccyx bone can be also regarded as important diagnostic signs [8, 9]. FTHS patients unfortunately die in infancy or in early childhood due to cardiovascular anomalies or respiratory infections. The most common underlying genetic defect in FTHS has recently been established through homozygosity mapping studies in patients, identifying homozygous mutations in the SH3PXD2B gene on chromosome 5q35.1 [9]. The analysis of patients identified four different intragenic mutations, and one complete deletion of SH3PXD2B [9]. A novel mutation in FTHS patients has also been described caused by the deletion of exon 13 of the SH3PXD2B gene [10]. Recently, two new homozygous loss-of-function mutations have been identified in the SH3PXD2B gene in patients with Borrone dermato-cardio-skeletal syndrome (BDSC syndrome) which is related to the FTHS [11]. SH3PXD2B null mice appear to share many of the skeletal, craniofacial, cardiac and ocular defects described in FTHS, supporting the link between this gene and the syndrome [9]. Interestingly, studying the spontaneous mouse mutant nee has revealed that those mice also exhibited runted growth, craniofacial and skeletal abnormalities, ocular anterior segment dysgenesis, and hearing impairment, similar to SH3PXD2B null mice [12]. Using genetic mapping and DNA sequencing, the cause of nee phenotypes was identified as a 1 bp deletion within the SH3PXD2B gene on mouse Chromosome 11 which causes a frameshift and a protein truncation altering a portion of the third SH3 domain and deleting all of the fourth SH3 domain [12].
The protein product of the SH3PXD2B gene is known as Tks4/HOFI/SH3PXD2B/fad49 (tyrosine kinase substrate with four SH3 domains/homolog of FISH/SH3 and PX domain-containing protein 2B/factor for adipocyte differentiation 49, hereafter termed Tks4). Tks4 has emerged as a candidate scaffold molecule that has the capability to regulate the actin cytoskeleton via Src and EGFR [13–15]. In addition, Tks4 was shown to play an important role in the formation of functional podosomes [16], production of reactive oxygen species (ROS) by tumor cells [17–19], and in the differentiation of white adipose tissue [20]. In 2010, Iqbal and colleagues have identified a family with FTHS whose SH3PXD2B gene contains a substitution mutation which results in the change of the conserved arginine 43 to tryptophan in the PX domain [9]. We have recently demonstrated that the R43W mutation seriously impairs the cellular expression and the function of Tks4 [14].
In the present study we further characterized the mutant Tks4R43W protein in COS7 cells. Here we show that mutant Tks4 is very likely misfolded since it is seen in the detergent-insoluble fraction of cell extracts. In addition, in some cells Tks4R43W colocalizes with microtubules and the perinuclear Tks4 aggregate formation depends on the intact microtubule network. The misfolded Tks4 mutant also colocalizes with the MTOC and its aggregate is encaged by the intermediate filament protein vimentin. Finally, pretreatment of cells with the proteasome inhibitor MG132 markedly increases the levels of aggresomes formed by Tks4R43W. Our results therefore suggest that the misfolded FTHS protein Tks4R43W is transported via the microtubular system to aggresomes localized at the juxtanuclear region.
Results
Tks4R43W displays characteristics of misfolding
In 2010, Iqbal and colleagues have identified a patient with FTHS whose SH3PXD2B gene contains a substitution mutation which results in the change of the conserved arginine 43 to tryptophan in the PX domain. Interestingly, the symptoms of this particular patient were indistinguishable of those who had more severe mutations leading to complete loss of Tks4 protein synthesis [9]. This finding suggests that the Tks4R43W protein is likely damaged or misfolded, leading to reduced intracellular protein concentration. We have previously expressed Tks4R43W in COS7 cells and found that the expression of the mutant protein was significantly decreased in cell lysates, it formed aggregates and accumulated around the nucleus [14]. In this study Tks4R43W protein was further characterized concerning its intracellular behavior. Damaged or misfolded proteins in aggresomes are often insoluble in non-denaturing detergents [4]. Therefore, we investigated the solubility of V5-tagged mutant Tks4 by sequential extraction into detergent-soluble and insoluble fractions. As shown in Fig. 1, wild type Tks4 was found predominantly in the detergent soluble fraction. However, Tks4R43W was sedimented almost exclusively in the detergent-insoluble pellet fraction. To exclude experimental artifact, the solubility of a well-characterized cytosolic protein was also determined. The E3 ubiquitin-protein ligase Cbl was detected completely in the soluble fractions (Fig. 1, [21, 22]). In addition, a marker of the pellet fraction (caveolin) and an aggresome marker (HDAC6) were also tested.
Fig. 1.
