Abstract
The steroid receptor-associated immunophilins FKBP51, FKBP52, CyP40 and PP5 have specific roles in steroid receptor function that impact steroid hormone-binding affinity, nucleocytoplasmic shuttling and transcriptional activation of target genes in a tissue-specific manner. Aberrant expression of these functionally unique immunophilins has the potential to cause steroid-based diseases, including breast and prostate cancer, diabetes and related metabolic disorders, male and female infertility and major depressive disorders. This review addresses the function of these proteins as co-chaperones in steroid receptor-Hsp90 complexes and extensively covers current knowledge of the link between the steroid receptor-associated immunophilins and human disease. An improved understanding of their mechanisms of action has revealed opportunities for molecular therapies to enhance or inhibit cellular processes under immunophilin control that contribute both to human health and disease.
Introduction
The receptors for the steroid hormones androgen (AR), oestrogen (ERα), glucocorticoid (GR), mineralocorticoid (MR) and progesterone (PR) belong to a sub-group of the nuclear receptor superfamily that act as ligand-regulated transcription factors to modulate the transcription of target genes.1 Cyclophilin 40 (CyP40), the FK506-binding proteins FKBP51 and FKBP52, and protein phosphatase 5 (PP5) have been identified as heat shock protein 90 (Hsp90) immunophilin co-chaperones within steroid receptor-Hsp90 heterocomplexes, where their regulatory control over receptor function has been best defined (reviewed in references 2–9). From their well-established effects on steroid receptors, these immunophilins have now emerged as potential drug targets in pathways associated with normal physiology (e.g. reproduction, gluconeogenesis, lipid metabolism and regulation of the stress response by the hypothalamic-pituitary-adrenal axis), as well as a variety of steroid-based diseases including infertility, breast and prostate cancer, depression and stress-related psychiatric disorders, insulin resistance and diabetes.
Discovery, Architecture of Functional Domains and Three-Dimensional Structures
Each of these co-chaperones contains a 3-unit tetratricopeptide repeat (TPR) structural motif connected to a domain mediating enzymatic function: peptidyl prolyl isomerase (PPIase) activity in CyP40, FKBP51 and FKBP52 and serine/threonine protein phosphatase activity in PP5 (Figure 1A). Hsp90 functions as a homodimeric ATP-dependent chaperone consisting of an N-terminal domain which contains the ATP-binding pocket and binds the co-chaperone p23, a middle domain which binds the co-chaperone activator of Hsp90 ATPase 1 (Aha1) and accommodates folding substrates such as steroid receptors, and a C-terminal dimerisation domain capped at the C-terminus by the MEEVD peptide motif which forms the docking site for TPR-containing co-chaperones, including the above immunophilins and heat shock organising protein (Hop) (Figure 2).10–12 Both Hop13–15 and p2313,16–17 inhibit Hsp90 ATPase activity, while Aha1 is a potent activator of Hsp90 ATPase function.18 A detailed model for client loading and completion of the chaperone cycle has recently been proposed in which monomeric Hop initially binds one MEEVD site to block Hsp90 ATPase activity and facilitate attachment of heat shock protein 70 (Hsp70) bound to client. PPIase co-chaperone binding to the second TPR acceptor site allows the synergistic interaction of Aha1, PPIase and ATP nucleotide to displace Hop, with Aha1 accelerating Hsp90 transition to an ATPase-competent conformation. Progression to a fully-closed intermediate then favours an exchange of p23 for Aha1 resulting in client protein maturation and its release from Hsp90 as an optimally folded protein.19 The TPR immunophilins thus have an important co-operative role with other co-chaperones which inhibit or accelerate Hsp90 ATPase activity during the chaperone reaction cycle.
Figure 1.
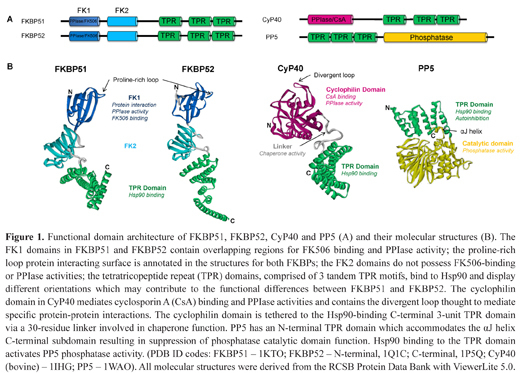
Functional domain architecture of FKBP51, FKBP52, CyP40 and PP5 (A) and their molecular structures (B). The FK1 domains in FKBP51 and FKBP52 contain overlapping regions for FK506 binding and PPIase activity; the proline-rich loop protein interacting surface is annotated in the structures for both FKBPs; the FK2 domains do not possess FK506-binding or PPIase activities; the tetratricopeptide repeat (TPR) domains, comprised of 3 tandem TPR motifs, bind to Hsp90 and display different orientations which may contribute to the functional differences between FKBP51 and FKBP52. The cyclophilin domain in CyP40 mediates cyclosporin A (CsA) binding and PPIase activities and contains the divergent loop thought to mediate specific protein-protein interactions. The cyclophilin domain is tethered to the Hsp90-binding C-terminal 3-unit TPR domain via a 30-residue linker involved in chaperone function. PP5 has an N-terminal TPR domain which accommodates the αJ helix C-terminal subdomain resulting in suppression of phosphatase catalytic domain function. Hsp90 binding to the TPR domain activates PP5 phosphatase activity. (PDB ID codes: FKBP51 – 1KTO; FKBP52 – N-terminal, 1Q1C; C-terminal, 1P5Q; CyP40 (bovine) – 1IHG; PP5 – 1WAO). All molecular structures were derived from the RCSB Protein Data Bank with ViewerLite 5.0.
Figure 2.
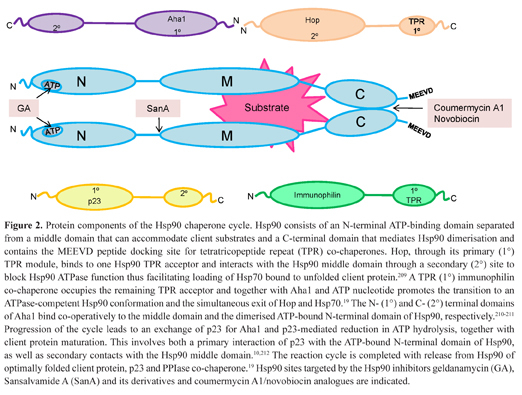
Protein components of the Hsp90 chaperone cycle. Hsp90 consists of an N-terminal ATP-binding domain separated from a middle domain that can accommodate client substrates and a C-terminal domain that mediates Hsp90 dimerisation and contains the MEEVD peptide docking site for tetratricopeptide repeat (TPR) co-chaperones. Hop, through its primary (1°) TPR module, binds to one Hsp90 TPR acceptor and interacts with the Hsp90 middle domain through a secondary (2°) site to block Hsp90 ATPase function thus facilitating loading of Hsp70 bound to unfolded client protein.209 A TPR (1°) immunophilin co-chaperone occupies the remaining TPR acceptor and together with Aha1 and ATP nucleotide promotes the transition to an ATPase-competent Hsp90 conformation and the simultaneous exit of Hop and Hsp70.19 The N- (1°) and C- (2°) terminal domains of Aha1 bind co-operatively to the middle domain and the dimerised ATP-bound N-terminal domain of Hsp90, respectively.210–211 Progression of the cycle leads to an exchange of p23 for Aha1 and p23-mediated reduction in ATP hydrolysis, together with client protein maturation. This involves both a primary interaction of p23 with the ATP-bound N-terminal domain of Hsp90, as well as secondary contacts with the Hsp90 middle domain.10,212 The reaction cycle is completed with release from Hsp90 of optimally folded client protein, p23 and PPIase co-chaperone.19 Hsp90 sites targeted by the Hsp90 inhibitors geldanamycin (GA), Sansalvamide A (SanA) and its derivatives and coumermycin A1/novobiocin analogues are indicated.
Human FKBP52, an Hsp90-associated component of receptors AR, ERα, GR and PR20 was identified as an FK506-binding PPIase by Peattie and colleagues.21 Simultaneously, purification of the intact ERα-Hsp90 complex led to the isolation of CyP40,22 belonging to the cyclophilin family of PPIases and a target for the immunosuppressant cyclosporin A (CsA).23 Sequence comparison between FKBP52 and CyP40, the first TPR-containing cyclophilin to be identified, confirmed the FKBP52 protein as a TPR immunophilin.22 FKBP51, a TPR immunophilin with high sequence similarity to FKBP52, was found by Smith and co-workers to accumulate in hormone-free PR-Hsp90 complexes.24–25 All three PPIases, FKBP51, FKBP52 and CyP40 were shown to bind to Hsp90 through their respective TPR domains.24,26–28 PP5, which displays low FK506-binding affinity,29 was shown to be present in GR-Hsp90 heterocomplexes through an interaction with Hsp90 mediated by its N-terminal TPR domain.30 A truncated version of PP5, containing only the TPR domain, behaved as a dominant negative mutant by blocking GR-mediated transactivation, thus providing the first evidence of a functional role of the TPR immunophilins in steroid receptor signalling in vivo.30 FKBP52 and PP5 displayed a predominantly nuclear localisation but were excluded from cell nucleoli, while CyP40 was initially seen to localise mainly within the nucleoli of rat pulmonary endothelial cells (reviewed in reference 31). The distribution pattern of these cochaperones varied between different cell lines. For example, in MCF-7 breast cancer cells both FKBP52 and CyP40 were localised throughout the cytoplasm, but were more strongly expressed within the nucleus and showed a distinct co-localisation within the nucleolus.32 On the other hand, CyP40 was almost exclusively located within the cytoplasm of porcine kidney LLC-PK cells.33 Accumulating evidence suggests that FKBP51 is located within the cytoplasmic compartment.34
CyP40 and the two FKBPs display a similar structural architecture, with an N-terminal PPIase domain overlapping a binding site for CsA or FK506, respectively, separated from a C-terminal 3-unit TPR domain via a flexible linker (Figure 1).35–37 The cyclophilin domain of CyP40 is similar to other single domain cyclophilins apart from a putative protein interaction loop typical of the divergent loop subclass of cyclophilins (Figure 1B).36 In FKBP51 and FKBP52, FK506 binds to the first of two FKBP-like domains, termed FK1, while the second domain, called FK2, lacks drug-binding activity. Differences in relative domain orientations are thought to contribute to the opposing influences of the two co-chaperones on steroid receptor activity (Figure 1B).37–38 Bound CsA and FK506, respectively inhibit the PPIase activity of the cyclophilin and FK1 domains, which may modulate target protein activity by direct or indirect association. The structural organisation of the full-length autoinhibited form of PP5 shows the N-terminal TPR domain linked to a C-terminal phosphatase domain followed by a short C-terminal subdomain (Figure 1B).39 In this inactive conformation, the TPR domain restricts target protein access to the catalytic site, and this structure is stabilised by the C-terminal subdomain. Suppression of catalytic activity can be overcome by disrupting the TPR-catalytic domain interface through an allosteric conformational change induced by the binding of polyunsaturated fatty acids or Hsp90 to the TPR domain.40–42
Preferential Association of Immunophilins with Specific Steroid Receptors
Factors that Help Determine Immunophilin Recruitment
The reversible targeting of the common Hsp90 acceptor site for TPR immunophilins supported the concept that association of steroid receptor-Hsp90 complexes with different TPR proteins might impact on receptor function resulting in differential modulation of hormone-binding affinity, nucleocytoplasmic localisation and receptor transactivation.24,26,28,30 While the binding affinities of the TPR immunophilins for Hsp90 and their relative cellular abundance might have some influence on their level of incorporation into steroid receptor-Hsp90 complexes, there is evidence of selective coupling of specific TPR immunophilins with individual receptors. Thus, both GR and PR preferentially associate with FKBP51 over FKBP52 and CyP40 in in vitro assembly reactions,24,43 whereas FKBP52 and PP5 were somewhat evenly distributed among GR-Hsp90 complexes recovered from mouse L cells.29 CyP40 protein was clearly the dominant TPR immunophilin in ERα-Hsp90 complexes purified from calf uterine cytosol,44 although FKBP52 was also recovered with ERα in these purified extracts.22
TPR immunophilin retention in receptor complexes might, in part, be driven by direct co-chaperone-receptor interaction.45 For example, results of structural studies have shown that deletion of the three residue insertion (D195, H196, D197) within the FKBP51 FK2 domain compromised assembly of this immunophilin into PR complexes, whereas removal of the corresponding FK2 insertion loop from FKBP52 had no effect on association with receptor.35 Thus, direct contact of the FKBP51 FK2 domain with PR might favour FKBP51 association over FKBP52 with this receptor.24 Additionally, although FKBP51 is the preferred TPR immunophilin in mature GR-Hsp90 complexes, the observed hormone-induced interchange of FKBP51 by FKBP52 in GR-Hsp90 complexes, resulting in the favoured nuclear translocation of receptor complexes,34 suggests that unique steroid receptor ligand-binding domain conformations might be an important determinant governing incorporation of specific TPR immunophilins within receptor-Hsp90 complexes. This is consistent with evidence that FKBP52 potentiation of GR transcriptional activity, together with increased GR hormone-binding affinity, is localised to the GR ligand-binding domain46 and is supported by results demonstrating that specific preferences for TPR immunophilins map to the ligand-binding domains of GR, PR and MR, with recruitment and cellular localisation of receptor-Hsp90-TPR immunophilin complexes being additionally controlled by ligand binding.29,47–49 Nuclear import of GR-Hsp90 heterocomplexes containing FKBP52 or PP5 TPR immunophilins is facilitated by the interaction of these complexes with key components of the nuclear pore complex, such as Nup62 and the transport receptor, importin β.50 MR behaves similarly to GR and after hormonal exposure accumulates in the nucleus as an intact receptor-Hsp90 complex associated with FKBP52.50–51 There is now accumulating evidence that steroid receptors, both in the absence of hormone and in hormone-bound heterocomplexes, shuttle freely between nuclear and cytoplasmic cellular locations.2,52–54
