Abstract
Background and purpose
Studies assessing the relationship between chronic post-stroke language impairment (aphasia), and ischemic brain damage usually rely on measuring the extent of brain necrosis observed on magnetic resonance image (MRI). Nonetheless, clinical observation suggests that patients can exhibit deficits that are more severe than what would be expected based on lesion location and size. This phenomenon is commonly explained as being the result of cortical disconnection. To understand if disconnection contributes to clinical symptoms, we assessed the relationship between language impairments and structural brain connectivity (the connectome) in patients with chronic aphasia after a stroke
Methods
Thirty-nine patients with chronic aphasia underwent language assessment and MRI scanning. Relying on MRI data, we reconstructed the individual connectome from T1 weighted and diffusion tensor imaging (DTI). Deterministic fiber tractography was employed to assess connectivity between each possible pair of cortical Brodmann areas (BA). Multiple linear regression analyses were performed to evaluate the relationship between language performance and cortical necrosis and cortical disconnection.
Results
We observed that structural disconnection of BA 45 (spared by the necrotic tissue) was independently associated with naming performance, controlling for the extent of BA 45 necrosis (F=4.62, p<0.01; necrosis: β=0.43; p=0.03; disconnection β=1.21; p<0.001).
Conclusions
We suggest that cortical disconnection, as measured by the structural connectome, is an independent predictor of naming impairment in patients with chronic aphasia. The full extent of clinically relevant brain damage after an ischemic stroke may be underappreciated by visual inspection of cortical necrosis alone.
Keywords: Aphasia, disconnection, stroke, connectome, diffusion tensor imaging
Introduction
The location of chronic post-stroke brain damage is typically based on T1-weighted, T2-weighted, or Fluid Attenuated Inversion Recovery (FLAIR) Magnetic Resonance Imaging (MRI) sequences1–3. These are principled techniques providing information about the extent of the cortical and subcortical lesions. However, it is important to note that these methods may fail to detect the full extent of white matter injury. It is well known from animal and human studies that cortical and subcortical strokes are associated with white matter injury and anterograde and retrograde Wallerian degeneration4–6. Therefore, cortical disconnections as a result of white matter injury may occur in areas not included in the frank lesion, but still account for behavioral impairment.
Aphasia is an impairment of language processing, commonly observed after a dominant hemisphere stroke7. The emergence of aphasia after a stroke is traditionally associated with structural damage affecting cortical regions related to language functions. Classically, deficits in speech production are expected to occur following lesions affecting pars opercularis and pars triangularis in the left inferior frontal gyrus (now referred to as Broca’s area, involving the cytoarchitectonic Brodmann areas [BA] 44 and 45). Conversely, deficits in speech comprehension are typically associated with damage affecting portions of the temporal lobe, especially BA 22 – Wernicke’s area8.
It is a caveat that much of what we know about brain-language relationships has been derived from lesion studies that may have underappreciated the extent of cortical disconnection. In fact, circuit disconnections could account for up to 26% of exceptions to the classical radiological-clinical correlations in aphasia9.
Newer imaging methodologies now enable the comprehensive mapping of all neural connections in the brain at medium and large scale (the brain connectome)10–15. The purpose of this study was to examine two independent but complementary forms of cortical damage - necrosis and disconnection – in relation to global aphasia measures and naming impairments in persons with aphasia. For this purpose, we performed a comprehensive assessment of aphasia severity and naming impairments in a series of subjects with chronic aphasia due to a left hemisphere stroke. We also assessed necrotic tissue damage and impaired neural connectivity from these subjects using high resolution MRI. Furthermore, we introduce new connectome-mapping techniques to accurately assess the anatomy of cortical and subcortical lesions in subjects with stroke.
Methods
Subjects
We studied thirty-nine individuals (mean age 62.7 ±12.8 years, 22 male, 16 female) who suffered a left hemisphere ischemic stroke at least six months prior to enrolling in this study (Table I in the Online Data Supplement). All subjects enrolled in this study were right handed prior to the stroke.
All subjects were recruited from the local community. They did not have a history or imaging evidence of other previous strokes, and had no history of other neurological illnesses. None of the subjects had a history of intellectual or learning disabilities. All subjects were able to ambulate either with no assistance, or with the use of a cane. Patients with a history of learning disability, with a history of uncontrolled seizures, or with more severe forms of motor impairment (i.e., not able to ambulate) were excluded from this study.
All subjects signed an informed consent to participate in this study. Subjects were enrolled at the Medical University of South Carolina (MUSC) or at the University of South Carolina (USC). The Institutional Review Boards at USC and at MUSC approved this study.