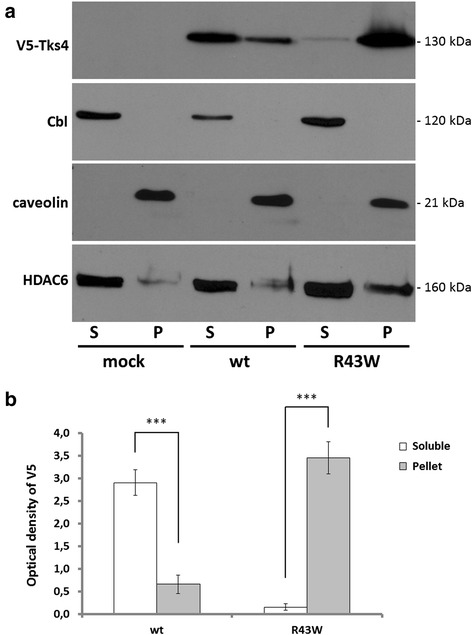
The Tks4R43W mutant protein is present in detergent-insoluble pellets. 1a, COS7 cells were transiently transfected with wild type V5-Tks4 or V5-Tks4R43W constructs. After 18 h the cells were lysed, and the lysates were separated into detergent-soluble (S) and insoluble pellet fractions (P) by centrifugation and analyzed by Western blotting for Tks4 (with anti-V5 antibody), Cbl, caveolin-1 and HDAC6, respectively. 1b, Wild type and mutant Tks4 protein levels were determined by densitometry. Error bars represent the standard deviation from three independent experiments. Asterisks indicate the level of significance (one asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001)
Different localization patterns of Tks4R43W protein in COS7 cells
We have recently demonstrated that the R43W mutant Tks4 aggregates in cells and the aggregate localizes at the juxtanuclear region [14]. However, only a part of Tks4R43W-expressing cells showed this localization pattern. Therefore, we transiently expressed the V5-tagged mutant Tks4 in cells, stained them with anti-V5 antibody, and categorized them based on the localization patterns of Tks4R43W. We can distinguish the following localization patterns: a, cells with uniform cytoplasmic distribution (Fig. 2a/e), b, cells with aggresomes (Fig. 2a/f), c, cells with filamentous staining (Fig. 2a/g), finally, d, cells with aggresomes and filamentous anti-V5 staining together (Fig. 2a/h). To calculate the percentage of cells showing different Tks4R43W localization, cells with V5-Tks4 immunreactivity were scrutinized under laser confocal microscope. We found that approximately 35 % of cells showed uniform cytoplasmic distribution, while the percentage of cells containing aggressomes was around 32 %. The percentage of cells with filamentous staining only or with aggresomes was significantly lower, approximately 18 and 15 %, respectively (Fig. 2b). Taken together, it seems that approximately half of the cells expressing Tks4R43W contain aggresomes. To ensure that we can detect Tks4R43W in the aggresomes, HDAC6, a marker of aggresomes, was also stained (Fig. 2c).
Fig. 2.
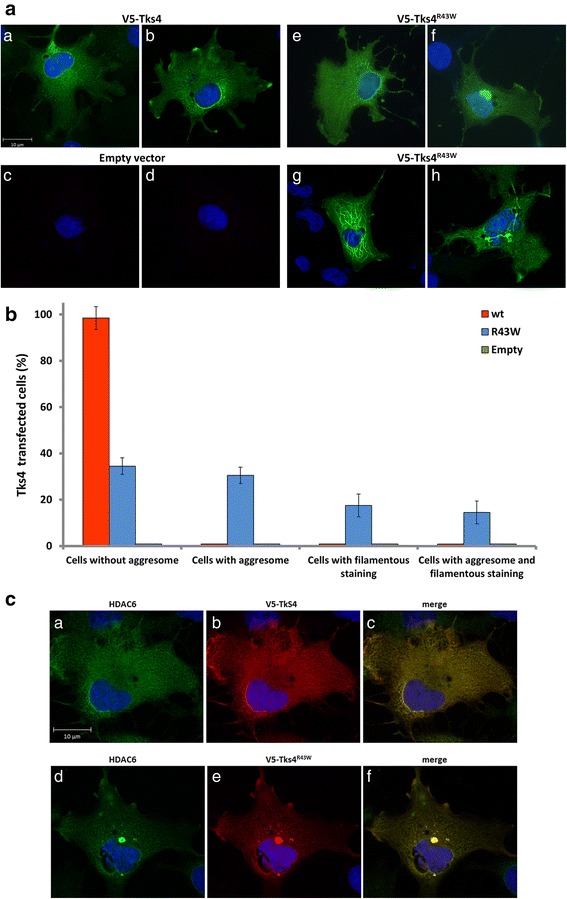
Different localization patterns of Tks4 and Tks4R43W proteins in cells. 2a, COS7 cells were transiently transfected with V5-Tks4, V5-Tks4R43W or V5-empty constructs. After 18 h, cells were fixed and stained for Tks4 with anti-V5 antibody. 2b, The percentage of aggresomes in cells was quantified by counting 300 cells/sample by using ImageJ software. Error bars represent the standard deviation from three independent experiments. 2c, Tks4R43W aggresomes colocalize with the HDAC6 aggresome marker. COS7 cells were transiently transfected with V5-Tks4 or V5-Tks4R43W constructs. After 18 h, cells were fixed and stained stained for Tks4 (with anti-V5 antibody, red) and HDAC6 (green)
The microtubule network delivers Tks4 R43W to aggresomes
Damaged or misfolded proteins are delivered via an HDAC6-dependent, microtubule-based retrograde transport toward the microtubule-organizing center (MTOC) into aggresomes [6]. Using anti-alfa-tubulin fluorescent staining we have shown that, in those cells where the mutant Tks4 shows filamentous staining, Tks4R43W partially colocalizes with the microtubule network, while the wild type protein shows a uniform cytoplasmic distribution (Fig. 3).
Fig. 3.
