Abstract
The vitamin D receptor (VDR), a nuclear transcription factor, elicits physiological regulation of gene transcription following binding of its ligand, 1,25-dihydroxyvitamin D. The major biological activities of vitamin D contribute to regulation of plasma calcium and phosphate homeostasis and bone remodeling, although recent evidence suggests that vitamin D, like other steroid hormone receptors, can regulate a diverse range of biological activities across many tissues. Such properties raise the notion that vitamin D deficiency may not only be detrimental to bone and muscular health, but also a risk factor for a number of adverse health outcomes including increased risk of cardiovascular disease, inflammation, immune system disorders and cancer. Advances in transcriptional research provide data not only on ligand-dependent activities of the VDR, but other activities of vitamin D extending to rapid modulation of intra-cellular signaling pathways as well as apparent ligand-independent interactions between the VDR and other transcriptionally active proteins. In this review, we detail the chief molecular activities of the VDR in regulating gene transcription, intracellular signaling and actions of VDR via binding to transcriptional regulating proteins. The breadth of biological activities attributed to vitamin D informs clinical biochemists and health care professionals on the implications of vitamin D deficiency for health.
Introduction
The vitamin D receptor (VDR) is a nuclear transcription factor responsible for the biological activity of vitamin D by binding its ligand, the active form of the vitamin D hormone, 1,25-dihydroxyvitamin D (1,25D). Present in a diverse range of tissues, the VDR has the ability to exert an extensive biological response, when activated by ligand-binding, via regulation of gene transcription and stimulation of intra-cellular signaling pathways. The dominant action of the VDR, as evidenced by genetic knockout models and clinical evidence,1–4 is to regulate plasma calcium and phosphate homeostasis via stimulation of intestinal calcium absorption, renal tubular reabsorption of calcium and resorption of bone. More recently, vitamin D has been shown to play a role in cell function outside of calcium and mineral homeostasis including inhibition of cellular proliferation and stimulation of cell maturation which may involve various tissues including skin, the immune system and possibly others such as colonic, breast and prostate cells. Moreover, vitamin D deficiency is associated with a number of adverse health outcomes including increased risk of cardiovascular disease, inflammation, diabetes and disturbed hair follicle cycling.5–7 Whether vitamin D deficiency exerts a causal role with these extraskeletal systems remains to be established.
Recent advances in transcriptional research, such as the development of chromatin immunoprecipitation (ChIP) analysis, have increased understanding of vitamin D’s ability to influence gene transcription via ligand-dependent actions.8 However, the ability for vitamin D to exert a diverse range of physiological activities extends beyond this classical interpretation of activity to facilitate the more recently discovered functions of 1,25D and VDR including regulation of gene expression via complex intracellular signaling pathways. Since vitamin D plays a key role in fundamental physiological processes and also in the pathophysiology of many disease states, it is important to understand the breadth of biological activities, which are under the control of vitamin D to better inform clinical biochemists and health care professionals of the implications of vitamin D deficiency.
Regulation of Gene Transcription
Binding of the ligand, 1,25D, to its receptor, VDR, initiates heterodimerisation with the retinoid-X receptor (RXR) at a vitamin D response element (VDRE), a specific DNA sequence located within the promoter region of vitamin D responsive genes.9 Binding of the liganded, heterodimerised VDR complex (VDR-RXR) to the VDRE directs the formation of a large protein complex that incorporates various co-regulatory molecules. The transcriptional complex activates gene transcription largely through chromatin remodeling and thus represents the specificity and sensitivity of the VDR. Chromatin remodeling is a central function of this transcriptional complex when bound to the VDRE. In the case of gene transcription, the complex recruits steroid receptor co-activators, such as steroid receptor coactivator-1 (SRC-1),10 vitamin D receptor interacting proteins (DRIP) such as MED111 and NCoA6212 in addition to histone acetyltransferases to derepress the locally condensed chromatin. The ultimate result is upregulation of a range of genes required for normal calcium and bone homeostasis.13–18 Furthermore, the VDR-RXR heterodimer may also act in unison with other corepressor molecules and potentiate repression of the expression of genes such as parathyroid hormone.19 After recruitment of the transcriptional complex, VDR-RXR synergises with vitamin D receptor interacting protein (DRIP) to recruit components of the basal molecular machinery, such as RNA polymerase II, necessary to initiate gene transcription (Figure 1).20
Figure 1.