Language testing
All subjects underwent comprehensive behavioral testing at the time of enrollment in the study. Testing was performed by a speech pathologist and included the Western Aphasia Battery16 (WAB) and the Philadelphia Naming Test17 (PNT).
The main purpose of language testing was to provide an assessment of: 1) aphasia severity, and 2) naming performance. Aphasia severity was determined using the WAB aphasia quotient (AQ), a measure aphasia severity ranging from 0 to 100 (> 93.8 indicates language abilities within normal limits).
Conversely, naming performance was determined based on PNT performance. The PNT is a computer-based assessment of naming in persons with aphasia17 and includes 175 pictures representing mid- and high-frequency nouns from a word frequency list compiled by Francis and Kucera18.
All subjects underwent a formal evaluation of speech apraxia based on the Subtest Six on the Apraxia Battery for Adults–Second Edition [ABA-2]19, to ensure that apraxia of speech was not a significant confounder accounting for naming difficulties.
MRI acquisition
All subjects underwent MRI scanning at USC or at MUSC. At both sites, MRI scanning was performed using the same type of MRI scanner, i.e., a 3T Siemens Trio equipped with a 12-channel head coil. The MRI scanning protocol is described in detail the Online Supplement.
Preprocessing of MRI data
In order to ensure adequate quantification of necrotic lesion burden and cortical connectivity, we optimized connectome-mapping methodology to evaluate structural brain properties while preserving anatomical authenticity in spite of the spatial distortions caused by the stroke lesion. MRI data preprocessing for connectome mapping involves multiple sequential steps10, namely: 1) segmentation of the cerebral cortex into multiple anatomically defined regions of interest (ROIS) and spatial registration of T1 weighted images; 2) transformation of white matter maps and cortical ROIs onto DTI space; 3) fiber tracking and connectome reconstruction; and 4) cortical connectivity assessment. These steps can be challenging when applied to brains with large anatomical distortions (due to mass effects or pathological atrophy) caused be structural lesions (i.e., necrotic tissue from the stroke).
Connectome methodology and necrotic lesion assessment are explained in the methods section of the Online Supplement, and in Figures 1 and 2.
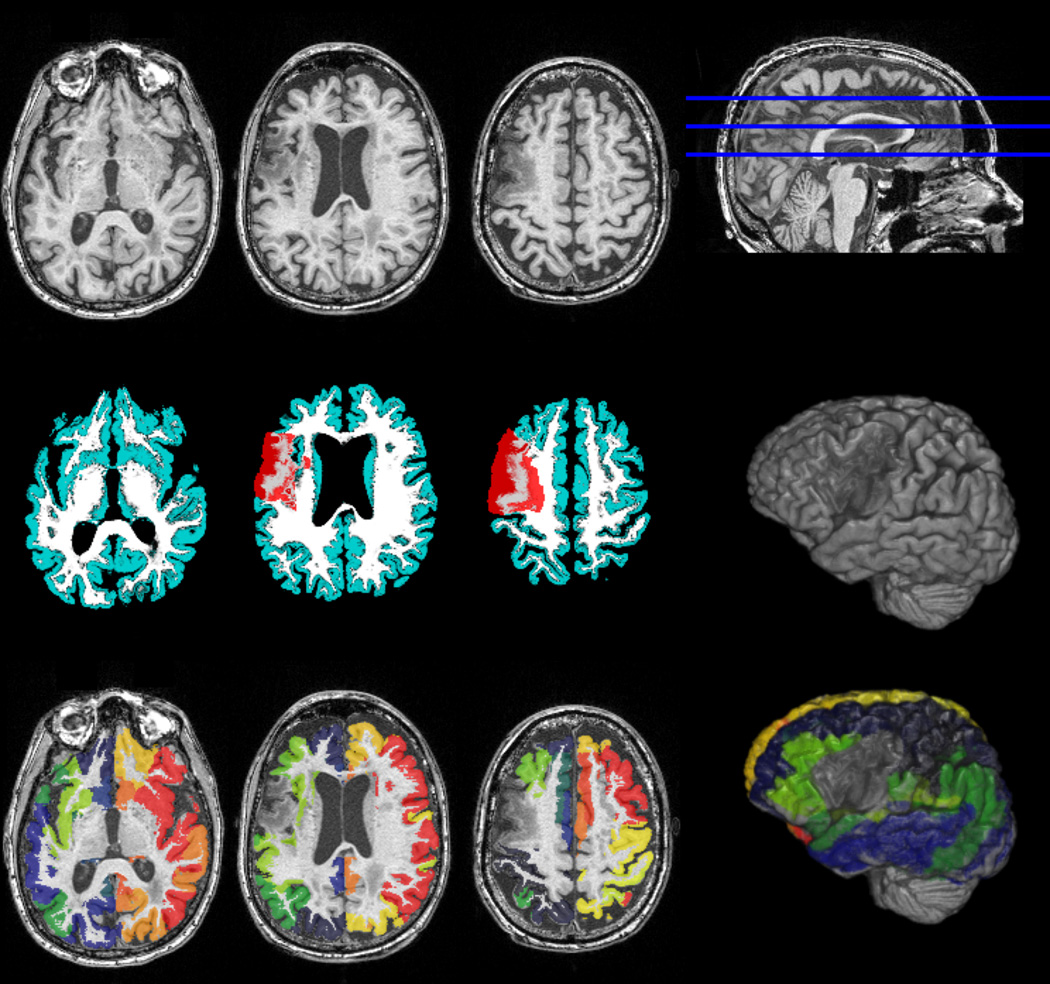
Figure 1.
