Abstract
The immunologic processes involved in autoimmune thyroid disease (AITD), particularly Graves’ disease (GD), are similar to other autoimmune diseases with the emphasis on the antibodies as the most unique aspect. These characteristics include a lymphocytic infiltrate at the target organs, the presence of antigen-reactive T and B cells and antibodies, and the establishment of animal models of GD by antibody transfer or immunization with antigen. Similar to other autoimmune diseases, risk factors for GD include the presence of multiple susceptibility genes, including certain HLA alleles, and the TSHR gene itself. In addition, a variety of known risk factors and precipitators have been characterized including the influence of sex and sex hormones, pregnancy, stress, infection, iodine and other potential environmental factors. The pathogenesis of GD is likely the result of a breakdown in the tolerance mechanisms, both at central and peripheral levels. Different subsets of T and B cells together with their regulatory populations play important roles in the propagation and maintenance of the disease process. Understanding different mechanistic in the complex system biology interplay will help to identify unique factors contributing to the AITD pathogenesis.
Keywords: AITD, GD, HT, Autoimmunity, TSHR, Autoantibody
Introduction
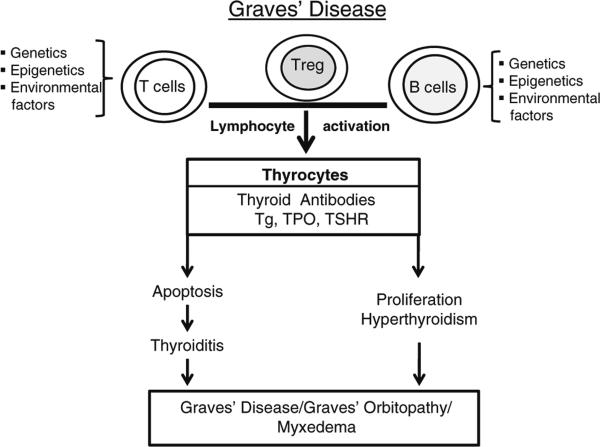
The autoimmune thyroid diseases (AITD) include Graves’ disease (GD), Hashimoto's thyroiditis (HT) and an overlap between these two entities. The hyperactivity of the thyroid gland is due to the presence of thyroid-stimulating antibodies, and these are now known to recognize and activate the thyroid-stimulating hormone receptor (TSHR). These TSHR-stimulating antibodies increase the growth and the function of the thyroid follicular cells leading to the excessive production of thyroid hormones (both T3 and T4) and symptoms of tachycardia, anxiety and weight loss among others. Pathologically, the disease is characterized by a heterogeneous lymphocytic infiltration of the thyroid parenchyma as well as infiltration of retro-orbital and dermal tissues [1–3]. Transplacental antibody transferred to the infants of affected mothers during pregnancy can induce symptoms similar to the mother by causing neonatal hyperthyroidism [4, 5]. Similarly, antibodies from patients with GD when injected into experimental animals or humans can induce thyroid activation [6]. On the other hand, HT is the most common inflammatory thyroid lesion and is the most frequent cause of hypothyroidism in adults in the developed world. The thyroid gland in HT is gradually destroyed by cell- and antibody-mediated immune processes and was the first human disorder to be recognized as an autoimmune disease [7]. The hallmark of HT is the presence of T cells and antibodies against thyroid peroxidase (TPO) and/or thyroglobulin (Tg) that are associated with the destruction of the thyroid follicles. It is also characterized by marked and diffuse lymphocytic infiltration of the thyroid parenchyma, causing a dense accumulation of lymphocytes, mainly T lymphocytes, plasma cells and germinal centers along with dendritic cells and macrophages. While extensive studies have been carried out to elucidate the immunopathogenesis of AITD, the mechanisms are far from being fully understood. However, significant progress has been made on the role of B and T cells, thyroid cell apoptosis and the signals induced by TSHR autoantibodies (Fig. 1). In this short review, the emphasis is on recent developments in our understanding of the immunology of GD.
Fig. 1.
An outline of the factors contributing to the development of Graves’ disease
Autoimmunity and AITD
The pathologic processes involved in AITD are similar to other autoimmune diseases with the emphasis on the antibodies as the most unique aspect. These characteristics include a lymphocytic infiltrate at the target organ and the presence of antigen-reactive T and B cells to very well-characterized thyroid antigens. In addition, animal models of GD and HT have been established and have illustrated important elements of the autoimmunology of these disorders. Similar to other autoimmune diseases, risk factors for AITD include the presence of multiple susceptibility genes common to many autoimmune diseases, such as certain human leukocyte antigen (HLA) alleles and CTLA-4 gene polymorphisms, and more specifically the Tg and TSHR genes [8–10]. In addition, the influence of sex and sex hormones, pregnancy, stress, infection, iodine and other potential environmental factors such as radiation have been recognized [11].
The resulting breakdown in thyroid tolerance is the likely result of errors in multiple protective immune mechanisms. Many self-specific T cells escape thymic deletion but are normally prevented from responding to self-antigen by several additional mechanisms such as clonal anergy and peripheral suppression [12]. B cells recognizing specific self-antigen in the secondary lymphoid organs are trapped in the T cell areas; if not activated by T cells available to provide help, the B cells normally die by apoptosis [13] while B cells that bind soluble self-antigen also undergo anergy; downregulate membrane IgM expression; and survive for only a short time. The mechanisms of B cell self-tolerance also include receptor editing and autoreactive B cell receptor (BCRs) allelic exclusion, as well as clonal ignorance (lack of recognition) [13].
The thyroid antigens
Tg and TPO
Tg is a 670 Kd glycoprotein secreted by the thyroid cell and forms the basis of thyroid colloid. It is on the Tg backbone that thyroid hormones are synthesized with the help of the membrane enzyme thyroid peroxidase (TPO). Tg and TPO-specific T cells and antibodies are found in patients with GD and HT, and it is well known that Tg-Ab and TPO-Ab may occur many years before disease onset. Such antibodies are polyclonal and are often found in very high titers in HT patients. Their presence correlates well with the degree of intrathyroidal lymphocytic infiltration [5]. Since most patients with GD have such thyroid antibodies, it is logical to consider that GD occurs on a background of thyroiditis. This logic is supported by animal models where immunization with the TSHR alone fails to induce a thyroid infiltrate.
The Tg antibodies appear to recognize the conformation of large fragments of TG and are directed against the same 4-6 B cell epitopes in both GD and HT [14]. TPO antibodies may be involved in complement/antibody-mediated cell cytotoxicity and similarly target conformational epitopes [15]. TSH, lectins and interferon-α can influence Tg and TPO expression [16], and both TPO-Ab and Tg-Ab may share common epitopes because of some, albeit limited, amino acid sequence homology [16]. Epitope mapping by monoclonal antibodies (mAbs) has revealed that antibodies to Tg and TPO may also be polyreactive [17].
The GD Autoantigen
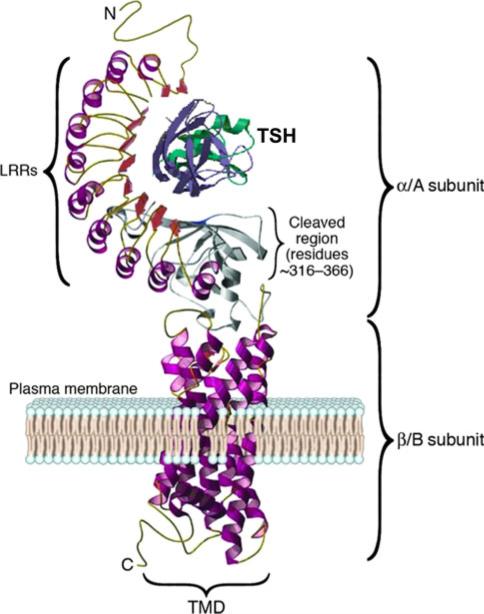
In GD, the main autoantigen is the TSHR that is expressed primarily in the thyroid but also on fibroblasts and adipocytes, bone cells and a variety of additional sites including the heart [18] (Fig. 2). The TSHR is a G-protein coupled receptor with seven transmembrane-spanning domains. TSH, acting via the TSHR, regulates thyroid growth and thyroid hormone production and secretion. The TSHR undergoes complex posttranslational processing involving dimerization and intramolecular cleavage; the latter modification leaves a two-subunit structural form of the receptor [19]. Data suggest that there is eventual shedding or degradation of the TSHR ectodomain [20–22] although this has not been confirmed in vivo. We have reviewed this antigen in detail elsewhere [23, 24]. Each of these post-translational events may influence the antigenicity of the receptor, and furthermore, this complex processing may contribute to the break in TSHR self-tolerance. For example, the affinity of TSHR antibodies for the TSHR ectodomain is greater than for the holoreceptor itself [20]. However, factors that contribute to TSHR presentation as a target for the immune system when expressed at extra-thyroidal sites are not well understood.
Fig. 2.
