Abstract
Parkinson’s disease (PD) is a neurodegenerative basal ganglia disease that disrupts cognitive control processes involved in response selection. The current study investigated the effects of PD on the ability to resolve conflicts during response selection when performance emphasized response speed versus response accuracy. Twenty-one (21) PD patients and 21 healthy controls (HC) completed a Simon conflict task, and a subset of 10 participants from each group provided simultaneous movement-related potential (MRP) data to track patterns of motor cortex activation and inhibition associated with the successful resolution of conflicting response tendencies. Both groups adjusted performance strategically to emphasize response speed or accuracy (i.e., speed-accuracy effect). For HC, interference from a conflicting response was reduced when response accuracy rather than speed was prioritized. For PD patients, however, there was a reduction in interference, but it was not statistically significant. The conceptual framework of the Dual-Process Activation-Suppression (DPAS) model revealed that the groups experienced similar susceptibility to making fast impulsive errors in conflict trials irrespective of speed-accuracy instructions, but PD patients were less proficient and delayed compared to HC at suppressing the interference from these incorrect response tendencies, especially under speed pressure. Analysis of MRPs on response conflict trials showed attenuated inhibition of the motor cortex controlling the conflicting impulsive response tendency in PD patients compared to HC. These results further confirm the detrimental effects of PD inhibitory control mechanisms and their exacerbation when patients perform under speed pressure. The results also suggest that a downstream effect of inhibitory dysfunction in PD is diminished inhibition of motor cortex controlling conflicting response tendencies.
Introduction
The spontaneous processing of irrelevant stimulus information can activate highly reflexive or overlearned responses that benefit or conflict with the performance of goal-determined responses. For example, when a response activated spontaneously by irrelevant information corresponds to the response signaled by goal-relevant stimulus information, the speed and accuracy of issuing the goal response may be facilitated. However, when irrelevant and goal-relevant stimulus information signal conflicting responses, the speed and accuracy of issuing the goal response is typically compromised. The situation involving conflict is of particular interest because it affords the opportunity to investigate speeded cognitive control processes engaged to resolve response conflict. Converging evidence suggests that control circuitries linking prefrontal cortices to the direct (action selection) and indirect (action suppression) basal ganglia pathways coordinate the selection and suppression of conflicting responses (Aron, Behrens, Smith, Frank, & Poldrack, 2007; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004; Redgrave, Prescott, & Gurney, 1999; Mink, 1996; Alexander, DeLong, & Strick, 1986). This has been especially insightful in understanding certain cognitive control deficits in Parkinson’s disease (PD). In PD, the degeneration of dopamine-producing neurons that modulate these pathways impairs the ability to resolve conflict during action selection and, in particular, the proficiency of suppressing conflicting responses (Wylie et al., 2009a b).
As we describe next, the proficiency of resolving response conflict is directly modified by strategies that prioritize speed or accuracy of performance. In a previous study, we demonstrated that performing under speed stress exacerbated deficits in conflict resolution and inhibitory control in PD (Wylie et al., 2009b). In the current investigation, we used a different experimental paradigm to confirm the reliability of these effects and included a novel extension that incorporated measurements of movement-related potentials (MRPs) to track the activation and suppression of M1 motor cortices controlling these responses.
Speed-Accuracy Strategy Modulates Conflict Resolution
The degree to which responses activated by irrelevant information facilitate or interfere with goal-directed behavior is highly sensitive to how much pressure there is to respond quickly or accurately to the stimulus event. The effects of time pressure on the speed and accuracy of reactions typically follow the aptly named Speed-Accuracy Tradeoff (SAT) function (Wickelgren, 1977): prioritizing the speed of reactions yields faster responses at the cost of making more errors, whereas prioritizing response accuracy produces fewer errors but slower responses. In conflict situations, these strategic adjustments in the SAT are particularly influential. Thus, focusing on speed in response conflict situations enhances interference effects and the susceptibility of acting on spontaneous, prepotent reactions in error (Wylie et al., 2009b). Conversely, focusing on accuracy in response conflict situations reduces interference effects and the tendency of reacting spontaneously in error. The dynamics involving the relationship between speed-accuracy tradeoff and the activation and suppression of spontaneous reactions are elegantly captured using distributional analytic techniques within the theoretical framework of the Dual-Process Activation-Suppression (DPAS) model (Ridderinkhof, 2002; Wylie et al., 2009a b; for a review see van den Wildenberg et al., 2010).
This model posits that the appearance of an imperative stimulus in situations in which it conveys conflicting response information activates two processing routes concurrently; an automatic route that is engaged directly by the conflicting stimulus information to activate the alternative, incorrect response and a deliberate controlled route that is responsible for identifying the target stimulus and selecting the response appropriate to it. It is assumed further that the activation associated with conflicting information is most influential early in processing and that over time, as processing unfolds, suppression mechanisms are engaged to inhibit this activation and facilitate selection of the correct response. The temporal dynamics of activation and suppression can be isolated using two sets of distributional analyses. Specifically, susceptibility to the activation of prepotent, erroneous responses (i.e., response capture) is revealed by plotting accuracy rates as a function of RT (i.e., conditional accuracy functions, CAFs) to determine the distribution of error rates from the fastest to the slowest response latencies. Most errors, what we call impulsive errors, occur at the fastest response latencies on conflict trials. The degree of proficiency in resolving interference by suppressing the activation of erroneous reactions, as an act of cognitive control, is revealed by plotting interference effects as a function of RT (i.e., delta plots) to determine the extent to which interference is reduced at slower response latencies, with steeper reductions in response interference reflecting more proficient inhibitory control. This conceptual-statistical approach to understanding the temporal dynamics of response conflict is gaining support in a growing body of research (e.g., Forstmann, Jahfari, et al., 2008; Forstmann, van den Wildenberg, & Ridderinkhof, 2008; see Ridderinkhof, Forstmann, Wylie, Burle, & van den Wildenberg, 2011, for a review).
The temporal dynamics approach of the DPAS is proving to be especially valuable in characterizing deficits in cognitive control in conflict tasks by individuals, like those with PD, who suffer from frontal basal ganglia dysfunction (Frank, 2005; Praamstra, Stegeman, Cools, & Horstink, 1998; Praamstra, Plat, Meyer, & Horstink, 1999; Praamstra & Plat, 2001; Voon & Fox, 2007; Wylie, Ridderinkhof, Bashore, & van den Wildenberg, 2010). In a recent study, for example, Wylie et al. (2009b) used an arrow variant of the Eriksen flanker task (Eriksen, B.A. & Eriksen, C.W., 1974) to examine if these deficits among PD patients are especially sensitive to SAT effects, predicting that they would be exacerbated by an emphasis on speed rather than accuracy in responding. In the Flanker task, visual distractors signal the same or a conflicting response as an imperative stimulus; conflict produces a slowing of RT and reduction in accuracy called the Flanker interference effect. Speed pressure was found by Wylie et al. (2009b) to exacerbate the Flanker interference effect more among PD patients than healthy controls (HC). Moreover, the proportion of fast errors on incongruent trials indicated that when PD patients and HC were pressed for speed they were equally susceptible to acting on the spontaneous response impulse signaled by the flankers. Most importantly, however, when looking at the dynamics of the interference effect, HC suppressed flanker interference to a similar degree of proficiency under speed and accuracy conditions, whereas PD patients showed diminished suppression overall that was even more pronounced when concerned about the speed of reacting rather than the accuracy of responding. Thus, impaired inhibitory action control in PD was exacerbated under speed stress.
Movement-Related Potentials: Tracking the Activation and Suppression of Responses
The current investigation aimed to replicate and extend response suppression dynamics in PD using the DPAS model in conjunction with a different, but equally sensitive, procedure for inducing response conflict, the Simon task (Simon, 1969). In a subset of participants, we also simultaneously recorded movement-related brain potential (MRP) activity to track psychophysiological indices of response activation-inhibition patterns at the level of the primary motor cortex (M1). Changes in the properties of one MRP component in particular, the lateralized readiness potential (LRP), have proven to be very sensitive indices of the differential activation of correct and incorrect responses in conflicting choice situations. That is, the LRP reflects the difference in motor cortex activation between two response alternatives (de Jong, Wierda, Mulder, & Mulder, 1988; Gratton, Coles, Sirevaag, Eriksen, C.W., & Donchin, 1988; Low, Miller, & Vierck, 2002; Miller, 2007; see early review in Coles, 1989). Specifically, on conflict trials, an initial, relatively small deflection of electrical activity, identified first by Gratton et al. (1988) in a flanker task, is apparent in the LRP that is thought to reflect a bias in activity in favor of the incorrect response. This deflection quickly reverses direction, transforming into a much larger signal of longer duration that is thought to reflect activation of the correct response.
Analyses of LRP activity in conjunction with behavioral measures have deepened our insights into the nature of response system activation deficits of PD patients in conflict tasks. For example, the exacerbated flanker effect in PD patients relative to HC was associated with the initial activation of an incorrect response, as reflected both in an earlier onset latency of the LRP and a larger and longer duration of the early deflection (Praamstra Stegeman, Cools, & Horstink, 1998; Praamstra, Plat, Meyer, & Horstink, 1999). However, although findings from LRP studies have augmented those from behavioral studies, there are nonetheless limitations in the exclusive use of this component to study Simon conflict effects. First, by virtue of the subtraction procedures used to derive the LRP, it represents the difference in brain electrical activity recorded from scalp sites over the motor cortices contralateral and ipsilateral to the movement. Thus, it is difficult to determine the relative contributions of each M1 motor area to the activation of the correct response and suppression of the incorrect response alternative. Several studies have shown that unilateral measures of the MRP may reveal the unique and specific contributions of the motor areas contra- and ipsilateral to the processes of activation and suppression, respectively, of the correct and incorrect response hand alternatives (Beste, 2009; Taniguchi, Burle, Vidal, & Bonnet, 2001). Second, suppression of motor cortex can be modulated independently from contralateral activation (Carbonnell, Hasbroucq, Grapperon, & Vidal, 2004; Tandonnet, 2003). Because the LRP is a subtraction or difference waveform, it cannot be used to dissociate the relative contributions of activation- and inhibition-related processes. This dissociation is important, especially in patient groups where these processes might be affected differentially. Third, the structural properties of the LRP are confounded by lateralized visual potentials generated from posterior sites that spread across the scalp when visual stimuli are presented in the visual half-field, as they are in the Simon task (for detailed discussion, see Praamstra, 2007).
An alternative transformation of the EEG that may circumvent these limitations is the response-locked surface Laplacian technique (Tanaguchi et al., 2001; Tandonnet, 2006; Vidal, Grapperon, Bonnet, & Hasbroucq, 2003). The Laplacian transformation of EEG acts as a spatial high pass filter, removing blur from current diffusions through the skull and thereby reducing contributions from remote sources (like the visual cortex) and enhancing spatial information (Babiloni, 2001; Nunez, 1981; Tandonnet, 2003). In manual choice reaction tasks, this spatial fidelity permits separate monitoring of the activity of each motor cortex just prior to the emission of an overt response. Specifically, in healthy young adults a negative-going wave begins to evolve in electrodes overlying motor cortex (e.g., C3, C4) contralateral to the responding hand within 200 ms prior to the recorded response. This negative wave is thought to reflect activation of the motor cortex that controls the goal-directed correct response (e.g., C3 for responses by the right hand). Concurrently, a positive-going wave develops at electrodes contralateral to the non-selected response hand (i.e., ipsilateral to the responding hand; e.g., C4 for responses by the right hand) that is thought to reflect the simultaneous inhibition of activity in the motor cortex that controls production of the incorrect response (for reviews see Burle, 2004; Carbonell et al., 2004; Meckler et al., 2010; Meynier, Burle, Possamai, Vidal, & Hasbroucq, 2009; van de Laar et al., 2012; Vidal et al., 2003). Given the deficits in inhibitory action control among PD patients, we reasoned that the response-locked surface Laplacian technique could provide new insights about the degree of suppression of conflicting motor responses at M1, especially in situations involving speed stress.