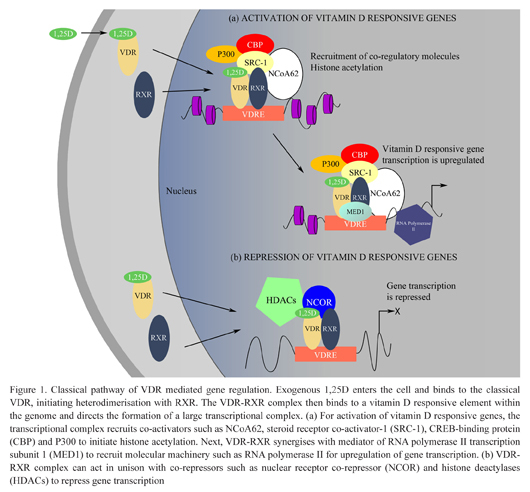
Classical pathway of VDR mediated gene regulation. Exogenous 1,25D enters the cell and binds to the classical VDR, initiating heterodimerisation with RXR. The VDR-RXR complex then binds to a vitamin D responsive element within the genome and directs the formation of a large transcriptional complex. (a) For activation of vitamin D responsive genes, the transcriptional complex recruits co-activators such as NCoA62, steroid receptor co-activator-1 (SRC-1), CREB-binding protein (CBP) and P300 to initiate histone acetylation. Next, VDR-RXR synergises with mediator of RNA polymerase II transcription subunit 1 (MED1) to recruit molecular machinery such as RNA polymerase II for upregulation of gene transcription. (b) VDRRXR complex can act in unison with co-repressors such as nuclear receptor co-repressor (NCOR) and histone deactylases (HDACs) to repress gene transcription
It is the totality of the transcriptional complexes which defines the specificity and sensitivity of vitamin D to regulate biological responses through a wide range of tissues. Currently we understand the contribution of at least four elements of the transcriptional complex. In the case of vitamin D, the nuclear receptor ligand, 1,25D, identifies the physiological specificity of the response. The VDRE identifies the genetic specificity of the response. The various co-activators and other proteins complexing to the liganded VDR-RXR heterodimer bound to the VDRE identify the cell specificity and finally, the vitamin D-responsive gene product identifies the biological response.21
To regulate the level of 1,25D, the ligand-bound VDR complex must regulate its biosynthesis and degradation. Central to this process are the enzymes 1,25 dihydroxyvitamin D 24-hydroxylase (24-OHase), encoded by the CYP24 gene and responsible for oxidation of 1,25D to water-soluble catabolites and 25 hydroxyvitamin 1alpha hydroxylase (1αOHase), encoded by the CYP27B1 gene and responsible for synthesis of 1,25D from 25-hydroxyvitamin D. As mentioned above, VDR-ligand interaction regulates expression of these genes allowing for exquisite control over the level of intracellular 1,25D. The 1,25D endocrine system arises from plasma levels of 1,25D, which in health are solely regulated through the expression of these genes in the kidney. Expression of these genes occurs across numerous tissues regulating 1,25D within each of these tissues for autocrine/paracrine activities.22
CYP24 is expressed, as far as is known, in every cell which expresses the VDR. It is highly sensitive to 1,25D activation, owing to the fact that it contains two VDREs in its promoter region23–25 although this varies between species.26 Intriguingly, ChIP-chip and ChIP-seq analyses have revealed that CYP24 also contains downstream enhancers some thousands of base pairs from the CYP24 gene that contribute to VDR gene transcription signalling and protein recruitment.27 The importance of VDR-ligand binding to 24-OHase levels is most apparent in mice in which the VDR gene has been deleted throughout the whole animal, the global-VDR knockout model, whereby levels of the water-soluble catabolites of 1,25D were found to be extremely low.28 When the CYP24 gene was deleted, mice displayed highly elevated plasma 1,25D levels and aberrant calcium homeostasis that greatly affected bone mineralisation processes.29 Moreover, altered CYP24 expression has been implicated in a wide variety of cancers, with the overarching suggestion that inhibition of CYP24 expression may be useful as an anti-cancer therapeutic.30 Recent data also suggest that 1,25D can also negatively regulate CYP24 transcription through epigenetic events involving histone methylation.31
Regulation of intestinal calcium absorption is one of the most-well recognised aspects of vitamin D activity. Calcium transport across intestinal epithelium is achieved through the ion channel transient receptor potential vanilloid type 6 (TRPV6).32 Seminal in vivo studies by Song et al in 2003 demonstrated that 1,25D regulates mRNA expression of this gene and later work by Meyer and colleagues in 2006 demonstrated that TRPV6 has multiple VDR binding sites that are necessary for transcriptional regulation of the gene via 1,25D.18, 33 As is the case with CYP24, the upregulation of TRPV6 requires VDR-RXR binding to several promoter region VDREs enabling histone acetylation to remodel the local chromatin environment, and enhance recruitment of coactivators to stimulate gene transcription. The ultimate physiological outcome has been well demonstrated in transgenic mice where over-expression of TRPV6 led to significant increases in intestinal calcium absorption and had a marked effect on bone volume.34 These results provide evidence that manipulation of gene expression is a key component of the physiological activity of the vitamin D hormone, particularly in relation to regulation of intestinal calcium absorption contributing to plasma calcium homeostasis.