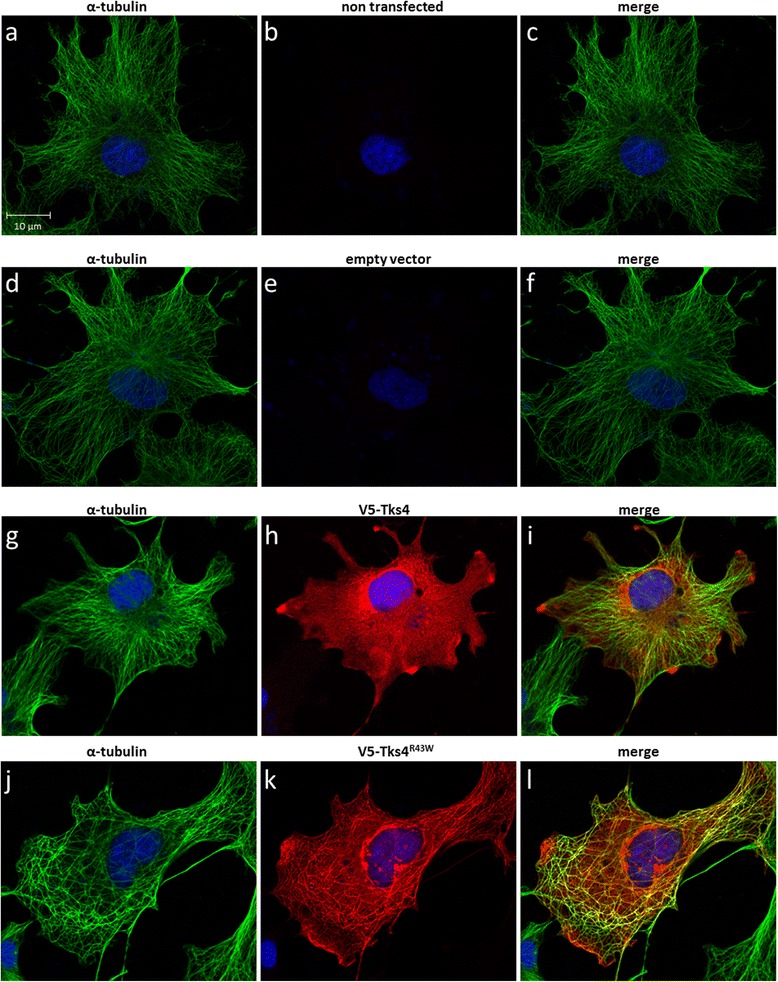
The Tks4R43W mutant protein colocalizes with the microtubule network of the cytoskeleton. COS7 cells were transiently transfected with wild type V5-Tks4 (3g, h, i), V5-Tks4R43W (3j, k, l) or V5-empty (3d, e, f) constructs. Non-transfected cells are also shown (3a, b,c). After 18 h, cells were fixed and stained for Tks4 (with anti-V5 antibody, red, 3b, e, j, k) and α-tubulin (green, 3a, d, g, j). Merged pictures are also shown (3c, f, i, l). Cell nuclei were visualized by DAPI staining. The scale bar represents 10 μm
In order to show that the transport of Tks4R43W is indeed microtubule dependent, we pretreated COS7 cells with 1.6 μM of the microtubule depolymerizing drug nocodazole for 16 h, which leads to destabilization of the microtubule network (Fig. 4a). In the presence of nocodazole, aggresome formation in Tks4R43W-expressing cells was largely inhibited (Fig. 4a/j-l, 4b). Interestingly, in those cells protein aggregates of the mutant Tks4 showed punctate structures throughout the cell, reflecting that without active microtubule network small aggregates of Tks4R43W cannot be delivered to the MTOC and assembled to large cytoplasmic inclusions. To prove the role of microtubules in the transport of Tks4R43W in another way, cells expressing Tks4R43W were treated with the histone deacetylase inhibitor Trichostatin A (TSA). TSA is a well-known inhibitor of HDAC6 that yields hyper-acetylated tubulin. It was found that hyper-acetylated microtubules observed after TSA treatment exhibited delayed depolymerization [23–25]. Figure 4c and Fig. 4d demonstrate that inhibition of HDAC6 in Tks4R43W–expressing cells prevented aggresome formation. Interestingly, misfolded Tks4 protein shows very similar staining in these cells as seen in cells treated with the microtubule depolymerizing drug nocodazole.
Fig. 4.
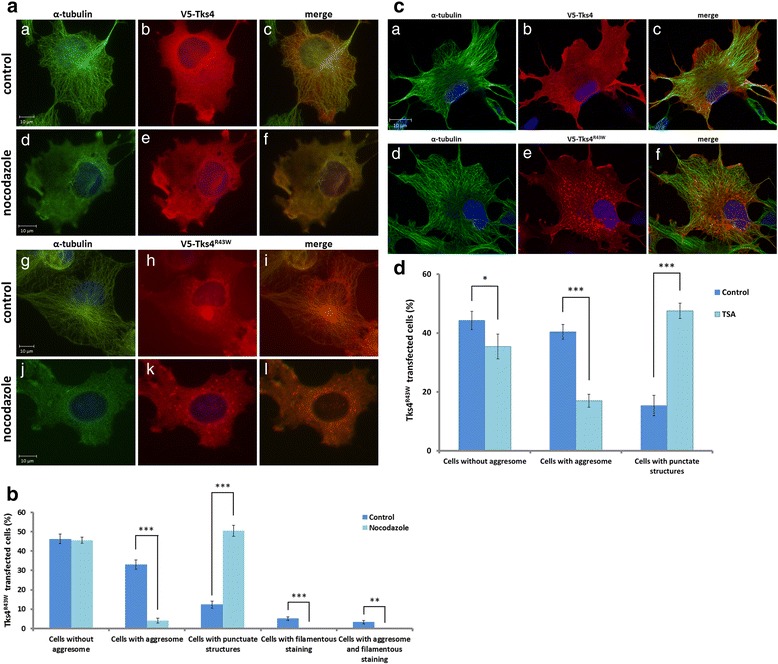
Perinuclear Tks4R43W aggregate formation is microtubule and HDAC6-dependent. 4a, COS7 cells were transiently transfected with wild type V5-Tks4 or V5-Tks4R43W constructs. After 4 h, the cells were treated with 1,6 μM nocodazole for 16 h. The cells were fixed and stained for Tks4 (with anti-V5 antibody, red) and α-tubulin (green). 4b, The percentage of aggresomes and punctate structures in cells was quantified by counting 300 cells/sample by using ImageJ software. 4c, COS7 cells were transiently transfected with wild type V5-Tks4 or V5-Tks4R43W constructs. After 4 h, the cells were treated with 1 μM Trichostatin A (TSA) for 16 h. The cells were fixed and stained for Tks4 (with anti-V5 antibody, red) and α-tubulin (green). 4d, The percentage of aggresomes and punctate structures in cells was quantified by counting 300 cells/sample by using ImageJ software. Error bars represent the standard deviation from three independent experiments. Asterisks indicate the level of significance (one asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001)
The aggresome formed by Tks4R43W colocalizes with the centrosome
As described above, aggresomes are typically formed at the MTOC at the juxtanuclear region. MTOC or centrosome contains several specific proteins responsible for microtubule nucleation and anchoring, including γ-tubulin, pericentrin, ninein and centrins [26]. To prove that Tks4R43W aggregates are accumulated at the MTOC, the centrosome marker protein centrin2 was fused to GFP and was co-expressed with the wild type Tks4 or Tks4R43W in COS7 cells. As demonstrated in Fig. 5, aggresome formed by mutant Tks4 showed clear colocalization with centrosome marker protein centrin2.