The Current Investigation
In this study, the effects of differences in speed-accuracy tradeoff on the proficiency and temporal dynamics of interference control in PD patients are examined for the first time using MRPs. The Simon effect is characterized by an increase in both RT and error rate when the spatial location (e.g., left visual half-field) of an imperative stimulus and the response it signals (e.g., blue circle indicating a right thumb press) conflict (i.e., are non-corresponding) as opposed to when they correspond (e.g., blue circle indicating a right thumb press presented in the right visual half-field). Our behavioral predictions were informed by previous studies of the Simon effect in PD patients (Wylie et al., 2010) and of the differential influence of variations in SAT instructions on flanker interference effects in PD patients and HC (Wylie et al., 2009b). In general, we expected that the Simon effect would be more dramatic in the speed than in the accuracy condition for both PD patients and HC. Consistent with the findings of Wylie et al. (2010), we expected that PD patients and HC would show similar mean Simon effects, but distributional analyses would reveal poorer ability to suppress interference among PD patients. Based on the findings of Wylie et al. (2009b) in the flanker task, we anticipated that PD patients would have greater difficulty suppressing incorrect response activations than HC when pressed for speed. However, we anticipated that differences might also emerge because the Simon and flanker tasks differ in the way in which incorrect response impulses are activated. In the former, conflict is generated by the spatial location of the stimulus, whereas in the latter conflict is generated by response-relevant information contained in the flankers. Thus, the current study allowed us to determine if emphasis on speed or on accuracy of responding impacts the ability of PD patients to activate and suppress incorrect response impulses differently in the Simon than in the Eriksen flanker task. We did not expect group differences in the strength of activation of prepotent erroneous responses, as reflected by the percentage of fast impulsive errors, a prediction that was also based on previous studies in our laboratory (Wylie et al., 2009, 2010).
Additionally, we measured changes in MRP activity in a subset of participants to determine if the impaired response suppression in PD we expected to observe in our behavioral measures, especially under speed stress, would be accompanied by electrophysiological changes reflective of diminished inhibition of the motor cortex controlling impulsive response tendencies on conflict trials. That is, when conflict occurs under speed stress, we expected PD participants, compared to HC, to show reduced buildup of the positive-going wave associated with inhibition of the incorrect impulsive response alternative just prior to execution of the correct response. Given previous data suggesting that PD patients and HC show similar choice reaction times and speed-accuracy adjustments, we made no a priori predictions about potential group differences regarding MRPs associated with the activation of the correct response hand.
Methods
Participants
Twenty-one (21) people with PD and 21 HC participated in the study and contributed to the final behavioral analyses. As shown in Table 1, the two groups were similar in age, gender, education, and Mini-Mental Status Exam scores (MMSE); Folstein, Folstein, & McHugh, 1975) Although PD patients scored slightly higher on a self-report measure of depression (CES-D) than HC (t (29)= −2.28, p <.05), the scores for both groups were well within the normal mood range and did not suggest depressed mood. Due to technical issues, only 15 PD and 16 HC participants provided potentially usable MRP data. Of these, 5 PD and 6 HC participants were further excluded from the MRP analysis either because artifactual noise corrupted greater than half of all trials or MRP signals were corrupted (i.e., deviated more than 2 SD deviations from the group MRP average) on more than two conditions. Thus, the final MRP analyses included two demographically similar groups comprised of 10 HC and 10 PD patients, respectively, who showed patterns of behavioral performance consistent with patterns obtained from the larger groups.
Table 1.
Participant demographics (averages).
| PD | HC | p-value | PD | HC | p-value | |
|---|---|---|---|---|---|---|
| Sample Size | 21 | 21 | – | 10 | 10 | – |
| Age | 63.5 | 62.9 | .79 | 63.1 | 61.0 | 0.54 |
| Education | 17.0 | 17.7 | .31 | 16.5 | 17.9 | 0.21 |
| Gender (M:F) | 13:8 | 13:8 | 6:4 | 4:6 | ||
| Depression Rating | 8.86 | 5.10 | .03 | 10.6 | 5.3 | 0.07 |
| MMSE | 29.1 | 29.5 | .26 | 29.2 | 29.6 | 0.29 |
| UPDRS | 12.8 | – | – | 13.2 | ||
| Disease Duration | 6.0 | – | – | 3.52 |
Participants with PD were recruited from a specialty Movement Disorders Clinic where they had been diagnosed by a neurologist who specializes in movement disorders. Disease severity in this patient sample ranged from mild to moderate, as indicated by the Hoehn and Yahr Scale (Hoehn, 1967). Nineteen of the 21 participants with PD were taking dopaminergic medication and were tested during the “on” state of their medication cycle. Healthy controls were recruited through community advertising or were spouses of the PD participants. Exclusionary criteria included history of other neurological conditions, unstable mood disorders, bipolar affective disorder, schizophrenia, or other psychiatric or medical conditions known to compromise executive cognitive functioning. Participation in the study was voluntary and informed consent, compliant with the standards of ethical conduct in human research as governed by the University of Virginia and Vanderbilt University human investigation committees, was obtained from all participants.
Design and Procedure
The Simon task was run on an IBM-compatible computer using Eprime software (PST 2.0). All stimuli were presented against a grey background on a 17-inch screen located approximately one meter from the participant and positioned such that the stimuli appeared at eye-level. On each trial, participants responded to the appearance of a blue or green circle that was presented either to the left or to the right of a centrally located, square-shaped black fixation mark. They were instructed to respond to the circle on the basis of its color (e.g., blue circle, left-hand response; green circle, right-hand response). The color-response direction mapping was reversed for half the participants in each group. Each block of trials was initiated by the onset of the fixation mark, which remained on the screen continuously until the block ended. One thousand (1000) milliseconds (ms) after the onset of the fixation mark the first green or blue circle appeared in the left or right visual half-field. It remained on the screen for 250 ms. Participants were given 1200 ms to respond. Fifty (50) ms after the RT limit was reached another green or blue circle appeared. Thus, the interstimulus interval (i.e., onset of circle N to onset of circle N+1) was 1250 ms; this time period also constituted the intertrial interval. Offset of the fixation mark signaled the end of a block of trials. Responses were made with the left or right thumb using a handheld button box placed comfortably in the participant’s lap.
Trials were defined as either corresponding (C) or non-corresponding (NC). Corresponding trials were those in which the stimulus appeared on the same side as the assigned response (e.g., blue circle requiring a left thumb press was presented in the left visual half-field). Non-corresponding trials were those in which the stimulus appeared on the side opposite the assigned response (e.g. blue circle requiring a left response was presented in the right visual half-field). Each type of trial occurred randomly, but with equal probability, within a block. Blocks were alternated between speed (SPD) and accuracy (ACC) instruction sets. During the accuracy instruction set, participants were encouraged to respond as accurately as possible without sacrificing too much speed. With speed instructions, participants were encouraged to respond as quickly as possible and to be more concerned about being fast than about making errors. At the end of each block, subjects received general feedback about their RT and accuracy to help ensure that they complied with the instructions for that block. At the beginning of each block, participants were reminded of the changed instructional set.
Each subject completed 160 practice trials, divided into 6 blocks, before beginning the data acquisition blocks. In the first block of 40 practice trials, the circle appeared at visual fixation so subjects could learn the color-response mapping. In the second block of 40 practice trials, the circle appeared either to the left or to the right of fixation. The final four practice blocks, each with 20 trials, alternated between speed and accuracy instructions that were presented on the screen. For data acquisition, participants performed 20 blocks, each consisting of 40 trials, thus resulting in 800 experimental trials. In total, participants completed 10 blocks that emphasized speed and 10 blocks that emphasized accuracy. Speed and accuracy blocks alternated across the 20-block session. Each block of trials lasted for about 2 minutes and breaks were offered after every block. The complete experimental session lasted about one hour and 15 minutes. Brain electrical activity was recorded at the scalp as the task was being performed.
Electroencephalograpy
EEG activity was recorded from 64 scalp sites using a hyroCel Geodesic Sensor Net with Ag/AgCl electrodes (EGI system, Eugene, OR). Impedances were kept below 40 kΩ. Eye movements were recorded with electrode pairs placed above and below the eye (vertical electro-oculogram) and from the outer canthi of each eye (horizontal electro-oculogram). During recording, EEG signals were referenced to the vertex (Cz). Offline, the signals were re-referenced to the average of the mastoids. Signals were amplified with a Geodesic EEG system amplifier (Net Amps 300) and recorded with Net Station acquisition software. The data were digitized at 1024 Hz.
Data Analysis
Behavioral data
The experimental design had one between-subjects factor with two levels, Group (PD, HC), and two within-subjects factors with two levels each, Instructions (Accuracy, Speed) and Correspondence (Corresponding, Non-corresponding). For every participant, RT values on any single trial within each factor level that were longer than four standard deviations above the mean or shorter than 150 ms (i.e., anticipatory responses) were discarded. Visual inspection of RTs ensured that the discarded trials were indeed outlying values. Less than 1% of trials were excluded per participant for each combination of instruction set and correspondence. Mean RTs and accuracy rates were then computed and analyzed. Accuracy rates were square-root transformed for analysis because they typically are not normally distributed. As reported previously (Wylie et al., 2010), the DPAS model provided the conceptual framework within which the temporal dynamics of response activation and suppression uncovered by the distributional analyses were interpreted. The single-trial RT data for each participant were rank-ordered from fastest to slowest and then divided into 10 equal-sized bins. CAFs for errors and delta plots for RT were computed from these data. For CAFs, the data from all trials, both correct and incorrect, were separated first by instruction set and then by correspondence. Mean accuracy rates were calculated for each bin and plotted as a function of the mean RT for each bin. The percentage of impulsive errors for the fastest RT bin was used to infer the strength of incorrect response activation, with higher rates associated with stronger incorrect response activation (Kornblum, Hasbroucq, & Osman, 1990). For the delta plots, the data from correct trials were separated by instruction set. The Simon effect (RTNC – RTC) was calculated for each bin and plotted as a function of the mean RT for each bin. According to past studies and the DPAS model, the slope value connecting the two slowest RT bins provides the most sensitive measure of the proficiency of response suppression (Burle, van den Wildenberg, & Ridderinkhof, 2005; Burle, Possamai, Vidal, Bonnet, & Hasbroucq, 2002; Wijnen, 2007; see detailed review in van den Wildenberg et al., 2010). The more negative-going the delta slope, the more proficient the suppression.
MRP data
Response-locked MRPs were calculated for correct response trials only. Epochs with amplitudes exceeding 150 μV or voltage steps of more than ±75 μV within a window of 200 ms were rejected from further analysis. Single-trial EEG activity was corrected for ocular artifacts using the procedure developed by Gratton, Coles, and Donchin (1983). Average MRPs were computed from this corrected activity for each stimulus and channel of interest. Analyses focused on activity measured at scalp sites C3 and C4 because they are located above the two motor cortices (Eimer, 1998; Leuthold, 2003; Spencer & Coles, 1999) and previous studies on inhibitory control and motor preparation have shown that these areas are involved in the activation and suppression of correct and incorrect motor responses (Burle, 2004; van de Laar, van den Wildenberg, van Boxtel, Huizenga, & van der Molen, 2012). The EEG activity recorded at these sites was filtered at a bandwidth of 1 Hz (high pass) to 30 Hz (low pass), and a roll-off of 24dB/octave. The ipsi- and contralateral segmentations were averaged across left- and right-hand responses, time-locked to the response, and baseline corrected −700 to −600 before the response was registered. To estimate current source densities (CSDs) and to improve the spatial resolution of the EEG signals (Nunez & Srinivasan, 2006), a Surface Laplacian transformation was applied (Perrin, Pernier, Bertrand, & Echallier, 1989). After the CSD transform, MRP averages were calculated at C3 and C4 for the different conditions (instruction, correspondence, and electrode-response side) and low-pass filtered at 15 Hz (van de Laar et al., 2012).