Greatly increased incorporation of FKBP51 into GR-Hsp90 complexes stabilises an inactive receptor conformation, causing a significant decrease in GR hormone-binding affinity.55–57 This inhibitory influence of FKBP51 on GR activity requires both FK domains, as well as Hsp90 binding, but is not reliant on FKBP51 PPIase activity.58 The FK506 drug-binding pocket of FKBP51 is inaccessible to FK506 in these low affinity hormone-binding GR heterocomplexes, but on dynamic assembly/disassembly of GR-Hsp90-FKBP51 complexes in receptor cytosols, exposure to FK506 prevented re-association of FKBP51 with receptor, correlating with a sharp increase in receptor hormone uptake and binding affinity. Similarly, treatment of L929 cells with FK506 increased GR hormone-binding affinity through a displacement of FKBP51 by PP5.59 In contrast, FK506 appeared to be equally recognised by FKBP52, whether as a component of mature, high affinity hormone-binding GR complexes or not.55,60 Furthermore, the immunosuppressant blocks FKBP52-mediated potentiation of GR activity.46 These differential actions of FK506 may likely arise from distinct domain orientations evident from recent structural analyses of these TPR immunophilins (Figure 1B).35,37–38
In a model for steroid receptor-TPR immunophilin selectivity, Hsp90 has been proposed, on the one hand, to undergo specific changes in conformation, allowing it to serve as a critical mediator for the differential recruitment of TPR immunophilins by receptor ligand-binding domains and to facilitate immunophilin control over receptor function on the other.47 Mutations in the C-terminal region of Hsp90, that includes the MEEVD peptide docking motif for TPR immunophilins, altered the interaction patterns for the TPR cochaperones, suggesting that they make distinct and extensive contacts leading to differential modulation of Hsp90 chaperone function.61–64 Additionally, sequences outside the core TPR domain of the immunophilins can impact high affinity Hsp90 interaction.28,43,62,65 Thus, the 30-residue linker immediately upstream of the TPR domain, that mediates CyP40 chaperone activity66 (Figure 1B), may help stabilise the TPR domain for enhanced Hsp90 binding or may, alternatively, lock in an Hsp90 conformation optimal for client protein function. Corresponding C-terminal regions flanking the TPR domains of CyP40, FKBP51 and FKBP52 include a final helix that contains the charge-Y Hsp90 interaction motif and forms part of an extended Hsp90-binding surface that may determine the Hsp90 binding specificities of these co-chaperones.62,65 Deletion of Cpr7, a CyP40 Saccharomyces cerevisiae homologue, compromised GR transcriptional activity which was partly restored by overexpression of a Cpr7 C-terminal TPR fragment that included the linker region deemed to be largely responsible for Cpr7’s potent chaperone activity.66–68 Significantly, it was speculated that in targeting the GR-Hsp90 complex, the TPR/linker region may act on Hsp90 to promote an effective chaperoning of the receptor to a more hormone-responsive conformation.67 Functional analysis of the protein phosphatase, Ppt1, the yeast homologue of PP5, provided further evidence for the modulation of Hsp90 chaperoning properties by TPR co-chaperones.69 Purified Ppt1 was shown to specifically dephosphorylate Hsp90 in vitro via a mechanism that required the binding of the Ppt1 TPR domain to Hsp90, while in vivo studies demonstrated a significantly reduced efficacy of the Hsp90 chaperone system in a Ppt1-deleted yeast strain that inhibited maturation and activation of Hsp90-dependent client proteins, including GR.69 It is worth noting that while Hsp90 as well as some of its co-chaperone components are conserved and highly homologous to their human counterparts, they are not equivalent. Thus, although yeast provides a very valuable model, especially for GR studies, subtle differences between the effects of the yeast and mammalian Hsp90 chaperone machines on GR function can contribute to significant differences in efficacy of specific corticosteroid ligands.70
TPR Immunophilins Function as Steroid Receptor Modulators
Functional interaction of steroid receptors with yeast Hsp90 allowed application of the yeast model to demonstrate a dependence of AR, ERα and GR on Hsp90 for hormonal signalling.71–72 Subsequent studies revealed that deletion of Cpr7, but not that of its companion CyP40 yeast homologue, Cpr6, adversely affected both ERα and GR transcriptional activity.73–75 Receptor function could be fully restored either with wild type Cpr7 or a Cpr7 lacking PPIase activity, confirming a dominant role for regions outside of the cyclophilin domain, such as the TPR/linker, in steroid receptor regulation.67 Interestingly, human CyP40 displays a higher catalytic activity than either of its yeast homologues, but has a chaperoning ability similar to Cpr7.76 Evidence from the Sanchez laboratory77 showed that both siRNA knockdown of CyP40 and exposure of prostate cancer LNCaP cells to CsA, dramatically inhibited AR activity. Moreover, CyP40 knockdown moderated the inhibitory effects of CsA, confirming the selective targeting of the immunophilin by CsA. In the context of prostate cancer cells, the results point to involvement of the CyP40 PPIase domain, but whether these effects on AR are mediated by CyP40 catalytic function or by direct contact with the AR ligand-binding domain (e.g. via the divergent loop within the PPIase domain5,36) remains to be determined.
FKBP51 is the preferred TPR immunophilin for mature GR-Hsp90 complexes and represses GR function, with FKBP51 over-expression resulting in a receptor with decreased corticosteroid sensitivity.55–58 Indeed, cortisol resistance in New World primates has been attributed directly to the overexpression of this immunophilin in primate lymphocytes.55,58 Furthermore, GR-mediated upregulation of FKBP51 expression in glucocorticoid target tissues provides an inhibitory feedback mechanism for decreasing glucocorticoid sensitivity.78–79 In contrast, FKBP52 selectively enhances GR transcriptional activity by increasing hormone-binding affinity and nuclear transport, although the closely related FKBP51 can attenuate this FKBP52-mediated potentiation.46,59,80 Since FKBP52 potentiation of GR signalling requires assembly with Hsp90 and is directed through the GR ligand-binding domain with the involvement of the FKBP52 FK1 domain, Riggs and co-workers46 proposed a model whereby the FK1 domains of FKBP52 and FKBP51 make distinct contacts with the GR ligand-binding domain, stimulating conformational changes that either increase or reduce hormone-binding affinity, respectively. Glucocorticoid resistance in the guinea pig is attributed to mutations within the helix 1 to helix 3 (H1–H3) loop of the guinea pig GR, prompting Fuller et al.81 to suggest that the loop might serve as a contact point for FKBP52 and/or FKBP51 with receptor. Functional studies however, have discounted a role for the loop in primary interactions with FKBP52 to potentiate receptor or with FKBP51 to repress receptor function.82 Instead, the GR H1–H3 loop is thought to transmit different folding signals from Hsp90-associated FKBP51 and FKBP52 to unliganded receptor resulting in ligand-binding domain conformational changes affecting both ligand-binding affinity and nuclear translocation.82
Studies with FKBP52 knockout mouse strains have extended the critical physiological role of FKBP52 to cellular responses controlled by both AR83 and PR,84–85 while a physiological impact of this TPR immunophilin on ERα46,85 and MR49 cellular activity was not observed, despite assembly of FKBP52 with Hsp90 containing these receptors. In studies aimed at understanding the mechanism mediating the specific control of FKBP52 over AR, GR and PR function, an extensive mutational analysis of the FKBP52 FK1 catalytic site excluded a role for FKBP52 PPIase activity and identified a loop overhanging the FK1 catalytic pocket as a structural feature important for AR (and GR/PR) potentiation.86 This proline-rich loop (FKBP52 sequence: 116AGS119PPKIPP124) is largely responsible for the functional difference between FKBP52 and FKBP51 relating to receptor potentiation and repression of hormone binding, respectively.86 The corresponding FKBP51 sequence (116AGS119LPKIPS124) differs at residues 119 and 124. It has been proposed that a critical proline (human FKBP52 Pro119) within this loop allows specific contact with a region of the AR ligand-binding domain, thus helping to stabilise a ligand-binding domain conformation favourable for high affinity hormone binding leading to efficient transcriptional activation.86 It is speculated that a leucine substitution within the corresponding FK1 sequence of FKBP51 alters the loop conformation sufficiently to disrupt this functionally important contact. The possibility exists that in AR-Hsp90 complexes containing FKBP52, Hsp90 orients the co-chaperone to achieve unique interactions with the receptor ligand-binding domain, allowing Hsp90 to facilitate optimal hormone binding and to further fine-tune the hormonal response. Proline substitution for Leu119 in FKBP51 converts the immunophilin to a potentiator of AR transcriptional activity, thus mimicking the role of FKBP52.86
PP5, as well as FKBP51, are the preferred TPR immunophilins in GR-Hsp90 complexes.47 Dexamethasone-induced transcription was shown to be enhanced with PP5 knockdown, although there was no apparent effect of reduced PP5 protein expression on hormone binding.48 Suppression of PP5 expression led to nuclear accumulation of GR in the absence of hormone, suggesting a role for PP5 in nucleocytoplasmic shuttling of the receptor.48 In addition, ligand-dependent replacement of FKBP51 by PP5 has been demonstrated for MR.49 Further studies have shown that, under basal conditions in the absence of ligand, GR is continuously phosphorylated and then dephosphorylated by phosphatases (including PP5) linked to receptor through Hsp90-dependent contacts with the ligand-binding domain.87 Selective depletion of PP5 followed by hormonal stimulation resulted in a differential effect on target gene expression. Together, the results were consistent with PP5-dependent modulation of GR phosphorylation and transcriptional activity.87 A yeast two-hybrid screen identified ERα as an interaction target for PP5 which was found to function as a negative regulator of ERα transcription in vivo by inhibiting epidermal growth factor (EGF)-dependent phosphorylation of Ser118 in the receptor N-terminal domain.88 Although a direct PP5-ERα interaction was indicated, suggesting the non-involvement of Hsp90, a role for this major molecular chaperone in the in vivo effects of PP5 on ERα function cannot be discounted. These findings identify PP5 as a critical regulator of ERα cellular function through its modulating role on both receptor phosphorylation states and transcriptional activity.88
Lipid metabolism is controlled by the reciprocal influences of GR and peroxisome proliferator-activated receptor-γ (PPARγ) signalling on the equilibrium between lipolysis and lipogenesis.89–90 PP5 helps to control this balance by preventing GR hyperphosphorylation and inhibiting GR activity at prolipogenic genes such as CD36.7,91 On the other hand, simultaneous PP5-mediated dephosphorylation of PPARγ, within the context of PPARγ-Hsp90-PP5 complexes,92 was shown to promote the lipogenic actions of PPARγ by increasing the transcriptional expression of CD36, as well as other lipogenic genes, e.g. aP2 (Figure 3).7,91 These findings place PP5 at a central point in nuclear receptor-mediated control of lipid metabolism.
Figure 3.

Role of TPR immunophilins in metabolic disorders. FKBP51, FKBP52 and PP5 are important modulators of glucocorticoid receptor (GR) activity with a preferential association of GR for FKBP51 and PP5 over FKBP52. Both FKBP51 and PP5 function as negative regulators of GR, while FKBP52 acts to enhance GR transcriptional activity. Glucocorticoids have a major role in promoting hepatic gluconeogenesis through a mobilisation of free fatty acids from peripheral adipose by stimulating fat breakdown and amino acids derived from protein degradation in muscle.89–90 Overstimulation by glucocorticoids leads to metabolic diseases such as obesity, fatty liver (steatosis), hyperglycaemia and insulin resistance.89–90 Sanchez and coworkers7,91 have revealed that, compared to wild type animals, FKBP51 knockout mice resisted weight gain when subjected to high-fat dietary stress. Exposed to a similar stress challenge FKBP52-deficient mice were susceptible to hyperglycaemia and hyperinsulinaemia correlating with insulin resistance and liver steatosis, both coupled to an underlying reduction in hepatic gluconeogenesis. A measured glucocorticoid response in the liver under metabolic stress was proposed to help reduce hepatic lipid load.7,91 PP5-mediated dephosphorylation of GR and PPARγ at specific serine residues caused a reduction of GR activity, while increasing PPARγ transcriptional activity at genes involved in lipid metabolism. Thus PP5 reciprocal modulation of these two receptors antagonises GR antilipogenic actions and at the same time promotes PPARγ adipogenic activity.133 In comparison, mice lacking PP5 were observed not to accumulate fat.135
Table 1 provides a summary of steroid hormone signalling pathway modulation by steroid receptor-associated immunophilins.
Table 1.