A computer model of the TSH receptor based on the crystal structure of the receptor ectodomain, while the seven transmembrane domain structure was derived from the rhodopsin receptor crystal. The large ectodomain is made of nine leucine-rich repeats (LRRs), which form the characteristic “horse shoe” shaped structure with a concave inner surface which harbors the major ligand and autoantibody binding regions. The hinge region and unique 50aa cleaved region in the ectodomain, of unknown structure, is shown in gray and is characteristic only of the TSHR among the glycoprotein hormone receptor family
TSH receptor antibodies
One of the unique characteristics of GD, neither found in normals nor in the entire animal kingdom, is the presence of TSHR antibodies (TSHR-Ab) that are easily detectable in the vast majority of patients [25]. B cells in lymph node germinal centers undergo somatic hypermutation and allow the survival of self-specific B cells that secrete antibodies to the TSHR. Furthermore, such B cells can also potentially present thyroid autoantigen to T cells and secrete proinflammatory cytokines [26]. Similarly, TSHR autoreactive T cells survive deletion and can be demonstrated in patients with GD [27]. These findings suggest, therefore, that both B cells and T cells play a central role not only in producing TSHR-Abs but also in mediating the chronic inflammatory changes of the disease seen in the thyroid gland and in the retro-orbital spaces (causing Graves’ orbitopathy) and the dermis (causing pre-tibial myxedema). Whether it is the B cells or the T cells that play a primary role in GD is unclear and of little import since most likely both are intimately involved [28].
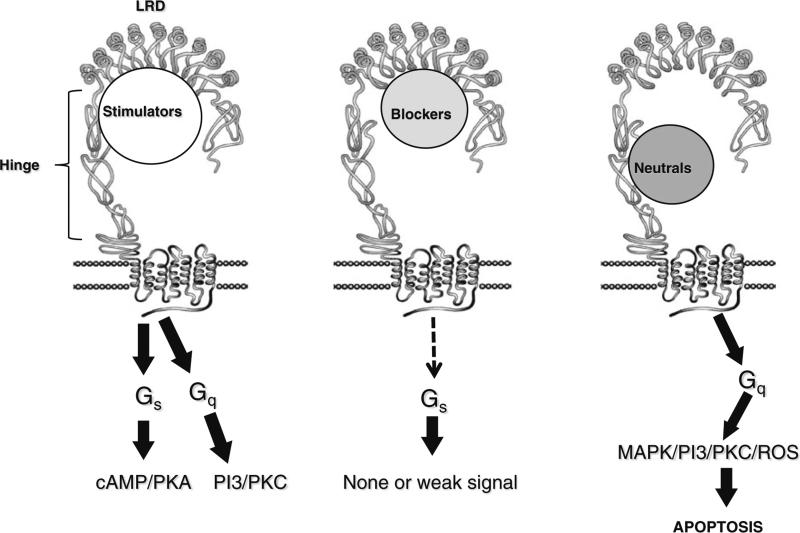
After many years of studying serum IgG from patients with GD, the most precise delineation of the characteristics of TSHR-Abs has resulted from the analysis of monoclonal antibodies to the TSHR derived from patients with GD or from rodents immunized with the TSHR [29–31]. Three varieties of TSHR-Ab are present in most patients with GD; stimulating, blocking and neutral antibodies (Fig. 3). Stimulating antibodies bind only to the naturally conformed TSHR, induce cAMP generation and by definition inhibit any simultaneous TSH-mediated activation of thyroid function [32]. In addition, TSHR blocking antibodies, whose primary biological action is to prevent TSH binding to the receptor to such an extent that they may induce hypothyroidism, may also act as weak TSH agonists [32]. Some such blocking antibodies are conformationally dependent, while others have a high affinity for reduced TSHR antigen and/or linear peptides (Fig. 4). On the other hand, neutral TSHR antibodies neither block TSH binding nor TSH action and they do not induce cAMP generation. Neutral TSHR-Abs bind exclusively with linear epitopes and are directed to the “unique region” of the receptor between amino acids 316-366 [33]. The presence of differing proportions of high-affinity TSHR-Abs with varied biological activity in patients with GD no doubt contributes to the clinical phenotype; varying from hyperthyroidism to hypothyroidism and vice versa. Thus, a classification of these antibodies based on function as suggested by us earlier seems more relevant than their ability or lack of ability in influencing TSH binding or cAMP generation [33].
Fig. 3.
The three different varieties of TSH receptor antibodies and their functional consequences
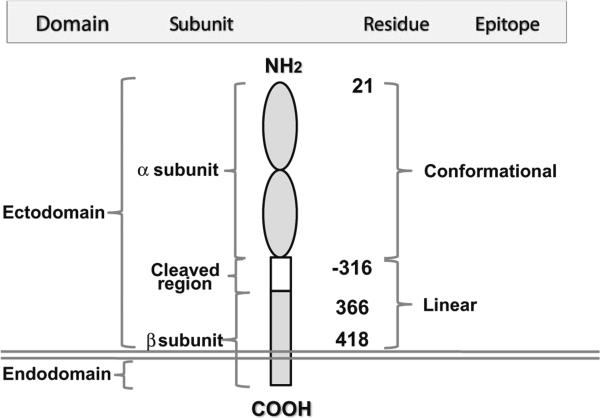
Fig. 4.
A model of the TSH receptor illustrating multiple domains, subunits and epitope distributions containing different amino acid residues. All stimulating and blocking antibodies are directed to the conformational epitopes whereas neutral variety is linear, mainly targeting cleavage region
Epitopes of TSH receptor antibodies
Monoclonal antibodies to the TSHR
Monoclonal antibodies to the TSHR raised against a variety of TSHR antigen preparations, including recombinant proteins and synthesized peptides, all proved to be of the blocking variety. Only when intact natural TSH receptors or genetic immunization were used, was it possible to induce thyroid-stimulating antibodies and an animal model of hyperthyroidism [34]. Subsequently, the rare B cells secreting TSHR antibodies with stimulating activity [30] were used to generate monoclonal antibodies including MS-1 raised in a hamster (Fig. 5). Later, mAbs were derived directly from human peripheral blood; for example, M-22 was obtained from a patient with severe hyperthyroid GD [35]. In addition, blocking and neutral mAbs have been well characterized from rodents and humans [35, 36].
Fig. 5.
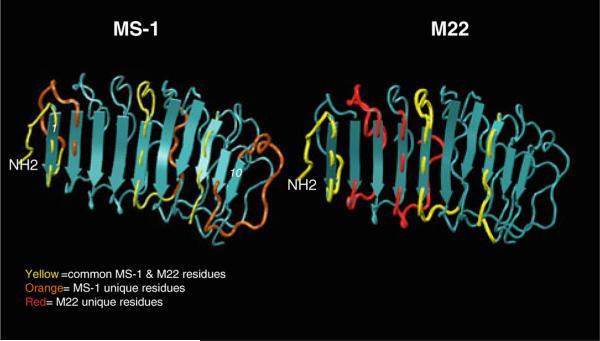
Contact residues of MS-1 and M22 mapped on TSHR LRD structure. We used epitope protection and mass spectrometry to identify the conformational epitopes of a hamster monoclonal antibody MS-1 and human stimulating antibody M22 [39]. The discontinuous epitopes of these two antibodies were then mapped to the partial TSHR ectodomain structure (PDB 3GO4). The leucine-rich domain (LRD) structure of the TSHR ectodomain is shown consisting of various α-helices and β-pleats (blue) that form the concave half-donut structure. On these structures marked are the amino acids sequences that were identified by the epitope protection method. Note the gross differences and similarities in the epitopes of the two stimulating antibodies (Color figure online)
Stimulating TSHR-Ab epitopes
The TSHR ectodomain has been crystallized with a stimulating monoclonal Fab fragment bound in situ [37]. Several amino acids distributed along an extensive part of the concave surface of the leucine-rich repeat region (LRR) of the TSHR ectodomain were found to be important for antibody binding (Fig. 3) [37]. Past and present studies have indicated that stimulating TSHR antibodies may harbor epitopes that are outside the LRR [38]. Recent studies from our laboratory looking at conformational epitopes using mass spectrometry [39] have indicated that epitopes exist outside the LRR for blocking as well as stimulating monoclonal antibodies [40]. The importance of the N terminal region of the extracellular domain (ECD) has been well illustrated as well as residues in the “hinge” (Fig. 3) region [20]. From these and other studies of TSHR antibodies [35, 41–43], it has been concluded that epitope differences may be responsible for their different biological activities. Our recent study of three stimulating TSHR-mAbs showed variation in their patterns of signal transduction and is consistent with this conclusion [32]. Further studies using mutational analyses are needed to relate epitope binding to signal transduction patterns (see below).
Blocking TSHR-Ab epitopes
Epitopes for TSHR blocking antibodies appear to be more widely distributed than for stimulating antibodies. Experimentally produced blocking TSHR-mAbs have been shown to bind to independent linear or conformational epitopes [44] on the α subunit although at least one linear epitope on the β subunit has been described [33]. TSHR autoantibodies from patients with GD or HT have been shown to compete with a blocking TSHR-mAb to the N-terminus of the TSHR β subunit (amino acids 382-415) [45]. Hence, blocking antibodies that cause hypothyroidism are heterogeneous, and their epitopes may lie broadly upstream of the α subunit or downstream to the β subunit [46]. Clearly, there appear to be multiple epitopes involved in this repertoire of antibodies. Recent crystallization of a human blocking TSHR-Ab was in agreement with this conclusion [47].
Neutral TSHR-Ab epitopes
Neutral TSHR antibodies were thought to have no influence on thyroid cell function but have now been shown to have signaling potential (see below). In animal models of GD [48, 49], the major linear epitopes recognized are mainly N-terminus peptides (~20 amino acids) and the TSHR cleavage region (Figs. 2 and 4) [24]. Neither of these epitopes are involved in the TSH binding pocket [50], and thus neutral TSHR-mAbs fail to block TSH binding to the receptor.