Both the vitamin D endocrine and autocrine/paracrine systems support the formation of healthy bones and each of the major bone cell types have the capability to respond to 1,25D to mediate their activities, as well as to synthesise 1,25D.35–37 Therefore, it is reasonable to suspect that 1,25D can regulate gene transcription of bone cell-specific factors. Indeed, seminal studies demonstrated 1,25D to upregulate expression of the gene coding for the protein receptor activator of nuclear factor kappa-B ligand (RANKL), which is a key controller of osteoclast differentiation.38 In vitro analyses of the gene responsible for RANKL, TNFSF11, identified VDR-RXR complex binding to a number of VDREs, including some up to -76kb upstream of the TNFSF11 promoter region in a portion of the genome termed the Distal Control Region (DCR).39, 40 In vivo evidence illustrated the physiological impact of deletion of the DCR, demonstrably blunting the response of 1,25D and PTH and increasing bone mass and strength in a murine model due to decreased bone resorption.41 Thus, VDR-ligand binding is important for RANKL gene expression and as such, plays a pivotal role in the regulation of plasma calcium and bone homeostatic processes. Moreover, 1,25D-induced transactivation of RANKL provides a very clear example of the regulatory networks that may be required for physiologically appropriate gene transcription.
Regulation of the calcium economy requires the cooperation of parathyroid hormone (PTH) and plasma 1,25D, with the latter having a powerful effect in decreasing transcription of the PTH gene as evidenced by early in vitro and in vivo studies.42–44 Notably, mice lacking the VDR demonstrate marked increases in PTH as transcriptional repression is lost.1 Although the biological response is quite clear, how the VDREs within the PTH gene control a negative molecular response is currently poorly understood, though several groups have proposed sites that may modulate negative regulation.19,45,46 By contrast, analysis of the VDRE in the avian PTH gene demonstrated that there may be several co-regulatory proteins that can in fact mediate a positive regulatory response and thus the VDR-RXR complex alone may not be the key to transcriptional regulation of PTH.47 At present, there is little credible evidence that adequately demonstrates how 1,25D can transcriptionally repress PTH and further investigation into this mechanism is necessary to unravel the enigma of 1,25D gene repression.
Non-Transcriptional Activities of Vitamin D Membrane-Bound Receptors for 1,25D
The traditional activities of 1,25D to modulate gene transcription are also complemented by its ability to regulate intracellular, extranuclear pathways and cytoplasmic signalling cascades. As opposed to the genomic activities described in the previous section which act over the course of hours to manifest changes in protein levels, these cytoplasmic actions are generally regarded to be rapid responses, taking place within seconds to minutes. A vast library of studies details the ability for 1,25D to affect intracellular calcium levels as well as intracellular signalling pathways involving phosphate kinases and phosphatases. Of note is the fact that activation of different signalling pathways are dependent on the cell type, thus providing an avenue to explain the pleiotropic effects of vitamin D.48–50
Nemere and colleagues have extensively researched the so-called non-genomic activities of 1,25D through investigation of membrane proteins and intracellular signalling. They have revealed that 1,25D can act through a distinct membrane-associated rapid-response steroid-binding (MARRS) protein51 to facilitate rapid responses. This protein was found to be identical to the multifunctional protein disulfide isomerise family A, member 3 (PDIA3), an enzyme of the endoplasmic reticulum. Antibody blocking this protein prevented calcium and phosphate transport across intestinal epithelial cell membranes.51–52 Further work also demonstrated PDIA3 can rapidly activate the protein kinase C pathway in chondrocytes and bone-forming osteoblasts.53–54 Further studies determined that PDIA3 is located in plasma membrane caveolae and that it physically interacts with scaffolding proteins present in this location to activate signalling cascades.55 In vivo ablation of the PDIA3 gene in mice confirmed the necessity of this protein in modulation of the rapid response to 1,25D.56 Notably, isolation of primary osteoblasts from calvaria of both wild type and global VDR null mice showed rapid activation of this pathway occurred in response to 1,25D in both genotypes, suggesting that these particular responses do not require the traditional nuclear VDR.57
Interestingly, several studies suggest the classical VDR can also associate with caveolae of the plasma membrane, like other steroid hormone receptors such as the estrogen receptor.58–61 There are several isomers of 1,25D, and to elicit these non-genomic actions through the classical VDR, 1,25D must be in the 6-s-cis conformational shape, as opposed to the 6-s-trans . The former is responsible for the rapid biological response associated with non-genomic activities, whereas the latter is the preferred conformation for the genomic activities.62 The importance of these conformation states has become more apparent subsequent to identification that the classical VDR may have two ligand-binding pockets63 lending credence to the wide variety of biological activities proposed for 1,25D. Moreover, the classical VDR does seem necessary to stimulate some non-genomic responses such as intracellular calcium ion flux, as was demonstrated in ROS17/2.8 osteosarcoma cells.64 Do the classical VDR and the PDIA3 protein at the plasma membrane work together to achieve biological activity? More recent data suggest that this may be the case as photoprotection of fibroblasts has been demonstrated to be a rapid non-genomic action of vitamin D that requires both proteins.65 Ultimately, the rapid responses provided by 1,25D regulation of non-genomic pathways demonstrate the extensive variety of actions that the vitamin D system is required to maintain. Coupled with regulation of genomic actions, the extent to which vitamin D can regulate physiological processes is becoming clearer.