Modulation of steroid hormone signalling by steroid receptor-associated immunophilins.
| TPR Immunophilin | Interaction target | Role |
|---|---|---|
| FKBP51 | GR | FKBP51 is the preferred TPR immunophilin (along with PP5) for GR-Hsp90 complexes. FKBP51 inhibits GR function by reducing receptor hormone binding affinity resulting in decreased glucocorticoid sensitivity 55–58 GR-mediated upregulation of FKBP51 results in an inhibitory feedback mechanism to further decrease glucocorticoid sensitivity.78–79 The FKBP51 FK1 domain is proposed to make direct contact with the GR ligand-binding domain to reduce hormone-binding activity.46 FKBP51 Leu119 in the corresponding proline-rich loop of the FKBP51 FK1 domain disrupts functional contact within AR, GR, PR ligand-binding domains.86 |
| FKBP51 | AR | FKBP51 overexpression increases AR transcriptional activity by promoting AR assembly with mature FKBP51-Hsp90-p23 complexes and higher levels of androgen-ligated AR.131 |
| FKBP52 | GR | FKBP52 enhances GR transcriptional activity by increasing hormone-binding affinity and nuclear transport.46,59,80 The FKBP52 FK1 domain is proposed to make direct contact with the GR ligand-binding domain to increase hormone-binding affinity 46 |
| FKBP52 | AR, PR | Mouse knockout studies have revealed a critical physiological role for FKBP52 in cellular responses controlled by AR, PR, but not ERα, MR.83–85 The proline-rich loop in the FKBP52 FK1 domain makes specific contact with the receptor ligand-binding domain to increase hormone-binding affinity and potentiation of AR, GR, PR. FKBP52 Pro119 has been identified as the critical contact proline. 86 |
| CyP40 | ERα | CyP40 is the preferred immunophilin for ERα.21,39 |
| Cpr7 | ERα, GR | Deletion of Cpr7 in yeast adversely affects ERα, GR transcriptional activity. Receptor regulation is delineated to the TPR domain.73–75 |
| CyP40 | AR | Knockdown of CyP40 or select targeting of CyP40 by CsA inhibits AR activity in LNCaP cells.72 |
| PP5 | GR | A preferred TPR immunophilin (together with FKBP51) for GR-Hsp90 complexes.47 Knockdown of PP5 enhances GR transcriptional activity without affecting hormone-binding affinity, pointing to a role for PP5 in GR nucleocytoplasmic shuttling. 48 There is evidence of PP5-dependent modulation of GR phosphorylation and transcriptional activity.87 PP5 controls the balance between lipolysis and lipogenesis by preventing GR hyperphosphorylation and inhibiting GR activity.7,89–91 |
| PP5 | MR | Ligand binding caused FKBP51 to be replaced by PP5 in MR-Hsp90 complexes.49 |
| PP5 | ERα | PP5 functions as a negative regulator of ERα transcriptional activity by inhibiting phosphorylation of specific residues in the receptor N-terminal domain. 88 |
AR, androgen receptor; CsA, cyclosporin A; CyP40, cyclophilin 40; ERα, oestrogen receptor; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; PP5, protein phosphatase 5; PR, progesterone receptor; TPR, tetratricopeptide repeat
Steroid Receptor-Associated Immunophilins in Health and Steroid-Based Diseases
Reproductive Physiology
The infertility phenotype of both FKBP52 knockout male and female mice demonstrates the critical connection of this TPR immunophilin with reproductive processes.83–84,93 Male mice deficient in FKBP52 displayed features (e.g. prostate dysgenesis, penile hypospadias) consistent with partial androgen insensitivity, reflecting the loss of FKBP52-mediated control over AR function,83,93 whereas parallel studies with FKBP51 knockout male animals revealed no apparent impact on AR signalling.93 Loss of FKBP52 also contributes to male infertility by compromising sperm fertilising capacity, suggesting a role for the co-chaperone in sperm function.94 Hypospadias is observed as a common birth defect in humans. In this regard, the developmentally controlled expression of FKBP52 appears to be essential for coordinating AR-mediated signalling with outgrowth of the developing penis.95 Female FKBP52 null mice displayed a maternal defect impeding successful pregnancy that traced to progesterone insensitivity in the maternal uterus, signifying a role for FKBP52 in PR-mediated support of blastocyst implantation84 (reviewed in Tranguch et al.96). Using a separate FKBP52-deficient mouse model, Sanchez and co-workers confirmed the dramatic role of FKBP52 in uterine reproductive physiology.85 The implantation failure in FKBP52 deficient female mice could be overcome with exogenous progesterone supplementation.97 Additional studies showed that FKBP52 deficiency98 conferred uterine resistance to progesterone during pregnancy, with increased progesterone levels being able to restore PR signalling sufficiently to sustain pregnancy to full-term.97 Clinical trials have confirmed the benefits of progesterone treatment in preventing pregnancy loss in women experiencing recurrent miscarriages.99
Endometriosis, a common oestrogen-dependent gynaecological disorder affecting the health and reproductive function of women of childbearing age, is caused by a hormonal imbalance derived from enhanced oestrogenic influence coupled with a reduction in progesterone responsiveness.100–102 FKBP52 protein expression was found to be significantly reduced in the eutopic endometrium of baboons induced with endometriosis103 and this observation was supported by findings in the FKBP52 knockout mouse model in which the uterine-specific progesterone resistance was shown to promote the growth of endometriotic lesions.104 Moreover, FKBP52 was found to be down-regulated in human endometriosis, thus underscoring the potential role of the cochaperone in the pathogenesis of the disease.104–105
Breast and Prostate Cancer
Although the TPR-containing immunophilins are ubiquitously expressed, changes in their protein expression levels leading to alterations in the balance of the co-chaperones available for incorporation into steroid receptor complexes could impact on receptor function. In this regard, hormone-dependent cancers of the breast and prostate, in which ERα and AR are the principal targets of anti-hormonal therapies, are of paramount interest. CyP40 and FKBP52 mRNA expression is up-regulated in breast cancer compared to normal tissue.106 A contrasting pattern of protein expression was seen for these two immunophilins in a panel of breast cancer cell lines, with FKBP52 being more highly variable and more highly expressed (by as much as 40-fold) than CyP40.106 The presence of ERα was strongly correlated with higher levels of FKBP52 in the cell lines. For example, the FKBP52:CyP40 molar ratio for the ERα-positive T47-D and MCF-7 cell lines was 38.3 and 34.9, respectively, while the corresponding ratio for the ERα-negative cell line, MDA-MB-231, was much lower at 2.5.
The CyP40 gene has been mapped to 4q31.3,107 within a region (4q25-q34) previously demonstrating loss of heterozygosity in cases of advanced breast cancer.108 A dinucleotide repeat polymorphic marker detected in the 5′-flanking region of the CyP40 gene allowed allelic loss at the CyP40 locus to be determined in tumours from a cohort of breast cancer patients.109 The possibility exists that genetic loss of CyP40 might compromise ERα function by altering immunophilin composition in receptor complexes, impacting anti-oestrogen resistance and resulting in phenotypic changes in breast cancer.
Down-regulation of FKBP51 protein expression has been observed in some breast cancer cell lines110–111 in which the immunophilin may be regulated in a reciprocal fashion via a feed-forward loop involving androgen signalling, on the one hand and negative feedback through glucocorticoid and progestin signalling on the other.78–79,112–115 Since FKBP51 acts as a negative regulator of Akt activation,111,116 low levels or absence of FKBP51 would lead to hyperactivation of Akt, thus predisposing to tumorigenesis and cancer cell resistance to chemotherapy (reviewed in Li et al.110). It is noteworthy that FKBP51 enhances NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) signalling through its interaction with IKKα (IκB kinase α) and IKKε (IκB kinase ε).117 NF-κB signalling is activated in a significant percentage of human cancers118 and specifically in breast cancers.119 Interestingly, IKKε has recently been identified as a kinase oncogene, being amplified and overexpressed in breast cancer cell lines and breast tumours.120 In some cell types, Akt and IKKε appear to be functionally related implicating the NF-κB pathway as a downstream mediator of Akt signalling.
Analysis of breast cancer tissue microarrays revealed elevated levels of PP5 protein expression in human breast cancer, with a highly significant correlation being observed between high levels of PP5 and invasive ductal carcinoma of the breast in patients presenting with metastatic disease at diagnosis.121 Observations in a xenograft mouse model for tumour development using MCF-7 cells stably expressing PP5 at different levels, confirmed that constitutive overexpression of PP5 was associated with accelerated tumour growth in animals under normal oestrogen conditions.121 These findings indicate that aberrant PP5 expression contributes to the development of human breast cancer. Surprisingly, the tissue microarray study failed to show a correlation between tumour PP5 levels and ERα expression.121 Endogenous expression of PP5 is oestrogen-regulated, with a consensus oestrogen response element (ERE) identified within the PP5 promoter.122 PP5 negatively regulates ERα-mediated transcription,88 yet depletion of PP5 negates oestrogen-mediated proliferation of MCF-7 cells without affecting the expression of immediate-early ERα-responsive genes such as c-myc.122 PP5 may therefore function as a feedback inhibitor to regulate ERα activity and may, at the same time, enhance tumour growth either downstream or independently of its effects on ERα.121 In MCF-7 and T47-D breast cancer cells, the oestrogen-promoted increases in PP5 expression result in dephosphorylation of the ligand-activated form of GR and a reduction in GR activity. Goleva and co-workers have demonstrated that depletion of PP5 can abolish this affect by enhancing corticosteroid efficacy and allowing activated GR to suppress oestrogen-induced cell proliferation.123 These results have clear implications for glucocorticoid therapy in breast cancer.
FKBP52 is much more highly expressed in prostate cancer cell lines in comparison to primary prostate cells124 and is upregulated in prostate cancer needle biopsies.125 FKBP52 potentiation of AR signalling may be of particular relevance in androgen ablation therapy, where androgen levels are markedly reduced, but can still effectively stimulate the receptor.126 In studies employing xenograft animal models, CWR22-R androgen-independent tumours that arose from androgen-dependent tumours (CWR22)127 expressed higher levels of FKBP51.128–129 In additional studies, FKBP51 expression decreased in the CWR22-R tumours of mice following androgen-ablation, with levels normalising over time and then becoming more elevated in mice exposed to androgens.128–129 Such dysregulated behaviour is highly suggestive of a direct role for FKBP51 in prostate cancer growth and progression to the highly invasive androgen-independent state. FKBP51 is recognised as a highly sensitive AR-regulated gene that functions as an important component of a feed-forward mechanism linked to the partial reactivation of AR-signalling pathways in the absence of androgens, leading to the outgrowth of androgen-independent tumours.112,114,128–130 Sanchez and co-workers have confirmed a significantly increased expression of FKBP51 in most prostate cancer tissues and in androgen-dependent and androgen-independent cell lines, suggesting that FKBP51 might have a critical role in prostate cancer growth and progression.77 FKBP51 overexpression was found to increase the AR transcriptional response by facilitating hormone-binding competence through the assembly of the AR ligand-binding domain with mature FKBP51-Hsp90-p23 complexes resulting in higher levels of androgen-liganded receptor and providing a pathway for AR-dependent signalling and growth in a low-androgen environment.131 Furthermore, overexpression of FKBP51 was shown to stimulate prostate cancer cell growth and severely affect the efficacy of bicalutamide, an antiandrogen used in patients undergoing androgen ablation therapy.131 Evidence of significantly increased expression of CyP40 in prostate cancer tissues and in androgen-dependent and -independent prostate cancer cell lines, suggests an important role for CyP40 in the regulation of prostate cancer growth.77 Depletion of CyP40 in androgen-dependent LNCaP prostate cancer cells strongly inhibited androgen-dependent transcriptional activity and cell growth, rendering the cells essentially unresponsive to androgen and restricting the cells to a basal growth pattern on exposure to hormone. The above findings identify all three TPR immunophilins – FKBP51, FKBP52 and CyP40, as positive regulators of AR-mediated prostate cancer cell growth. The combined effects of these co-chaperones may contribute to a unified mechanism that promotes increased FKBP51 expression in prostate cancer cells, further impacting on AR transcriptional activity.
Diabetes and Metabolic Disorders
A major function of glucocorticoids is to promote gluconeogenesis in the liver in response to metabolic stress, and overstimulation by glucocorticoids has been linked to specific hallmarks of diabetes and metabolic syndrome, including central obesity, steatosis and insulin resistance.90,132 The negative regulatory influences of FKBP51 suppress GR hormonal responses. FKBP51 knockout mice therefore displayed increased glucocorticoid sensitivity on exposure to dexamethasone agonist with elevated GR markers being expressed in relevant tissues such as liver, muscle and adipose and the animals demonstrating resistance to weight gain on a high-fat diet (Figure 3).7,91 As already described, FKBP52 is a positive regulator of GR transcriptional activity through its potentiation of GR-ligand binding affinity and promotion of receptor nuclear translocation. FKBP52+/− mice subjected to high-fat dietary stress developed glucocorticoid resistance from a loss of hepatic GR activity.133 Thus, decreased expression of FKBP52 causing a reduced GR control of gluconeogenesis may, under dietary stress, switch metabolism in the liver from glucose production to lipogenesis resulting in hepatic steatosis (Figure 3).7,133
In addition to enhancing hepatic gluconeogenesis, glucocorticoids induce insulin resistance and impair pancreatic β-cell function, physiological outcomes consistent with a pro-diabetic affect.134 As already noted, PP5 has a central role in lipid metabolism through its reciprocal modulation of GR and PPARγ activity.7,91 PP5-deficient mice fed a high-fat diet gained strikingly less weight and displayed improved insulin sensitivity with no significant development of hyperinsulinaemia when compared with wild type mice on the same dietary regimen (Figure 3).135 Additional data supports a protective role for PP5 in β-cells against the cytotoxic effects of glucocorticoids.136 Together, these data highlight PP5 as a potential pharmacological target against the development of obesity-induced insulin resistance.