In choice RT tasks, activity in the motor cortices starts to develop −200 to −150 ms before onset of the mechanical response (Burle et al., 2004; Carbonnell et al., 2004; Vidal et al., 2003). The slope of the development of these potentials has provided a sensitive baseline free measure of the degree to which motor cortices become activated or suppressed; steeper slopes reflect a higher degree of activation or inhibition. Consistent with previous studies, we analyzed the slopes of these potentials in a primary window of −150 to −75 ms. This window was also selected to reduce overlap with earlier, adjacent waveforms, reduce variability associated with early and late tails of averaged waveforms without sacrificing sensitivity to factor effects, and to avoid inclusion of late portions of the motor potentials that return to baseline around the time of EMG onset (~75 ms prior to a response; see Luck, 2006; see also discussion by Praamstra, 2007, emphasizing the importance of reducing the influence of overlapping potentials in the Simon task). As a secondary analysis, we also explored group differences in the early and late portions of the motor potentials by analyzing adjacent time windows from −225 to −150 ms and from −75 ms to the overt response.
Motor potentials associated with correct hand activation and impulsive hand suppression were analyzed separately using repeated measures analysis of variance. Experimental factors included a between-subjects factor of Group (PD, HC) and two within-subjects factors, Instructions (Accuracy, Speed) and Correspondence (Corresponding, Non-corresponding). Our a priori predictions concerned the effects of PD on the suppression of the motor cortex controlling the incorrect response hand on conflict trials; thus, after performing the omnibus analysis, we conducted a focused analysis of non-corresponding trials that compared motor potentials associated with response inhibition between PD and HC groups directly.
Results
Behavior
Mean RT and Accuracy Rates
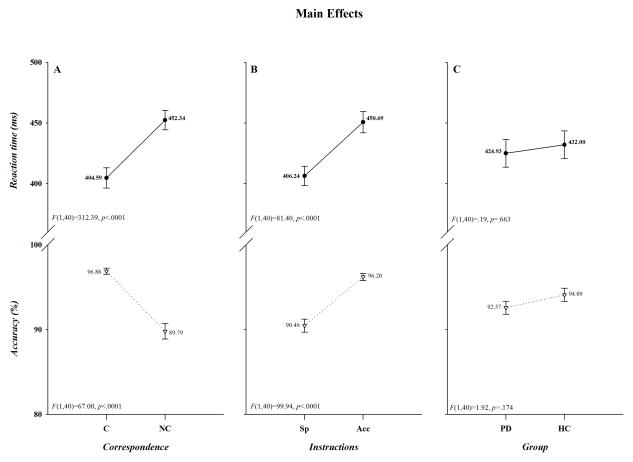
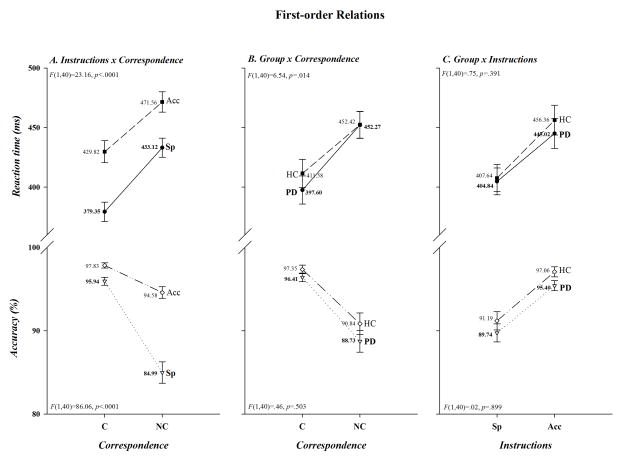
As illustrated in Figure 1, overall mean response latencies were faster and more accurate to spatially corresponding than to non-corresponding stimuli (Correspondence, F(1,40): RT, F=312.39, p<.0001; Acc, F=67.00, p<.0001; panel A), whereas they were faster but less accurate when the instructions emphasized speed rather than accuracy in responding (Instructions, F(1,40): RT, F=81.40, p<.0001; Acc, F=99.94, p<.0001; panel B). Thus, the task produced a robust Simon effect and subjects adjusted their performance to comply with the instructional set. It is also apparent in Figure 1C that overall mean response speed and accuracy did not differ between the two groups (Group, F(1,40): RT, F=.19, p=.663; Acc, F=1.92, p=.174,). As depicted in Figure 2A, however, the cost of non-correspondence was smaller under accuracy than speed instructions for both RT (41.74 vs. 53.77 ms) and accuracy (−3.25 vs. −10.95%)(Instructions x Correspondence, F(1,40): RT, F=23.16, p<.0001; Acc, F=86.06, p<.0001,); and, as shown in Figure 2B, the Simon effect was reduced on RT, but not on accuracy, in HC compared to PD patients (HC 40.84 ms; PD 54.67 ms; HC −6.51%; PD −7.68%)(Group x Correspondence, F(1,40): RT, F=6.54, p=.014; Acc, F=.46, p=.503). In contrast, as depicted in Figure 2C, both response latency and accuracy decreased comparably in the two groups when instructions emphasized speed of responding (Group x Instructions, F(1, 40): RT, F=.75, p=.391; Acc, F =.02, p=.899).
Figure 1.
The effects of Correspondence (panel A), Instructions (panel B), and Group (panel C) on RT (upper half of each panel) and accuracy (lower half of each panel). The F ratios and p values associated with each main effect are shown in the lower left of each half-panel. Note that in this and subsequent figures “Acc” and “Sp” designate accuracy and speed instructions; “HC” and “PD” identify healthy controls and patients with Parkinson’s disease; and RT and accuracy values are shown for each data point.
Figure 2.
First-order relations are illustrated in panels A, B, and C for both RT (upper half-panels) and accuracy (lower half-panels). Both significant and non-significant interactions are given for illustrative clarity. The F ratios and p values associated with each interaction are shown in the upper left half-panel for RT and the lower left half-panel for accuracy.
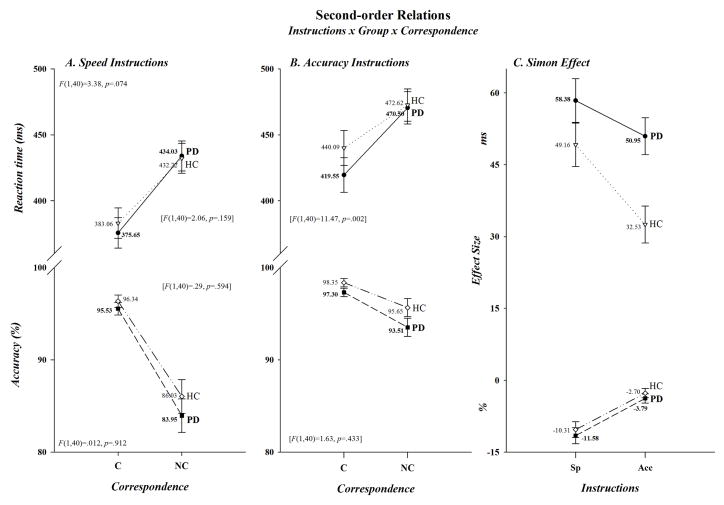
Even though differences between PD patients and HC in the size of the Simon effect only tended to be associated with variations in speed/accuracy instructions for RT, not for accuracy (Instructions x Correspondence x Group, F(1,40): RT, F=3.38, p=.074; Acc, F=.012, p=.912), the smaller effect on RT among HC in the Group x Correspondence interaction was suggestive. To look more closely at these relationships, we completed separate analyses within each instructional set. As can be seen in Figure 3A, when encouraged to emphasize speed of responding, patients and controls showed comparable Simon effects on both RT and accuracy (58.38 vs. 49.16 ms; −3.79 vs. −2.70%)(F(1,40): RT, F=2.06, p=.159; Acc, F=.29, p=.594). However, as is evident in Figure 3B, the Simon effect was smaller on RT, but not on accuracy, in HC than PD patients when instructions emphasized accuracy of responding (32.53 vs. 50.95 ms; −2.70 vs −3.79%)(F(1,40): RT, F=11.47, p=.002; Acc, F=1.63, p=.433,). For illustrative clarity, the magnitudes of the Simon effect in the two groups under speed and accuracy instructions are plotted in Figure 3C. Analyses within each subject group revealed that among PD patients the cost of non-correspondence tended to be reduced on RT and was reduced very significantly on accuracy under accuracy as opposed to speed instructions (50.95 vs. 58.38 ms; −3.79 vs. −11.58%)(Instructions x Correspondence, F(1,40): RT, F=3.47, p=.077; Acc, F=47.32, p<.0001), whereas the cost was reduced dramatically among HC on both RT and accuracy (32.53 vs. 50.95 ms; −2.70 vs. −10.31%)(Instructions x Correspondence, F(1,40): RT, F=30.56, p<.0001; Acc, F=39.31, p<.0001). Note, as can be seen in Figure 3B, the reduced cost of non-correspondence in HC under accuracy instructions was produced by a relative slowing in RT when making corresponding responses, not by any relative change in RT when non-corresponding responses were made. However, analysis of the difference in RT between the two groups revealed that the slowing was not statistically reliable (Group, F(1,40)=1.23, p=.275).
Figure 3.
Second-order relations are depicted for both RT (upper half-panels) and accuracy (lower half-panels). For visual inspection purposes the effects of speed and accuracy instructions are presented in separate graphs, panels A and B, respectively. Both significant and non-significant interactions are given once again for illustrative clarity. The F ratios and p values are shown for the three-way interaction in the upper left (RT) and lower left (accuracy) half-panel of panel A. They are also shown in brackets for the Group x Correspondence interaction revealed by the separate analyses done within each level of Instructions. To better visualize the cost of non-correspondence on both RT and accuracy in the two groups, we have re-plotted the data from panels A and B in panel C to show the size of the Simon effect in each group for each instructional set.
Central to this work is isolation of temporal processing differences between the two groups that may contribute to producing the Simon effect and to mediating its reduction in HC, but not in PD patients, when the concern is with response accuracy rather than speed. As we have reported previously (e.g., Wylie et al., 2009a, b), distributional analyses can expose differences in processing dynamics between patients and controls as well as uncover processing deficits among patients that are not evident in mean behavioral measures. We turn to those analyses to determine the degree to which they are revelatory.