Image preprocessing steps employed during the construction of the connectome. The upper row demonstrates T1-weighted images from a representative subject. Necrotic tissue can be observed in the left hemisphere, affecting the insula and pre-central dorsolateral regions. The middle row demonstrates the probabilistic maps of gray (cyan) and white matter (white), and their relationship with the necrotic lesion mask (in red, manually drawn on T2 images). The bottom row demonstrates the probabilistic map of gray matter segmented in accordance with the Brodmann area Atlas. Note the exclusion of the necrotic tissue from the segmented cortical map, as demonstrated by the three-dimensional reconstructions on the third column.
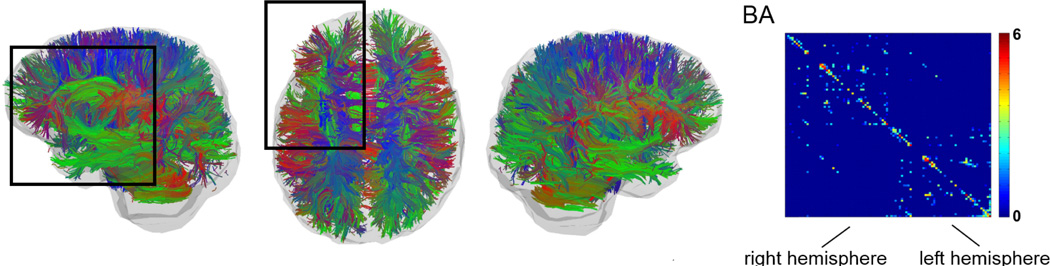
Figure 2.
Deterministic tractography results from a representative subject (same subject as in Figure 1). The left panels show the tracts with color indicating the mean direction of each fiber. Note the relative reduction in tracked fibers in the left frontal lobe (highlighted by the dark rectangle), compared with the right side. The resulting connectome from this subject is demonstrated on the right side of the figure. The scale bar represents log(number of fibers).
It should be noted that this method does not take into consideration physiological asymmetries between hemispheres. Our group previously assessed the degree of physiological asymmetries in the connectome in healthy older adults20. None of the ROIs tested in this study are associated with a significant asymmetry in healthy subjects, except for a part of BA 45 (i.e., the opercularis component of the inferior frontal gyrus), which demonstrates a leftward asymmetry. These findings are in accordance with previous literature21. Thus, left sided fiber reductions are not likely to represent a physiological pattern.
Statistical Analyses
We performed multiple linear regression analyses with the performance on naming measures defined as the dependent variable, with the following independent variables: 1) percentage of damage to each BA area, 2) percentage number of fibers of each BA area. Each BA area was analyzed separately. The explanatory power of the resulting regression model was determined as R2 (proportional reduction in error) with the explanatory factors of interest entered into the regression analysis using a stepwise approach. The level of statistical significance was set at p<0.05, adjusted through Bonferroni correction based on the number of regressions investigated.
Results
Language - aphasia severity and naming performance
Aphasia severity: the mean AQ for the group of subjects was 57.94 (SD = 28.27). Performances on WAB subtests were as follows (mean ±SD): comprehension = 7.67 ±1.96; fluency = 5.23 ±3.32; speech repetition = 5.25 ±3.71; information content = 5.69 ±3.20. Aphasia types were distributed as follows: 38% (15/39) anomic aphasia; 38% (15/39) Broca’s aphasia; 5% (2/39) Wernicke’s aphasia; 10% (4/39) with conduction aphasia; 8% (3/39) with global aphasia. Naming performance: subjects correctly named an average of 51 ±33% of the PNT items (Table I in the Online Data Supplement).