Depression and Stress-Related Disorders
The hypothalamic-pituitary-adrenal axis regulates response to stress by releasing corticosteroid hormones resulting in GR transactivation and the targeting of numerous genes that serve to control neuronal responses underlying behavioural adaptation.137 In some individuals, an imbalance in these control mechanisms may skew responses towards stress-related brain disease. Binder and co-workers were the first to report on the association between SNPs in the FKBP5 gene, encoding FKBP51, with outcome of antidepressant treatment and recurrence of major depressive disease.138 Their study also revealed that risk allele SNPs were correlated with increases in FKBP51 protein expression leading to a suppression of GR activity and a change in hypothalamic-pituitary-adrenal axis regulation corresponding to a reduced protective response. Prolonged GR resistance, signifying a dysregulated stress response, would likely predispose to stress-related psychiatric disorders. This is supported by findings that alleles associated with enhanced FKBP51 expression occur in individuals with major depression, bipolar disorder and post-traumatic stress disorder (reviewed by Binder139). A separate study involving an analysis of non-psychotic major depressive disorders has revealed a significant association of the marker rs1360780 with disease status, but only in the white non-Hispanic racial group.140 Such an association was not reported previously from results of the initial Binder study.138 Although replication in a different population sample is required, Suzuki and co-workers have reported that the same FKBP5 polymorphism may affect personality traits, as measured by harm avoidance and co-operativeness, in a gender-specific manner – harm avoidance in females; co-operativeness in males.141 A connection between hypothalamic-pituitary-adrenal axis function and gender-specific personality has been previously reported.142
An assessment of the effect of chronic exposure to corticosterone in mice detected decreases in DNA methylation within the functionally relevant intron 1 and 5 regions of FKBP5,78–79 in hippocampal and hypothalamic tissues and in blood.143 These changes were associated with increased FKBP5 expression, suggesting that epigenetic influences may impact on stress-induced glucocorticoid regulation of the hypothalamic-pituitary-adrenal axis. A number of studies have demonstrated a relationship between FKBP5 polymorphisms and prediction of post-traumatic stress disorder, major depression and suicide following early trauma or childhood abuse.144–146 Binder and co-workers now have evidence of long-term epigenetic modifications that, in the context of the FKBP5 gene, link childhood trauma with differential FKBP5 allele-specific (intron 2) GR activation to changes in DNA methylation in intron 7 accompanied by further increases in the differential responsiveness of FKBP5 to GR activation.147
Studies in mouse brain of GR-mediated FKBP5 gene expression, as well as its regulation in response to restrained stress and food deprivation showed that regions with high basal expression levels were associated with an attenuated response, thus confirming FKBP5 as a modulator of GR function.148 FKBP5−/− mice exposed to sufficiently intense, acute stressors displayed a more active coping behaviour, as well as significantly lower hypothalamic-pituitary-adrenal axis reactivity, strongly supporting the results of human studies identifying FKBP5 as a critical factor in the regulation of stress-related diseases.149 Aged FKBP5−/− mice were shown to be resistant to stress-induced depressive-like activity, and displayed reduced levels of circulating corticosterone following stress, in comparison to wild type control animals.150 Furthermore, a reduced anxiety-like behaviour was noted in the FKBP5-ablated mice. Importantly, the absence of cognitive impairment and behavioural anomalies, together with the lack of apparent pathological alterations in these knockout animals93 suggest that therapeutic targeting of FKBP51 may not be associated with overly deleterious consequences. These outcomes were supported by studies with younger FKBP5 knockout mice which appeared to be less vulnerable to the effects of prolonged chronic social defeat stress and showed a reduced response to acute stimuli, as well as a more active stress-coping ability.151 Interestingly, heterozygous knockout (FKBP52+/−) mice displayed a mixture of increased stress sensitivity and stress resilience to different behavioural and neuroendocrine paradigms, observations that may be explained by the down-regulation of FKBP51 in some tissues, similar to the phenotype of FKBP5−/− mice.152
Regulation of Expression and Function of Steroid Receptor-Associated Immunophilins
Transcriptional Regulation
All four TPR co-chaperones are hormonally regulated. As already described above, FKBP51 expression is upregulated by androgens, glucocorticoids and progestins78–79,112–115 through the interaction of their respective receptors with a distal enhancer element within intron 5 of the FKBP5 gene.79,114–115 Expression of FKBP51 was also strongly upregulated by aldosterone in the distal colon, a major target tissue for this hormone.153 Ectopic expression of microRNA-100 and microRNA-99a led to a suppression of FKBP51 protein levels in Jurkat cells, identifying the FKBP co-chaperone as a novel target for both microRNAs.154 PP5 expression is increased by oestradiol stimulation in the ERα-positive MCF-7 breast cancer cell line.122 Characterisation of the human PP5 promoter has confirmed the presence of a single consensus ERE together with a consensus hypoxia-inducible factor-1 (HIF-1) response element and heat shock factor 1 (HSF1) binding sites within the 5′-untranslated region of the PP5 gene, supporting the induction of PP5 expression by hypoxia and heat shock, respectively.155 Oestradiol has also been shown to upregulate CyP40 and FKBP52 mRNA and protein expression in MCF-7 cells.156 Since potential EREs could not be defined within a 5-kilobase 5′-flanking region of the CyP40 promoter, the oestrogenic influence appears to be indirect and may result from mitogenic signals integrated through GA-binding protein, an Ets-related transcription factor identified as a key regulator of CyP40 expression.157 JunB interacts with a functional AP-1 site within the CyP40 promoter to induce the potent expression of CyP40 in Karpas 299 ALK+ ALCL (anaplastic lymphoma kinase positive, anaplastic large-cell lymphoma) cells, proliferation of which is controlled by activation of the NPM-ALK (nucleophosminanaplastic lymphoma kinase) oncogenic kinase.153 Heat shock and chemical stress increased CyP40 mRNA and protein turnover rate, identifying the co-chaperone as a heat shock protein;32 putative heat-shock elements are located within the first intron of human CyP40.159 FKBP52 has previously been determined to be heat-inducible,160 consistent with the presence of consensus heat-shock elements within the FKBP52 promoter.161 Progesterone and oestradiol were shown to differentially regulate FKBP52 in the uterus in a cell-specific manner.162 Thus, oestrogen induction of FKBP52 expression was primarily restricted to luminal and glandular epithelial cells, whereas progestins increased FKBP52 levels in stromal cells via a mechanism requiring progesterone-mediated activation of the Hoxa10 transcription factor.162 Interestingly, FKBP52 has been identified as a direct target of the c-myc protooncogene,163 itself an oestrogen early-response gene induced via direct co-operative regulation between ERα and AP-1 of a distal enhancer containing an ERE half-site and an AP-1 site.164–165
Modulation of Immunophilin Function by Targeting the TPR Domain
Structural studies of the full length autoinhibited conformation of PP5 have revealed that the catalytic domain C-terminal segment containing the αJ helix docks with the TPR domain through a region that partially overlaps the Hsp90-binding groove (Figure 1B).39 Mutation of the key Hsp90 binding residues of the TPR domain had no influence on PP5 basal phosphatase activity providing evidence that the TPR-phosphatase domain interface differs from that involved in Hsp90 binding.166 This partial blockade of the TPR binding site still enables Hsp90 to competitively disrupt TPR-phosphatase domain contacts allowing access to the catalytic site and activation of PP5 catalytic activity.39,41 Full length Hsp90 is a more potent activator of PP5 phosphatase activity than the C-terminal Hsp90 peptide indicating that residues lying N-terminal of the MEEVD motif contribute significantly to high affinity Hsp90-PP5 interactions.39,64 Hsp70 binding also stimulates PP5 phosphatase activity, although the C-terminal Hsp70 IEEVD TPR interaction motif has a lower binding affinity than the corresponding Hsp90 MEEVD peptide for the PP5 TPR domain.167 Interestingly, CyP40 was also shown to bind Hsp90 preferentially over Hsp70, while FKBP52 was unable to compete with CyP40 for Hsp70 binding suggesting that FKBP52 discriminates better between the Hsp90 and Hsp70 TPR acceptor sites.168 The TPR domain structures of both PP5 and CyP40 are stabilised by non-physiological intermolecular associations within their crystallographic forms.36,169 Moreover, the existence of two alternate docking orientations for the Hsp90 MEEVD peptide in the CyP40 TPR domain, one of which corresponded to that defined for the Hop-MEEVD interaction,170 revealed a degree of functional flexibility for the peptide-binding groove.36,171 Recent NMR data have revealed that the isolated PP5 TPR domain is substantially unfolded at physiological temperatures and that interaction with the Hsp90 MEEVD peptide stabilises a folded, ordered structure leading to the suggestion that a coupled folding/binding mechanism might be a common feature of protein interaction with TPR domains.172–173
The differential stimulation of PP5 phosphatase activity by the binding of Hsp90, arachidonic acid and a range of long-chain fatty acid derivatives to the PP5 TPR domain suggested that the level of activation was associated with induction of different conformations.41 Recent structural data has confirmed that arachidonoyl-CoA ester binds and stabilises an alternate conformation of the TPR domain, thus restricting the ability of the TPR domain to form the specific contacts required for inhibition of catalytic activity.39 On the other hand, the action of arachidonic acid to open up the catalytic site of PP5 triggers KLHDC10 (kelch domain-containing protein 10) interaction with the phosphatase domain resulting in a suppression of PP5 catalytic activity.174 The association of S100 proteins with PP5 provides a Ca2+-dependent mechanism for regulating the phosphorylation of PP5 target proteins, either through its phosphatase activity or through PP5 interactions with its protein binding partners.175 S100 proteins bind to the PP5 TPR domain through an interaction mode that differs from the two-carboxylate clamp established for Hsp90 interaction with TPR co-chaperones,170 yet they clearly inhibit Hsp90 binding to PP5, CyP40, FKBP52 and Hop.175–177 Evidence suggests that these co-chaperones have a preferential association with specific S100 proteins175 and mediate these interactions by accommodating a Ca2+-induced hydrophobic binding surface within their TPR domains.178 PP5 catalytic activity was more strongly activated by S100 protein interaction compared to Hsp90, resulting in greatly increased dephosphorylation of phospho-tau, both in vitro and in vivo.175 Additionally, S100A1 protein inhibited apoptosis signal-regulating kinase 1 (ASK1)-PP5 interaction in a dose-dependent manner.175 Since PP5 acts as a negative regulator of ASK1,179 this finding suggests that S100 proteins may potentially interfere with cellular signalling via this stress response pathway. The observed adaptability of the PP5 TPR domain could well be extended to the TPR domains of CyP40, FKBP51 and FKBP52.