The role of thyroid-specific B cells and their control
In the last five years, insight into the contribution of autoreactive B cells to the normal human B cell repertoire and their regulation at self-tolerance checkpoints has come from the analysis of monoclonal antibodies cloned from single purified B cells at different stages during their development [13]. Since diversity by V(D)J recombination and somatic hypermutation provides protective humoral immunity and also generates potentially harmful autoreactive B cell clones, attempts to thwart autoimmunity are ensured by several checkpoints at which developing autoreactive B cells are counter-selected. Thus, defects in central and peripheral checkpoints for B cell tolerance may also be involved in the autoimmunity of GD. Indeed, B cell survival factors such as B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) have been shown to be important in an induced Graves’ animal model [51]. Blockade of both BAFF and B cell maturation antigen (BCMA) using soluble decoy receptors ameliorated hyperthyroid GD in mice induced by TSHR immunization indicating that such treatment may lead to targeted therapy for GD. Therefore, the concept that B cells may play a central role in balancing autoimmune thyroid reactivity is clinically important. Indeed, recent studies using gene silencing, targeting BAFF, inhibited proinflammatory cytokine expression, suppressed plasma cell generation and Th17 cells and caused marked amelioration in autoimmune arthritis [52]. Similar early clinical studies using B cell suppression in Graves’ Ophthalmopathy with anti-CD20 (Rituximab) add further support to this concept [53]. These findings clearly indicate that targeting B cells and factors that regulate B cell function may be highly applicable to the modulation of clinical GD.
Signal induction by TSHR antibodies
To help determine the functional consequences of TSHR-Abs, we have recently probed the signaling pathways using a well-characterized rat thyroid cell model[32]. These studies demonstrated that stimulating TSHR antibodies used signaling pathways similar to TSH for cell activation and growth (Fig. 6). However, some TSHR blocking and neutral TSHR-mAbs also resulted in signal responses demonstrating that such TSHR-mAbs may be weak agonists or inverse agonists [32]. These observations help explain how TSHR-Abs may contribute to different clinical phenotypes in autoimmune thyroid disease. Most striking was that two of the blocking TSHR antibodies we examined resulted not only in signal initiation but they showed different pathway dominance [32]. Also of general interest was our characterization of two neutral TSHR-Ab signaling cascades and downstream effectors that identified two separate signaling mechanisms. For example, one antibody showed suppression of signaling activity, including cell proliferation, whereas the other neutral antibody activated cell signaling and even induced some cell proliferation. By definition, both failed to inhibit TSH binding and both failed to activate cAMP generation. The amino acid sequences for these neutral antibody binding sites [33] showed an overlap of 5 amino acids (337 – 341 residues), and we deduced that the last sixteen amino acids (341 – 356) may, therefore, be critical to signal-activating events. These findings suggest that epitope-specific functional patterns may exist even for linear epitopes. A conformational monoclonal antibody with a binding site that includes the hinge region has also been described as an inverse agonist that suppressed TSHR constitutive activity [54] as assessed by cAMP generation. These findings raise the possibility that multiple domains in the ectodomain of the TSHR may exist that suppress or stimulate the TSHR by initiating conformational changes in the TSHR structure.
Fig. 6.
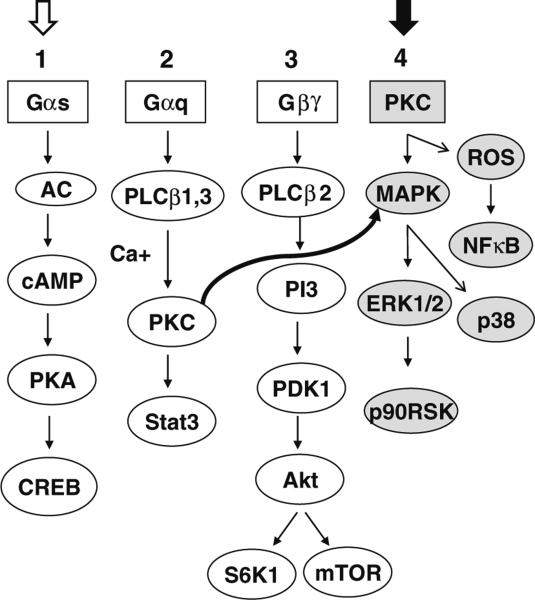
Simplified diagrammatic illustration of the major signaling pathways used by TSHR antibodies. In FRTL-5 thyrocytes, TSHR-stimulating antibodies and TSH activate major signaling cascades numbered 1, 2, and 3, whereas neutral antibodies activate 2, 3, and 4. Signaling cascades 1 is exclusively used by TSH or stimulating antibodies (open arrow) and 4 exclusively by the neutral antibodies (closed arrow). In our model system, Gαs activates cAMP/PKA/CREB via adenyl cyclase activation, and Gαq induces PKC/Stat3 via activation of PLCβ1,3 kinases with Ca + [40]. Similarly, Gβγ activates PI3 kinase and downstream molecules PDK1/Akt/mTOR/S6K1 via PLCβ2 kinase. PKC (curved arrow) once activated can induce different MAPKs including ERK1/2/p38/p90RSK and ROS/NFκB signaling cascades [61]. Note the pathways from left to right
Apoptosis in GD
There is increasing evidence showing that apoptosis plays a role in the development of AITD. In early investigations, antibody- and T-cell-mediated death mechanisms were proposed as being responsible for autoimmune thyrocyte depletion in HT, but later, areas of apoptosis were recognized in thyroid tissue from patients with GD [55]. Studies on apoptosis have since provided new insights into autoimmune target destruction, indicating the involvement of death receptors and cytokine-regulated apoptotic pathways in the likely pathogenesis of thyroid autoimmunity. Although apoptosis appears to play a role in the pathogenesis of GD, the mechanisms that mediate this process remain unclear but there is evidence that thyrocyte apoptosis in GD seems to be antibody induced [56]. In addition, a defect in CD4(+)CD25(+) T regulatory cells would break the immunologic tolerance of the host and induce an abnormal production of cytokines, but this would also facilitate the initiation of Treg apoptosis itself and thus lead to immune activation by decreasing the number of Treg cells as recently described in GD patients [57].
While studying apoptotic proteins, Bossowski et al. suggested that the changes in the expression of the Bcl-2 regulatory protein family in the thyroid follicular cells were indicative of their involvement in the pathogenesis of GD [58, 59]. The same group indicated that the changes in the expression of apoptotic molecules (Fas/FasL and caspase 8) on the surface of T lymphocytes and thyroid follicular cells in patients with AITD also reflected their substantial involvement in the pathogenesis of GD and HT [58]. Eight tagged SNPs representing the majority of common variation in the programmed cell death 1 gene (PDCD1) within a large UK Caucasian GD data set revealed significant associations indicating that PDCD1 may contribute toward the development of GD. Replication of this result is now needed to confirm such findings [60]. Clearly, death receptors/ligands appear to play a regulatory role in apoptosis, but caspase-independent mechanisms may also coexist and contribute to thyroid cell death in GD.
Thyrocyte oxidative stress and apoptosis induced by TSHR antibodies
Thyrocyte stimulation studies with TSHR-mAbs of different specificities revealed induction of cell proliferation by stimulating-mAbs which paralleled cAMP induction. However, certain neutral mAbs were found to activate multiple stress signaling cascades [61] and induced apoptosis (Fig. 3). These antibodies activated multiple oncogenes including p53, p73 and Rb and induced reactive oxygen species (ROS). Moreover, they induced endoplasmic reticulum stress protein (grp98) and also increased the expression of heat shock proteins (p27 and p107), heme oxygenase (HO) and superoxide dismutase (SOD). These data suggested that stress signaling may be present in Graves’ thyroid cells. In our study, cell death via apoptosis was confirmed by both morphological staining (annexin V and propidium iodide) and quantitative flow cytometry assay [61] and appeared to confirm the previously described histological evidence of apoptosis in thyroid tissue from patients with GD. These findings indicated that stress signaling cascades were involved although it remains unclear whether oxidative stress alone or cell-specific signaling molecules induced such apoptosis. Either way, these findings indicate that neutral TSHR-mAbs have an ability to aggravate the local inflammatory infiltrate within the thyroid by inducing cellular apoptosis; a phenomenon known to activate innate and bystander immune-reactivity via DNA release from the apoptotic cells [62]. This phenomenon may also be involved in Graves’ orbitopathy via activation-induced fibroblast death, since these cells express TSHR abundantly (see below). Future in vivo studies will help to address these issues.
Humoral immunity to other antigens in GD
Rodents subjected to TSHR immunization do not express an intrathyroidal infiltrate unless outbred animals (such as hamsters) are used. This suggests that GD is a complex genetic disorder, which normally occurs on the background of a concomitant autoimmune thyroiditis. Factors in favor of this explanation include the finding that none of the thyroid-specific antibodies can transfer the intrathyroidal thyroid infiltrate [63]. Antibodies to pendrin, the sodium iodide symporter [64, 65], thyroxine or triiodothyronine [66], tubulin, megalin, calmodulin, and DNA or DNA-associated proteins [67–69] have all been reported to be present in AITD, but their clinical and pathogenic significance are lacking.
The IGF-1 receptor is widely expressed but may be overexpressed in B cells and fibroblasts from GD patients [70]. Synergism between antibodies to the TSHR and the IGF-1 receptor has been suggested as an important concept in activating the thyrocyte. This requires further investigation since it is well known that TSH and IGF-1 or insulin are interdependent in inducing thyroid cell proliferation [71].
Susceptibility genes in GD
Early observations following the discovery of the HLA system soon found the association between different HLA polymorphisms and autoimmune diseases. This association has been known for many years for GD [8]. Although the association with GD has been widely reproducible, the risk ratio has always been low (3.0-4.0) and there was no linkage with HLA [72] unlike that seen for Type 1 diabetes. With the identification of thyroid cell expression of MHC class 2 genes in the glands of affected patients and evidence of the necessity of coincidental MHC expression in immunization of mice with TSHR expressing cells to induce hyperthyroidism in rodents [73], it became clear that the MHC played a mechanistic role in GD (see below). However, the identification of the Arg74 residue as an important determinant of thyroid antigenic peptide binding in the MHC binding groove [74] allowed the derivation of higher risk associations, but these nevertheless remained modest indicating that additional important genetic and environmental influences are involved rather than just the MHC.