Response Capture
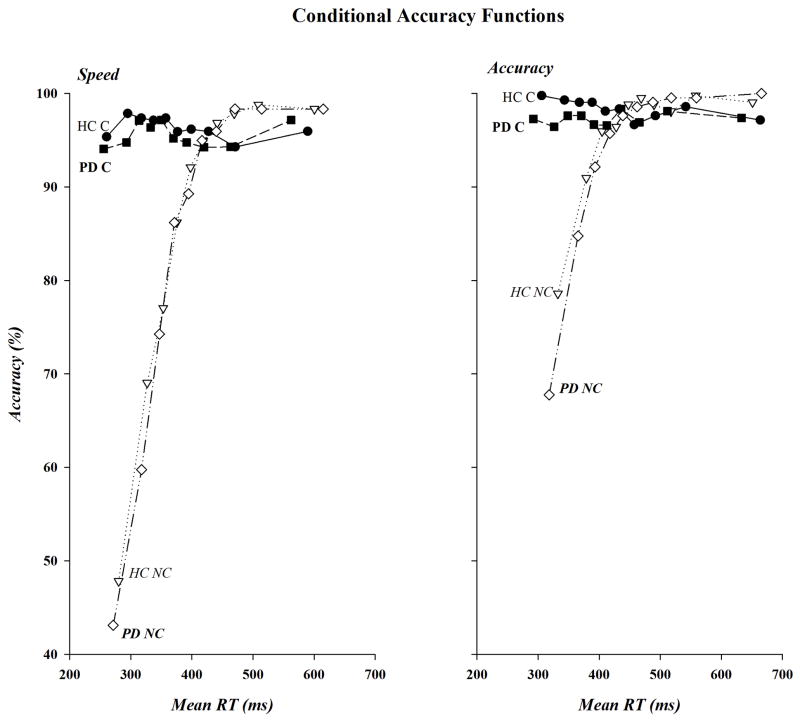
The CAFs for PD and HC under speed and accuracy instructions are shown in Figure 4A and 4B, respectively. It is readily apparent in these functions that the preponderance of errors were made to non-corresponding stimuli, irrespective of instructional set, that these impulsive errors were more likely when speed as opposed to accuracy in responding was emphasized, and that the patterns of error rates were similar in the two groups. Strong statistical support was found for these relationships. The percentage of correct responses for the fastest RT bin was used for the analysis. Impulsive errors in this bin, as reflected in low accuracy rates, were higher on non-corresponding than on corresponding trials (59.33 vs. 96.61%)(Correspondence, F(1,40)=173.96, p<.0001) and when subjects were pressed for speed as opposed to accuracy (70.09 vs. 85.85%)(Instructions, F(1,40)=79.53, p<.0001). Moreover, the decrease in accuracy for fast non-corresponding responses was larger when speed (−49.23%) rather than accuracy (−25.34%) of responding was stressed (Instructions x Correspondence, F(1,40)=47.29, p<.0001). However, PD patients and HC were equally likely to commit fast impulsive errors (75.55 vs. 80.39%, Group, F(1, 40)=2.68, p=.110), irrespective of variations in Correspondence or Instructions or in their combined variation (F(1,40): Correspondence x Group, F=1.08, p=.305; Instructions x Group, F=1.07, p=.307; Correspondence x Instructions x Group, F=.51, p=.482). Note, separate analyses completed on non-corresponding responses within each group revealed that fast impulsive errors were more likely to occur under speed than accuracy conditions for both HC (47.83 vs. 78.60%; Instructions, F(1,20)=43.15, p<.0001) and PD patients (43.12 vs. 67.76%; Instructions, F(1,20)=25.35, p<.0001).
Figure 4.
CAFs for PD patients and HC under speed (left panel) and accuracy (right panel) instructions for corresponding (C) and non-corresponding (NC) responses. Accuracy, shown on the ordinate, is plotted against mean bin RT, shown on the abscissa, for the fastest (bin 1) to the slowest (bin 10) bins.
Interference Suppression
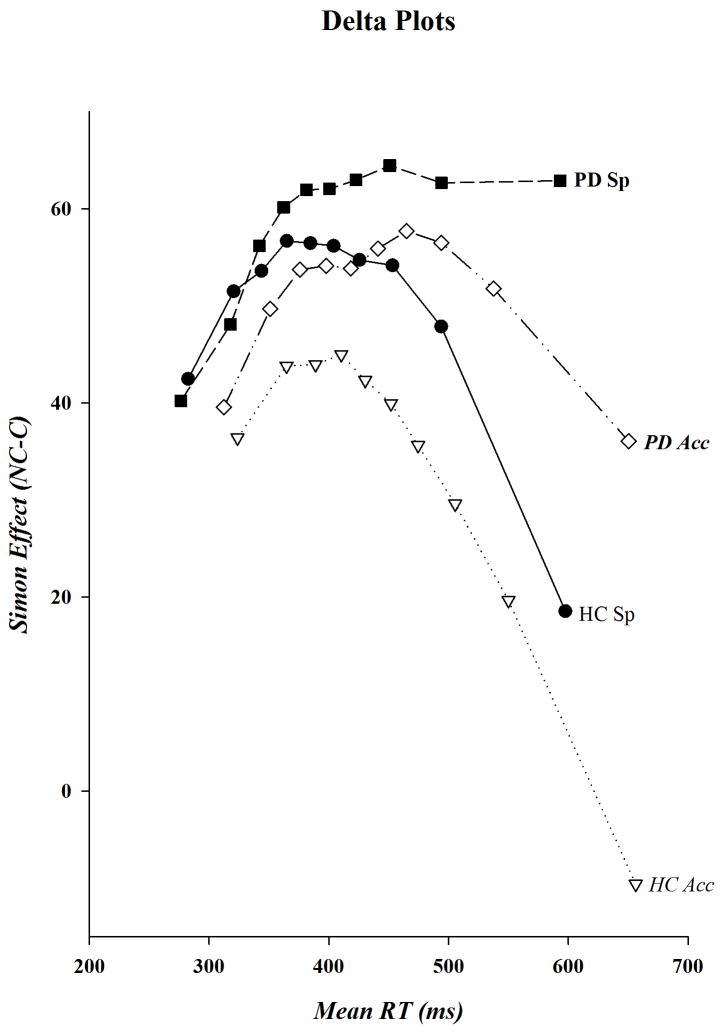
The delta plots for HC and PD participants are shown in Figure 5. Visual inspection of these plots reveals a striking departure in their shapes that supports our a priori prediction that deficits in inhibitory processes among PD patients are exacerbated when they encounter response conflict under speed stress. As is readily evident, when speed of responding was stressed the delta plot for PD patients, in contrast to the other three plots, does not have a steep negative-going final slope, the absence of which suggests that the growth of response suppression is diminished among PD patients when they are pressed for speed. Confirming these patterns, analyses restricted to the final slope revealed that its steepness was significantly less negative-going in PD patients than HC (Group, F(1,40)=6.21, p=.017), irrespective of instructional set (Instructions, F(1,40)=2.22, p=.144; Instructions x Group, F(1,40)=1.64, p=.208). Analyses done separately within each level of instruction revealed that the steepness of the final slope did not differ between the two groups under accuracy instructions, whereas it was significantly more negative-going in HC than PD patients when speed of responding was stressed (Group, F(1,40): Acc, F=1.79, p=.188; Sp, F=6.09, p=.018). Indeed, analyses done separately within each group uncovered no differences in the steepness of the final slope in HC associated with response instruction, but did reveal that the final slope was less negative-going in PD patients when speed of responding was stressed (Instructions, F(1,20): HC, F=.02, p=.896; PD, F=5.29, p=.032). These findings expose what may be a fundamental deficit in PD patients, a dramatically diminished capacity to suppress incorrect response impulses when attempting to maximize their rate of performing a speeded decision-making task.
Figure 5.
Delta plots for PD patients and HC under speed and accuracy instructions. The size of the Simon effect, shown on the ordinate, is plotted against mean bin RT, shown on the abscissa, for the fastest (bin 1) to the slowest (bin 10) bins.
In addition to assessing differences in the final slope, we explored apparent group- and instruction-related differences in the temporal pattern and size of Simon effects across the RT distribution that were suggested in our visual examination of the shapes of the delta plots.
Transition from “activation to suppression”
As visible in Figure 5, the transition into a negative-going function appears to emerge later (i.e., a delayed suppression effect) among PD patients than HC under either instructional set, and the transition appears to occur earlier in HC under accuracy as compared to speed instructions, suggesting that one effect of focusing on response accuracy might be the earlier, and consequently more complete, suppression of incorrect response impulses. A separate analysis of the bin values supports the observation that interference suppression is delayed in PD patients and that suppression occurs earlier in HC under accuracy than speed instructions.
Visual inspection of the delta plots suggests that the Simon effect peaks by the 4th bin in HC under both sets of instructions and by the 7th bin in PD patients under accuracy instructions. Hence, we used each one of these bins to assess reductions in the effect across the remaining bins. Within HC we found that, in comparison to Bin 4, the Simon effect was reduced by an increasing size through each of the remaining bins when accuracy was the focus (Bin, F(1,20): Fs 4.78 to 38.98, ps .041 to <.0001), but that the reduction was not significant until the 10th bin under speed instructions (Bin, F(1,20): bin 4 vs. bins 5 to 9, Fs .03 to 2.12; ps .872 to .153; Bin 10, F=10.16, p=.005). (The bin analyses were not corrected for multiple comparisons so p-values larger than 0.008 should be interpreted with caution.) Among PD patients, however, a significant reduction from the peak at Bin 7 did not occur until Bin 10 (Bin, F(1,20): bin 7 vs. 8, F=.39, p=.539; bin 7 vs. 9, F=2.51, p=.129; bin 7 vs. 10, F=9.67, p=.006). Complementarily, comparisons of adjacent bins under accuracy instructions within each group determined that the reductions in the Simon effect in HC were increasingly significant for each pairwise comparison after the peak 4th bin (Bin, F(1,20): bin 5 vs. 6, F=4.75, p=.041; bin 6 vs. 7, F=6.66, p=.018; bin 7 vs. 8, F=6.94, p=.016; bin 8 vs. 9, F=11.00, p=.003; bin 9 vs. 10, F=23.90, p<.0001). The bin analyses were not corrected for multiple comparisons so p-values larger than 0.02 should be interpreted with caution.)The Simon effect was reduced in PD patients as well following the peak 7th bin; however, the reduction was less dramatic than that seen in HC (Bin, F(1,20): bin 8 vs. 9, F=4.13, p=.056; bin 9 vs. 10, F=6.71, p=.018). Thus, the reduction in the size of the Simon effect occurred earlier under accuracy compared to speed instructions in HC, and was both delayed and less pronounced among PD patients under accuracy conditions.
Global reduction of Simon effect under accuracy instructions restricted to HC
In Figure 5 the magnitude of the Simon effect appears to be reduced under accuracy instructions for HC across the entire RT distribution, a difference that is consistent with the results from the analyses on the mean behavioral data. In contrast, the magnitude of the Simon effect appears to be similar in PD for the two instructional sets across the RT distribution until the slowest response latencies when a transition into a negative-going final slope emerges under accuracy, but not speed, instructions. Thus, in PD patients, focusing on response accuracy appears not to have produced a global reduction of interference effects on both accuracy and RT like that apparent in HC. Analyses restricted to the HC group revealed that the size of the Simon effect was indeed smaller across all 10 latency bins when accuracy as opposed to speed of responding was stressed (Instructions, F(1,20): Fs 5.56 to 35.51, ps .029 to <.0001), whereas only marginally smaller Simon effects were evident in PD patients in the 4th, 5th, and 9th bins and statistically reliable reductions did not appear under accuracy instructions until the 10th bin (Instructions, F(1,40): bins 1 to 4, 6 to 8, Fs .02 to 1.68, ps .884 to .210; Bin 4, F=3.23, p=.087, Bin 5, F=4.16, p=.055; Bin 9, F=3.20, p=.089, Bin 10, F=6.70, p=.018). Again note that after correction for multiple comparisons, only the HC group will show significant differences.
Summary of Behavioral Results
Analyses on the mean behavioral data revealed comparable Simon effects on RT and accuracy rates in the two groups when speed of responding was emphasized; however, when accuracy of responding was stressed there was a larger reduction in the Simon effect on RT, but not on accuracy, in HC than PD patients. Analyses of the CAFs revealed that impulsive response errors were high and comparable between the two groups for non-corresponding responses under speed instructions; however, instructions to be accurate reduced impulsive errors in both groups, but this reduction tended to be less dramatic among PD patients than HD. Analyses of the final delta plot slopes revealed that PD patients and HC were equally proficient at suppressing response conflict under accuracy instructions (although the magnitude of the Simon effect was significantly larger in PD patients). However, whereas HC were equally proficient at suppressing response conflict under speed and accuracy conditions, PD patients were seriously compromised in suppressing this conflict when speed of responding was stressed. Indeed, response suppression, as expressed both in slope values and Simon effect sizes, was minimal among PD patients under speed instructions. In addition, distributional differences in the magnitude of the Simon effect indicated that suppression occurred earlier in HC than PD, as it did in HC under accuracy as opposed to speed conditions. Moreover, the breadth of the benefit that accrued to HC, but not to PD patients, when focusing on response accuracy was suggested in reductions in the size of the Simon effect they evinced under accuracy instructions across the entire RT distribution that were not evident in PD patients until the last (i.e., slowest) response bin.