Modulation of Immunophilin Function by Disrupting Interaction with Hsp90
Hsp90 is often present in an activated multichaperone complex in cancer cells, thus facilitating cell growth by protecting key oncoprotein clients such as kinases and transcription factors from degradation and allowing malignant transformation and progression.180–181 Given the association of ERα with Hsp90 and that the receptor tyrosine kinase, human epidermal growth factor 2 (HER2), is one of the most Hsp90-dependent client proteins known, breast cancer is a prime target for Hsp90 inhibitors.182 Targeting Hsp90 in prostate cancer is a particularly attractive therapeutic strategy, as Hsp90 is commonly over expressed in prostate tumour cells and AR, the key driver of prostate cancer progression, is a sensitive Hsp90 client protein.183–185 Derivatives of the naturally occurring Hsp90 inhibitor, geldanamycin, such as 17-AAG, have been pathfinder molecules in animal models and early clinical trials, leading to the development of synthetic small-molecule Hsp90 inhibitors, some of which have progressed to the clinic.186–187 These inhibitors bind the Hsp90 ATP-binding site to block the ATPase-coupled chaperone cycle leading to the depletion of Hsp90 client proteins, cell-cycle arrest and apoptosis (Figure 2).186–187
A second druggable site has been identified in the Hsp90 C-terminal domain that is targeted by coumarin-based antibiotics such as novobiocin188 which bind at the interface between two monomers (Figure 2).189 Improvements in the affinity of these compounds for Hsp90 have allowed them to induce apoptosis in cancer cells and in some cases demonstrate superior efficacy compared to 17-AAG.190 We have shown that novobiocin blocks the binding of TPR immunophilins to Hsp90.65 Novobiocin appears to act allosterically to induce global conformational changes within Hsp90. Peptide analogues of the natural product Sansalvamide A, bind at a novel site between the N-terminal and middle domains of Hsp90 (Figure 2) and allosterically inhibit the binding of Hsp90 to TPR immunophilins by blocking their interaction with the MEEVD acceptor site.191–192 The Sansalvamide A derivative, SM145, has a unique molecular profile in that it inhibits Hsp90 function, leading to decreased expression of steroid receptor levels and potently blocks Hsp90 interaction with several TPR co-chaperones including CyP40, FKBP51 and FKBP52.192
Targeting Immunophilin Interaction with Steroid Receptors
The binding function 3 (BF3) surface within the AR ligand-binding domain has the ability to allosterically alter the activation function 2 (AF2) co-activator binding pocket.193 BF3 residues altered through natural mutations linked to androgen insensitivity and those associated with prostate cancer either diminish or enhance AR AF2 activity, respectively, underlining the importance of the BF3 surface for AR function.193 Cox and co-workers have recently identified small molecule inhibitors of FKBP52-enhanced AR function in prostate cancer cells that target a region of the AR ligand-binding domain overlapping the BF3 surface.194 Multiple residues that contribute to the FKBP52 sensitivity of AR, some of which form part of the binding site for MJC13, the lead compound, have been identified.194 Since MJC13 helps to maintain an intact ARHsp90-FKBP52 complex at low hormone concentrations, it is possible that the inhibitor interferes with a critical next step - a hormone-induced, FKBP52-dependent change in AR conformation necessary for nuclear translocation. Sequence comparisons have revealed some conservation of BF3 residues within the ligand-binding domains for AR, GR, MR and PR, suggesting the presence of BF3-like regulatory domains in each receptor.193 A very limited conservation of these residues is apparent in ERα, suggesting the formation of a BF3 type surface that is unique to this receptor.193 Both ERα and MR behave differently to AR, GR and PR, through their inability to respond to FKBP52. Certain structural differences within their ligand-binding domains distinguish these two receptors from the other members of this subfamily.194 Since FKBP52 also regulates GR and PR activity, most likely through specific BF3 surfaces, there is the potential for the development of FKBP52-specific inhibitors targeting GR and PR function to treat a range of steroid hormone-based diseases.195 The BF3 pocket is a potential target for second-site modulators that can allosterically block agonist-activated AR function to inhibit prostate cancer cell growth.196
Steroid Receptor-Associated Immunophilins as Drug Targets
CyP40 is one of 17 members of the human cyclophilin PPIase family that catalyse the cis-trans isomerisation of peptide-proline bonds, an activity that may be central to the numerous roles ascribed to cyclophilins in biological processes that include protein folding, chaperone activity, signalling, mitochondrial function, the stress response, gene expression and regulation of kinase activity.197–198 By targeting the active site, CsA universally blocks cyclophilin isomerase activity, and its specific interaction with the prototypical cyclophilin, CyP18, yields a heteromeric complex that binds and inhibits the calcineurin phosphatase, forming the basis of immunosuppression by the drug.197–198 Within the CyP40 N-terminal cyclophilin domain, residues important for PPIase activity and CsA binding are identical to CyP18, except for His141 which, although conserved among CyP40 homologues, remains a tryptophan residue in CyP18 and other cyclophilins, a modification that may account for the decreased catalytic activity and CsA-binding affinity of CyP40.199–200 Although clinically useful as an immunosuppressive agent, CsA binds nonselectively to most cyclophilins. With the development of fluorescent probes to examine ligand specificity,201 studies are currently underway to define more selective inhibitors to increase the potential of CyP40 as a therapeutic target. Cyclophilin PPIase domains contain two pockets near the active site that may contribute to substrate specificity, with the first accommodating the target proline. A detailed structural and biochemical analysis of human cyclophilins has identified so-called ‘gatekeeper’ surfaces that surround or guard entrance to a secondary pocket that interacts with substrate residues adjacent to the substrate proline.23 These gatekeeper surfaces are unique to individual cyclophilins, displaying distinct molecular features and sufficient chemical diversity to make this region highly suitable for drug targeting and likely to result in compounds with greater isoform specificity.23
Different isoforms of the FKBP protein family modulate diverse cellular pathways providing therapeutic opportunities in cancer, neurodegenerative diseases and psychiatric disorders. Since FK506 mediates non-covalent interactions with the most conserved residues in FKBP PPIase domains, chemical scaffolds based on this drug will likely hit multiple targets, making it difficult to target specific cellular pathways. Novel drug scaffolds then appear to be of paramount importance in order to achieve significant improvements in inhibitor specificity.202 The association of FKBP51 with stress-related disorders and neurodegenerative diseases, together with the protective effects seen with FKBP51 depletion in animal models of depression and anxiety make this co-chaperone a promising drug target. Although synthetic analogues of FK506 continue to be evaluated,203–204 to date the affinities of FKBP51 and FKBP52 appear to be very similar for most FKBP ligands.202,205 Since FKBP51 and FKBP52 have distinct physiological roles (e.g. in reproduction and regulation of the hypothalamic-pituitary-adrenal axis through their differential modulation of steroid receptor function) selective inhibition of FKBP51 will be necessary for the treatment of neurological disorders. A novel conceptual approach is the potential pharmacological targeting of the proline-rich loop within the FK1 domains of FKBP51 and FKBP52 in which the L/P119 interchange is predominantly responsible for their opposing influences.9,86,203,206 Pronounced structural differences arising from an invariably cis conformation of the L119–P120 peptide in FKBP51, compared to the P119–P120 peptide in FKBP52 which can exist in a trans orientation, forms the underlying basis for this potential drug development strategy.
Conclusions
Since the identification of FKBP51, FKBP52, CyP40 and PP5, as components of steroid receptor-Hsp90 complexes, significant progress has been made in defining their roles in receptor function and the associated mechanisms involved. Biochemical studies, together with careful examination of phenotypes displayed by mouse knockout models have revealed that these Hsp90 co-chaperones are themselves hormonally-regulated and have the capacity to exert cell-specific, and in some instances, opposing influences (e.g. FKBP52 vs FKBP51) over receptor activity. Dysregulated expression of TPR immunophilins may potentially be linked to steroid-based diseases, including breast and prostate cancer, diabetes and related metabolic disorders, male and female infertility and major depression and neurodegenerative disorders. The continued validation of the cellular functions of the TPR immunophilins, together with an improved understanding of their mechanisms of action have opened opportunities for targeting these protein modulators either through their enzyme functions (PPIase activity in FKBP51, FKBP52 and CyP40; phosphatase activity in PP5) and/or their TPR domains (e.g. disruption of interactions with Hsp90, other protein partners), as well as by specific targeting of their contact domains with steroid receptors (e.g. FKBP52 FK1 proline-rich loop interaction with the BF3 allosteric domain in the AR ligand-binding domain). Molecular therapies to enhance or inhibit some cellular processes controlled by TPR immunophilins already exist, while for others development is underway. Conditional knockout mouse models may allow a more detailed characterisation of the tissue- and cell-specific actions of these co-chaperones. This may also be facilitated by targeted deletion of individual TPR immunophilins in human cells using the CRISPR/Cas9 system.207 Although CyP40 knockout mice have been available for some time, there currently exists a gap in our knowledge of the physiological role played by CyP40 and novel findings in this area are eagerly awaited. The TPR co-chaperones are potential targets for cancer therapy and may predict the likely prognosis of certain malignancies.208 Immunostaining of a breast cancer tissue microarray for PP5 revealed a positive correlation between PP5 overexpression and ductal carcinoma in situ.121 Similar analyses in breast cancer are certainly warranted for the other TPR immunophilins, with staining intensity linked to data for ERα, PR and HER2, as well as disease progression and treatment outcomes.
Acknowledgments
For research in the Ratajczak laboratory, we wish to acknowledge support from the National Health & Medical Research Council of Australia, the National Breast Cancer Foundation, the Cancer Council of Western Australia, the Prostate Cancer Foundation of Australia, the Sir Charles Gairdner Hospital Research Fund and the Harry Perkins Institute of Medical Research.
Footnotes
Competing Interests: None declared.
References
- 1.Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–64. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 2.Echeverria PC, Picard D. Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim Biophys Acta. 2010;1803:641–9. doi: 10.1016/j.bbamcr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Picard D. Chaperoning steroid hormone action. Trends Endocrinol Metab. 2006;17:229–35. doi: 10.1016/j.tem.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Pratt WB, Galigniana MD, Harrell JM, DeFranco DB. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal. 2004;16:857–72. doi: 10.1016/j.cellsig.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Ratajczak T, Ward BK, Cluning C, Allan RK. Cyclophilin 40: an Hsp90-cochaperone associated with apo-steroid receptors. Int J Biochem Cell Biol. 2009;41:1652–5. doi: 10.1016/j.biocel.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Ratajczak T, Ward BK, Minchin RF. Immunophilin chaperones in steroid receptor signalling. Curr Top Med Chem. 2003;3:1348–57. doi: 10.2174/1568026033451934. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez ER. Chaperoning steroidal physiology: lessons from mouse genetic models of Hsp90 and its cochaperones. Biochim Biophys Acta. 2012;1823:722–9. doi: 10.1016/j.bbamcr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith DF. Tetratricopeptide repeat cochaperones in steroid receptor complexes. Cell Stress Chaperones. 2004;9:109–21. doi: 10.1379/CSC-31.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DF, Toft DO. Minireview: the intersection of steroid receptors with molecular chaperones: observations and questions. Mol Endocrinol. 2008;22:2229–40. doi: 10.1210/me.2008-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, et al. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440:1013–7. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer P, Prodromou C, Liao C, Hu B, Roe SM, Vaughan CK, et al. Structural basis for recruitment of the ATPase activator Aha1 to the Hsp90 chaperone machinery. EMBO J. 2004;23:1402–10. doi: 10.1038/sj.emboj.7600141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–94. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin SH, Smith HW, Jackson SE. Stimulation of the weak ATPase activity of human hsp90 by a client protein. J Mol Biol. 2002;315:787–98. doi: 10.1006/jmbi.2001.5245. [DOI] [PubMed] [Google Scholar]
- 14.Prodromou C, Siligardi G, O’Brien R, Woolfson DN, Regan L, Panaretou B, et al. Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones. EMBO J. 1999;18:754–62. doi: 10.1093/emboj/18.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter K, Muschler P, Hainzl O, Reinstein J, Buchner J. Sti1 is a non-competitive inhibitor of the Hsp90 ATPase. Binding prevents the N-terminal dimerization reaction during the atpase cycle. J Biol Chem. 2003;278:10328–33. doi: 10.1074/jbc.M213094200. [DOI] [PubMed] [Google Scholar]
- 16.Richter K, Walter S, Buchner J. The Co-chaperone Sba1 connects the ATPase reaction of Hsp90 to the progression of the chaperone cycle. J Mol Biol. 2004;342:1403–13. doi: 10.1016/j.jmb.2004.07.064. [DOI] [PubMed] [Google Scholar]