Fig. 5.
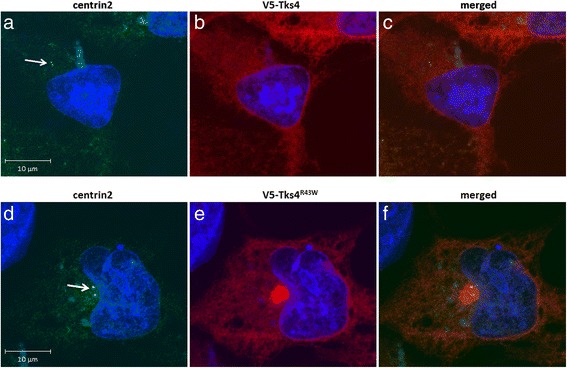
Aggresomes formed by Tks4R43W mutant colocalizes with the centrosome. COS7 cells were transiently transfected with hCent2-pEGFP-C1 and V5-Tks4 (5a, b, c) or hCent2-pEGFP-C1 and V5-Tks4R43W (5d, e, f) constructs. After 18 h, cells were fixed and stained for Tks4 with anti-V5 antibody (red). Cell nuclei were visualized by DAPI staining. The arrow indicates the localization of centrin2 present at the juxtanuclear region. The scale bar represents 10 μm
The Tks4R43W aggresome is encaged by vimentin
One of the most characteristic features of aggresomes is the localization of the intermediate filament protein vimentin which changes its normal fibrillar distribution to form a cage surrounding the aggregates [3, 4]. To further verify that the observed pericentriolar Tks4R43W aggregate corresponds to aggresomes, subcellular localization of vimentin was examined in cell expressing wild type and mutant Tks4. In cells expressing wild type Tks4, vimentin distribution was filamentous and dispersed throughout the cell (Fig. 6a and c). However, in cells transfected with TKS4R43W vimentin filaments were seen to form a ring-like structure around the aggregated proteins in the juxtanuclear region (Fig. 6d and f).
Fig. 6.
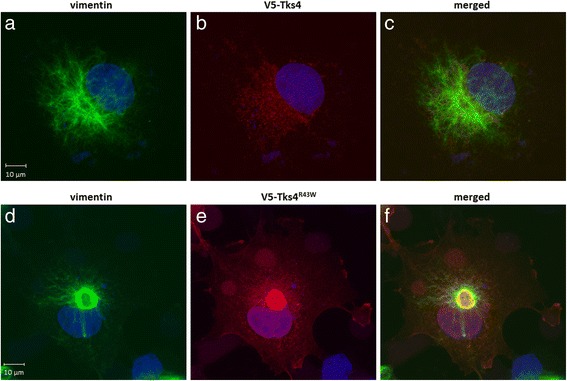
Tks4R43W aggresome is encaged by vimentin. COS7 cells were transiently transfected with wild type V5-Tks4 (6a, b, c) or V5-Tks4R43W (6d, e, f) constructs. After 18 h, cells were fixed and stained for Tks4 (with anti-V5 antibody, red) and vimentin (green). Cell nuclei were visualized by DAPI staining. The scale bar represents 10 μm
The proteasome inhibitor MG132 increases the level of aggresome formation by Tks4R43W
In our previous experiments we have shown that Tks4R43W is transported to the centrosome where it can be sequestered in aggresomes. Nevertheless, other mechanisms, such as proteasomal degradation may also contribute to the clearance of this misfolded protein. To test if the proteasomal system significantly contributes to the elimination of Tks4R43W, we challenged the cells with the cell-permeable proteasome inhibitor MG132. Figure 7a and b show that pretreatment of the cells with 5 μM MG132 for 20 h significantly increased the percentage of cells with perinuclear aggregates. To see the effect of MG132 on protein levels too, cells treated with the inhibitor were separated into detergent soluble and insoluble fractions, as performed in Fig. 1. Figure 7c and d demonstrates that the protein levels present in the pellet fractions of MG132-treated cells were significantly increased in cells expressing the mutant Tks4 proteins. These findings suggest that the ubiquitin-proteasome system also contributes to the elimination of the mutant Tks4 proteins.
Fig. 7.