VDR Binding to Intracellular Proteins
The VDR also exerts biological activities by directly binding to intracellular proteins to either stimulate VDRE-mediated genomic activity or by influencing the activity of other transcriptional regulating proteins. One of the most extensively researched proteins is β-catenin which has a dual function contributing to cell-cell adhesion when located at adherens junctions of the plasma membrane and to regulation of gene transcription. Β-catenin functions in the Wnt signaling pathway as a regulator of gene transcription and has long been implicated in hair follicle cycling, malignancy and more recently, bone mineral homeostasis.66–68 The activity of Wnt signaling is to mobilise β-catenin from the cytoplasm, where it is bound to the linker protein E-cadherin, to the nucleus allowing it to bind transcription factors of the T-cell factor (TCF) and lymphoid enhancer factor (LEF) families to promote gene expression. The vitamin D system can antagonise this Β-catenin transcriptional process via two methods. The first is to reduce β-catenin signaling indirectly by genomic regulation of CDH1, encoding for E-cadherin. In a human colorectal cancer cell line expressing the VDR (SW480-ADH), 1,25D markedly increased the level of E-cadherin expression via upregulating transcription of the E-cadherin gene. This coincided with disturbed β-catenin distribution, whereby 1,25D increased nuclear export and localisation to the plasma membrane69, 70 thus reducing proliferation of this cancer cell model. On the other hand, VDR can influence β-catenin signaling independent of E-cadherin upregulation, via direct binding69 which relies upon the activator function-2 (AF-2) domain of the VDR.70 Shah and colleagues revealed 1,25D represses β-catenin signaling equally in a colorectal cancer cell line with and without homozygous E-cadherin deletion demonstrating that the biological outcomes are a result of VDR-β-catenin interaction.71
β-catenin-VDR interactions have also been implicated as necessary factors in hair follicle cycling.5 Mutations in the VDR gene giving rise to a defective receptor result in disordered calcium homeostasis and skeletal growth as well as alopecia in both mice and man.1 While a high-calcium diet prevents the metabolic abnormalities, it does not reverse the alopecia.72 In contrast, mice with deletion of CYP27B1 do not display alopecia despite also displaying disordered calcium homeostasis, suggesting that the defect is not a result of loss of 1,25D.73 When the VDR was specifically reconstituted into keratinocytes of VDR null (−/−) mice, the alopecia was reversed, yet the metabolic abnormalities could not be reversed, demonstrating for the first time that keratinocyte VDR is necessary for normal hair growth74 and this occurs in an apparent ligand-independent manner.75 The accumulating evidence indicate that the VDR can act independently of 1,25D however, further studies will be necessary to elucidate whether VDR must bind ligands other than 1,25D to mediate these biological actions.
In contrast to its effects in colorectal cancer cells, activation of the Wnt/β-catenin signaling pathway is responsible for increasing acquisition of bone. This is achieved through stimulation of the canonical Wnt signaling pathway by ligands such as LRP5, while decreasing levels of DKK-1 and SFRP2 which inhibit this pathway, ultimately increasing proliferation of osteoblasts and stimulating bone accrual. Of note, Wnt/β-catenin signaling is inhibited via the actions of sclerostin, a protein produced by osteocytes, demonstrating the importance of the canonical pathway to modulate bone homeostasis. There is potential for VDR and β-catenin to interact within bone cells to modulate bone formation, as preliminary in vitro evidence, using a human osteoblast-like osteosarcoma cell line, suggests that β-catenin modulation of gene transcription is affected by the unliganded VDR.76 The weight of evidence suggests that VDR-β-catenin interactions are another activity of the vitamin D system and some of these activities can occur without traditional VDR-1,25D binding. Again it is unknown whether these 1,25D-independent activities of VDR require another, so far unidentified, vitamin D metabolite as a ligand or are truly ligand independent.
The interplay between the Wnt signaling pathway and vitamin D is just one of several pathways through which vitamin D exerts physiological effects within the body. Several other transcription factors and proteins are now being examined for similar interactions with the VDR lending further evidence to the fundamental role of vitamin D in cell processes such as cell proliferation and differentiation. One of these, the Class O Forkhead box (FoxO) proteins, are important transcription factors that, among their varied roles, control organism longevity and tumour suppression.77 FoxO transcriptional regulation is activated by both deacetylation and dephosphorylation, ultimately resulting in transcriptional output. It is the activity of the cofactor Sirtuin 1 (Sirt1), a class III histone deacetylase, in addition to the VDR-RXR complex and the catalytic subunit of protein phosphatase 1 (PP1c) which directs this process. Intriguingly, both Sirt1 and PP1c can interact directly with the VDR, independent of 1,25D, though addition of the hormone provides enhancement of Sirt1 recruitment.78 As such, the ligand-bound VDR is able to rapidly induce deacetylation and dephosphorylation, allowing for FoxO-mediated gene transcription to occur.79
Conclusion
As with other steroid hormone receptors, the VDR exerts powerful and diverse physiological effects due to its capability to influence gene transcription in a number of ways. The vitamin D endocrine system has long been recognised as a major component of the regulation of plasma calcium and phosphate homeostasis which enables adequate muscular function, bone growth and mineralisation. These activities arise from plasma 1,25D activating the VDR in intestinal, renal and bone tissues to regulate gene transcription, predominantly through direct VDR-1,25D-chromatin interactions. More recently in the 21st century autocrine/paracrine sources of 1,25D have been confirmed to initiate activities in tissues including skin and bone. The vitamin D effects on gene transcription through direct binding to chromatin can be enhanced through activation of cytoplasmic intracellular or ‘second messenger’ signaling pathways regulated by the classical nuclear VDR or other membrane bound vitamin D receptors. Furthermore, VDR can directly interact with proteins to modulate gene transcription without directly binding to chromatin. These latter activities appear to be either dependent or independent of 1,25D binding. Whether those activities which are independent of 1,25D binding require another vitamin D metabolite to activate the VDR or are truly ligand independent is unknown at this time. Thus our understanding of the molecular underpinnings of the vitamin D system has increased exponentially in line with the development of new technologies and methods. This knowledge extends the range of effects we attribute to vitamin D activity into the realms of immunity and tumor prevention.