Using candidate gene approaches and whole genome screening technologies (including linkage analysis and genome-wide association study or GWAS), investigators have found a variety of genes associated with GD. Table 1 lists the confirmed genes associated with the AITD: HLA [75], CTLA4 [76], PTPN22 [77], CD40 [78], IL2RA (CD25) [79], FCRL3 [80], TG [81] and the TSHR [77, 82]. Of particular interest has been the specific association of intronic polymorphisms of the TSHR gene with GD [82]. We used TSHR-SNP-rs2268458 and genotyped 200 patients with GD, 83 patients with Hashimoto's thyroiditis (HT) and 118 healthy controls (all female Caucasians). The allele and genotype frequencies from GD patients, but not HT patients, were significantly different to controls [82]. The frequency of the combined genotype (allele) CC + TC was also significantly higher in GD patients versus controls, suggesting that the C containing genotype increased the risk for GD in a dominant manner (p = 0.018, OR = 1.8). It remains to be determined how a TSHR intronic polymorphism associates with GD and how the immune regulatory genes contribute to disease mechanisms.
So far, the identified genes linked to and/or associated with GD are each small contributors to genetic risk. Multiple gene polymorphisms (combinations of haplotypes) appear to be needed to develop GD. And the genetic risk, epigenetic influences and environmental factors must also interact and contribute to the onset and development of disease (Fig. 1).
T cells and AITD
The different phenotypic expression of thyroid autoimmunity can be simplistically viewed as a balance of Th1 versus Th2 immune responses. A predominantly Th1-mediated immune activity may promote apoptotic pathways on thyroid follicular cells leading to thyroid cell destruction. Conversely, predominance of Th2-mediated immune responses may induce antigen-specific B lymphocytes to produce TSHR-Abs causing thyroid cell growth and over activity. A variety of factors have been implicated that may influence these explanations. For example, clinical evidence supports the hypothesis that stress may influence the expression of thyroid autoimmunity in susceptible individuals favoring the development of GD by shifting the Th1-Th2 balance away from Th1 and toward Th2. Conversely, recovery from stress or the immune suppressive effect of pregnancy may induce a Th2 to Th1 “return shift” leading to autoimmune postpartum thyroiditis [83].
Antigen-specific T cells
T cells play an important role in the maintenance of chronic inflammation in GD [52–56] as reflected by the presence in the intrathyroidal infiltrate of both Th1 and Th2 cells [84, 85] and their reactivity to TSHR antigen and TSHR peptides [84–89]. Oligoclonal use of T cell receptor (TCR) genes has been identified in the T cells infiltrating the thyroid gland (and retro-orbital tissues) from patients with GD [84, 85] indicative of clonal expansion and suggesting the primary nature of the T cell responses. However, variation in specific TCR use is seen among different patients [86, 90] as would be expected since T cells recognize the TSHR only in the context of MHC molecules. Studies with TSHR peptides in patients with GD have likewise shown a variety of T cell epitopes with none dominant overall [90]. However, some restriction related to particular HLA types has been reported [89].
Regulatory T cells
Recently much attention has been focused on regulatory CD4 + T cells (Tregs). These cells express CD25 (α chain of the IL-2 receptor) on their cell surface and also express FoxP3 [91], a critically important transcription factor of the forkhead family which is necessary for the development and maintenance of Treg function. Tregs have suppressive functions on effector T cells, and the lack of these cells allows the development of autoimmunity [92]. Treg function in animal models of AITD has been explored, and recent data suggest similar suppressive effects [93–95]. However, the relevance of such immunization-induced models to immune regulation in human disease is questionable. Increased numbers of Tregs within the thyroid as well as in peripheral blood have been reported in patients with GD and to have defective suppressive immune function [96], indicating that Tregs may be deficient in their downregulatory effects in GD. Of note, however, is uncertainty over whether the FOXP3 locus is associated with GD [97] or not [98].
Th17 cells
Recent evidence indicates that naïve CD4 + T cells can be differentiated into Th1, Th2, Th17 and Tregs depending on the local cytokine milieu. Both in vitro and in vivo murine and human CD4 + T cells document polarization of certain cytokines toward Th17 cells [99–101] that produce IL-17 cytokine. In these studies, IL-12 skews toward Th1, IL-4 toward Th2, TGF-β toward Tregs, and IL-6 plus TGF-β toward Th17 [102]. It has been proposed that Th17 or Th1 responses rather than Treg differentiation may be responsible for the development and/or progression of autoimmune disease [102]. Recently, the role of Th17 cells in GD has been described in a patient who underwent CD34 + selected autologous stem-cell transplantation and developed GD [103]. The same group identified an additional two patients with GD out of 25 cases who underwent autologous hematopoietic stem-cell transplantation [104]. Increases in IL-6 and TGF-β cytokines preceded increased Th17 levels in this patient indicating that priming of these cells by both IL-6 and TGF-β was required to induce pathogenic Th17 cells and subsequent development of TSHR-specific autoreactive B cells.
Intrathyroidal antigen presentation in GD
There are at least three clear routes for TSHR antigen to be presented to thyroid-specific T cells; by the thyroid cells themselves, via intrathyroidal or extrathyroidal dendritic cells and using B cells directly. Patients with GD demonstrate increases in overall anti-apoptotic Bcl-2 expression and a reduced apoptotic Fas expression on thyrocytes while exhibiting opposite effects on the infiltrating lymphocytes [55, 105, 106]. These findings indicate that the induction of lymphocyte death helps thyrocyte survival possibly by down-modulating inflammatory cytokines. However, there remains an element of ongoing apoptosis of thyroid cells in GD glands [107] providing ample thyroid antigen for presentation in addition to the secretion of Tg and the shedding of the TSHR ectodomain [55, 105]. Clearly, these observations highlight the important role of regulating antigen presentation and [105] apoptosis in thyroid autoimmunity.
Thyrocytes as antigen-presenting cells
It has been known for some time now that thyroid epithelial cells can express MHC class I and class 2 antigens in AITD [108]. MHC antigen expression endows the ability to present viral antigen to T cells [108] and thyroid antigen to thyroid-specific T cells [109, 110]. One of the animal models of hyperthyroidism is dependent on MHC antigen expression [73]. What remains unclear is how important this mechanism is in the context of human thyroid autoimmunity.
Intrathyroidal dendritic cells
Dendritic cells within the thyroid gland itself have been implicated in AITD, in thyroiditis more than GD, but there have been surprisingly limited numbers of studies. For example, necrotic thyrocytes facilitated maturation of dendritic cells in a mouse model of thyroiditis [111], and T cell interactions with dendritic cells have been implicated in intrathyroidal lymphoid hyperplasia [112]. Immunization of mice with TSHR-loaded dendritic cells has been successful in inducing hyperthyroidism [113]. Furthermore, characterization of thyroidal dendritic cells in GD has shown their adherence to thyrocytes [114, 115]. It is, therefore, to be assumed that such cells have an important role in thyroid antigen presentation in GD.
The CD1 molecule, which resembles MHC I, is a family of four cell surface proteins (CD1 a, b, c and d) [116]. Both dendritic and B cells express CD1 molecules and present lipid antigens to T cells to regulate antimicrobial, and antitumor responses, and the balance between tolerance and autoimmunity [116]. To determine whether CD1 played a role in the generation of thyroid autoimmunity, Roura-Mir and colleagues [117] studied CD1+ APCs (antigen-presenting cells) and CD1-restricted T cells within the thyroid glands of affected GD and HT patients. CD1-expressing APCs infiltrated the thyroid gland in large numbers during the acute and chronic stages of both patient groups. Fresh thyroid-derived lymphocytes and short-term T cell lines isolated from the glands were able to lyse target cells that expressed CD1 proteins. These findings indicate that CD1-restricted intrathyroidal T cells may orchestrate inflammasomes that include both innate and adaptive immunity [118] during human AITD.
Antigen presentation by B cells
Tg antigen has been shown to be presented by B cells in animal models [119], but to date, there are no data on TSHR presentation relevant to GD. However, as with all antigens, B cells are powerful antigen presenters and are able by their antibody specificity to capture specific antigens, even those in sparse supply such as the TSHR.
Graves’ orbitopathy
The retro-orbital inflammatory response associated with GD has no unique genetic etiology [120] and appears to be just a manifestation of a more severe form of the disease. The presentation of pre-tibial myxedema is unusual without eye disease so the two abnormalities are thought to have common characteristics (Fig. 1) [121]. The immunopathology of this disorder has been the subject of recent reviews [121] and can be summarized as a local immune response to the TSHR that is widely expressed on fibroblasts and adipocytes at both these anatomical sites [122] There is a widespread inflammatory infiltrate in the tissues and muscles involving T cells, B cells, dendritic cells and macrophages [121] with both cytokine and chemokine involvement. The degree of GO tends to correlate with the titer of TSHR-Abs, and the disease responds well to B cell depletion therapy with steroids or Rituximab [123, 124].
Of special interest to us has been that the rate of apoptosis is higher in the orbital fibroadipose tissue of GO patients, compared to normal, and is related to the clinical features of the disease [125]. Whether such observations can be blamed on neutral TSHR-Abs, as described earlier, remains to be determined, but neutral antibodies can induce apoptosis of fibroblasts under appropriate conditions [40].