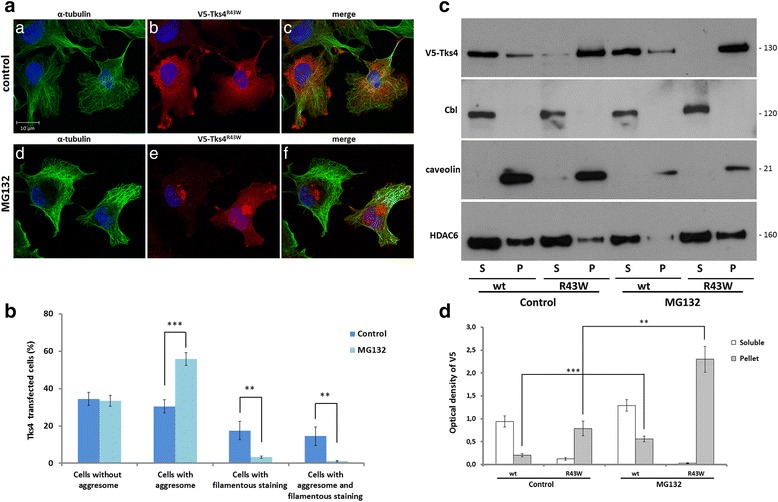
The proteasome inhibitor MG132 increases the level of aggresome formation by the Tks4R43W mutant. 7a, COS7 cells were transiently transfected with a V5-Tks4 and V5-Tks4R43W construct. After 18 h, the cells were treated with 5 μM MG132 for 20 h. The cells were fixed and stained for Tks4 (with anti-V5 antibody, red) and α-tubulin (green). Cell nuclei were visualized by DAPI staining. 7b, The percentage of cells with aggresomes was quantified by counting 300 cells/sample by using the ImageJ software. 7c, COS7 cells were transiently transfected with V5-Tks4 and V5-Tks4R43W construct. After 18 h the cells were treated with 5 μM MG132 for 20 h or left untreated. Cells were then lysed, and the lysates were separated into detergent-soluble (S) and insoluble pellet fractions (P) by centrifugation and analyzed by Western blotting for Tks4 (with anti-V5 antibody), Cbl, caveolin-1 and HDAC6, respectively. 7d, The Tks4 protein levels were determined by densitometry. Error bars represent the standard deviation from three independent experiments. Asterisks indicate the level of significance (one asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001)
Other mutations of the FTHS patients also result in aberrant expression of Tks4 proteins
In addition to the R43W mutation, Iqbal et al. described two further mutations in the SH3PXD2B gene of FTHS patients. A homozygous insertion c.147insT was detected which predicted the creation of an immediate stop in the same codon resulting a 48 amino acid long protein (Tks41–48). In other patients, a homozygous 1 bp deletion c.969delG was identified which predicted a frameshift followed by a premature stop codon (p.G323fsX19). The expected protein possesses 341 amino acids (Tks41–341) and the truncation occurs after the second SH3 domain in the structure of Tks4 [9]. To check the expression and the intracellular localization of these mutants, cDNA constructs were generated as described in the Methods. V5- Tks41–48 and V5- Tks41–341 were transiently expressed in COS7 cells, protein lysates were then subjected to SDS-PAGE and immunoblot with an anti-V5 monoclonal antibody. As demonstrated in Fig. 8a, wild type Tks4 and Tks4R43W can be detected with an apparent molecular weight of 140 kDa. It is worth to note that due to the over-expression of the proteins some degradation can be seen at lower molecular weights. Interestingly, the shorter Tks4 mutant, Tks41–48, shows no expression at all. Finally, expression of the Tks41–341 can be detected with an approximate molecular weight of 45 kDa. Next, we transiently expressed the V5-tagged Tks4 proteins in cells. As Fig. 8b demonstrates, a portion of Tks4R43W localizes at the juxtanuclear region in the aggresome, as described above (Fig. 2a). Tks41–48, as expected, did not express in the cell. Finally, Tks41–341 shows an interesting picture accumulating in the nuclei of the cells. Taken together, while Tks41–48 shows no expression in the cells, the other two mutants, Tks4R43W and Tks41–341, show abnormal intracellular localizations.
Fig. 8.
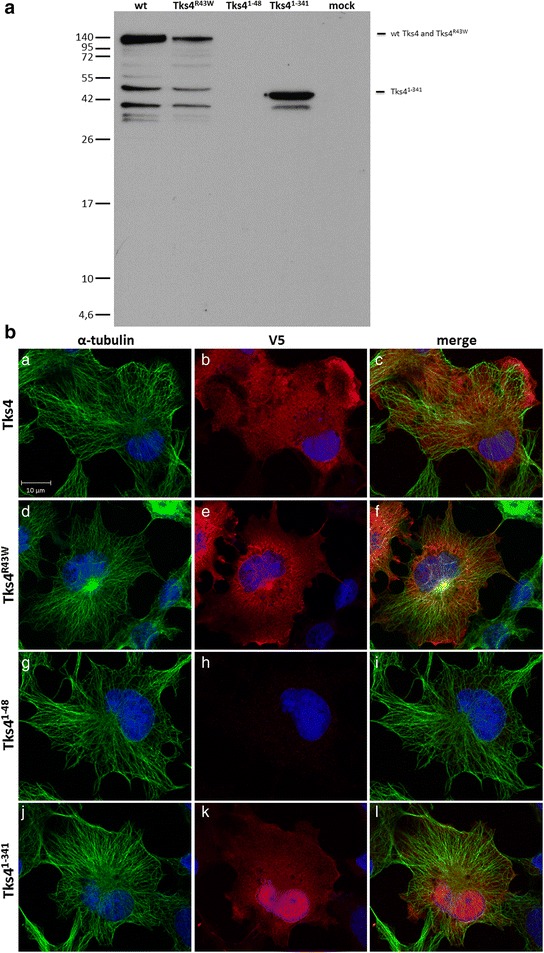
The expression patterns of Tks4 mutant proteins. 8a, COS7 cells were transiently transfected with V5-Tks4, V5-Tks4R43W, V5-Tks41–48 and V5-Tks41–341 constructs. After 18 h the cells were lysed, the lysates were separated by centrifugation and the supernatant analyzed by Western blotting for Tks4 with anti-V5 antibody. 8b, COS7 cells were transiently transfected with V5-Tks4, V5-Tks4R43W, V5-Tks41–48 and V5-Tks41–341 constructs. After 18 h, cells were fixed and stained for Tks4 (with anti-V5 antibody, red) and α-tubulin (green). Cell nuclei were visualized by DAPI staining. The scale bar represents 10 μm
Discussion
FTHS is an autosomal-recessive disorder characterized by abnormalities that affect bone, heart, and eye development. Patients usually die in infancy or in early childhood because of the cardiovascular anomalies, respiratory infections or unknown causes [9]. Iqbal and colleagues have investigated several families with FTHS and revealed five different homozygous mutations in the SH3PXD2B gene encoding for Tks4 [9]. One particularly interesting mutation of them is the change of the highly conserved arginine 43 to tryptophan within the PX domain of Tks4 [9]. We have recently demonstrated that the R43W mutation seriously impairs the lipid-binding of the PX domain and the cellular expression of Tks4 [14].