In light of these findings, the importance of maintaining adequate vitamin D levels is now more pertinent than ever. The full implications of vitamin D deficiency go beyond regulation of vitamin D responsive genes and affect a variety of systems necessary for cell proliferation, growth, maturation and differentiation. Further investigations of the non-classical pathways regulated by vitamin D – whether mediated by liganded- or unliganded-VDR – are necessary for elucidating the vitamin D endocrine and autocrine/paracrine systems contributing to human health.
Acknowledgments
The authors acknowledge the support of the University of South Australia for an Australian Postgraduate Award to support the studies of Jackson Ryan and to the National Health and Medical Research Council for Project Grant funding which supported primary research studies during the preparation of this manuscript.
Footnotes
Competing Interests: None declared.
References
- 1.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94:9831–5. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen JF, Fleischman AR, Finberg L, Hamstra A, DeLuca HF. Rickets with alopecia: an inborn error of vitamin D metabolism. J Pediatr. 1979;94:729–35. doi: 10.1016/s0022-3476(79)80139-0. [DOI] [PubMed] [Google Scholar]
- 3.Pike JW, Dokoh S, Haussler MR, Liberman UA, Marx SJ, Eil C. Vitamin D3—resistant fibroblasts have immunoassayable 1,25-dihydroxyvitamin D3 receptors. Science. 1984;224:879–81. doi: 10.1126/science.6326262. [DOI] [PubMed] [Google Scholar]
- 4.Balsan S, Garabedian M, Liberman UA, Eil C, Bourdeau A, Guillozo H, et al. Rickets and alopecia with resistance to 1,25-dihydroxyvitamin D: two different clinical courses with two different cellular defects. J Clin Endocrinol Metab. 1983;57:803–11. doi: 10.1210/jcem-57-4-803. [DOI] [PubMed] [Google Scholar]
- 5.Bikle DD. Vitamin D and the skin: Physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Intermountain Heart Collaborative (IHC) Study Group Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–8. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–5. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 8.Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). (table of contents) Endocrinol Metab Clin North Am. 2010;39:255–69. doi: 10.1016/j.ecl.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haussler MR, Haussler CA, Bartik L, Whitfield GK, Hsieh JC, Slater S, et al. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr Rev. 2008;66(Suppl 2):S98–112. doi: 10.1111/j.1753-4887.2008.00093.x. [DOI] [PubMed] [Google Scholar]
- 10.Oñate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–7. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 11.Rachez C, Lemon BD, Suldan Z, Bromleigh V, Gamble M, Näär AM, et al. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–8. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 12.Baudino TA, Kraichely DM, Jefcoat SC, Jr, Winchester SK, Partridge NC, MacDonald PN. Isolation and characterization of a novel coactivator protein, NCoA-62, involved in vitamin D-mediated transcription. J Biol Chem. 1998;273:16434–41. doi: 10.1074/jbc.273.26.16434. [DOI] [PubMed] [Google Scholar]
- 13.Anderson PH, O’Loughlin PD, May BK, Morris HA. Quantification of mRNA for the vitamin D metabolizing enzymes CYP27B1 and CYP24 and vitamin D receptor in kidney using real-time reverse transcriptase- polymerase chain reaction. J Mol Endocrinol. 2003;31:123–32. doi: 10.1677/jme.0.0310123. [DOI] [PubMed] [Google Scholar]
- 14.Shinki T, Jin CH, Nishimura A, Nagai Y, Ohyama Y, Noshiro M, et al. Parathyroid hormone inhibits 25-hydroxyvitamin D3-24-hydroxylase mRNA expression stimulated by 1 alpha,25-dihydroxyvitamin D3 in rat kidney but not in intestine. J Biol Chem. 1992;267:13757–62. [PubMed] [Google Scholar]
- 15.Kerner SA, Scott RA, Pike JW. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989;86:4455–9. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noda M, Vogel RL, Craig AM, Prahl J, DeLuca HF, Denhardt DT. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 receptor and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl Acad Sci U S A. 1990;87:9995–9. doi: 10.1073/pnas.87.24.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitazawa S, Kajimoto K, Kondo T, Kitazawa R. Vitamin D3 supports osteoclastogenesis via functional vitamin D response element of human RANKL gene promoter. J Cell Biochem. 2003;89:771–7. doi: 10.1002/jcb.10567. [DOI] [PubMed] [Google Scholar]
- 18.Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol. 2006;20:1447–61. doi: 10.1210/me.2006-0031. [DOI] [PubMed] [Google Scholar]