MRP results
The patterns that emerged in the delta plots in this current study confirm our previous work and offer further support for the conclusion that when performing under speed stress PD patients are less proficient than HC at suppressing interference from incorrect response impulses. The primary aim of our MRP analyses was to test the specific prediction that when pressed for speed and faced with conflict from an incorrect response impulse the capacity of PD patients to inhibit the motor cortex controlling the incorrect response impulse is reduced. A brief comment about this approach is necessary, however, before describing the results of our analyses. While our intention here is to begin to bridge behavioral findings of reduced suppression inferred from the delta plot with diminished inhibition of motor cortex inferred from neurophysiological patterns, the translation between the two provided by the current data set is not straightforward. The delta plot patterns that evince suppression emerge at the slow end of the RT distribution, whereas the MRPs reported here are based on averaged waveforms collapsed across the entire RT distribution. Comparable binning of the MRP and the RT data used to generate delta plots would require substantially more trials than were collected in this experiment to produce reliable waveforms, particularly at the slow end of the RT distribution where variability is greatest. This direct comparison awaits future investigation. Nonetheless, the results of the analyses we describe below reveal important differences in averaged inhibitory MRPs between the two groups under speed stress that confirm predicted patterns.
A subset of participants (10 from each group) produced high quality MRP data that allowed investigation of group differences in inhibitory MRPs across corresponding and non-corresponding trials. The response-locked Laplacian transformed waveforms for corresponding and non-corresponding trials are plotted for speed and accuracy instructions in Figures 6 and 7, respectively. As can be seen in these plots, a clear divergence between MRPs associated with correct and incorrect response hands evolves less than 200 ms before a correct overt response is issued. A negative-going MRP develops at electrode sites above the motor cortex contralateral to the correct response hand, signaling activation of the motor area controlling this response. Coincidently, a positive-going MRP develops at electrode sites above the motor cortex contralateral to the incorrect response hand, signaling inhibition of motor cortex controlling the incorrect response. This pattern of MRP activity replicates that seen for bimanual choice reaction tasks in previous studies (Burle et al., 2004; Vidal et al., 2003; Carbonnell et al, 2004). Our description of the results of a series of analyses we completed on this diverging pattern of MRP activity begins with the combined effects of variations in Group, Correspondence, and Instructions on the steepness of the slope of the developing inhibitory MRP. Next, we describe the results of specific analyses we did on the influence of speed stress on inhibitory MRPs in situations in which response conflict must be suppressed. We then describe the results of our analyses of the MRPs associated with activation and execution of the correct response. Table 2 contains a list of the slopes of the developing positive- and negative-going waveforms across all conditions and groups for the primary and secondary MRP windows of interest.
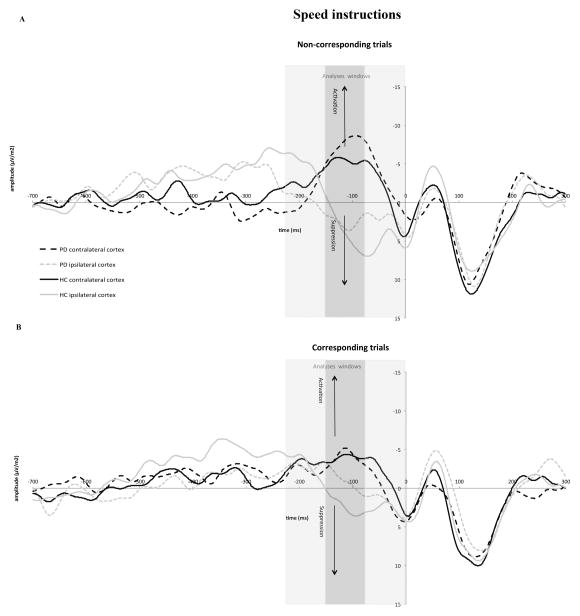
Figure 6.
Response-locked movement-related brain potential (MRP) activity recorded at C3 and C4 under speed instructions for non-corresponding (panel A) and corresponding (panel B) responses. Activation of motor cortex contralateral to the correct response is identified by the upward pointing arrows and suppression of activity in motor cortex ipsilateral to the correct hand is identified by downward pointing arrows. The former is associated with production of the correct response, and the latter is associated with inhibition of the incorrect response. Gray bars represent the different windows used in the data analyses to capture activation and inhibition processes before the correct response was emitted (−225 to −150 ms, −150 to −75 ms, and −75 to 0 ms). The window used in the primary analysis was −150 to −75 ms.
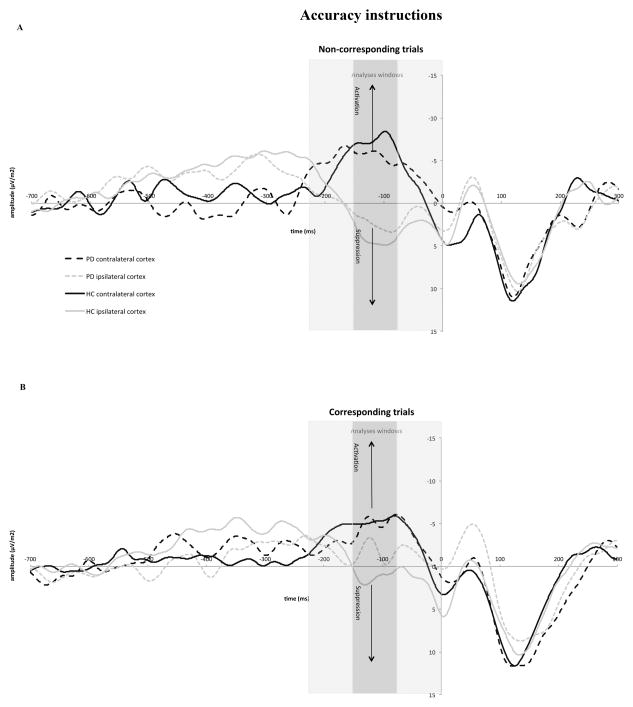
Figure 7.
Response-locked movement-related brain potential (MRP) activity recorded at C3 and C4 under accuracy instructions for non-corresponding (panel A) and corresponding (panel B) responses. As in Figure 6, gray bars represent the different windows used in the data analyses to capture activation and inhibition processes before the correct response was emitted (−225 to −150 ms. −150 to −75 ms, and −75 to 0 ms). The window used in the primary analyses was −150 to −75 ms.
Table 2.
Means and Standard Errors of inhibition slopes ipsilateral to the correct response for three windows of analysis starting at −225 ms before the response until response onset (0).
| PD | HC | |||||
|---|---|---|---|---|---|---|
| −225 to −150 | −150 to −75 | −75 to 0 | −225 to −150 | −150 to −75 | −75 to 0 | |
| Speed Instructions | ||||||
| Corresponding | 0.00(0.03) | 0.06(0.04) | 0.05(0.05) | 0.06(0.03) | 0.04(0.04) | 0.01(0.05) |
| Non-Corresponding | 0.03(0.04) | 0.00(0.04) | 0.03(0.03) | 0.07(0.04) | 0.11(0.04) | −0.03(0.03) |
| Accuracy Instructions | ||||||
| Corresponding | 0.05(0.04) | 0.01(0.02) | 0.04(0.03) | 0.04(0.03) | −0.02(0.02) | 0.07(0.03) |
| Non-Corresponding | 0.03(0.03) | 0.03(0.02) | 0.01(0.04) | 0.09(0.03) | 0.01(0.02) | 0.01(0.04) |
Analyses of Positive-going MRPs: Inhibition of Incorrect Motor Cortex
Primary analysis window (−150 to −75 ms)
Factor effects on inhibition of motor cortex controlling the incorrect response
Activation of this inhibitory process, as reflected in the development of a positive-going MRP at the scalp site contralateral to the incorrect response hand, occurred within a time window similar to that reported by other investigators, −150 to −75 ms before the correct response (Carbonnell et al., 2004; Tandonnet et al., 2006; Vidal et al., 2003). The slope of this signal was steeper under speed (.05) than accuracy (.01) instructions (Instructions, F(1,18)=11.55, p<.0001), indicating that focusing on speed induces stronger inhibition of the motor cortex controlling the incorrect response. However, there were neither slope differences between either HC and PD patients (Group, F(1,18)=.13, p=.721) nor between corresponding and non-corresponding responses (Correspondence, F(1,18)=.45, p=.511). Although there was an apparent reversal between the two groups in the effect of correspondence on the slope of the MRP, with it being larger in HC on non-corresponding trials (.06 vs. .02) and larger in PD patients on corresponding trials (.04 vs. .01), the interaction did not achieve statistical significance (Correspondence x Group, F(1,18)=2.60, p=.124). In contrast, the relative influence of instructional set on the steepness of the positive slope did differ between the two groups (Instructions x Group, F(1,18)=7.25, p=.015). Under speed instructions, HC showed a steeper positive MRP than did PD patients (.08 vs. .03), whereas under accuracy instructions the slope was steeper in PD patients than in HC (.02 vs. −.001). None of the remaining interactions involving Correspondence, Group, and Instructions were statistically significant (ps>.10).
Focused analysis of inhibition of motor cortex controlling the incorrect response in the face of response conflict
The need for response suppression is greatest in the face of conflict between an incorrect motor impulse and a goal response, such as occurs on non-corresponding trials. Moreover, this conflict is particularly pronounced when the respondent is most concerned about response speed. Our a priori hypothesis was that the reduction in inhibitory control in PD patients inferred from behavioral data would be revealed neurophysiologically by a reduction in the positive-going MRP reflecting inhibition of the incorrect motor cortex on conflict trials under speed stress. A separate analysis of the steepness of this positivity on non-corresponding conflict trials revealed that the slope tended to be more positive when speed rather than accuracy of responding was stressed (Instructions, F(1,18)=2.91, p=.105). Most importantly, there was a clear difference between the two groups in the influence of instructional set on the steepness of the positive slope (Instruction x Group, (F(1,18)=9.02, p<.0001). It was much steeper among HC (i.e., inhibition of the incorrect motor cortex was stronger) when they focused on speed (.11) rather than accuracy (.01), (t(9) = −4.13, p= 0.003) of responding, and the steepness of this speed-related slope was greater than that measured in PD patients (one sided t-test, t(18)=−1.87, p=.038). In other words, PD patients showed reduced inhibition of the motor cortex controlling the impulsive, conflicting response hand under speed stress. Additionally, they showed a different pattern whereby the steepness of the slope was more positive when focusing on accuracy (.03) rather than speed (.004) of responding although this was not significantly different ((t (9) = 0.79, p=0.450). Thus, PD patients showed less proficient inhibition of incorrect motor cortex when they focused on speed as opposed to accuracy of responding, a pattern that resembled the behavioral findings.
Secondary analysis windows
Analyses of an earlier and later time window, −225 to −150 ms and −75 to 0 ms, respectively, did not disclose group or other factor effects on the motor potential associated with the motor cortex controlling the impulsive response hand (all ps for main and interaction effects >.10). Our failure to find differences in the later window was not surprising given that the MRPs begin to return to baseline shortly before a correct response is emitted.
Analyses of Negative-going MRPs: Activation of Motor Cortex Controlling the Correct Response
Recall, we had no a priori expectations regarding group differences in the steepness of the slope of the negative-going MRP, which signals the activation strength of motor cortex controlling the correct response hand, because previous data have shown that the choice RTs of PD patients and HC are comparable and the two groups adjust their response speeds similarly when instructed to respond with greater concern for speed or for accuracy. Factor effects on these slopes and the time windows we used in our analyses are shown in Table 3.
Table 3.