In the present study we have further characterized the mutant Tks4R43W protein and studied the mechanism by which the misfolded Tks4 is sequestered. We prove here that the Tks4R43W protein is transported via the microtubule network to aggresomes. This is based on the following findings: a, Tks4R43W shows partial colocalization with the microtubule network; b, both nocodazole, a microtubule-depolymerizing drug, and Trichostatin A, a histone deacetylase inhibitor, inhibit aggresome formation in cells expressing the mutant Tks4; c, Tks4R43W shows colocalization with the centrosome-specific centrin2; d, finally, the intermediate filament protein vimentin forms a ring-like structure around the Tks4R43W aggregates.
Much evidence has accumulated to indicate that the aggresome represents a special protective response to proteotoxic stresses [1, 2, 4, 27]. However, it is not clear if aggresome formation is an independent process or it is activated only when the degradative capacity of the ubiquitin-proteasome system is overwhelmed. Our data based on the characteristics of the Tks4R43W favor the second alternative. Pretreatment of cells with the cell-permeable proteasome inhibitor MG132 resulted in the dramatic increase in the number of Tks4R43W expressing cells with perinuclear aggregates. Therefore, it is likely that mutant Tks4 is also eliminated by the ubiquitin-proteasome system. However, immunoblotting of the immunopericipated Tks4R43W with specific anti-ubiquitin antibody did not detect the ubiquitination of the mutant Tks4 (data not shown). Further experiment will therefore be required to establish the precise mechanism by which the proteasome system eliminates the misfolded Tks4.
Finally, we have constructed two other mutant Tks4 proteins which were predicted earlier by studying FTHS patients [9]. We wished to investigate how they express in cells and what their intracellular localization is. The V5-tagged, 48 amino acid long protein (Tks41–48) showed no expression at all in cells studied either by immunoblot or by confocal microscopy. However, when the V5-tagged, 341 amino acid long truncation mutant (Tks41–341) were transiently expressed in COS7 cell, and cell lysates were prepared and subjected to anti-V5 immunoblot, a specific band was detected with an approximate molecular weight of 45 kDa. Interestingly, Tks41–341 was localized primarily in the nuclei of the cells. The expression levels of the shorter truncation mutant Tks4 (c.147insT, Tks41–48) were analyzed earlier by immunoblot of total cell lysates of human primary dermal fibroblasts isolated from FTHS patient. While Tks4 signal was detected in control fibroblasts, no signal was seen in cells of FTHS patients [9]. Our finding using transiently over-expressed cell system is in agreement with the above finding, namely that we could also not detected the expression of Tks41–48 protein in cells. Interestingly, Iqbal et al. detected normal levels of SH3PXD2B transcript in FTHS families with the c.147insT (F49X) mutation, excluding that the premature stop codon introduced by this mutation results in nonsense-mediated RNA decay. They guessed that the truncation resulted in an unstable protein [9]. Taken together, lack of expression of Tks41–48 or aberrant intracellular expressions of Tks4R43W and Tks41–341 strongly suggest that these mutations result in dysfunctional proteins which are not capable of operating properly, leading to the development of FTHS.
It has been well established that both Tks4 and Tks5 play important roles in the regulation and formation of podosomes [16, 28, 29]. Podosomes represent transient subcellular structures that are actin-rich membrane protrusions found on the ventral surface of the cells facing the extracellular matrix. The migration of normal cells is driven by podosomes when these specialized structures allow the cells to adhere and invade in the surroundings [29]. Since podosomes are necessary for the adhesion and migration of a variety of cell types, including macrophages, dendritic cells, osteoclasts, vascular smooth muscle, and endothelial cells [9], it is likely that dysfunctional Tks4 proteins are implicated in the development of FTHS phenotype.
Conclusions
In summary, we show that the mutant FTHS protein Tks4R43W is found in the detergent-insoluble cell fractions. The misfolded protein seems to be transported to the aggresomes via the microtubule system. Lack of expression of Tks41–48 or aberrant intracellular expressions of Tks4R43W and Tks41–341 strongly suggest that these mutations result in dysfunctional proteins which are not capable of operating properly, leading to the development of FTHS.
Methods
Antibodies, constructs and reagents
Monoclonal and polyclonal antibodies against the V5 epitope (R96025 and AB3792) were purchased from Invitrogen (Carlsbad, CA, USA) and Millipore (Billerica, MA, USA), respectively. Antibody against α-tubulin (DM1A) and vimentin (V6630) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibody against Cbl (sc-170) was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Antibody against HDAC6 (7558) and caveolin-1 (3267) were obtained from Cell Signaling Technology (Danvers, MA, USA). The anti-rabbit and anti-mouse HRP-linked antibodies (NA934V, NA931V) were purchased from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom). Alexa Fluor 488 anti-rabbit (A11034), Alexa Fluor 488 anti-mouse (A11029), Alexa Fluor 546 anti-rabbit (A11035), and Alexa Fluor 546 anti-mouse (A11030) antibodies were purchased from Invitrogen (Carlsbad, CA, USA). The generation of V5 epitope tagged Tks4 and the V5-Tks4R43W mutant was described previously [13, 14]. To generate the V5-tagged Tks41–48 and Tks41–341 proteins [9], the shorter Tks4 coding sequences was amplified by PCR and subcloned into BamHI/XbaI sites of pcDNA3.1/TOPO-V5-His plasmid (Life Technologies, Carlsbad, CA, USA). The single guanine change was introduced into the c.969delG deletion mutant-V5 construct (V5- Tks41–341) using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies). The constructs were verified by sequencing. The human centrin2 containing hCent2-pEGFP-C1 construct was a kind gift of Jeffrey L. Salisbury (Tulane University, USA). Stock solutions of MG132 (474790, Calbiochem), nocodazole (M1404, Sigma-Aldrich) and Trichostatin A (T8552, Sigma-Aldrich) were prepared according to the manufacturer’s instructions. Pfu DNA polymerase (EP0501) was purchased from Thermo Scientific (Waltham, MA, USA). The BamHI (1010A) and XbaI (1093A) enzymes were purchased from Takara Bio Inc (Otsu, Shiga, Japan).