More recent work has also suggested that antibodies to the IGF-1 receptor are present in the serum of patients with GD, especially those with GO [126, 127], and may be involved in enhancing the effects of TSHR-Abs.
Concluding remarks
Autoimmunity represents a collection of heterogeneous disorders often controlled by complex genetic and environmental factors and stochastic contributions. A common view of GD is that TSHR-Abs promote the disease by enhancing thyroid antigen expression. However, T cell activation and subsequent thyroid infiltration in GD patients are not just the result of direct autoantibody-induced mechanisms. In fact, GD appears to develop on a background of concurrent autoimmune thyroiditis. The pathogenesis of GD is, therefore, likely the result of a breakdown in the mechanisms controlling tolerance at different levels (central and peripheral) and involving different cellular subsets (B and T cells) and antigens. The likely scenario for T cell activation and recruitment of inflammatory cells into the gland of patients with GD may be the production of a cocktail of cytokines and chemokines released via by-stander T cells along with antibody-mediated activation of thyrocytes. The released TSHR, via shedding or from dead thyrocytes, further activates T, B and antigen-presenting (dendritic) cells further expanding cellular subsets to perpetuate the disease process. Hence, there remains a need to study innate and adaptive immunity in the context of the TSHR autoantigen in GD. This would provide the basis for the description of the roles that different subsets of T and B cells, and their cytokine and autoantibody production, play in disease pathogenesis and would be greatly helped by more developed rodent models involving the thyroid gland and extra-thyroidal tissues. A possible framework may then emerge that would delineate how different inappropriate immune responses can lead to clinical GD and its varying phenotypes. The emerging role of regulatory cells in different immune compartments and the role of antigen-presenting cells may underlie future premises in our understanding. Detailed studies of these cells in a cellular context-dependent manner will provide a more clear understanding of GD pathogenesis leading to potential therapeutic opportunities for active immune regulation and long-term tolerance induction.
Acknowledgments
Supported in part by NIH grants DK069713, DK052464 and the VA Merit Award Program. We thank Dr. Xiaoming Yin for help with the manuscript.
Biography
 Syed A. Morshed
Syed A. Morshed
References
- 1.Adams DD, Purves HD. Abnormal responses in the assay of thyrotropin. Proc Univ Otago Med School. 1956;34:11–2. [Google Scholar]
- 2.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43(1):55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 3.McIver B, Morris JC. The pathogenesis of Graves’ disease. Endocrinol Metab Clin North Am. 1998;27(1):73–89. doi: 10.1016/s0889-8529(05)70299-1. [DOI] [PubMed] [Google Scholar]
- 4.Bahn RS, Heufelder AE. Pathogenesis of Graves’ ophthalmopathy. N Engl J Med. 1993;329(20):1468–75. doi: 10.1056/NEJM199311113292007. [DOI] [PubMed] [Google Scholar]
- 5.LiVolsi VA. The pathology of autoimmune thyroid disease: a review. Thyroid. 1994;4(3):333–9. doi: 10.1089/thy.1994.4.333. [DOI] [PubMed] [Google Scholar]
- 6.Rees SB, McLachlan SM, Furmaniak J. Autoantibodies to the thyrotropin receptor. Endocr Rev. 1988;9(1):106–21. doi: 10.1210/edrv-9-1-106. [DOI] [PubMed] [Google Scholar]
- 7.Witebsky E, Rose NR, Terplan K, Paine JR, Egan RW. Chronic thyroiditis and autoimmunization. J Am Med Assoc. 1957;164(13):1439–47. doi: 10.1001/jama.1957.02980130015004. Epub 1957/07/27. [DOI] [PubMed] [Google Scholar]
- 8.Tomer Y. Genetic susceptibility to autoimmune thyroid disease: past, present, and future. Thyroid. 2010;20(7):715–25. doi: 10.1089/thy.2010.1644. Epub 2010/07/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomer Y, Menconi F, Davies TF, Barbesino G, Rocchi R, Pinchera A, et al. Dissecting genetic heterogeneity in autoimmune thyroid diseases by subset analysis. J Autoimmun. 2007;29(2–3):69–77. doi: 10.1016/j.jaut.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson EM, Tomer Y. The genetic basis of thyroid autoimmunity. Thyroid. 2007;17(10):949–61. doi: 10.1089/thy.2007.0153. Epub 2007/09/11. [DOI] [PubMed] [Google Scholar]
- 11.Davies TF. Infection and autoimmune thyroid disease. J Clin Endocrinol Metab. 2008;93(3):674–6. doi: 10.1210/jc.2008-0095. [DOI] [PubMed] [Google Scholar]
- 12.Ladygina N, Martin BR, Altman A. Dynamic palmitoylation and the role of DHHC proteins in T cell activation and anergy. Adv Immunol. 2011;109:1–44. doi: 10.1016/B978-0-12-387664-5.00001-7. Epub 2011/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20(6):632–8. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Latrofa F, Ricci D, Grasso L, Vitti P, Masserini L, Basolo F, et al. Characterization of thyroglobulin epitopes in patients with autoimmune and non-autoimmune thyroid diseases using recombinant human monoclonal thyroglobulin autoantibodies. J Clin Endocrinol Metab. 2008;93(2):591–6. doi: 10.1210/jc.2007-1199. Epub 2007/11/22. [DOI] [PubMed] [Google Scholar]
- 15.Rebuffat SA, Nguyen B, Robert B, Castex F, Peraldi-Roux S. Antithyroperoxidase antibody-dependent cytotoxicity in auto-immune thyroid disease. J Clin Endocrinol Metab. 2008;93(3):929–34. doi: 10.1210/jc.2007-2042. Epub 2007/12/13. [DOI] [PubMed] [Google Scholar]
- 16.Kohno Y, Naito N, Hiyama Y, Shimojo N, Suzuki N, Tarutani O, et al. Thyroglobulin and thyroid peroxidase share common epitopes recognized by autoantibodies in patients with chronic autoimmune thyroiditis. J Clin Endocrinol Metab. 1988;67(5):899–907. doi: 10.1210/jcem-67-5-899. Epub 1988/11/01. [DOI] [PubMed] [Google Scholar]
- 17.Latrofa F, Pichurin P, Guo J, Rapoport B, McLachlan SM. Thyroglobulin-thyroperoxidase autoantibodies are polyreactive, not bispecific: analysis using human monoclonal autoantibodies. J Clin Endocrinol Metab. 2003;88(1):371–8. doi: 10.1210/jc.2002-021073. Epub 2003/01/10. [DOI] [PubMed] [Google Scholar]
- 18.Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: potential autoantigen in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1998;83(3):998–1002. doi: 10.1210/jcem.83.3.4676. [DOI] [PubMed] [Google Scholar]
- 19.Misrahi M, Ghinea N, Sar S, Saunier B, Jolivet A, Loosfelt H, et al. Processing of the precursors of the human thyroid-stimulating hormone receptor in various eukaryotic cells (human thyrocytes, transfected L cells and baculovirus-infected insect cells). Eur J Biochem. 1994;222(2):711–9. doi: 10.1111/j.1432-1033.1994.tb18916.x. [DOI] [PubMed] [Google Scholar]
- 20.Chazenbalk GD, Pichurin P, Chen CR, Latrofa F, Johnstone AP, McLachlan SM, et al. Thyroid-stimulating autoantibodies in Graves disease preferentially recognize the free A subunit, not the thyrotropin holoreceptor. J Clin Invest. 2002;110(2):209–17. doi: 10.1172/JCI15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajita Y, Rickards CR, Buckland PR, Howells RD, Rees SB. Analysis of thyrotropin receptors by photoaffinity labelling. Orientation of receptor subunits in the cell membrane. Biochem J. 1985;227(2):413–20. doi: 10.1042/bj2270413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loosfelt H, Pichon C, Jolivet A, Misrahi M, Caillou B, Jamous M, et al. Two-subunit structure of the human thyrotropin receptor. Proc Natl Acad Sci USA. 1992;89(9):3765–9. doi: 10.1073/pnas.89.9.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latif R, Morshed SA, Zaidi M, Davies TF. The thyroid-stimulating hormone receptor: impact of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on multi-merization, cleavage, and signaling. Endocrinol Metab Clin North Am. 2009;38(2):319–41. doi: 10.1016/j.ecl.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Davies TF, Ando T, Lin RY, Tomer Y, Latif R. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest. 2005;115(8):1972–83. doi: 10.1172/JCI26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlase H, Davies TF. Insights into the molecular mechanisms of the autoimmune thyroid diseases. In: Eisenbarth GS, editor. Endocrine and organ specific autoimmunity. R.G. Landes Co.; CA: 1999. pp. 98–132. [Google Scholar]