- 17.Siligardi G, Hu B, Panaretou B, Piper PW, Pearl LH, Prodromou C. Co-chaperone regulation of conformational switching in the Hsp90 ATPase cycle. J Biol Chem. 2004;279:51989–98. doi: 10.1074/jbc.M410562200. [DOI] [PubMed] [Google Scholar]
- 18.Panaretou B, Siligardi G, Meyer P, Maloney A, Sullivan JK, Singh S, et al. Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1. Mol Cell. 2002;10:1307–18. doi: 10.1016/s1097-2765(02)00785-2. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Richter K, Reinstein J, Buchner J. Integration of the accelerator Aha1 in the Hsp90 co-chaperone cycle. Nat Struct Mol Biol. 2013;20:326–31. doi: 10.1038/nsmb.2502. [DOI] [PubMed] [Google Scholar]
- 20.Tai PK, Maeda Y, Nakao K, Wakim NG, Duhring JL, Faber LE. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986;25:5269–75. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- 21.Peattie DA, Harding MW, Fleming MA, DeCenzo MT, Lippke JA, Livingston DJ, et al. Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 1992;89:10974–8. doi: 10.1073/pnas.89.22.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratajczak T, Carrello A, Mark PJ, Warner BJ, Simpson RJ, Moritz RL, et al. The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59) J Biol Chem. 1993;268:13187–92. [PubMed] [Google Scholar]
- 23.Davis TL, Walker JR, Campagna-Slater V, Finerty PJ, Jr, Paramanathan R, Bernstein G, et al. Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases. PLoS Biol. 2010;8:e1000439. doi: 10.1371/journal.pbio.1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair SC, Rimerman RA, Toran EJ, Chen S, Prapapanich V, Butts RN, et al. Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with Hsp90 and progesterone receptor. Mol Cell Biol. 1997;17:594–603. doi: 10.1128/mcb.17.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith DF, Albers MW, Schreiber SL, Leach KL, Deibel MR., Jr FKBP54, a novel FK506-binding protein in avian progesterone receptor complexes and HeLa extracts. J Biol Chem. 1993;268:24270–3. [PubMed] [Google Scholar]
- 26.Owens-Grillo JK, Hoffmann K, Hutchison KA, Yem AW, Deibel MR, Jr, Handschumacher RE, et al. The cyclosporin A-binding immunophilin CyP-40 and the FK506-binding immunophilin hsp56 bind to a common site on hsp90 and exist in independent cytosolic heterocomplexes with the untransformed glucocorticoid receptor. J Biol Chem. 1995;270:20479–84. doi: 10.1074/jbc.270.35.20479. [DOI] [PubMed] [Google Scholar]
- 27.Radanyi C, Chambraud B, Baulieu EE. The ability of the immunophilin FKBP59-HBI to interact with the 90-kDa heat shock protein is encoded by its tetratricopeptide repeat domain. Proc Natl Acad Sci U S A. 1994;91:11197–201. doi: 10.1073/pnas.91.23.11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratajczak T, Carrello A. Cyclophilin 40 (CyP-40), mapping of its hsp90 binding domain and evidence that FKBP52 competes with CyP-40 for hsp90 binding. J Biol Chem. 1996;271:2961–5. doi: 10.1074/jbc.271.6.2961. [DOI] [PubMed] [Google Scholar]
- 29.Silverstein AM, Galigniana MD, Chen M-S, Owens-Grillo JK, Chinkers M, Pratt WB. Protein phosphatase 5 is a major component of glucocorticoid receptor. hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem. 1997;272:16224–30. doi: 10.1074/jbc.272.26.16224. [DOI] [PubMed] [Google Scholar]
- 30.Chen M-S, Silverstein AM, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem. 1996;271:32315–20. doi: 10.1074/jbc.271.50.32315. [DOI] [PubMed] [Google Scholar]
- 31.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–60. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 32.Mark PJ, Ward BK, Kumar P, Lahooti H, Minchin RF, Ratajczak T. Human cyclophilin 40 is a heat shock protein that exhibits altered intracellular localization following heat shock. Cell Stress Chaperones. 2001;6:59–70. doi: 10.1379/1466-1268(2001)006<0059:hciahs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokoi H, Kondo H, Furuya A, Hanai N, Ikeda JE, Anazawa H. Characterization of cyclophilin 40: highly conserved protein that directly associates with Hsp90. Biol Pharm Bull. 1996;19:506–11. doi: 10.1248/bpb.19.506. [DOI] [PubMed] [Google Scholar]
- 34.Davies TH, Ning Y-M, Sánchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- 35.Sinars CR, Cheung-Flynn J, Rimerman RA, Scammell JG, Smith DF, Clardy J. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 2003;100:868–73. doi: 10.1073/pnas.0231020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor P, Dornan J, Carrello A, Minchin RF, Ratajczak T, Walkinshaw MD. Two structures of cyclophilin 40: folding and fidelity in the TPR domains. Structure. 2001;9:431–8. doi: 10.1016/s0969-2126(01)00603-7. [DOI] [PubMed] [Google Scholar]
- 37.Wu B, Li P, Liu Y, Lou Z, Ding Y, Shu C, et al. 3D structure of human FK506-binding protein 52: implications for the assembly of the glucocorticoid receptor/Hsp90/immunophilin heterocomplex. Proc Natl Acad Sci U S A. 2004;101:8348–53. doi: 10.1073/pnas.0305969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cioffi DL, Hubler TR, Scammell JG. Organization and function of the FKBP52 and FKBP51 genes. Curr Opin Pharmacol. 2011;11:308–13. doi: 10.1016/j.coph.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Roe SM, Cliff MJ, Williams MA, Ladbury JE, Cohen PT, et al. Molecular basis for TPR domain-mediated regulation of protein phosphatase 5. EMBO J. 2005;24:1–10. doi: 10.1038/sj.emboj.7600496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen MX, Cohen PT. Activation of protein phosphatase 5 by limited proteolysis or the binding of polyunsaturated fatty acids to the TPR domain. FEBS Lett. 1997;400:136–40. doi: 10.1016/s0014-5793(96)01427-5. [DOI] [PubMed] [Google Scholar]
- 41.Ramsey AJ, Chinkers M. Identification of potential physiological activators of protein phosphatase 5. Biochemistry. 2002;41:5625–32. doi: 10.1021/bi016090h. [DOI] [PubMed] [Google Scholar]
- 42.Skinner J, Sinclair C, Romeo C, Armstrong D, Charbonneau H, Rossie S. Purification of a fatty acid-stimulated protein-serine/threonine phosphatase from bovine brain and its identification as a homolog of protein phosphatase 5. J Biol Chem. 1997;272:22464–71. doi: 10.1074/jbc.272.36.22464. [DOI] [PubMed] [Google Scholar]
- 43.Barent RL, Nair SC, Carr DC, Ruan Y, Rimerman RA, Fulton J, et al. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol. 1998;12:342–54. doi: 10.1210/mend.12.3.0075. [DOI] [PubMed] [Google Scholar]
- 44.Ratajczak T, Hlaing J, Brockway MJ, Hähnel R. Isolation of untransformed bovine estrogen receptor without molybdate stabilization. J Steroid Biochem. 1990;35:543–53. doi: 10.1016/0022-4731(90)90197-z. [DOI] [PubMed] [Google Scholar]
- 45.Silverstein AM, Galigniana MD, Kanelakis KC, Radanyi C, Renoir J-M, Pratt WB. Different regions of the immunophilin FKBP52 determine its association with the glucocorticoid receptor, hsp90, and cytoplasmic dynein. J Biol Chem. 1999;274:36980–6. doi: 10.1074/jbc.274.52.36980. [DOI] [PubMed] [Google Scholar]
- 46.Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, et al. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003;22:1158–67. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banerjee A, Periyasamy S, Wolf IM, Hinds TD, Jr, Yong W, Shou W, et al. Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins. Biochemistry. 2008;47:10471–80. doi: 10.1021/bi8011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dean DA, Urban G, Aragon IV, Swingle M, Miller B, Rusconi S, et al. Serine/threonine protein phosphatase 5 (PP5) participates in the regulation of glucocorticoid receptor nucleocytoplasmic shuttling. BMC Cell Biol. 2001;2:6. doi: 10.1186/1471-2121-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallo LI, Ghini AA, Piwien Pilipuk G, Galigniana MD. Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry. 2007;46:14044–57. doi: 10.1021/bi701372c. [DOI] [PubMed] [Google Scholar]
- 50.Echeverría PC, Mazaira G, Erlejman A, Gomez-Sanchez C, Piwien Pilipuk G, Galigniana MD. Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Mol Cell Biol. 2009;29:4788–97. doi: 10.1128/MCB.00649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galigniana MD, Erlejman AG, Monte M, Gomez-Sanchez C, Piwien-Pilipuk G. The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Mol Cell Biol. 2010;30:1285–98. doi: 10.1128/MCB.01190-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galigniana MD, Echeverría PC, Erlejman AG, Piwien-Pilipuk G. Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore. Nucleus. 2010;1:299–308. doi: 10.4161/nucl.1.4.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guiochon-Mantel A, Lescop P, Christin-Maitre S, Loosfelt H, Perrot-Applanat M, Milgrom E. Nucleocytoplasmic shuttling of the progesterone receptor. EMBO J. 1991;10:3851–9. doi: 10.1002/j.1460-2075.1991.tb04954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madan AP, DeFranco DB. Bidirectional transport of glucocorticoid receptors across the nuclear envelope. Proc Natl Acad Sci U S A. 1993;90:3588–92. doi: 10.1073/pnas.90.8.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–13. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84:663–9. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- 57.Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol. 2001;124:152–65. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- 58.Denny WB, Prapapanich V, Smith DF, Scammell JG. Structure-function analysis of squirrel monkey FK506-binding protein 51, a potent inhibitor of glucocorticoid receptor activity. Endocrinology. 2005;146:3194–201. doi: 10.1210/en.2005-0027. [DOI] [PubMed] [Google Scholar]
- 59.Davies TH, Ning YM, Sánchez ER. Differential control of glucocorticoid receptor hormone-binding function by tetratricopeptide repeat (TPR) proteins and the immunosuppressive ligand FK506. Biochemistry. 2005;44:2030–8. doi: 10.1021/bi048503v. [DOI] [PubMed] [Google Scholar]
- 60.Tai PK, Albers MW, Chang H, Faber LE, Schreiber SL. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992;256:1315–8. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- 61.Chen S, Sullivan WP, Toft DO, Smith DF. Differential interactions of p23 and the TPR-containing proteins Hop, Cyp40, FKBP52 and FKBP51 with Hsp90 mutants. Cell Stress Chaperones. 1998;3:118–29. doi: 10.1379/1466-1268(1998)003<0118:diopat>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheung-Flynn J, Roberts PJ, Riggs DL, Smith DF. C-terminal sequences outside the tetratricopeptide repeat domain of FKBP51 and FKBP52 cause differential binding to Hsp90. J Biol Chem. 2003;278:17388–94. doi: 10.1074/jbc.M300955200. [DOI] [PubMed] [Google Scholar]
- 63.Prodromou C, Pearl LH. Structure and functional relationships of Hsp90. Curr Cancer Drug Targets. 2003;3:301–23. doi: 10.2174/1568009033481877. [DOI] [PubMed] [Google Scholar]
- 64.Ramsey AJ, Russell LC, Whitt SR, Chinkers M. Overlapping sites of tetratricopeptide repeat protein binding and chaperone activity in heat shock protein 90. J Biol Chem. 2000;275:17857–62. doi: 10.1074/jbc.M001625200. [DOI] [PubMed] [Google Scholar]
- 65.Allan RK, Mok D, Ward BK, Ratajczak T. Modulation of chaperone function and cochaperone interaction by novobiocin in the C-terminal domain of Hsp90: evidence that coumarin antibiotics disrupt Hsp90 dimerization. J Biol Chem. 2006;281:7161–71. doi: 10.1074/jbc.M512406200. [DOI] [PubMed] [Google Scholar]
- 66.Mok D, Allan RK, Carrello A, Wangoo K, Walkinshaw MD, Ratajczak T. The chaperone function of cyclophilin 40 maps to a cleft between the prolyl isomerase and tetratricopeptide repeat domains. FEBS Lett. 2006;580:2761–8. doi: 10.1016/j.febslet.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 67.Duina AA, Marsh JA, Kurtz RB, Chang H-C, Lindquist S, Gaber RF. The peptidyl-prolyl isomerase domain of the CyP-40 cyclophilin homolog Cpr7 is not required to support growth or glucocorticoid receptor activity in Saccharomyces cerevisiae. J Biol Chem. 1998;273:10819–22. doi: 10.1074/jbc.273.18.10819. [DOI] [PubMed] [Google Scholar]
- 68.Mayr C, Richter K, Lilie H, Buchner J. Cpr6 and Cpr7, two closely related Hsp90-associated immunophilins from Saccharomyces cerevisiae, differ in their functional properties. J Biol Chem. 2000;275:34140–6. doi: 10.1074/jbc.M005251200. [DOI] [PubMed] [Google Scholar]
- 69.Wandinger SK, Suhre MH, Wegele H, Buchner J. The phosphatase Ppt1 is a dedicated regulator of the molecular chaperone Hsp90. EMBO J. 2006;25:367–76. doi: 10.1038/sj.emboj.7600930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garabedian MJ, Yamamoto KR. Genetic dissection of the signaling domain of a mammalian steroid receptor in yeast. Mol Biol Cell. 1992;3:1245–57. doi: 10.1091/mbc.3.11.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang Y, Fliss AE, Robins DM, Caplan AJ. Hsp90 regulates androgen receptor hormone binding affinity in vivo. J Biol Chem. 1996;271:28697–702. doi: 10.1074/jbc.271.45.28697. [DOI] [PubMed] [Google Scholar]
- 72.Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–8. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 73.Duina AA, Chang H-C, Marsh JA, Lindquist S, Gaber RF. A cyclophilin function in Hsp90-dependent signal transduction. Science. 1996;274:1713–5. doi: 10.1126/science.274.5293.1713. [DOI] [PubMed] [Google Scholar]
- 74.Duina AA, Marsh JA, Gaber RF. Identification of two CyP-40-like cyclophilins in Saccharomyces cerevisiae, one of which is required for normal growth. Yeast. 1996;12:943–52. doi: 10.1002/(sici)1097-0061(199608)12:10<943::aid-yea997>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 75.Warth R, Briand PA, Picard D. Functional analysis of the yeast 40 kDa cyclophilin Cyp40 and its role for viability and steroid receptor regulation. Biol Chem. 1997;378:381–91. doi: 10.1515/bchm.1997.378.5.381. [DOI] [PubMed] [Google Scholar]
- 76.Pirkl F, Buchner J. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J Mol Biol. 2001;308:795–806. doi: 10.1006/jmbi.2001.4595. [DOI] [PubMed] [Google Scholar]