Cell lines, transfection, and inhibition
COS7 cells were purchased from American Type Culture Collection and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal calf serum (Invitrogen, Carlsbad, CA, USA), penicillin (100 units/ml), and streptomycin (100 μg/ml). All cell lines were transiently transfected with Lipofectamine (Invitrogen) according to the manufacturer’s instructions. In the experiments where indicated, transfected cells were pretreated with 5 μM MG132 for 20 h, 1.6 μM nocodazole for 16 h or 1 μM Trichostatin A for 16 h after the transfection.
Confocal microscopy
COS7 cells plated on glass cover slips were transiently transfected with different constructs as indicated and then incubated for 4 or 18 h. When indicated 5 μM MG132 were added 18 h after transfection for 20 h. In other experiments cells were treated with 1.6 μM nocodazole or with 1 μM Trichostatin A for 16 h starting 4 h after transfection. The cells were fixed in 4 % paraformaldehyde-PBS for 15 min or in −20 °C methanol for 5 min (in centrosome colocalization experiment), permeabilized in 0.2 % Triton X-100 in PBS for 5 min, and blocked with 1 % BSA in PBS for 20 min. Anti-V5 polyclonal rabbit and anti-α-tubulin antibody was applied in 1:1000 dilution for 30 min. After washing with PBS the samples were incubated with Alexa Fluor 488 (or 546) labeled anti-mouse or anti-rabbit secondary antibody for 30 min in 1:1000 dilution. After 30 min of washing with PBS cover slips were mounted onto slides in a 100 mM Tris–HCl buffer, pH 8.5, containing 10 % Mowiol 4–88 (Calbiochem), 25 % glycerol, and 2.5 % 1,4-diazobicyclo- [2.2.2]octane (DABCO, Sigma-Aldrich, St. Louis, MO, USA). The pictures of fixed samples were acquired on a Zeiss LSM710 inverted confocal microscope with 63X objective (Carl Zeiss, Jena, Germany). To minimize the cross-talk between imaged channels, sequential image collection was used. Cells are shown as single confocal section. All images were processed using ZEN software (Carl Zeiss, Jena, Germany).
Characterization of aggregates and western blotting
The sequential extraction of aggregates was performed according to [30]. Equal volumes of protein extracts from each fraction (supernatant, pellet) were subjected to SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). Membranes were then blocked with 5 % skim milk powder, incubated with monoclonal anti-V5, Cbl, caveolin-1 or HDAC6 primary antibodies, and horseradish peroxidase-conjugated secondary antibodies, and developed using ECL detection reagents (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Densitometry on scanned images was done using the ImageJ 1.46x software.
Statistics
The number of aggresomes was quantified by counting 300 cells/sample by using ImageJ software. Quantitative results are presented as mean and s.d. of at least three independent experiments. Statistical differences between two groups of data were analyzed by Student’s t-test.
Acknowledgements
We are grateful to Jeffrey L. Salisbury (Department of Biochemistry and Molecular Biology, Mayo Clinic College of Medicine, Rochester, Minnesota, Tulane University, USA) for providing the human centrin2 containing hCent2 pEGFP-C1 construct. The work was supported by a grant from the Hungarian Research Fund OTKA (K 83867), the MedinProt program of the Hungarian Academy of Sciences, and the “Lendület” grant from the Hungarian Academy of Sciences (L.B.).
Abbreviations
- Tks4
Tyrosine kinase substrate with four SH3 domains
- HDAC6
Histone deacetylase 6
- MTOC
Microtubule-organizing center
- TSA
Trichostatin A
- GFP
Green fluorescent protein
- PX domain
Phox homology domain
- SH3 domain
Src homology domain
Footnotes
Csaba Ádám and Anna Fekete contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CA, AF, GB, and ZN performed and designed the experiments. NT took the pictures with the confocal microscope. JO and MG provided several reagents. SP helped to maintain the cells. LB, the corresponding author, supervised the experiments with LK and wrote the manuscript with the help of CA. All authors read and approved the final manuscript.
Contributor Information
Csaba Ádám, Email: buday.laszlo@ttk.mta.hu.
Anna Fekete, Email: buday.laszlo@ttk.mta.hu.
Gábor Bőgel, Email: buday.laszlo@ttk.mta.hu.
Zsuzsanna Németh, Email: buday.laszlo@ttk.mta.hu.
Natália Tőkési, Email: buday.laszlo@ttk.mta.hu.
Judit Ovádi, Email: buday.laszlo@ttk.mta.hu.
Károly Liliom, Email: buday.laszlo@ttk.mta.hu.
Szabolcs Pesti, Email: buday.laszlo@ttk.mta.hu.
Miklós Geiszt, Email: buday.laszlo@ttk.mta.hu.
László Buday, Email: buday.laszlo@ttk.mta.hu.