Means and Standard Errors of activation slopes contralateral to the correct response for three windows of analysis starting at −225 ms before the response until response onset (0).
| PD | HC | |||||
|---|---|---|---|---|---|---|
| −225 to −150 | −150 to −75 | −75 to 0 | −225 to −150 | −150 to −75 | −75 to 0 | |
| Speed Instructions | ||||||
| Corresponding | 0.01(0.03) | 0.00(0.04) | 0.12(0.05) | −0.01(0.03) | −0.01(0.04) | 0.11(0.05) |
| Non-Corresponding | −0.09(0.02) | −0.04(0.04) | 0.13(0.04) | −0.03(0.02) | 0.00(0.04) | 0.16(0.04) |
| Accuracy Instructions | ||||||
| Corresponding | −0.02(0.04) | −0.02(0.04) | 0.10(0.04) | −0.05(0.04) | −0.02(0.04) | 0.13(0.04) |
| Non-Corresponding | −0.04(0.03) | 0.02(0.04) | 0.07(0.06) | −0.09(0.03) | −0.01(0.04) | 0.13(0.06) |
Primary analysis window (−150 to −75 ms)
The steepness of the negative-going slope was not altered by instructional set, spatial correspondence, or group membership (all main effect ps>.10), suggesting the absence of factor influences on the activation of the motor cortex controlling the correct response hand. Additionally, none of the higher-order interactions between these factors was significant. Thus, in the primary analysis window, PD patients and HC show similar development of motor cortex activation controlling the correct response. Inspection of the waveforms, however, suggests that the MRPs associated with activation of the correct response developed earlier than those associated with inhibition of the incorrect response; thus, we completed an analysis of an earlier time window, −225 to −150 ms, to determine if factor effects would be disclosed. In addition, we completed an analysis on the time window shortly before the response, −75 to 0 ms.
Secondary analysis windows
−225 to −150 ms
A steeper slope was observed on non-corresponding (−.06) than on corresponding (−.02) trials (Correspondence, F(1,18)=8.02, p=.011), whereas the slopes did not differ between the two groups (Group, F(1,18)=0.06, p=0.809) or between speed/accuracy instructions (Instructions, F(1,18)=2.99, p=0.101). However, differential influences of speed or accuracy instructions on the negative-going MRP were evident in the two groups (Instructions x Group, F(1,18)=5.4, p=.032). When focusing on responding accurately, HCs showed steeper increases in motor cortex activation (HC −.07) than PD patients (PD −.03). In contrast, this pattern reversed when the focus was on response speed; PD patients showed steeper increases in motor cortex activation than HCs (HC −.02, PD −.04). However, group differences in motor cortex activation depended on both instructional set and the presence or absence of conflict (Instructions x Correspondence x Group, F(1,18)=4.60, p=.046). Deconstruction of this interaction revealed that group differences in activating motor cortex controlling the correct response on conflict trials were particularly sensitive to whether instructions stressed speed or accuracy of responding. When response speed was emphasized, PD patients showed steeper development of the negative-going MRP than HCs (PD −.09, HC −.03), a pattern that reversed completely when the focus was on responding accurately (HC −.09, PD −.04; Instructions x Group, F(1,18)=10.69, p<.0001). Further analysis of these patterns showed that the group difference was driven primarily by motor cortex activation when the focus was on speed of responding (HC −.03, PD −.08; (18)=−2.09, p=.05). Thus, compared to PD patients, HCs appeared to require less activation of motor cortex to issue correct responses when focusing on speed of responding.
−75 to 0 ms
There were no factor effects on the negative-going motor potential shortly before the response was emitted (all ps for main and interaction effects >.10).
Summary of MRP Findings
The neurophysiological data revealed reduced inhibition of the motor cortex controlling the impulsive incorrect response tendency in PD patients that was especially pronounced under speed instructions and is compatible with reduced suppression of impulsive responses inferred behaviorally. PD patients showed relatively stronger activation of the motor cortex controlling the goal-directed correct response when pressed for speed rather accuracy of responding, whereas HC showed an opposite pattern of relatively stronger activation when focusing on response accuracy rather than speed.
Discussion
An emerging literature indicates that PD patients have difficulty resolving interference in the face of response conflict. The current study of PD expanded this work in novel ways by investigating SAT effects in a Simon conflict task and, in a subset of participants, by recording MRPs to track the putative neural activation and suppression of the motor cortices (i.e., M1) that control goal-directed correct and impulsive incorrect responses, respectively. We first discuss the behavioral findings before turning to insights provided by the analysis of MRP activity.
Mean Speed-Accuracy Tradeoff and Conflict Effects in PD
PD patients and HC alike showed typical speed-accuracy tradeoff and mean Simon conflict effects. When instructions emphasized response speed, participants in both groups had faster RTs and made more overall errors than when response accuracy was emphasized. Similarly, interference on non-corresponding trials slowed RT and increased error rates compared to corresponding trials, which were associated with faster RTs and higher accuracy rates. The mean Simon effects on RT and accuracy were also sensitive to speed-accuracy instructions in expected ways. For HC, Simon effects were reduced on both RT and accuracy rate under accuracy as compared to speed instructions. While PD patients showed a similar reduction of response errors when focused on performing accurately, the mean Simon effect on RT was not reduced significantly. In fact, this non-significant reduction appeared to be driven by a subtle decline in capacity among PD patients to regulate their response speed on non-conflict, but not on conflict, trials when focused on response accuracy. Importantly, however, interpretations of mean effects are limited as they mask the dynamics of the activation and suppression of incorrect response tendencies in the Simon task. We next turn to results from analytical methods that provide deeper insight into the dynamics of interference control.
Speed Pressure Exacerbates Inhibitory Control Deficits in PD
To separate the strength of initial response capture by impulsive actions from cognitive control processes engaged subsequently to suppress the interference from these impulses, we applied the theoretical-statistical framework of the DPAS model. First, a typical pattern of stronger incorrect response capture (i.e., more fast impulsive errors) was seen on non-corresponding than on corresponding trials, replicating an earlier finding from our laboratory in which 52 patients were tested in the Simon task (Wylie et al., 2010). Moreover, incorrect response capture on non-corresponding trials was greater when participants focused on their speed rather than on their accuracy of responding. Importantly, HC and PD patients showed similar patterns of response capture, replicating our previous findings for the flanker task (Wylie et al., 2009b). Thus, across two conflict paradigms, PD does not appear to have a differential impact on the strength of initial capture by impulsive actions, even under conditions in which there is pressure to maximize performance speed. It should be kept in mind, however, that there was a tendency for PD patients to make more fast, impulsive errors than HC when accuracy of responding was stressed.
Second, the delta plots for RT confirmed a pattern of increasing interference across fast response latencies that, with the exception of PD patients under speed instructions, decreased dramatically at slower response latencies. This decline in the interference effect at the slow end of the RT distribution is argued to reflect the effect of an inhibitory control mechanism that suppresses incorrect response activation (Ridderinkhof, 2002). While in HC this mechanism was clearly engaged under both sets of instructions, in PD patients it appeared to have been engaged only under accuracy instructions. Moreover, irrespective of instructions, PD patients took longer and were less proficient in suppressing interference from the incorrect response impulse. With respect to the effects of instructions, HC achieved comparable final levels of proficiency in suppressing incorrect response impulses under speed and accuracy conditions (i.e., the negative-going final slopes of the delta plot were similar). However, engagement of this inhibitory control mechanism, as revealed in reductions in the Simon effect, occurred earlier in HC when they focused on responding accurately. In contrast, PD patients had considerable difficulty suppressing incorrect response impulses when pressed for speed as opposed to accuracy; indeed, they showed little evidence of inhibition under speed instructions. Despite better suppression under accuracy instructions, suppression was delayed in PD patients compared to HC under either instruction set.
As was the case for the CAFs, our finding that the final slope of the delta plot was reduced in PD patients, suggesting they are less able than HC to suppress incorrect response activation in the Simon task, replicates the finding of Wylie et al. (2010). In addition, the current study extends this finding by showing that the deficit in response suppression is especially pronounced when patients perform under speed stress. Similarly, the current results are in accord with findings from a previous study in which we found that PD patients experienced larger flanker interference effects and poorer suppression under speed as compared to accuracy instructions (Wylie et al., 2009b). Thus, the current study replicates and extends two previous findings, which further confirms that PD patients are less effective at suppressing incorrect response impulses, and this deficit is even more pronounced under speed pressure.
Speed Pressure Reduces the Inhibition of Incorrect Motor Cortex Activation in PD
Previous ERP studies of conflict effects in PD have provided important insights into the neural mechanisms that may underlie interference control deficits. For example, using a flanker task, and colleagues (Praamstra, Stegeman, Cools and Horstink, 1998; Praamstra, Plat, Meyer and Horstink 1999) showed that, compared to HC, increased interference effects in medication-withdrawn PD patients were accompanied by specific changes in the LRP on incongruent trials: the initial deflection of the stimulus-locked LRP, which reflects activity biased in favor of the incorrect response tendency, was larger in amplitude among PD patients. As these investigators reasoned, this amplitude difference supports the conclusion that stronger activation and interference occurs in PD at the level of motor cortex controlling the incorrect response tendency. However, in a study of medicated PD patients using a another variant of the flanker task Falkenstein, Willemssen, Hohnsbein and Hielscher (2006) found no evidence of differential interference effects, as reflected in LRP patterns, between PD patients and healthy controls. The failure to find a difference suggests that dopaminergic medication may reduce or even eliminate this activation deficit.
In a later study using a Simon task to assess impulse control deficits in medication-withdrawn PD patients, Praamstra and Plat (2001) also observed an abnormal enhancement of attention-related activity at scalp sites over motor cortex among the patients that they interpreted as expressive of a disinhibition of prepotent visually-activated response tendencies.
Together, previous findings suggest that problems resolving response conflict in PD may also be accompanied by altered patterns of motor cortex activity associated with the conflicting response hands. The current study extends these findings by providing insight into the dynamic interplay between response-related excitatory and inhibitory processing within each motor cortex just prior to an issued response, which cannot be dissociated using conventional LRP methods. Importantly, the pattern of motor potential activity described here bears a very close resemblance to patterns reported previously in bimanual choice reaction tasks (Carbonnell et al., 2004; Tandonnet et al., 2006; Vidal et al., 2003). A clear negative-going MRP developed contralateral to the correct response hand around 200 ms before a correct overt response that lasted about 150 ms; this negativity signaled activation of the motor cortex controlling the correct response. Concurrently, a positive-going MRP emerged over motor cortex contralateral to the incorrect response hand around 150 ms before a correct response, indicating suppression of neural elements controlling the impulsive response tendency. Thus, both groups showed dynamic patterns of activating motor cortex controlling the correct hand with simultaneous suppression of motor cortex controlling the competing/conflicting hand just prior to producing a correct overt response.
Consistent with previous studies (Carbonnell et al., 2004; Tandonnet et al., 2006; Vidal et al., 2003), we used the slope of the developing positive MRP to evaluate group differences in the strength of inhibition of underlying M1 motor cortex controlling an incorrect response impulse. These analyses revealed that, relative to HCs, PD patients showed a significant reduction in the positivity of this slope, supporting the inference that inhibition of the motor cortex associated with the impulsive response was diminished during conflict trials, especially when they were pressed for speed rather than accuracy of responding. In contrast, group differences in the slope of the positive MRP disappeared when the focus was on responding accurately. These findings provide initial electrophysiological evidence that resolution of response conflict in PD is related in part to less effective inhibition of motor cortex controlling incorrect or impulsive response tendencies.
While patterns of negative-going MRP activity associated with activation of motor cortex controlling the correct response hand were of secondary interest, they nonetheless provided additional insight into processing differences between the two groups. Differences in the slopes of the negative MRPs emerged slightly earlier than did slope differences for the positive MRPs; in the 150–225 ms range before a correct overt response. For HC, when faced with conflict, a steeper negative-going MRP slope was observed when the focus was on responding accurately rather than quickly. That is, the strength of correct motor cortex activation appears higher when HC are focused on being accurate. PD patients, in contrast, showed an opposite pattern, a steeper negative-going slope when focusing on speed, not accuracy, of responding. These patterns fit well with ideas about processing dynamics governing speed-accuracy tradeoffs, to which we turn next.