- 26.Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001;2(9):764–6. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 27.De Groot LJ, Shin YH, Pan D, Gopalakrishnan G, Hennessey JV. Evaluation of T cell stimulation by thyrotropin-receptor epitopes in Graves’ disease. J Endocrinol Invest. 2009;32(1):52–6. doi: 10.1007/BF03345679. Epub 2009/04/02. [DOI] [PubMed] [Google Scholar]
- 28.Caturegli P, Kimura H, Rocchi R, Rose NR. Autoimmune thyroid disease. Curr Opin Rheumatol. 2007;19(1):44–8. doi: 10.1097/BOR.0b013e3280113d1a. [DOI] [PubMed] [Google Scholar]
- 29.Ando T, Davies TF. Monoclonal antibodies to the thyrotropin receptor. Clin Dev Immunol. 2005;12(2):137–43. doi: 10.1080/17402520500078238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ando T, Latif R, Pritsker A, Moran T, Nagayama Y, Davies TF. A monoclonal thyroid-stimulating antibody. J Clin Invest. 2002;110(11):1667–74. doi: 10.1172/JCI16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ando T, Latif R, Davies TF. Antibody-induced modulation of TSH receptor post-translational processing. J Endocrinol. 2007;195(1):179–86. doi: 10.1677/JOE-07-0058. [DOI] [PubMed] [Google Scholar]
- 32.Morshed SA, Latif R, Davies TF. Characterization of thyrotropin receptor antibody-induced signaling cascades. Endocrinology. 2009;150(1):519–29. doi: 10.1210/en.2008-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ando T, Latif R, Daniel S, Eguchi K, Davies TF. Dissecting linear and conformational epitopes on the native thyrotropin receptor. Endocrinology. 2004;145(11):5185–93. doi: 10.1210/en.2004-0789. [DOI] [PubMed] [Google Scholar]
- 34.Davies TF, Bobovnikova Y, Weiss M, Vlase H, Moran T, Graves PN. Development and characterization of monoclonal antibodies specific for the murine thyrotropin receptor. Thyroid. 1998;8(8):693–701. doi: 10.1089/thy.1998.8.693. [DOI] [PubMed] [Google Scholar]
- 35.Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Kiddie A, et al. Characteristics of a human monoclonal auto-antibody to the thyrotropin receptor: sequence structure and function. Thyroid. 2004;14(8):560–70. doi: 10.1089/1050725041692918. [DOI] [PubMed] [Google Scholar]
- 36.Nagy EV, Burch HB, Mahoney K, Lukes YG, Morris JC, III, Burman KD. Graves’ IgG recognizes linear epitopes in the human thyrotropin receptor. Biochem Biophys Res Commun. 1992;188(1):28–33. doi: 10.1016/0006-291x(92)92345-x. [DOI] [PubMed] [Google Scholar]
- 37.Sanders J, Chirgadze DY, Sanders P, Baker S, Sullivan A, Bhardwaja A, et al. Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid. 2007;17(5):395–410. doi: 10.1089/thy.2007.0034. [DOI] [PubMed] [Google Scholar]
- 38.Sanders J, Bolton J, Sanders P, Jeffreys J, Nakatake N, Richards T, et al. Effects of TSH receptor mutations on binding and biological activity of monoclonal antibodies and TSH. Thyroid. 2006;16(12):1195–206. doi: 10.1089/thy.2006.16.1195. [DOI] [PubMed] [Google Scholar]
- 39.Latif R, Michalek K, Teixeira A, Davies TF. Protecting TSH receptor antibody binding pockets reveals both leucine rich repeats and non-leucine rich domains.. 81st Annual Meeting of the American Thyroid Association; CA, USA. 2011. [Google Scholar]
- 40.Morshed SA, Latif R, Davies TF. TSHR antibodies induce fibroblast proliferation and apoptosis: implications for Graves’ disease.. 81st Annual Meeting of the American Thyroid Association; CA, USA.. 2011. [Google Scholar]
- 41.Minich WB, Lenzner C, Morgenthaler NG. Antibodies to TSH-receptor in thyroid autoimmune disease interact with monoclonal antibodies whose epitopes are broadly distributed on the receptor. Clin Exp Immunol. 2004;136(1):129–36. doi: 10.1111/j.1365-2249.2004.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanders J, Jeffreys J, Depraetere H, Richards T, Evans M, Kiddie A, et al. Thyroid-stimulating monoclonal antibodies. Thyroid. 2002;12(12):1043–50. doi: 10.1089/105072502321085135. [DOI] [PubMed] [Google Scholar]
- 43.Schwarz-Lauer L, Pichurin PN, Chen CR, Nagayama Y, Paras C, Morris JC, et al. The cysteine-rich amino terminus of the thyrotropin receptor is the immunodominant linear antibody epitope in mice immunized using naked deoxyribonucleic acid or adenovirus vectors. Endocrinology. 2003;144(5):1718–25. doi: 10.1210/en.2002-0069. [DOI] [PubMed] [Google Scholar]
- 44.Ando T, Latif R, Davies TF. Concentration-dependent regulation of thyrotropin receptor function by thyroid-stimulating antibody. J Clin Invest. 2004;113(11):1589–95. doi: 10.1172/JCI21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minich WB, Loos U. Detection of functionally different types of pathological autoantibodies against thyrotropin receptor in Graves’ patients sera by luminescent immunoprecipitation analysis. Exp Clin Endocrinol Diabetes. 2000;108(2):110–9. doi: 10.1055/s-2000-5804. [DOI] [PubMed] [Google Scholar]
- 46.Kakinuma A, Chazenbalk GD, Tanaka K, Nagayama Y, McLachlan SM, Rapoport B. An N-linked glycosylation motif from the noncleaving luteinizing hormone receptor substituted for the homologous region (Gly367 to Glu369) of the thyrotropin receptor prevents cleavage at its second, downstream site. J Biol Chem. 1997;272(45):28296–300. doi: 10.1074/jbc.272.45.28296. [DOI] [PubMed] [Google Scholar]
- 47.Sanders P, Young S, Sanders J, Kabelis K, Baker S, Sullivan A, et al. Crystal structure of the TSH receptor (TSHR) bound to a blocking-type TSHR autoantibody. J Mol Endocrinol. 2011;46(2):81–99. doi: 10.1530/JME-10-0127. Epub 2011/01/21. [DOI] [PubMed] [Google Scholar]
- 48.Costagliola S, Rodien P, Many MC, Ludgate M, Vassart G. Genetic immunization against the human thyrotropin receptor causes thyroiditis and allows production of monoclonal antibodies recognizing the native receptor. J Immunol. 1998;160(3):1458–65. [PubMed] [Google Scholar]
- 49.Nagayama Y, Kita-Furuyama M, Ando T, Nakao K, Mizuguchi H, Hayakawa T, et al. A novel murine model of Graves’ hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol. 2002;168(6):2789–94. doi: 10.4049/jimmunol.168.6.2789. [DOI] [PubMed] [Google Scholar]
- 50.Jeffreys J, Depraetere H, Sanders J, Oda Y, Evans M, Kiddie A, et al. Characterization of the thyrotropin binding pocket. Thyroid. 2002;12(12):1051–61. doi: 10.1089/105072502321085144. [DOI] [PubMed] [Google Scholar]
- 51.Gilbert JA, Kalled SL, Moorhead J, Hess DM, Rennert P, Li Z, et al. Treatment of autoimmune hyperthyroidism in a murine model of Graves’ disease with tumor necrosis factor-family ligand inhibitors suggests a key role for B cell activating factor in disease pathology. Endocrinology. 2006;147(10):4561–8. doi: 10.1210/en.2006-0507. [DOI] [PubMed] [Google Scholar]
- 52.Lai Kwan LQ, King Hung KO, Zheng BJ, Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc Natl Acad Sci USA. 2008;105(39):14993–8. doi: 10.1073/pnas.0806044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salvi M, Vannucchi G, Campi I, Rossi S, Bonara P, Sbrozzi F, et al. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur J Endocrinol. 2006;154(4):511–7. doi: 10.1530/eje.1.02119. [DOI] [PubMed] [Google Scholar]
- 54.Chen CR, McLachlan SM, Rapoport B. Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology. 2007;148(5):2375–82. doi: 10.1210/en.2006-1754. [DOI] [PubMed] [Google Scholar]
- 55.Stassi G, De MR. Autoimmune thyroid disease: new models of cell death in autoimmunity. Nat Rev Immunol. 2002;2(3):195–204. doi: 10.1038/nri750. [DOI] [PubMed] [Google Scholar]
- 56.Wang SH, Baker JR. The role of apoptosis in thyroid autoimmunity. Thyroid. 2007;17(10):975–9. doi: 10.1089/thy.2007.0208. Epub 2007/09/29. [DOI] [PubMed] [Google Scholar]
- 57.Mao C, Wang S, Xiao Y, Xu J, Jiang Q, Jin M, et al. Impairment of regulatory capacity of CD4+ CD25+ regulatory T cells mediated by dendritic cell polarization and hyperthyroidism in Graves’ disease. J Immunol. 2011;186(8):4734–43. doi: 10.4049/jimmunol.0904135. Epub 2011/03/15. [DOI] [PubMed] [Google Scholar]
- 58.Bossowski A, Czarnocka B, Bardadin K, Stasiak-Barmuta A, Urban M, Dadan J, et al. Identification of apoptotic proteins in thyroid gland from patients with Graves’ disease and Hashimoto's thyroiditis. Autoimmunity. 2008;41(2):163–73. doi: 10.1080/08916930701727749. Epub 2008/03/08. [DOI] [PubMed] [Google Scholar]
- 59.Bossowski A, Czarnocka B, Bardadin K, Urban M, Niedziela M, Dadan J. Expression of Bcl-2 family proteins in thyrocytes from young patients with immune and nonimmune thyroid diseases. Horm Res. 2008;70(3):155–64. doi: 10.1159/000145017. Epub 2008/07/30. [DOI] [PubMed] [Google Scholar]