- 77.Periyasamy S, Hinds T, Jr, Shemshedini L, Shou W, Sanchez ER. FKBP51 and Cyp40 are positive regulators of androgen-dependent prostate cancer cell growth and the targets of FK506 and cyclosporin A. Oncogene. 2010;29:1691–701. doi: 10.1038/onc.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hubler TR, Scammell JG. Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress Chaperones. 2004;9:243–52. doi: 10.1379/CSC-32R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paakinaho V, Makkonen H, Jääskeläinen T, Palvimo JJ. Glucocorticoid receptor activates poised FKBP51 locus through long-distance interactions. Mol Endocrinol. 2010;24:511–25. doi: 10.1210/me.2009-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wochnik GM, Rüegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–16. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 81.Fuller PJ, Smith BJ, Rogerson FM. Cortisol resistance in the New World revisited. Trends Endocrinol Metab. 2004;15:296–9. doi: 10.1016/j.tem.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Cluning C, Ward BK, Rea SL, Arulpragasam A, Fuller PJ, Ratajczak T. The helix 1-3 loop in the glucocorticoid receptor LBD is a regulatory element for FKBP cochaperones. Mol Endocrinol. 2013;27:1020–35. doi: 10.1210/me.2012-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19:1654–66. doi: 10.1210/me.2005-0071. [DOI] [PubMed] [Google Scholar]
- 84.Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, et al. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 2005;102:14326–31. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, et al. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol Endocrinol. 2006;20:2682–94. doi: 10.1210/me.2006-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J, Smith DF. Noncatalytic role of the FKBP52 peptidylprolyl isomerase domain in the regulation of steroid hormone signaling. Mol Cell Biol. 2007;27:8658–69. doi: 10.1128/MCB.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Z, Chen W, Kono E, Dang T, Garabedian MJ. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol Endocrinol. 2007;21:625–34. doi: 10.1210/me.2005-0338. [DOI] [PubMed] [Google Scholar]
- 88.Ikeda K, Ogawa S, Tsukui T, Horie-Inoue K, Ouchi Y, Kato S, et al. Protein phosphatase 5 is a negative regulator of estrogen receptor-mediated transcription. Mol Endocrinol. 2004;18:1131–43. doi: 10.1210/me.2003-0308. [DOI] [PubMed] [Google Scholar]
- 89.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–9. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 90.Vegiopoulos A, Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol. 2007;275:43–61. doi: 10.1016/j.mce.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 91.Hinds TD, Jr, Stechschulte LA, Cash HA, Whisler D, Banerjee A, Yong W, et al. Protein phosphatase 5 mediates lipid metabolism through reciprocal control of glucocorticoid receptor and peroxisome proliferator-activated receptor-γ (PPARγ) J Biol Chem. 2011;286:42911–22. doi: 10.1074/jbc.M111.311662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sumanasekera WK, Tien ES, Davis JW, 2nd, Turpey R, Perdew GH, Vanden Heuvel JP. Heat shock protein-90 (Hsp90) acts as a repressor of peroxisome proliferator-activated receptor-alpha (PPARalpha) and PPARbeta activity. Biochemistry. 2003;42:10726–35. doi: 10.1021/bi0347353. [DOI] [PubMed] [Google Scholar]
- 93.Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, Li W, et al. Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem. 2007;282:5026–36. doi: 10.1074/jbc.M609360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hong J, Kim ST, Tranguch S, Smith DF, Dey SK. Deficiency of co-chaperone immunophilin FKBP52 compromises sperm fertilizing capacity. Reproduction. 2007;133:395–403. doi: 10.1530/REP-06-0180. [DOI] [PubMed] [Google Scholar]
- 95.Chen H, Yong W, Hinds TD, Jr, Yang Z, Zhou Y, Sanchez ER, et al. Fkbp52 regulates androgen receptor transactivation activity and male urethra morphogenesis. J Biol Chem. 2010;285:27776–84. doi: 10.1074/jbc.M110.156091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tranguch S, Smith DF, Dey SK. Progesterone receptor requires a co-chaperone for signalling in uterine biology and implantation. Reprod Biomed Online. 2006;13:651–60. doi: 10.1016/s1472-6483(10)60655-4. [DOI] [PubMed] [Google Scholar]
- 97.Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117:1824–34. doi: 10.1172/JCI31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greig KT, Carotta S, Nutt SL. Critical roles for c-Myb in hematopoietic progenitor cells. Semin Immunol. 2008;20:247–56. doi: 10.1016/j.smim.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 99.Oates-Whitehead RM, Haas DM, Carrier JA. Progestogen for preventing miscarriage. Cochrane Database Syst Rev. 2003;4:CD003511. doi: 10.1002/14651858.CD003511. [DOI] [PubMed] [Google Scholar]
- 100.Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 101.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 102.Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol. 2002;83:149–55. doi: 10.1016/s0960-0760(02)00260-1. [DOI] [PubMed] [Google Scholar]
- 103.Jackson KS, Brudney A, Hastings JM, Mavrogianis PA, Kim JJ, Fazleabas AT. The altered distribution of the steroid hormone receptors and the chaperone immunophilin FKBP52 in a baboon model of endometriosis is associated with progesterone resistance during the window of uterine receptivity. Reprod Sci. 2007;14:137–50. doi: 10.1177/1933719106298409. [DOI] [PubMed] [Google Scholar]
- 104.Hirota Y, Tranguch S, Daikoku T, Hasegawa A, Osuga Y, Taketani Y, et al. Deficiency of immunophilin FKBP52 promotes endometriosis. Am J Pathol. 2008;173:1747–57. doi: 10.2353/ajpath.2008.080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang H, Zhou Y, Edelshain B, Schatz F, Lockwood CJ, Taylor HS. FKBP4 is regulated by HOXA10 during decidualization and in endometriosis. Reproduction. 2012;143:531–8. doi: 10.1530/REP-11-0438. [DOI] [PubMed] [Google Scholar]
- 106.Ward BK, Mark PJ, Ingram DM, Minchin RF, Ratajczak T. Expression of the estrogen receptor-associated immunophilins, cyclophilin 40 and FKBP52, in breast cancer. Breast Cancer Res Treat. 1999;58:267–80. doi: 10.1023/a:1006390804515. [DOI] [PubMed] [Google Scholar]
- 107.Ratajczak T, Woollatt E, Kumar P, Ward BK, Minchin RF, Baker E. Cyclophilin 40 (PPID) gene map position 4q31.3. Chromosome Res. 1997;5:151. doi: 10.1023/a:1018434628294. [DOI] [PubMed] [Google Scholar]
- 108.O’Connell P, Pekkel V, Fuqua S, Osborne CK, Allred DC. Molecular genetic studies of early breast cancer evolution. Breast Cancer Res Treat. 1994;32:5–12. doi: 10.1007/BF00666201. [DOI] [PubMed] [Google Scholar]
- 109.Ward BK, Kumar P, Turbett GR, Edmondston JE, Papadimitriou JM, Laing NG, et al. Allelic loss of cyclophilin 40, an estrogen receptor-associated immunophilin, in breast carcinomas. J Cancer Res Clin Oncol. 2001;127:109–15. doi: 10.1007/s004320000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li L, Lou Z, Wang L. The role of FKBP5 in cancer aetiology and chemoresistance. Br J Cancer. 2011;104:19–23. doi: 10.1038/sj.bjc.6606014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–66. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Febbo PG, Lowenberg M, Thorner AR, Brown M, Loda M, Golub TR. Androgen mediated regulation and functional implications of fkbp51 expression in prostate cancer. J Urol. 2005;173:1772–7. doi: 10.1097/01.ju.0000155845.44729.ba. [DOI] [PubMed] [Google Scholar]
- 113.Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–7. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- 114.Magee JA, Chang LW, Stormo GD, Milbrandt J. Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology. 2006;147:590–8. doi: 10.1210/en.2005-1001. [DOI] [PubMed] [Google Scholar]
- 115.Makkonen H, Kauhanen M, Paakinaho V, Jääskeläinen T, Palvimo JJ. Long-range activation of FKBP51 transcription by the androgen receptor via distal intronic enhancers. Nucleic Acids Res. 2009;37:4135–48. doi: 10.1093/nar/gkp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fabian AK, März A, Neimanis S, Biondi RM, Kozany C, Hausch F. InterAKTions with FKBPs—mutational and pharmacological exploration. PLoS One. 2013;8:e57508. doi: 10.1371/journal.pone.0057508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bouwmeester T, Bauch A, Ruffner H, Angrand P-O, Bergamini G, Croughton K, et al. A physical and functional map of the human TNF-α/NF-κ B signal transduction pathway. Nat Cell Biol. 2004;6:97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]
- 118.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 119.Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB, et al. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci U S A. 2004;101:10137–42. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–79. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 121.Golden T, Aragon IV, Rutland B, Tucker JA, Shevde LA, Samant RS, et al. Elevated levels of Ser/Thr protein phosphatase 5 (PP5) in human breast cancer. Biochim Biophys Acta. 2008;1782:259–70. doi: 10.1016/j.bbadis.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Urban G, Golden T, Aragon IV, Scammell JG, Dean NM, Honkanen RE. Identification of an estrogen-inducible phosphatase (PP5) that converts MCF-7 human breast carcinoma cells into an estrogen-independent phenotype when expressed constitutively. J Biol Chem. 2001;276:27638–46. doi: 10.1074/jbc.M103512200. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y, Leung DY, Nordeen SK, Goleva E. Estrogen inhibits glucocorticoid action via protein phosphatase 5 (PP5)-mediated glucocorticoid receptor dephosphorylation. J Biol Chem. 2009;284:24542–52. doi: 10.1074/jbc.M109.021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Periyasamy S, Warrier M, Tillekeratne MP, Shou W, Sanchez ER. The immunophilin ligands cyclosporin A and FK506 suppress prostate cancer cell growth by androgen receptor-dependent and -independent mechanisms. Endocrinology. 2007;148:4716–26. doi: 10.1210/en.2007-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin JF, Xu J, Tian HY, Gao X, Chen QX, Gu Q, et al. Identification of candidate prostate cancer biomarkers in prostate needle biopsy specimens using proteomic analysis. Int J Cancer. 2007;121:2596–605. doi: 10.1002/ijc.23016. [DOI] [PubMed] [Google Scholar]
- 126.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–41. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 127.Nagabhushan M, Miller CM, Pretlow TP, Giaconia JM, Edgehouse NL, Schwartz S, et al. CWR22: the first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 1996;56:3042–6. [PubMed] [Google Scholar]
- 128.Amler LC, Agus DB, LeDuc C, Sapinoso ML, Fox WD, Kern S, et al. Dysregulated expression of androgen-responsive and nonresponsive genes in the androgen-independent prostate cancer xenograft model CWR22-R1. Cancer Res. 2000;60:6134–41. [PubMed] [Google Scholar]
- 129.Mousses S, Wagner U, Chen Y, Kim JW, Bubendorf L, Bittner M, et al. Failure of hormone therapy in prostate cancer involves systematic restoration of androgen responsive genes and activation of rapamycin sensitive signaling. Oncogene. 2001;20:6718–23. doi: 10.1038/sj.onc.1204889. [DOI] [PubMed] [Google Scholar]
- 130.Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 131.Ni L, Yang C-S, Gioeli D, Frierson H, Toft DO, Paschal BM. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol Cell Biol. 2010;30:1243–53. doi: 10.1128/MCB.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 133.Warrier M, Hinds TD, Jr, Ledford KJ, Cash HA, Patel PR, Bowman TA, et al. Susceptibility to diet-induced hepatic steatosis and glucocorticoid resistance in FK506-binding protein 52-deficient mice. Endocrinology. 2010;151:3225–36. doi: 10.1210/en.2009-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Di Dalmazi G, Pagotto U, Pasquali R, Vicennati V. Glucocorticoids and type 2 diabetes: from physiology to pathology. J Nutr Metab. 2012;2012:525093. doi: 10.1155/2012/525093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grankvist N, Honkanen RE, Sjöholm Å, Ortsäter H. Genetic disruption of protein phosphatase 5 in mice prevents high-fat diet feeding-induced weight gain. FEBS Lett. 2013;587:3869–74. doi: 10.1016/j.febslet.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 136.Fransson L, Rosengren V, Saha TK, Grankvist N, Islam T, Honkanen RE, et al. Mitogen-activated protein kinases and protein phosphatase 5 mediate glucocorticoid-induced cytotoxicity in pancreatic islets and β-cells. Mol Cell Endocrinol. 2014;383:126–36. doi: 10.1016/j.mce.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 137.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 138.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Pütz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–25. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 139.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–95. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 140.Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, et al. The FKBP5-gene in depression and treatment response—an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol Psychiatry. 2008;63:1103–10. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shibuya N, Suzuki A, Sadahiro R, Kamata M, Matsumoto Y, Goto K, et al. Association study between a functional polymorphism of FK506-binding protein 51 (FKBP5) gene and personality traits in healthy subjects. Neurosci Lett. 2010;485:194–7. doi: 10.1016/j.neulet.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 142.Oswald LM, Zandi P, Nestadt G, Potash JB, Kalaydjian AE, Wand GS. Relationship between cortisol responses to stress and personality. Neuropsychopharmacology. 2006;31:1583–91. doi: 10.1038/sj.npp.1301012. [DOI] [PubMed] [Google Scholar]