- 19.Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM. Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1992;89:8097–101. doi: 10.1073/pnas.89.17.8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiba N, Suldan Z, Freedman LP, Parvin JD. Binding of liganded vitamin D receptor to the vitamin D receptor interacting protein coactivator complex induces interaction with RNA polymerase II holoenzyme. J Biol Chem. 2000;275:10719–22. doi: 10.1074/jbc.275.15.10719. [DOI] [PubMed] [Google Scholar]
- 21.Morris HA, Anderson PH, Nordin BE, Vitamin D. Activities for bone health. In: Morris HA, Anderson PH, Nordin BEC, editors. The Physiological Basis of Metabolic Bone Disease. Boca Raton, FL: CRC Press; 2014. pp. 167–86. [Google Scholar]
- 22.Anderson PH, O’Loughlin PD, May BK, Morris HA. Modulation of CYP27B1 and CYP24 mRNA expression in bone is independent of circulating 1,25(OH)2D3 levels. Bone. 2005;36:654–62. doi: 10.1016/j.bone.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Kerry DM, Dwivedi PP, Hahn CN, Morris HA, Omdahl JL, May BK. Transcriptional synergism between vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase (CYP24) promoter. J Biol Chem. 1996;271:29715–21. doi: 10.1074/jbc.271.47.29715. [DOI] [PubMed] [Google Scholar]
- 24.Chen KS, DeLuca HF. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim Biophys Acta. 1995;1263:1–9. doi: 10.1016/0167-4781(95)00060-t. [DOI] [PubMed] [Google Scholar]
- 25.Zierold C, Darwish HM, DeLuca HF. Two vitamin D response elements function in the rat 1,25-dihydroxyvitamin D 24-hydroxylase promoter. J Biol Chem. 1995;270:1675–8. doi: 10.1074/jbc.270.4.1675. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Iachini DN, Neilsen PM, Kaplan J, Michalakas J, Anderson PH, et al. Systematic characterisation of the rat and human CYP24A1 promoter. Mol Cell Endocrinol. 2010;325:46–53. doi: 10.1016/j.mce.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Meyer MB, Goetsch PD, Pike JW. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25-dihydroxyvitamin D3. J Biol Chem. 2010;285:15599–610. doi: 10.1074/jbc.M110.119958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–6. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 29.St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, et al. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141:2658–66. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- 30.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 31.Seth-Vollenweider T, Joshi S, Dhawan P, Sif S, Christakos S. Novel mechanism of negative regulation of 1,25-dihydroxyvitamin D3 induced 25-hydroxyvitamin D3 24-hydroxylase (CYP24A1) transcription: epigenetic modification involving crosstalk between protein arginine methyltransferase 5 and the SWI/SNF complex. J Biol Chem. 2014;289:33958–70. doi: 10.1074/jbc.M114.583302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, et al. Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem. 1999;274:8375–8. doi: 10.1074/jbc.274.13.8375. [DOI] [PubMed] [Google Scholar]
- 33.Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, et al. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–94. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- 34.Cui M, Li Q, Johnson R, Fleet JC. Villin promoter-mediated transgenic expression of transient receptor potential cation channel, subfamily V, member 6 (TRPV6) increases intestinal calcium absorption in wild-type and vitamin D receptor knockout mice. J Bone Miner Res. 2012;27:2097–107. doi: 10.1002/jbmr.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kogawa M, Findlay DM, Anderson PH, Ormsby R, Vincent C, Morris HA, et al. Osteoclastic metabolism of 25(OH)-vitamin D3: a potential mechanism for optimization of bone resorption. Endocrinology. 2010;151:4613–25. doi: 10.1210/en.2010-0334. [DOI] [PubMed] [Google Scholar]
- 36.Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, et al. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone. 2007;40:1517–28. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Lieben L, Carmeliet G. Vitamin D signaling in osteocytes: effects on bone and mineral homeostasis. Bone. 2013;54:237–43. doi: 10.1016/j.bone.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–57. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Yamazaki M, Zella LA, Shevde NK, Pike JW. Activation of receptor activator of NF-kappaB ligand gene expression by 1,25-dihydroxyvitamin D3 is mediated through multiple long-range enhancers. Mol Cell Biol. 2006;26:6469–86. doi: 10.1128/MCB.00353-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martowicz ML, Meyer MB, Pike JW. The mouse RANKL gene locus is defined by a broad pattern of histone H4 acetylation and regulated through distinct distal enhancers. J Cell Biochem. 