- 60.Newby PR, Roberts-Davies EL, Brand OJ, Heward JM, Frank-lyn JA, Gough SCL, et al. Tag SNP screening of the PDCD1 gene for association with Graves’ disease. Clin Endocrinol. 2007;67(1):125–8. doi: 10.1111/j.1365-2265.2007.02848.x. [DOI] [PubMed] [Google Scholar]
- 61.Morshed SA, Ando T, Latif R, Davies TF. Neutral antibodies to the TSH receptor are present in Graves’ disease and regulate selective signaling cascades. Endocrinology. 2010;151(11):5537–49. doi: 10.1210/en.2010-0424. Epub 2010/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawashima A, Tanigawa K, Akama T, Wu H, Sue M, Yoshihara A, et al. Fragments of genomic DNA released by injured cells activate innate immunity and suppress endocrine function in the thyroid. Endocrinology. 2011;152(4):1702–12. doi: 10.1210/en.2010-1132. Epub 2011/02/10. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura H, Usa T, Motomura M, Ichikawa T, Nakao K, Kawasaki E, et al. Prevalence of interrelated autoantibodies in thyroid diseases and autoimmune disorders. J Endocrinol Invest. 2008;31(10):861–5. doi: 10.1007/BF03346432. [DOI] [PubMed] [Google Scholar]
- 64.Endo T, Kogai T, Nakazato M, Saito T, Kaneshige M, Onaya T. Autoantibody against Na+/I- symporter in the sera of patients with autoimmune thyroid disease. Biochem Biophys Res Commun. 1996;224(1):92–5. doi: 10.1006/bbrc.1996.0989. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida A, Hisatome I, Taniguchi S, Shirayoshi Y, Yamamoto Y, Miake J, et al. Pendrin is a novel autoantigen recognized by patients with autoimmune thyroid diseases. J Clin Endocrinol Metab. 2009;94(2):442–8. doi: 10.1210/jc.2008-1732. [DOI] [PubMed] [Google Scholar]
- 66.Benvenga S, Trimarchi F, Robbins J. Circulating thyroid hormone autoantibodies. J Endocrinol Invest. 1987;10(6):605–19. doi: 10.1007/BF03347008. Epub 1987/12/01. [DOI] [PubMed] [Google Scholar]
- 67.Tachi J, Amino N, Tamaki H, Aozasa M, Iwatani Y, Miyai K. Long term follow-up and HLA association in patients with postpartum hypothyroidism. J Clin Endocrinol Metab. 1988;66(3):480–4. doi: 10.1210/jcem-66-3-480. Epub 1988/03/01. [DOI] [PubMed] [Google Scholar]
- 68.Katakura M, Yamada T, Aizawa T, Hiramatsu K, Yukimura Y, Ishihara M, et al. Presence of antideoxyribonucleic acid antibody in patients with hyperthyroidism of Graves’ disease. J Clin Endocrinol Metab. 1987;64(3):405–8. doi: 10.1210/jcem-64-3-405. Epub 1987/03/01. [DOI] [PubMed] [Google Scholar]
- 69.Marino M, Chiovato L, Friedlander JA, Latrofa F, Pinchera A, McCluskey RT. Serum antibodies against megalin (GP330) in patients with autoimmune thyroiditis. J Clin Endocrinol Metab. 1999;84(7):2468–74. doi: 10.1210/jcem.84.7.5837. Epub 1999/07/15. [DOI] [PubMed] [Google Scholar]
- 70.Douglas RS, Naik V, Hwang CJ, Afifiyan NF, Gianoukakis AG, Sand D, et al. B cells from patients with Graves’ disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol. 2008;181(8):5768–74. doi: 10.4049/jimmunol.181.8.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev. 2010;62(2):199–236. doi: 10.1124/pr.109.002469. Epub 2010/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davies TF, Yin X, Latif R. The genetics of the thyroid stimulating hormone receptor: history and relevance. Thyroid. 2010;20(7):727–36. doi: 10.1089/thy.2010.1638. Epub 2010/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kong YC, Flynn JC, Wan Q, David CS. HLA and H2 class II transgenic mouse models to study susceptibility and protection in autoimmune thyroid disease. Autoimmunity. 2003;36(6–7):397–404. doi: 10.1080/08916930310001603028. Epub 2003/12/13. [DOI] [PubMed] [Google Scholar]
- 74.Menconi F, Huber A, Osman R, Concepcion E, Jacobson EM, Stefan M, et al. Tg.2098 is a major human thyroglobulin T-cell epitope. J Autoimmun. 2010;35(1):45–51. doi: 10.1016/j.jaut.2010.01.004. Epub 2010/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ban Y, Tomer Y. The contribution of immune regulatory and thyroid specific genes to the etiology of Graves’ and Hashimoto's diseases. Autoimmunity. 2003;36(6–7):367–79. doi: 10.1080/08916930310001603037. Epub 2003/12/13. [DOI] [PubMed] [Google Scholar]
- 76.Han S, Zhang S, Zhang W, Li R, Li Y, Wang Z, et al. CTLA4 polymorphisms and ophthalmopathy in Graves’ disease patients: association study and meta-analysis. Hum Immunol. 2006;67(8):618–26. doi: 10.1016/j.humimm.2006.05.003. Epub 2006/08/19. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Zahir N, Jiang Q, Miliotis H, Heyraud S, Meng X, et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011;43(9):902–7. doi: 10.1038/ng.904. Epub 2011/08/16. [DOI] [PubMed] [Google Scholar]
- 78.Ban Y, Tozaki T, Taniyama M, Tomita M. Association of a C/T single-nucleotide polymorphism in the 50 untranslated region of the CD40 gene with Graves’ disease in Japanese. Thyroid. 2006;16(5):443–6. doi: 10.1089/thy.2006.16.443. Epub 2006/06/08. [DOI] [PubMed] [Google Scholar]
- 79.Chistiakov DA, Chistiakova EI, Voronova NV, Turakulov RI, Savost'anov KV. A variant of the Il2ra/Cd25 gene predisposing to graves’ disease is associated with increased levels of soluble interleukin-2 receptor. Scand J Immunol. 2011;74(5):496–501. doi: 10.1111/j.1365-3083.2011.02608.x. Epub 2011/08/06. [DOI] [PubMed] [Google Scholar]
- 80.Simmonds MJ, Heward JM, Carr-Smith J, Foxall H, Franklyn JA, Gough SC. Contribution of single nucleotide polymorphisms within FCRL3 and MAP3K7IP2 to the pathogenesis of Graves’ disease. J Clin Endocrinol Metab. 2006;91(3):1056–61. doi: 10.1210/jc.2005-1634. Epub 2005/12/31. [DOI] [PubMed] [Google Scholar]
- 81.Ban Y, Greenberg DA, Concepcion E, Skrabanek L, Villanueva R, Tomer Y. Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine auto-immune thyroid disease. Proc Natl Acad Sci USA. 2003;100(25):15119–24. doi: 10.1073/pnas.2434175100. Epub 2003/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yin X, Latif R, Bahn R, Tomer Y, Davies TF. Influence of the TSH receptor gene on susceptibility to Graves’ disease and Graves’ ophthalmopathy. Thyroid. 2008;18(11):1201–6. doi: 10.1089/thy.2008.0098. Epub 2008/10/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsatsoulis A. The role of stress in the clinical expression of thyroid autoimmunity. Ann N Y Acad Sci. 2006;1088:382–95. doi: 10.1196/annals.1366.015. Epub 2006/12/29. [DOI] [PubMed] [Google Scholar]
- 84.Davies TF, Martin A, Concepcion ES, Graves P, Cohen L, Ben-Nun A. Evidence of limited variability of antigen receptors on intrathyroidal T cells in autoimmune thyroid disease. N Engl J Med. 1991;325(4):238–44. doi: 10.1056/NEJM199107253250404. [DOI] [PubMed] [Google Scholar]
- 85.Davies TF, Martin A, Concepcion ES, Graves P, Lahat N, Cohen WL, et al. Evidence for selective accumulation of intrathyroidal T lymphocytes in human autoimmune thyroid disease based on T cell receptor V gene usage. J Clin Invest. 1992;89(1):157–62. doi: 10.1172/JCI115556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dayan CM, Londei M, Corcoran AE, Grubeck-Loebenstein B, James RF, Rapoport B, et al. Autoantigen recognition by thyroid-infiltrating T cells in Graves disease. Proc Natl Acad Sci USA. 1991;88(16):7415–9. doi: 10.1073/pnas.88.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jackson RA, Haynes BF, Burch WM, Shimizu K, Bowring MA, Eisenbarth GS. Ia+ T cells in new onset Graves’ disease. J Clin Endocrinol Metab. 1984;59(2):187–90. doi: 10.1210/jcem-59-2-187. [DOI] [PubMed] [Google Scholar]
- 88.Wall JR, Baur R, Schleusener H, Bandy-Dafoe P. Peripheral blood and intrathyroidal mononuclear cell populations in patients with autoimmune thyroid disorders enumerated using monoclonal antibodies. J Clin Endocrinol Metab. 1983;56(1):164–9. doi: 10.1210/jcem-56-1-164. [DOI] [PubMed] [Google Scholar]
- 89.Martin A, Nakashima M, Zhou A, Aronson D, Werner AJ, Davies TF. Detection of major T cell epitopes on human thyroid stimulating hormone receptor by overriding immune heterogeneity in patients with Graves’ disease. J Clin Endocrinol Metab. 1997;82(10):3361–6. doi: 10.1210/jcem.82.10.4299. [DOI] [PubMed] [Google Scholar]
- 90.Martin A, Barbesino G, Davies TF. T-cell receptors and auto-immune thyroid disease—signposts for T-cell-antigen driven diseases. Int Rev Immunol. 1999;18(1–2):111–40. doi: 10.3109/08830189909043021. [DOI] [PubMed] [Google Scholar]