- 143.Lee RS, Tamashiro KL, Yang X, Purcell RH, Harvey A, Willour VL, et al. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010;151:4332–43. doi: 10.1210/en.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Appel K, Schwahn C, Mahler J, Schulz A, Spitzer C, Fenske K, et al. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology. 2011;36:1982–91. doi: 10.1038/npp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–83. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scharf SH, Liebl C, Binder EB, Schmidt MV, Müller MB. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS One. 2011;6:e16883. doi: 10.1371/journal.pone.0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Touma C, Gassen NC, Herrmann L, Cheung-Flynn J, Büll DR, Ionescu IA, et al. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol Psychiatry. 2011;70:928–36. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 150.O’Leary JC, 3rd, Dharia S, Blair LJ, Brady S, Johnson AG, Peters M, et al. A new anti-depressive strategy for the elderly: ablation of FKBP5/FKBP51. PLoS One. 2011;6:e24840. doi: 10.1371/journal.pone.0024840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang XD, Wolf M, et al. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2012;62:332–9. doi: 10.1016/j.neuropharm.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 152.Hartmann J, Wagner KV, Dedic N, Marinescu D, Scharf SH, Wang XD, et al. Fkbp52 heterozygosity alters behavioral, endocrine and neurogenetic parameters under basal and chronic stress conditions in mice. Psychoneuroendocrinology. 2012;37:2009–21. doi: 10.1016/j.psyneuen.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 153.Petrovich E, Asher C, Garty H. Induction of FKBP51 by aldosterone in intestinal epithelium. J Steroid Biochem Mol Biol. 2014;139:78–87. doi: 10.1016/j.jsbmb.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 154.Li XJ, Luo XQ, Han BW, Duan FT, Wei PP, Chen YQ. MicroRNA-100/99a, deregulated in acute lymphoblastic leukaemia, suppress proliferation and promote apoptosis by regulating the FKBP51 and IGF1R/mTOR signalling pathways. Br J Cancer. 2013;109:2189–98. doi: 10.1038/bjc.2013.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou G, Golden T, Aragon IV, Honkanen RE. Ser/Thr protein phosphatase 5 inactivates hypoxia-induced activation of an apoptosis signal-regulating kinase 1/MKK-4/JNK signaling cascade. J Biol Chem. 2004;279:46595–605. doi: 10.1074/jbc.M408320200. [DOI] [PubMed] [Google Scholar]
- 156.Kumar P, Mark PJ, Ward BK, Minchin RF, Ratajczak T. Estradiol-regulated expression of the immunophilins cyclophilin 40 and FKBP52 in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2001;284:219–25. doi: 10.1006/bbrc.2001.4952. [DOI] [PubMed] [Google Scholar]
- 157.Kumar P, Ward BK, Minchin RF, Ratajczak T. Regulation of the Hsp90-binding immunophilin, cyclophilin 40, is mediated by multiple sites for GA-binding protein (GABP) Cell Stress Chaperones. 2001;6:78–91. doi: 10.1379/1466-1268(2001)006<0078:rothbi>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pearson JD, Mohammed Z, Bacani JT, Lai R, Ingham RJ. The heat shock protein-90 co-chaperone, Cyclophilin 40, promotes ALK-positive, anaplastic large cell lymphoma viability and its expression is regulated by the NPM-ALK oncoprotein. BMC Cancer. 2012;12:229. doi: 10.1186/1471-2407-12-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yokoi H, Shimizu Y, Anazawa H, Lefebvre CA, Korneluk RG, Ikeda JE. The structure and complete nucleotide sequence of the human cyclophilin 40 (PPID) gene. Genomics. 1996;35:448–55. doi: 10.1006/geno.1996.0384. [DOI] [PubMed] [Google Scholar]
- 160.Sanchez ER. Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes. J Biol Chem. 1990;265:22067–70. [PubMed] [Google Scholar]
- 161.Scammell JG, Hubler TR, Denny WB, Valentine DL. Organization of the human FK506-binding immunophilin FKBP52 protein gene (FKBP4) Genomics. 2003;81:640–3. doi: 10.1016/s0888-7543(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 162.Daikoku T, Tranguch S, Friedman DB, Das SK, Smith DF, Dey SK. Proteomic analysis identifies immunophilin FK506 binding protein 4 (FKBP52) as a downstream target of Hoxa10 in the periimplantation mouse uterus. Mol Endocrinol. 2005;19:683–97. doi: 10.1210/me.2004-0332. [DOI] [PubMed] [Google Scholar]
- 163.Coller HA, Grandori C, Tamayo P, Colbert T, Lander ES, Eisenman RN, et al. Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling, and adhesion. Proc Natl Acad Sci U S A. 2000;97:3260–5. doi: 10.1073/pnas.97.7.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dubik D, Dembinski TC, Shiu RP. Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cells. Cancer Res. 1987;47:6517–21. [PubMed] [Google Scholar]
- 165.Wang C, Mayer JA, Mazumdar A, Fertuck K, Kim H, Brown M, et al. Estrogen induces c–myc gene expression via an upstream enhancer activated by the estrogen receptor and the AP-1 transcription factor. Mol Endocrinol. 2011;25:1527–38. doi: 10.1210/me.2011-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kang H, Sayner SL, Gross KL, Russell LC, Chinkers M. Identification of amino acids in the tetratricopeptide repeat and C-terminal domains of protein phosphatase 5 involved in autoinhibition and lipid activation. Biochemistry. 2001;40:10485–90. doi: 10.1021/bi010999i. [DOI] [PubMed] [Google Scholar]
- 167.Connarn JN, Assimon VA, Reed RA, Tse E, Southworth DR, Zuiderweg ER, et al. The molecular chaperone Hsp70 activates protein phosphatase 5 (PP5) by binding the tetratricopeptide repeat (TPR) domain. J Biol Chem. 2014;289:2908–17. doi: 10.1074/jbc.M113.519421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Carrello A, Allan RK, Morgan SL, Owen BA, Mok D, Ward BK, et al. Interaction of the Hsp90 cochaperone cyclophilin 40 with Hsc70. Cell Stress Chaperones. 2004;9:167–81. doi: 10.1379/CSC-26R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Das AK, Cohen PW, Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 1998;17:1192–9. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, et al. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- 171.Ward BK, Allan RK, Mok D, Temple SE, Taylor P, Dornan J, et al. A structure-based mutational analysis of cyclophilin 40 identifies key residues in the core tetratricopeptide repeat domain that mediate binding to Hsp90. J Biol Chem. 2002;277:40799–809. doi: 10.1074/jbc.M207097200. [DOI] [PubMed] [Google Scholar]
- 172.Cliff MJ, Harris R, Barford D, Ladbury JE, Williams MA. Conformational diversity in the TPR domain-mediated interaction of protein phosphatase 5 with Hsp90. Structure. 2006;14:415–26. doi: 10.1016/j.str.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 173.Cliff MJ, Williams MA, Brooke-Smith J, Barford D, Ladbury JE. Molecular recognition via coupled folding and binding in a TPR domain. J Mol Biol. 2005;346:717–32. doi: 10.1016/j.jmb.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 174.Sekine Y, Hatanaka R, Watanabe T, Sono N, Iemura S, Natsume T, et al. The Kelch repeat protein KLHDC10 regulates oxidative stress-induced ASK1 activation by suppressing PP5. Mol Cell. 2012;48:692–704. doi: 10.1016/j.molcel.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 175.Yamaguchi F, Umeda Y, Shimamoto S, Tsuchiya M, Tokumitsu H, Tokuda M, et al. S100 proteins modulate protein phosphatase 5 function: a link between CA2+ signal transduction and protein dephosphorylation. J Biol Chem. 2012;287:13787–98. doi: 10.1074/jbc.M111.329771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shimamoto S, Kubota Y, Tokumitsu H, Kobayashi R. S100 proteins regulate the interaction of Hsp90 with Cyclophilin 40 and FKBP52 through their tetratricopeptide repeats. FEBS Lett. 2010;584:1119–25. doi: 10.1016/j.febslet.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 177.Shimamoto S, Takata M, Tokuda M, Oohira F, Tokumitsu H, Kobayashi R. Interactions of S100A2 and S100A6 with the tetratricopeptide repeat proteins, Hsp90/Hsp70-organizing protein and kinesin light chain. J Biol Chem. 2008;283:28246–58. doi: 10.1074/jbc.M801473200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem J. 2006;396:201–14. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Morita K, Saitoh M, Tobiume K, Matsuura H, Enomoto S, Nishitoh H, et al. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 2001;20:6028–36. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L, Gomes DaGama EM, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011;7:818–26. doi: 10.1038/nchembio.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Beliakoff J, Whitesell L. Hsp90: an emerging target for breast cancer therapy. Anticancer Drugs. 2004;15:651–62. doi: 10.1097/01.cad.0000136876.11928.be. [DOI] [PubMed] [Google Scholar]
- 183.Azad AA, Zoubeidi A, Gleave ME, Chi KN. Targeting heat shock proteins in metastatic castration-resistant prostate cancer. Nat Rev Urol. 2015;12:26–36. doi: 10.1038/nrurol.2014.320. [DOI] [PubMed] [Google Scholar]
- 184.Cardillo MR, Ippoliti F. IL-6, IL-10 and HSP-90 expression in tissue microarrays from human prostate cancer assessed by computer-assisted image analysis. Anticancer Res. 2006;26(5A):3409–16. [PubMed] [Google Scholar]
- 185.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8:440–8. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2012;1823:742–55. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Travers J, Sharp S, Workman P. HSP90 inhibition: two-pronged exploitation of cancer dependencies. Drug Discov Today. 2012;17:242–52. doi: 10.1016/j.drudis.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 188.Marcu MG, Schulte TW, Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J Natl Cancer Inst. 2000;92:242–8. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 189.Matts RL, Dixit A, Peterson LB, Sun L, Voruganti S, Kalyanaraman P, et al. Elucidation of the Hsp90 C-terminal inhibitor binding site. ACS Chem Biol. 2011;6:800–7. doi: 10.1021/cb200052x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Matthews SB, Vielhauer GA, Manthe CA, Chaguturu VK, Szabla K, Matts RL, et al. Characterization of a novel novobiocin analogue as a putative C-terminal inhibitor of heat shock protein 90 in prostate cancer cells. Prostate. 2010;70:27–36. doi: 10.1002/pros.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ardi VC, Alexander LD, Johnson VA, McAlpine SR. Macrocycles that inhibit the binding between heat shock protein 90 and TPR-containing proteins. ACS Chem Biol. 2011;6:1357–66. doi: 10.1021/cb200203m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.McConnell JR, Alexander LA, McAlpine SR. A heat shock protein 90 inhibitor that modulates the immunophilins and regulates hormone receptors without inducing the heat shock response. Bioorg Med Chem Lett. 2014;24:661–6. doi: 10.1016/j.bmcl.2013.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Estébanez-Perpiñá E, Arnold LA, Nguyen P, Rodrigues ED, Mar E, Bateman R, et al. A surface on the androgen receptor that allosterically regulates coactivator binding. Proc Natl Acad Sci U S A. 2007;104:16074–9. doi: 10.1073/pnas.0708036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.De Leon JT, Iwai A, Feau C, Garcia Y, Balsiger HA, Storer CL, et al. Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc Natl Acad Sci U S A. 2011;108:11878–83. doi: 10.1073/pnas.1105160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Moore TW, Mayne CG, Katzenellenbogen JA. Minireview: Not picking pockets: nuclear receptor alternate-site modulators (NRAMs) Mol Endocrinol. 2010;24:683–95. doi: 10.1210/me.2009-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Joseph JD, Wittmann BM, Dwyer MA, Cui H, Dye DA, McDonnell DP, et al. Inhibition of prostate cancer cell growth by second-site androgen receptor antagonists. Proc Natl Acad Sci U S A. 2009;106:12178–83. doi: 10.1073/pnas.0900185106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Galat A. Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity—targets—functions. Curr Top Med Chem. 2003;3:1315–47. doi: 10.2174/1568026033451862. [DOI] [PubMed] [Google Scholar]
- 198.Wang P, Heitman J. The cyclophilins. Genome Biol. 2005;6:226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hoffmann K, Kakalis LT, Anderson KS, Armitage IM, Handschumacher RE. Expression of human cyclophilin-40 and the effect of the His141—>Trp mutation on catalysis and cyclosporin A binding. Eur J Biochem. 1995;229:188–93. doi: 10.1111/j.1432-1033.1995.tb20454.x. [DOI] [PubMed] [Google Scholar]
- 200.Ratajczak T, Carrello A, Minchin RF. Biochemical and calmodulin binding properties of estrogen receptor binding cyclophilin expressed in Escherichia coli. Biochem Biophys Res Commun. 1995;209:117–25. doi: 10.1006/bbrc.1995.1478. [DOI] [PubMed] [Google Scholar]
- 201.Gaali S, Kozany C, Hoogeland B, Klein M, Hausch F. Facile synthesis of a fluorescent cyclosporin a analogue to study cyclophilin 40 and cyclophilin 18 ligands. ACS Med Chem Lett. 2010;1:536–9. doi: 10.1021/ml1001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Blackburn EA, Walkinshaw MD. Targeting FKBP isoforms with small-molecule ligands. Curr Opin Pharmacol. 2011;11:365–71. doi: 10.1016/j.coph.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 203.Bracher A, Kozany C, Hähle A, Wild P, Zacharias M, Hausch F. Crystal structures of the free and ligand-bound FK1-FK2 domain segment of FKBP52 reveal a flexible inter-domain hinge. J Mol Biol. 2013;425:4134–44. doi: 10.1016/j.jmb.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 204.Gopalakrishnan R, Kozany C, Gaali S, Kress C, Hoogeland B, Bracher A, et al. Evaluation of synthetic FK506 analogues as ligands for the FK506-binding proteins 51 and 52. J Med Chem. 2012;55:4114–22. doi: 10.1021/jm201746x. [DOI] [PubMed] [Google Scholar]
- 205.Gaali S, Gopalakrishnan R, Wang Y, Kozany C, Hausch F. The chemical biology of immunophilin ligands. Curr Med Chem. 2011;18:5355–79. doi: 10.2174/092986711798194342. [DOI] [PubMed] [Google Scholar]
- 206.Schmidt MV, Paez-Pereda M, Holsboer F, Hausch F. The prospect of FKBP51 as a drug target. ChemMedChem. 2012;7:1351–9. doi: 10.1002/cmdc.201200137. [DOI] [PubMed] [Google Scholar]
- 207.Zheng Q, Cai X, Tan MH, Schaffert S, Arnold CP, Gong X, et al. Precise gene deletion and replacement using the CRISPR/Cas9 system in human cells. Biotechniques. 2014;57:115–24. doi: 10.2144/000114196. [DOI] [PubMed] [Google Scholar]
- 208.Calderwood SK. Molecular cochaperones: tumor growth and cancer treatment. Scientifica (Cairo) 2013;2013:217513. doi: 10.1155/2013/217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schmid AB, Lagleder S, Gräwert MA, Röhl A, Hagn F, Wandinger SK, et al. The architecture of functional modules in the Hsp90 co-chaperone Sti1/Hop. EMBO J. 2012;31:1506–17. doi: 10.1038/emboj.2011.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Koulov AV, LaPointe P, Lu B, Razvi A, Coppinger J, Dong MQ, et al. Biological and structural basis for Aha1 regulation of Hsp90 ATPase activity in maintaining proteostasis in the human disease cystic fibrosis. Mol Biol Cell. 2010;21:871–84. doi: 10.1091/mbc.E09-12-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Retzlaff M, Hagn F, Mitschke L, Hessling M, Gugel F, Kessler H, et al. Asymmetric activation of the hsp90 dimer by its cochaperone aha1. Mol Cell. 2010;37:344–54. doi: 10.1016/j.molcel.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 212.Karagöz GE, Duarte AM, Ippel H, Uetrecht C, Sinnige T, van Rosmalen M, et al. N-terminal domain of human Hsp90 triggers binding to the cochaperone p23. Proc Natl Acad Sci U S A. 2011;108:580–5. doi: 10.1073/pnas.1011867108. [DOI] [PMC free article] [PubMed] [Google Scholar]