2011;112:2030–45. doi: 10.1002/jcb.23123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galli C, Zella LA, Fretz JA, Fu Q, Pike JW, Weinstein RS, et al. Targeted deletion of a distant transcriptional enhancer of the receptor activator of nuclear factor-kappaB ligand gene reduces bone remodeling and increases bone mass. Endocrinology. 2008;149:146–53. doi: 10.1210/en.2007-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silver J, Russell J, Sherwood LM. Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proc Natl Acad Sci U S A. 1985;82:4270–3. doi: 10.1073/pnas.82.12.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantley LK, Russell J, Lettieri D, Sherwood LM. 1,25-Dihydroxyvitamin D3 suppresses parathyroid hormone secretion from bovine parathyroid cells in tissue culture. Endocrinology. 1985;117:2114–9. doi: 10.1210/endo-117-5-2114. [DOI] [PubMed] [Google Scholar]
- 44.Silver J, Naveh-Many T, Mayer H, Schmelzer HJ, Popovtzer MM. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest. 1986;78:1296–301. doi: 10.1172/JCI112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu SM, Koszewski N, Lupez M, Malluche HH, Olivera A, Russell J. Characterization of a response element in the 5′-flanking region of the avian (chicken) PTH gene that mediates negative regulation of gene transcription by 1,25-dihydroxyvitamin D3 and binds the vitamin D3 receptor. Mol Endocrinol. 1996;10:206–15. doi: 10.1210/mend.10.2.8825560. [DOI] [PubMed] [Google Scholar]
- 46.Russell J, Ashok S, Koszewski NJ. Vitamin D receptor interactions with the rat parathyroid hormone gene: synergistic effects between two negative vitamin D response elements. J Bone Miner Res. 1999;14:1828–37. doi: 10.1359/jbmr.1999.14.11.1828. [DOI] [PubMed] [Google Scholar]
- 47.Koszewski NJ, Ashok S, Russell J. Turning a negative into a positive: vitamin D receptor interactions with the avian parathyroid hormone response element. Mol Endocrinol. 1999;13:455–65. doi: 10.1210/mend.13.3.0249. [DOI] [PubMed] [Google Scholar]
- 48.Wali RK, Baum CL, Bolt MJ, Brasitus TA, Sitrin MD. 1,25-dihydroxyvitamin D3 inhibits Na(+)-H+ exchange by stimulating membrane phosphoinositide turnover and increasing cytosolic calcium in CaCo-2 cells. Endocrinology. 1992;131:1125–33. doi: 10.1210/endo.131.3.1324151. [DOI] [PubMed] [Google Scholar]
- 49.Sergeev IN, Rhoten WB. 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. Endocrinology. 1995;136:2852–61. doi: 10.1210/endo.136.7.7789310. [DOI] [PubMed] [Google Scholar]
- 50.Dwivedi PP, Hii CS, Ferrante A, Tan J, Der CJ, Omdahl JL, et al. Role of MAP kinases in the 1,25-dihydroxyvitamin D3-induced transactivation of the rat cytochrome P450C24 (CYP24) promoter. Specific functions for ERK1/ERK2 and ERK5. J Biol Chem. 2002;277:29643–53. doi: 10.1074/jbc.M204561200. [DOI] [PubMed] [Google Scholar]
- 51.Nemere I, Farach-Carson MC, Rohe B, Sterling TM, Norman AW, Boyan BD, et al. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc Natl Acad Sci U S A. 2004;101:7392–7. doi: 10.1073/pnas.0402207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nemere I, Dormanen MC, Hammond MW, Okamura WH, Norman AW. Identification of a specific binding protein for 1 alpha,25-dihydroxyvitamin D3 in basal-lateral membranes of chick intestinal epithelium and relationship to transcaltachia. J Biol Chem. 1994;269:23750–6. [PubMed] [Google Scholar]
- 53.Nemere I, Schwartz Z, Pedrozo H, Sylvia VL, Dean DD, Boyan BD. Identification of a membrane receptor for 1,25-dihydroxyvitamin D3 which mediates rapid activation of protein kinase C. J Bone Miner Res. 1998;13:1353–9. doi: 10.1359/jbmr.1998.13.9.1353. [DOI] [PubMed] [Google Scholar]
- 54.Boyan BD, Bonewald LF, Sylvia VL, Nemere I, Larsson D, Norman AW, et al. Evidence for distinct membrane receptors for 1 alpha,25-(OH)(2)D(3) and 24R,25-(OH) (2)D(3) in osteoblasts. Steroids. 2002;67:235–46. doi: 10.1016/s0039-128x(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 55.Chen J, Olivares-Navarrete R, Wang Y, Herman TR, Boyan BD, Schwartz Z. Protein-disulfide isomerase-associated 3 (Pdia3) mediates the membrane response to 1,25-dihydroxyvitamin D3 in osteoblasts. J Biol Chem. 2010;285:37041–50. doi: 10.1074/jbc.M110.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nemere I, Garbi N, Hammerling G, Hintze KJ. Role of the 1,25D3-MARRS receptor in the 1,25(OH)2D3-stimulated uptake of calcium and phosphate in intestinal cells. Steroids. 2012;77:897–902. doi: 10.1016/j.steroids.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Wali RK, Kong J, Sitrin MD, Bissonnette M, Li YC. Vitamin D receptor is not required for the rapid actions of 1,25-dihydroxyvitamin D3 to increase intracellular calcium and activate protein kinase C in mouse osteoblasts. J Cell Biochem. 2003;88:794–801. doi: 10.1002/jcb.10432. [DOI] [PubMed] [Google Scholar]