- 91.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 92.Sakaguchi S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 93.McLachlan SM, Nagayama Y, Pichurin PN, Mizutori Y, Chen CR, Misharin A, et al. The link between Graves’ disease and Hashimoto's thyroiditis: a role for regulatory T cells. Endocrinology. 2007;148(12):5724–33. doi: 10.1210/en.2007-1024. [DOI] [PubMed] [Google Scholar]
- 94.Saitoh O, Nagayama Y. Regulation of Graves’ hyperthyroidism with naturally occurring CD4+ CD25+ regulatory T cells in a mouse model. Endocrinology. 2006;147(5):2417–22. doi: 10.1210/en.2005-1024. [DOI] [PubMed] [Google Scholar]
- 95.Saitoh O, Abiru N, Nakahara M, Nagayama Y. CD8+ CD122+ T cells, a newly identified regulatory T subset, negatively regulate Graves’ hyperthyroidism in a murine model. Endocrinology. 2007;148(12):6040–6. doi: 10.1210/en.2007-0300. [DOI] [PubMed] [Google Scholar]
- 96.Marazuela M, Garcia-Lopez MA, Figueroa-Vega N, de la FH, varado-Sanchez B, Monsivais-Urenda A, et al. Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab. 2006;91(9):3639–46. doi: 10.1210/jc.2005-2337. [DOI] [PubMed] [Google Scholar]
- 97.Owen CJ, Eden JA, Jennings CE, Wilson V, Cheetham TD, Pearce SH. Genetic association studies of the FOXP3 gene in Graves’ disease and autoimmune Addison's disease in the United Kingdom population. J Mol Endocrinol. 2006;37(1):97–104. doi: 10.1677/jme.1.02072. [DOI] [PubMed] [Google Scholar]
- 98.Ban Y, Tozaki T, Tobe T, Jacobson EM, Concepcion ES, Tomer Y. The regulatory T cell gene FOXP3 and genetic susceptibility to thyroid autoimmunity: an association analysis in Caucasian and Japanese cohorts. J Autoimmun. 2007;28(4):201–7. doi: 10.1016/j.jaut.2007.02.016. Epub 2007/04/10. [DOI] [PubMed] [Google Scholar]
- 99.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 100.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, it-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lubberts E, Joosten LA, van de Loo FA, Schwarzenberger P, Kolls J, Van Den Berg WB. Overexpression of IL-17 in the knee joint of collagen type II immunized mice promotes collagen arthritis and aggravates joint destruction. Inflamm Res. 2002;51(2):102–4. doi: 10.1007/BF02684010. [DOI] [PubMed] [Google Scholar]
- 102.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin ExpImmunol. 2007;148(1):32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bohgaki T, Atsumi T, Koike T. Autoimmune disease after autologous hematopoietic stem cell transplantation. Autoimmun Rev. 2008;7(3):198–203. doi: 10.1016/j.autrev.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 104.Bohgaki T, Atsumi T, Koike T. Multiple autoimmune diseases after autologous stem-cell transplantation. N Engl J Med. 2007;357(26):2734–6. doi: 10.1056/NEJMc076383. [DOI] [PubMed] [Google Scholar]
- 105.Baker JR., Jr The nature of apoptosis in the thyroid and the role it may play in autoimmune thyroid disease. Thyroid. 2001;11(3):245–7. doi: 10.1089/105072501750159633. [DOI] [PubMed] [Google Scholar]
- 106.Giordano C, Richiusa P, Bagnasco M, Salmaso C, Pizzolanti G, Galluzzo A. Thyrocytes–not innocent bystanders in autoimmune disease. Nat Immunol. 2001;2(3):183. doi: 10.1038/85224. [DOI] [PubMed] [Google Scholar]
- 107.Giordano C, Richiusa P, Bagnasco M, Pizzolanti G, Di BF, Sbriglia MS, et al. Differential regulation of Fas-mediated apoptosis in both thyrocyte and lymphocyte cellular compartments correlates with opposite phenotypic manifestations of autoimmune thyroid disease. Thyroid. 2001;11(3):233–44. doi: 10.1089/105072501750159615. [DOI] [PubMed] [Google Scholar]
- 108.Hamilton F, Black M, Farquharson MA, Stewart C, Foulis AK. Spatial correlation between thyroid epithelial cells expressing class II MHC molecules and interferon-gamma-containing lymphocytes in human thyroid autoimmune disease. Clin Exp Immunol. 1991;83(1):64–8. doi: 10.1111/j.1365-2249.1991.tb05589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sawai Y, DeGroot LJ. Binding of human thyrotropin receptor peptides to a Graves’ disease-predisposing human leukocyte antigen class II molecule. J Clin Endocrinol Metab. 2000;85(3):1176–9. doi: 10.1210/jcem.85.3.6376. [DOI] [PubMed] [Google Scholar]
- 110.McKenzie JM, Zakarija M. Fetal and neonatal hyperthyroidism and hypothyroidism due to maternal TSH receptor antibodies. Thyroid. 1992;2(2):155–9. doi: 10.1089/thy.1992.2.155. [DOI] [PubMed] [Google Scholar]
- 111.Lira SA, Martin AP, Marinkovic T, Furtado GC. Mechanisms regulating lymphocytic infiltration of the thyroid in murine models of thyroiditis. Crit Rev Immunol. 2005;25(4):251–62. doi: 10.1615/critrevimmunol.v25.i4.10. [DOI] [PubMed] [Google Scholar]
- 112.Martin AP, Coronel EC, Sano G, Chen SC, Vassileva G, Canasto-Chibuque C, et al. A novel model for lymphocytic infiltration of the thyroid gland generated by transgenic expression of the CC chemokine CCL21. J Immunol. 2004;173(8):4791–8. doi: 10.4049/jimmunol.173.8.4791. [DOI] [PubMed] [Google Scholar]
- 113.Kita-Furuyama M, Nagayama Y, Pichurin P, McLachlan SM, Rapoport B, Eguchi K. Dendritic cells infected with adenovirus expressing the thyrotrophin receptor induce Graves’ hyperthyroidism in BALB/c mice. ClinExp Immunol. 2003;131(2):234–40. doi: 10.1046/j.1365-2249.2003.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kabel PJ, Voorbij HA, De HM, van der Gaag RD, Drexhage HA. Intrathyroidal dendritic cells. J Clin Endocrinol Metab. 1988;66(1):199–207. doi: 10.1210/jcem-66-1-199. [DOI] [PubMed] [Google Scholar]
- 115.Quadbeck B, Eckstein AK, Tews S, Walz M, Hoermann R, Mann K, et al. Maturation of thyroidal dendritic cells in Graves’ disease. Scand J Immunol. 2002;55(6):612–20. doi: 10.1046/j.1365-3083.2002.01066.x. [DOI] [PubMed] [Google Scholar]
- 116.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–90. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 117.Roura-Mir C, Catalfamo M, Cheng TY, Marqusee E, Besra GS, Jaraquemada D, et al. CD1a and CD1c activate intrathyroidal T cells during Graves’ disease and Hashimoto's thyroiditis. J Immunol. 2005;174(6):3773–80. doi: 10.4049/jimmunol.174.6.3773. [DOI] [PubMed] [Google Scholar]
- 118.Moody DB. TLR gateways to CD1 function. Nat Immunol. 2006;7(8):811–7. doi: 10.1038/ni1368. [DOI] [PubMed] [Google Scholar]
- 119.Hutchings P, Rayner DC, Champion BR, Marshall-Clarke S, Macatonia S, Roitt I, et al. High efficiency antigen presentation by thyroglobulin-primed murine splenic B cells. Eur J Immunol. 1987;17(3):393–8. doi: 10.1002/eji.1830170314. [DOI] [PubMed] [Google Scholar]
- 120.Villanueva R, Inzerillo AM, Tomer Y, Barbesino G, Meltzer M, Concepcion ES, et al. Limited genetic susceptibility to severe Graves’ ophthalmopathy: no role for CTLA-4 but evidence for an environmental etiology. Thyroid. 2000;10(9):791–8. doi: 10.1089/thy.2000.10.791. Epub 2000/10/21. [DOI] [PubMed] [Google Scholar]
- 121.Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726–38. doi: 10.1056/NEJMra0905750. Epub 2010/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang L, Grennan-Jones F, Lane C, Rees DA, Dayan CM, Ludgate M. Adipose tissue depot-specific differences in the regulation of hyaluronan production of relevance to Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97(2):653–62. doi: 10.1210/jc.2011-1299. [DOI] [PubMed] [Google Scholar]
- 123.Gerding MN, van der Meer JW, Broenink M, Bakker O, Wiersinga WM, Prummel MF. Association of thyrotrophin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 2000;52(3):267–71. doi: 10.1046/j.1365-2265.2000.00959.x. Epub 2000/03/16. [DOI] [PubMed] [Google Scholar]
- 124.Vannucchi G, Campi I, Bonomi M, Covelli D, Dazzi D, Curro N, et al. Rituximab treatment in patients with active Graves’ orbitopathy: effects on proinflammatory and humoral immune reactions. Clin Exp Immunol. 2010;161(3):436–43. doi: 10.1111/j.1365-2249.2010.04191.x. Epub 2010/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Konuk O, Hondur A, Akyurek N, Unal M. Apoptosis in orbital fibroadipose tissue and its association with clinical features in Graves’ ophthalmopathy. Ocul Immunol Inflamm. 2007;15(2):105–11. doi: 10.1080/09273940601186735. Epub 2007/06/15. [DOI] [PubMed] [Google Scholar]
- 126.Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168(2):942–50. doi: 10.4049/jimmunol.168.2.942. [DOI] [PubMed] [Google Scholar]
- 127.Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170(12):6348–54. doi: 10.4049/jimmunol.170.12.6348. [DOI] [PubMed] [Google Scholar]