Potential Mechanisms Underlying SAT Effects on Inhibitory Control in PD
Focus on response speed produces sustained activity of brain areas involved in general motor preparation (Forstmann et al., 2008, 2011; van Veen et al., 2008). This enhanced preparation of the motor system is thought to shorten the distance between the individual’s state of response readiness and the motor threshold for triggering a specific overt movement. In this prepared state, it is argued that less motor activation is required to boost a selected response to threshold. However, activation of an incorrect response impulse is also more likely to reach motor threshold; thus, enhanced preparation also places greater demands on inhibition mechanisms in times of conflict in order to reduce interference from impulsive response tendencies. In other words, when response speed is emphasized, a bias toward acting is created and incorrect action impulses place greater demands on reactive inhibitory control to suppress these incorrect response tendencies.
The patterns of MRP activity in conflict trials in HC were congruent with these ideas. When speed was emphasized, MRP activity associated with activation of the correct response was reduced and MRP activity associated with inhibition of the incorrect response was enhanced. In PD patients, poor inhibition of M1 motor cortex, as reflected in reduced MRP activity associated with inhibition of the incorrect response, disrupts these dynamics. Thus, deficient suppression of the impulsive response may have required compensatory overreliance on activation of the correct response, as reflected in increased MRP activity associated with its production, to boost the signal strength of this response above the interference generated by activity associated with the impulsive response tendency.
When response accuracy is emphasized during task performance, the motor system is held in check proactively and a reduction in baseline M1 motor cortex activity is observed (van Veen et al. 2008). This state of motor preparation increases the distance between response readiness and the threshold triggering overt movements. A consequence of proactive suppression of the motor system is the necessary enhancement of motor cortex activation to boost selection of the correct response to threshold. Additionally, given the state of global motor system suppression, selective inhibition of the motor cortex controlling the incorrect response in conflict situations should manifest as a reduction in the slope of the positive-going (i.e., inhibition) MRP. In HC, the putative proactive suppression of motor responses led to both reduced overall interference from incorrect response impulses and earlier effects of selective suppression in the delta plots (i.e., earlier transition to a negative-going delta slope). Moreover, the MRP patterns also conformed to these predictions, showing that, relative to speed emphasis trials, a focus on accuracy led to an increase in the slope of the negative-going (i.e., activation) MRP coupled with a reduction in the slope of the positive-going MRP.
Again, inhibitory deficits in PD appeared to disrupt these dynamics. First, the absence of a reduction in interference under accuracy instructions, as revealed in the delta plots, suggested that proactive inhibitory control of the motor system was incomplete. As a result, compared to HC, PD patients showed greater reliance on selective inhibition of the incorrect motor cortex to facilitate correct response selection. Inhibition was improved in PD patients under accuracy compared to speed instructions, but relative to HC, remained less proficient and delayed in its effect on interference behaviorally.
The neural mechanisms underlying action selection and inhibition, as well as speed-accuracy tradeoff dynamics, in motor control are being linked increasingly to frontal-basal ganglia circuitries. Thus studies of interactions between these forms of action control hold promise in deepening our understanding of how PD disrupts voluntary action control. Recent studies of healthy adults posit very specific links between speed-accuracy adjustments in motor decision-making and basal ganglia activity (Bogacz & Gurney, 2007; Forstmann et al., 2008; Frank, 2006; Lo & Wang, 2006; van Veen et al., 2008). For example, functional imaging studies demonstrate that under speed and accuracy instructions, patterns of cortical input to the striatum of the basal ganglia modulate just prior to critical stimulus events, the net result of which are changes in basal ganglia inhibition over motor pathways and adjustments to putative response thresholds (Bogacz et al., 2010; Forstmann et al., 2008, 2011). How PD disrupts these processes is yet to be determined, but the current results are suggestive that interactions between these adaptive motor threshold processes and response selection and inhibition processes long demonstrated to depend on basal ganglia pathways are altered by PD.
In addition to a putative role of frontal-striatal circuitries in speed-accuracy modulation of motor thresholds and selection processes, the subthalamic nucleus (STN) has also been postulated to contribute to motor initiation adjustments and inhibitory motor processes (Albin et al., 1989; DeLong, 1990; Mink, 1996; van den Wildenberg et al., 2006). Theories have linked STN activity to mechanisms that delay or hold decisions and responses in check to prevent premature outcomes and buy time for additional decision-making processes to arrive at a desired or correct action (Frank et al., 2006, Frank, Samanta, Moustafa, & Sherman 2007). Deep brain stimulation of STN also modulates susceptibility to impulsive reactions in conflict tasks, further suggesting the role of these basal ganglia structures in response thresholds (Wylie et al., 2010). Future studies of how STN stimulation impacts speed-accuracy adjustments, particularly in situations of response conflict, will be especially informative.
Study Limitations and Extant Issues
A limitation of the current study is that PD patients were only tested in their medicated state, thus it remains uncertain how dopamine medications affect the reported behavioral and neurophysiological patterns. Our confidence in the findings as being suggestive of potentially robust markers of PD pathology is strengthened by the fact that the current behavioral findings replicate several studies of conflict effects in PD, including one that manipulated SAT in a different conflict task (Wylie et al., 2009b, Wylie et al. 2010, Praamstra et al. 1998, 1999). A previous study of PD reported the absence of dopamine medication modulation on conflict effects in a flanker and Simon task, although this study only analyzed mean interference effects and was focused primarily on error-related brain potentials (Falkenstein et al., 2001). Investigation of dopamine medication effects on MRPs and distributional behavioral patterns while PD patients perform conflict tasks under varying speed-accuracy instructions will inform several lines of investigation.
Fatigue is an important consideration and potential limitation in studies of PD patients and older adults. In the current study, participants completed 800 total trials across 20 experimental blocks (approximately 80 minutes). Several factors argue against fatigue as a significant problem or difference between groups. First, no group differences in overall RT or in RT associated with the slowest delta bins were measured. Thus, even the slowest RTs, which arguably are most vulnerable to fatigue effects, were similar between the groups. In addition, the ranges of RTs across the 10 bins for the CAFs under both speed and accuracy instructions were comparable and the group pairwise comparisons revealed no differences in bin speeds for any comparison. Second, blocks of trials were kept short and rest breaks were given after each block to minimize fatigue. Rest breaks also provided time for performance feedback and verbal encouragement to participants. Finally, both PD and HC groups complied with speed-accuracy instructions in similar ways, suggesting that compliance with adjustments in performance strategies was similar between the groups.
In Simon tasks, elicitation of potentials at posterior electrode sites over cortical areas associated with the deployment of visual attention to either visual field can overlap and confound interpretation of potentials measured from electrodes overlying the motor cortices (Praamstra, 2007). While the response-locked surface Laplacian technique used in the current study enhances the spatial fidelity of motor potentials and likely minimizes the posterior potential influences, the possibility of overlap cannot be ruled out entirely (Babiloni et al., 2001; Nunez, 1981; Tandonnet et al., 2003). With respect to those posterior components, a post-hoc analyses of the stimulus-locked potentials showed no effect of any of the experimental factors on early visual components.1
The fact that the MRPs reported here look very similar to those reported in previous choice reaction studies provides additional confidence in the motor-related nature of the measured potentials. Additionally, the findings that speed-accuracy instructions, which would not be expected to vary with early visual attention potentials, alter patterns of activity in the MRPs associated with activation and inhibition of the correct and incorrect responses, respectively, in meaningful ways within and between groups further underscores the motor-related nature of the measured waveforms. Future studies that systematically vary the deployment of visual attention to either visual half-field in the Simon task or include a central fixation condition for comparison purposes could further address this issue.
An important point to emphasize is that the measure of suppression inferred behaviorally from the delta plot (i.e., the final delta slope) is not functionally equivalent to the inhibition of motor M1 cortex revealed by averages of the positive-going MRPs on conflict trials. The suppression portion of the delta plot function is measured at the slowest response latencies, whereas the positive-going MRP reflects motor inhibition across all trials, irrespective of reaction time. Therefore, the MRPs do not directly relate to the suppression in the final bin of the reaction time distribution (or to the fast impulsive errors as measured by the CAFs). However, they inform us about the average motor cortex activation and suppression under conflict (i.e. the non corresponding trials) within each condition. The MRPs corroborate the behavioral findings of reduced suppression in the delta slopes: the suppression slope on the ipsilateral motor cortex was less steep under speed pressure in PD. We would expect that the reduced suppression slope on the ipsilateral motor cortex in PD would become even more pronounced in the final bin of the reaction time distribution. Establishing functional links between the structural properties of the delta plot and of the MRP, both in terms of timing and strength of activation, awaits further investigation. One difficulty is the necessity of large trial numbers in order to partition the averaged MRPs for corresponding and non-corresponding trial types into discrete RT distribution bins.
Conclusions
PD patients are quite capable of making strategic speed-accuracy adjustments during choice reaction tasks. However, these adjustments expose detrimental interactions with other aspects of action control. Pressing for response speed exacerbated PD patients’ difficulty inhibiting interference produced by conflicting action impulses. Focusing on responding accurately improved inhibitory control in PD, but its engagement remained delayed and less proficient compared to HC. It is not difficult to imagine how changes in these key aspects of action control might disrupt task performance across everyday situations that require speeded decisions to control actions (e.g., driving). The results also revealed that PD patients show reductions in neurophysiological inhibition of the motor cortex controlling an impulsive response tendency under speed stress. MRP activity associated with activation and inhibition goal-directed and impulsive incorrect responses computed using the surface Laplacian technique coupled with analytical techniques guided by the DPAS model may provide valuable tools for tracking the emergence of action control deficits and the effects of therapeutic interventions in PD and other diseases that disrupt frontal-basal ganglia circuitries.
Highlights.
Parkinson’s disease (PD) disrupts certain aspects of cognitive control.
The current study investigated the effects of PD on the ability to resolve response conflict under speed and accuracy instructions by means of a Simon task and ERPs
PD patients were less proficient at suppressing incorrect response impulses compared to HC under speed instructions
Likewise, the ERPs showed reduced suppression of the incorrect response alternative under speed instructions for PD patients compared to HC.
Acknowledgments
This work was supported by the National Institute of Aging (the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Aging or the National Institute of Health) by grant K23AG028750 (to S.A.W.), the American Academy of Neurology Clinical Research Fellowship (to D.O.C), The Netherlands Organization for Scientific Research Grants (to W.P.M v.d.W), and the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., grant (to T.R.B.).