- 58.Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol. 2004;18:2660–71. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- 59.Capiati D, Benassati S, Boland RL. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem. 2002;86:128–35. doi: 10.1002/jcb.10191. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen TM, Lieberherr M, Fritsch J, Guillozo H, Alvarez ML, Fitouri Z, et al. The rapid effects of 1,25-dihydroxyvitamin D3 require the vitamin D receptor and influence 24-hydroxylase activity: studies in human skin fibroblasts bearing vitamin D receptor mutations. J Biol Chem. 2004;279:7591–7. doi: 10.1074/jbc.M309517200. [DOI] [PubMed] [Google Scholar]
- 61.Norman AW, Olivera CJ, Barreto Silva FR, Bishop JE. A specific binding protein/receptor for 1alpha,25-dihydroxyvitamin D(3) is present in an intestinal caveolae membrane fraction. Biochem Biophys Res Commun. 2002;298:414–9. doi: 10.1016/s0006-291x(02)02482-8. [DOI] [PubMed] [Google Scholar]
- 62.Norman AW, Okamura WH, Hammond MW, Bishop JE, Dormanen MC, Bouillon R, et al. Comparison of 6-s-cis- and 6-s-trans-locked analogs of 1alpha,25-dihydroxyvitamin D3 indicates that the 6-s-cis conformation is preferred for rapid nongenomic biological responses and that neither 6-s-cis- nor 6-s-trans-locked analogs are preferred for genomic biological responses. Mol Endocrinol. 1997;11:1518–31. doi: 10.1210/mend.11.10.9993. [DOI] [PubMed] [Google Scholar]
- 63.Mizwicki MT, Keidel D, Bula CM, Bishop JE, Zanello LP, Wurtz JM, et al. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1alpha,25(OH)2-vitamin D3 signaling. Proc Natl Acad Sci U S A. 2004;101:12876–81. doi: 10.1073/pnas.0403606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bravo S, Paredes R, Izaurieta P, Lian JB, Stein JL, Stein GS, et al. The classic receptor for 1alpha,25-dihydroxy vitamin D3 is required for non-genomic actions of 1alpha,25-dihydroxy vitamin D3 in osteosarcoma cells. J Cell Biochem. 2006;99:995–1000. doi: 10.1002/jcb.21031. [DOI] [PubMed] [Google Scholar]
- 65.Sequeira VB, Rybchyn MS, Tongkao-On W, Gordon-Thomson C, Malloy PJ, Nemere I, et al. The role of the vitamin D receptor and ERp57 in photoprotection by 1α,25-dihydroxyvitamin D3. Mol Endocrinol. 2012;26:574–82. doi: 10.1210/me.2011-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515–24. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, et al. Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development. 2008;135:2161–72. doi: 10.1242/dev.017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 69.Pálmer HG, González-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–87. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah S, Hecht A, Pestell R, Byers SW. Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem. 2003;278:48137–45. doi: 10.1074/jbc.M307154200. [DOI] [PubMed] [Google Scholar]
- 71.Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, et al. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 72.Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139:4391–6. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- 73.Dardenne O, Prud’homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001;142:3135–41. doi: 10.1210/endo.142.7.8281. [DOI] [PubMed] [Google Scholar]
- 74.Chen CH, Sakai Y, Demay MB. Targeting expression of the human vitamin D receptor to the keratinocytes of vitamin D receptor null mice prevents alopecia. Endocrinology. 2001;142:5386–9. doi: 10.1210/endo.142.12.8650. [DOI] [PubMed] [Google Scholar]
- 75.Skorija K, Cox M, Sisk JM, Dowd DR, MacDonald PN, Thompson CC, et al. Ligand-independent actions of the vitamin D receptor maintain hair follicle homeostasis. Mol Endocrinol. 2005;19:855–62. doi: 10.1210/me.2004-0415. [DOI] [PubMed] [Google Scholar]
- 76.Haussler MR, Haussler CA, Whitfield GK, Hsieh JC, Thompson PD, Barthel TK, et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J Steroid Biochem Mol Biol. 2010;121:88–97. doi: 10.1016/j.jsbmb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Zhou Y, Graves DT. FOXO transcription factors: their clinical significance and regulation. Biomed Res Int. 2014;2014:925350. doi: 10.1155/2014/925350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dimitrov V, Salehi-Tabar R, An BS, White JH. Non-classical mechanisms of transcriptional regulation by the vitamin D receptor: insights into calcium homeostasis, immune system regulation and cancer chemoprevention. J Steroid Biochem Mol Biol. 2014;(144 Pt A):74–80. doi: 10.1016/j.jsbmb.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 79.An BS, Tavera-Mendoza LE, Dimitrov V, Wang X, Calderon MR, Wang HJ, et al. Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Mol Cell Biol. 2010;30:4890–900. doi: 10.1128/MCB.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