References
- 1.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 2.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–6. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Mata R, Gao YS, Sztul E. Hassles with taking out the garbage: aggravating aggresomes. Traffic. 2002;3:388–96. doi: 10.1034/j.1600-0854.2002.30602.x. [DOI] [PubMed] [Google Scholar]
- 4.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–98. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Gonzalez A, Lin T, Ikeda AK, Simms-Waldrip T, Fu C, Sakamoto KM. Role of the aggresome pathway in cancer: targeting histone deacetylase 6-dependent protein degradation. Cancer Res. 2008;68:2557–60. [DOI] [PubMed]
- 6.Chin LS, Olzmann JA, Li L. Parkin-mediated ubiquitin signalling in aggresome formation and autophagy. Biochem Soc Trans. 2010;38:144–9. doi: 10.1042/BST0380144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–66. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 8.Maas SM, Kayserili H, Lam J, Apak MY, Hennekam RC. Further delineation of Frank-ter Haar syndrome. Am J Med Genet A. 2004;131:127–33. doi: 10.1002/ajmg.a.30244. [DOI] [PubMed] [Google Scholar]
- 9.Iqbal Z, Cejudo-Martin P, de Brouwer A, van der Zwaag B, Ruiz-Lozano P, Scimia MC, et al. Disruption of the podosome adaptor protein TKS4 (SH3PXD2B) causes the skeletal dysplasia, eye, and cardiac abnormalities of Frank-Ter Haar Syndrome. Am J Hum Genet. 2010;86:254–61. [DOI] [PMC free article] [PubMed]
- 10.Bendon CL, Fenwick AL, Hurst JA, Nurnberg G, Nurnberg P, Wall SA, et al. Frank-ter Haar syndrome associated with sagittal craniosynostosis and raised intracranial pressure. BMC Med Genet. 2012;13:104. [DOI] [PMC free article] [PubMed]
- 11.Wilson GR, Sunley J, Smith KR, Pope K, Bromhead CJ, Fitzpatrick E, et al. Mutations in SH3PXD2B cause Borrone dermato-cardio-skeletal syndrome. Eur J Hum Genet. 2013 [DOI] [PMC free article] [PubMed]
- 12.Mao M, Thedens DR, Chang B, Harris BS, Zheng QY, Johnson KR, et al. The podosomal-adaptor protein SH3PXD2B is essential for normal postnatal development. Mamm Genome. 2009;20:462–75. [DOI] [PMC free article] [PubMed]
- 13.Lanyi A, Barath M, Peterfi Z, Bogel G, Orient A, Simon T, et al. The Homolog of the Five SH3-Domain Protein (HOFI/SH3PXD2B) Regulates Lamellipodia Formation and Cell Spreading. PLoS One. 2011;6:e23653. [DOI] [PMC free article] [PubMed]
- 14.Bogel G, Gujdar A, Geiszt M, Lanyi A, Fekete A, Sipeki S, et al. Frank-ter Haar syndrome protein Tks4 regulates epidermal growth factor-dependent cell migration. J Biol Chem. 2012;287:31321–9. [DOI] [PMC free article] [PubMed]
- 15.Fekete A, BG G, Pesti S, Peterfi Z, Geiszt M, Buday L. EGF regulates tyrosine phosphorylation and membrane-translocation of the scaffold protein Tks5. J Mol Signal. 2013;8:8. [DOI] [PMC free article] [PubMed]
- 16.Buschman MD, Bromann PA, Cejudo-Martin P, Wen F, Pass I, Courtneidge SA. The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol Biol Cell. 2009;20:1302–11. [DOI] [PMC free article] [PubMed]
- 17.Gianni D, Diaz B, Taulet N, Fowler B, Courtneidge SA, Bokoch GM. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal 2009;2:ra54. [DOI] [PMC free article] [PubMed]
- 18.Gianni D, Taulet N, DerMardirossian C, Bokoch GM. c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol Biol Cell. 2010;21:4287–98. doi: 10.1091/mbc.E10-08-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianni D, DerMardirossian C, Bokoch GM. Direct interaction between Tks proteins and the N-terminal proline-rich region (PRR) of NoxA1 mediates Nox1-dependent ROS generation. Eur J Cell Biol. 2011;90:164–71. doi: 10.1016/j.ejcb.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hishida T, Eguchi T, Osada S, Nishizuka M, Imagawa M. A novel gene, fad49, plays a crucial role in the immediate early stage of adipocyte differentiation via involvement in mitotic clonal expansion. Febs J. 2008;275:5576–88. doi: 10.1111/j.1742-4658.2008.06682.x. [DOI] [PubMed] [Google Scholar]
- 21.Buday L, Khwaja A, Sipeki S, Farago A, Downward J. Interactions of Cbl with two adapter proteins, Grb2 and Crk, upon T cell activation. J Biol Chem. 1996;271:6159–63. doi: 10.1074/jbc.271.11.6159. [DOI] [PubMed] [Google Scholar]
- 22.Mohapatra B, Ahmad G, Nadeau S, Zutshi N, An W, Scheffe S, et al. Protein tyrosine kinase regulation by ubiquitination: critical roles of Cbl-family ubiquitin ligases. Biochim Biophys Acta. 2013;1833:122–39. [DOI] [PMC free article] [PubMed]
- 23.Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–31. [DOI] [PMC free article] [PubMed]
- 24.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–8. [DOI] [PubMed]
- 25.Tran AD, Marmo TP, Salam AA, Che S, Finkelstein E, Kabarriti R, et al. HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J Cell Sci. 2007;120:1469–79. [DOI] [PubMed]
- 26.Rieder CL, Faruki S, Khodjakov A. The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol. 2001;11:413–9. doi: 10.1016/S0962-8924(01)02085-2. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Mata R, Bebok Z, Sorscher EJ, Sztul ES. Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J Cell Biol. 1999;146:1239–54. doi: 10.1083/jcb.146.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seals DF, Azucena Jr EF, Pass I, Tesfay L, Gordon R, Woodrow M, et al. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–65. [DOI] [PubMed]
- 29.Courtneidge SA. Cell migration and invasion in human disease: the Tks adaptor proteins. Biochem Soc Trans. 2012;40:129–32. doi: 10.1042/BST20110685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arslan MA, Chikina M, Csermely P, Soti C. Misfolded proteins inhibit proliferation and promote stress-induced death in SV40-transformed mammalian cells. FASEB J. 2012;26:766–77. doi: 10.1096/fj.11-186197. [DOI] [PubMed] [Google Scholar]