Footnotes
We analyzed stimulus-locked early visual potentials to determine condition and group effects. Specifically, we measured amplitude differences in N1 and P2 components measured from the most appropriate posterior electrode sites on our cap, PO3 and PO4. In summary, the amplitudes of these components did not vary as a function of group, correspondence, or instructions, suggesting that none of the experimental factors impacted early visual processing and attention to the Simon stimulus.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni F, Cincotti F, Carducci F, Rossini PM, Babiloni C. Spatial enhancement of EEG data by surface Laplacian estimation: the use of magnetic resonance imaging-based head models. Clinical Neurophysiology. 2001;112(5):724–727. doi: 10.1016/S1388-2457(01)00494-1. [DOI] [PubMed] [Google Scholar]
- Beste C, Konrad C, Saft C, Ukas T, Andrich J, Pfleiderer B, Falkenstein M. Alterations in voluntary movement execution in Huntington’s disease are related to the dominant motor system - Evidence from event-related potentials. Experimental Neurology. 2009;216(1):148–157. doi: 10.1016/j.expneurol.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Bogacz R, Gurney K. The basal ganglia and cortex implement optimal decision making between alternative actions. Neural Comput. 2007;19(2):442–477. doi: 10.1162/neco.2007.19.2.442. [DOI] [PubMed] [Google Scholar]
- Bogacz R, Wagenmakers EJ, Forstmann BU, Nieuwenhuis S. The neural basis of the speed-accuracy tradeoff. Trends Neurosci. 2010;33(1):10–16. doi: 10.1016/j.tins.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Brown P, Eusebio A. Paradoxes of functional neurosurgery: clues from basal ganglia recordings. Mov Disord. 2008;23(1):12–20. doi: 10.1002/mds.21796. quiz 158. [DOI] [PubMed] [Google Scholar]
- Burle B, Possamai CA, Vidal F, Bonnet M, Hasbroucq T. Executive control in the Simon effect: an electromyographic and distributional analysis. Psychological Research-Psychologische Forschung. 2002;66(4):324–336. doi: 10.1007/S00426-002-0105-6. [DOI] [PubMed] [Google Scholar]
- Burle B, Vidal F, Tandonnet C, Hasbroucq T. Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn. 2004;56(2):153–164. doi: 10.1016/j.bandc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Carbonnell L, Hasbroucq T, Grapperon J, Vidal F. Response selection and motor areas: a behavioural and electrophysiological study. Clinical Neurophysiology. 2004;115(9):2164–2174. doi: 10.1016/J.Clinph.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Coles MGH. Modern Mind-Brain Reading - Psychophysiology, Physiology, and Cognition. Psychophysiology. 1989;26(3):251–269. doi: 10.1111/J.1469-8986.1989.Tb01916.X. [DOI] [PubMed] [Google Scholar]
- de Jong R, Wierda M, Mulder G, Mulder LJ. Use of partial stimulus information in response processing. J Exp Psychol Hum Percept Perform. 1988;14(4):682–692. doi: 10.1037//0096-1523.14.4.682. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Eimer M. The lateralized readiness potential as an on-line measure of central response activation processes. Behavior Research Methods Instruments & Computers. 1998;30(1):146–156. doi: 10.3758/Bf03209424. [DOI] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of Noise Letters Upon Identification of a Target Letter in a Nonsearch Task. Perception & Psychophysics. 1974;16(1):143–149. doi: 10.3758/Bf03203267. [DOI] [Google Scholar]
- Eusebio A, Thevathasan W, Doyle Gaynor L, Pogosyan A, Bye E, Foltynie T, Brown P. Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry. 2011;82(5):569–573. doi: 10.1136/jnnp.2010.217489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Willemssen R, Hohnsbein J, Hielscher H. Effects of stimulus-response compatibility in Parkinson’s disease: a psychophysiological analysis. J Neural Transm. 2006;113(10):1449–1462. doi: 10.1007/s00702-005-0430-1. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WPM, Ridderinkhof KR. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: A model-based approach. Journal of Neuroscience. 2008;28(39):9790–9796. doi: 10.1523/jneurosci.1465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Tittgemeyer M, Wagenmakers EJ, Derrfuss J, Imperati D, Brown S. The speed-accuracy tradeoff in the elderly brain: a structural model-based approach. J Neurosci. 2011;31(47):17242–17249. doi: 10.1523/JNEUROSCI.0309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, van den Wildenberg WPM, Ridderinkhof KR. Neural mechanisms, temporal dynamics, and individual differences in interference control. Journal of Cognitive Neuroscience. 2008;20(10):1854–1865. doi: 10.1162/jocn.2008.20122. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: A neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. Journal of Cognitive Neuroscience. 2005;17(1):51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006;19(8):1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318(5854):1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles M, Sirevaag E, Eriksen C, Donchin E. Prestimulus and poststimulus activation of response channels – a psychophysiological analysis. Journal of Experimental Psychology- Human Perception and Performance. 1988;14(3):331–344. doi: 10.1037//0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A New Method for Off-Line Removal of Ocular Artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Ceballos-Baumann AO. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain. 2001;124(Pt 3):558–570. doi: 10.1093/brain/124.3.558. [DOI] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A. Dimensional Overlap - Cognitive Basis for Stimulus-Response Compatibility - a Model and Taxonomy. Psychological Review. 1990;97(2):253–270. doi: 10.1037/0033-295x.97.2.253. [DOI] [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197(4305):792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Leuthold H. Programming of expected and unexpected movements: effects on the onset of the lateralized readiness potential. Acta Psychologica. 2003;114(1):83–100. doi: 10.1016/S0001-6918(03)00051-9. [DOI] [PubMed] [Google Scholar]
- Lo CC, Wang XJ. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci. 2006;9(7):956–963. doi: 10.1038/nn1722. [DOI] [PubMed] [Google Scholar]
- Low KA, Miller J, Vierck E. Response slowing in Parkinson’s disease. Brain. 2002;125:1980–1994. doi: 10.1093/Brain/Awf206. [DOI] [PubMed] [Google Scholar]
- Meckler C, Allain S, Carbonnell L, Hasbroucq T, Burle B, Vidal F. Motor inhibition and response expectancy: A Laplacian ERP study. Biological Psychology. 2010;85(3):386–392. doi: 10.1016/J.Biopsycho.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Meynier C, Burle B, Possamai CA, Vidal F, Hasbroucq T. Neural inhibition and interhemispheric connections in two-choice reaction time: A Laplacian ERP study. Psychophysiology. 2009;46(4):726–730. doi: 10.1111/J.1469-8986.2009.00818.X. [DOI] [PubMed] [Google Scholar]
- Miller J. Contralateral and ipsilateral motor activation in visual simple reaction time: a test of the hemispheric coactivation model. Experimental Brain Research. 2007;176(4):539–558. doi: 10.1007/s00221-006-0641-1. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Nunez PL. A study of origins of the time dependencies of scalp EEG: i--theoretical basis. IEEE Trans Biomed Eng. 1981a;28(3):271–280. doi: 10.1109/tbme.1981.324700. [DOI] [PubMed] [Google Scholar]
- Nunez PL. A study of origins of the time dependencies of scalp EEG: ii--experimental support of theory. IEEE Trans Biomed Eng. 1981b;28(3):281–288. doi: 10.1109/TBME.1981.324701. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. A theoretical basis for standing and traveling brain waves measured with human EEG with implications for an integrated consciousness. Clin Neurophysiol. 2006;117(11):2424–2435. doi: 10.1016/j.clinph.2006.06.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Praamstra P. Do’s and don’ts with lateralized event-related brain potentials. Journal of Experimental Psychology-Human Perception and Performance. 2007;33(2) doi: 10.1037/0096-1523.33.2.497. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Stegeman DF, Cools AR, Horstink M. Reliance on external cues for movement initiation in Parkinson’s disease - Evidence from movement-related potentials. Brain. 1998;121 doi: 10.1093/brain/121.1.167. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Plat EM, Meyer AS, Horstink M. Motor cortex activation in Parkinson’s disease: Dissociation of electrocortical and peripheral measures of response generation. Movement Disorders. 1999;14(5) doi: 10.1002/1531-8257(199909)14:5<790::aid-mds1011>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Plat FM. Failed suppression of direct visuomotor activation in Parkinson’s disease. J Cogn Neurosci. 2001;13(1):31–43. doi: 10.1162/089892901564153. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. The basal ganglia: A vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR. Micro- and macro-adjustments of task set: activation and suppression in conflict tasks. Psychol Res. 2002;66(4):312–323. doi: 10.1007/s00426-002-0104-7. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Forstmann BU, Wylie SA, Burle B, van den Wildenberg WPM. Neurocognitive mechanisms of action control: resisting the call of the Sirens. Wiley Interdisciplinary Reviews-Cognitive Science. 2011;2(2):174–192. doi: 10.1002/Wcs.99. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cognition. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Ann Neurol. 1995;37(2):181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Rascol O. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain. 2000;123(Pt 2):394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Coles MG. The lateralized readiness potential: relationship between human data and response activation in a connectionist model. Psychophysiology. 1999;36(3):364–370. doi: 10.1017/s0048577299970749. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Valzania F, Nassetti SA, Tropeani A, Bisulli A, Santangelo M, Tassinari CA. Effects of chronic levodopa and pergolide treatment on cortical excitability in patients with Parkinson’s disease: a transcranial magnetic stimulation study. Clin Neurophysiol. 2000;111(7):1198–1202. doi: 10.1016/s1388-2457(00)00316-3. [DOI] [PubMed] [Google Scholar]
- Tandonnet C, Burle B, Vidal F, Hasbroucq T. The influence of time preparation on motor processes assessed by surface Laplacian estimation. Clin Neurophysiol. 2003;114(12):2376–2384. doi: 10.1016/s1388-2457(03)00253-0. [DOI] [PubMed] [Google Scholar]
- Tandonnet C, Burle B, Vidal F, Hasbroucq T. Knowing when to respond and the efficiency of the cortical motor command: A Laplacian ERP study. Brain Research. 2006;1109 doi: 10.1016/j.brainres.2006.06.052. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Burle B, Vidal F, Bonnet H. Deficit in motor cortical activity for simultaneous bimanual responses. Experimental Brain Research. 2001;137(3–4):259–268. doi: 10.1007/s002210000661. [DOI] [PubMed] [Google Scholar]
- Turner RS, Grafton ST, McIntosh AR, DeLong MR, Hoffman JM. The functional anatomy of parkinsonian bradykinesia. Neuroimage. 2003;19(1):163–179. doi: 10.1016/s1053-8119(03)00059-4. [DOI] [PubMed] [Google Scholar]
- van de Laar MC, van den Wildenberg WP, van Boxtel GJ, Huizenga HM, van der Molen MW. Lifespan changes in motor activation and inhibition during choice reactions: a Laplacian ERP study. Biol Psychol. 2012;89(2):323–334. doi: 10.1016/j.biopsycho.2011.11.005. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WPM, van Boxtel GJM, van der Molen MW, Bosch DA, Speelman JD, Brunia CHM. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. Journal of Cognitive Neuroscience. 2006;18(4):626–636. doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, Wylie SA, Forstmann BU, Burle B, Hasbroucq T, Ridderinkhof KR. To head or to heed? Beyond the surface of selective action inhibition: a review. Front Hum Neurosci. 2010;4:222. doi: 10.3389/fnhum.2010.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Krug MK, Carter CS. The neural and computational basis of controlled speed-accuracy tradeoff during task performance. J Cogn Neurosci. 2008;20(11):1952–1965. doi: 10.1162/jocn.2008.20146. [DOI] [PubMed] [Google Scholar]
- Vidal F, Grapperon J, Bonnet M, Hasbroucq T. The nature of unilateral motor commands in between-hand choice tasks as revealed by surface Laplacian estimation. Psychophysiology. 2003;40(5):796–805. doi: 10.1111/1469-8986.00080. [DOI] [PubMed] [Google Scholar]
- Voon V, Fox SH. Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol. 2007;64(8):1089–1096. doi: 10.1001/archneur.64.8.1089. [DOI] [PubMed] [Google Scholar]
- Wickelgren WA. Speed-Accuracy tradeoff and information-processing dynamics. Acta Psychologica. 1977;41(1):67–85. doi: 10.1016/0001-6918(77)90012-9. [DOI] [Google Scholar]
- Wijnen JG, Ridderinkhof KR. Response inhibition in motor and oculomotor conflict tasks: Different mechanisms, different dynamics? Brain and Cognition. 2007;63(3):260–270. doi: 10.1016/J.Bandc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Bashore TR, van den Wildenberg WPM. The effect of Parkinson’s disease on the dynamics of on-line and proactive cognitive control during action selection. J Cogn Neurosci. 2010;22(9):2058–2073. doi: 10.1162/jocn.2009.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, van den Wildenberg WPM, Ridderinkhof KR, Bashore TR, Powell VD, Manning CA, Wooten GF. The effect of Parkinson’s disease on interference control during action selection. Neuropsychologia. 2009a;47(1):145–157. doi: 10.1016/j.neuropsychologia.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, van den Wildenberg WPM, Ridderinkhof KR, Bashore TR, Powell VD, Manning CA, Wooten GF. The effect of speed-accuracy strategy on response interference control in Parkinson’s disease. Neuropsychologia. 2009b;47(8–9):1844–1853. doi: 10.1016/j.neuropsychologia.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]