Necrotic lesion location
All subjects exhibited a cortical/subcortical lesion in the left hemisphere, broadly distributed within the vascular territory of the left MCA. A considerable variability in lesion location was observed within the MCA territory. The insula and the subcortical aspect of the frontal operculum were the locations of maximal lesion overlap (Figure I in the Online Data Supplement).
Connectome
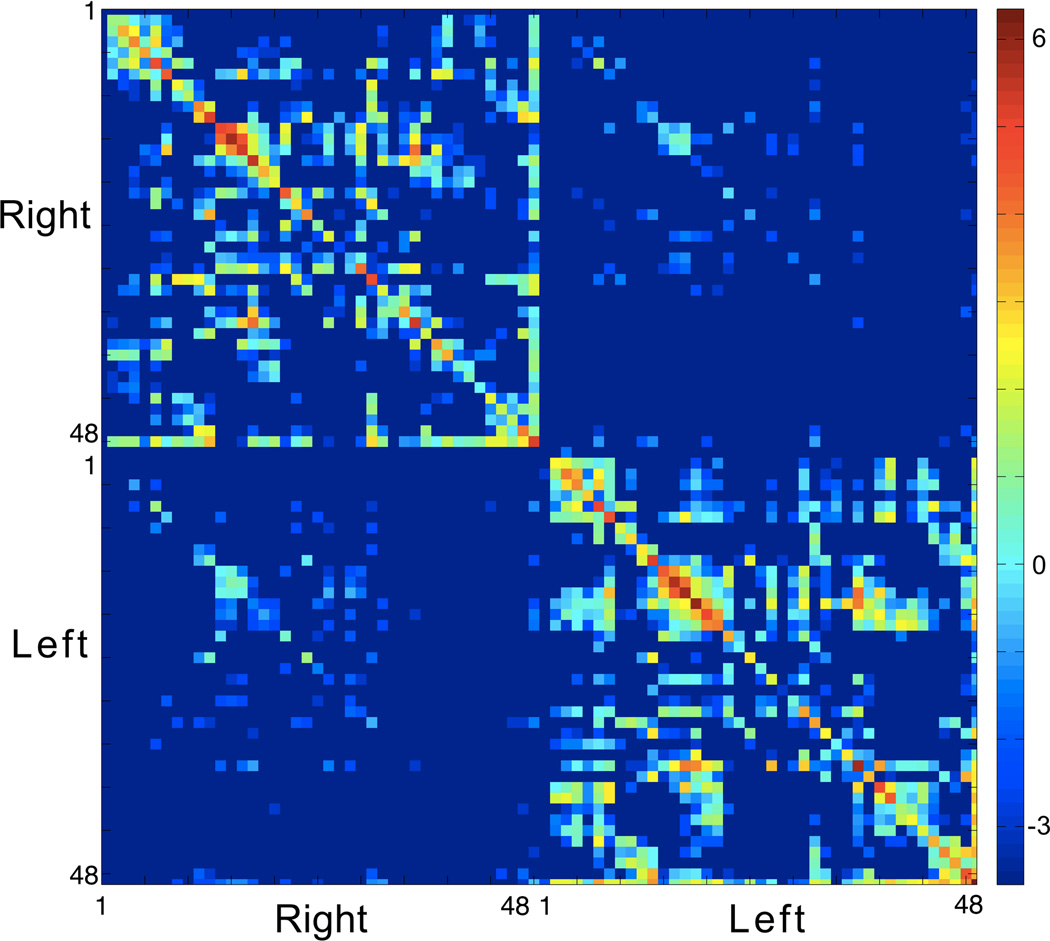
The average connectome from all subjects is demonstrated in Figure 3. The relative reduction in connectivity for the BAs in the left hemisphere can be observed by the relative proportion of connectivity weight per BA in the left hemisphere, compared with the right hemisphere (Figure II in the Online Data Supplement). This analysis excluded reciprocal connections to the region itself. Figure III in the Online Data supplement demonstrates the extent of necrosis of each BA. Interestingly, comparing the data from Figures II and III in the Online Data Supplement, reduction in connectivity was observed in regions with a higher percentage of damage, but it was not a perfect match, indicating that connectivity damage and cortical necrosis are complementary factors. This relationship can be appreciated in Figure IV in the Online Data Supplement demonstrating the correlation between necrosis and connectivity, for each ROI.
Figure 3.
The average connectome from all subjects. The scale bar represents log(number of fibers).
Overall necrosis extent and language impairment
We did not observe a relationship between overall lesion size and AQ on the WAB (r=−0.16, p=0.33); the WAB naming subscore (r=0.04, p=0.84); and correctly named items on the PNT (r=−0.07, p=0.67).
Regional necrosis and connectivity and language impairment
We focused our analyses on general aphasia measures (assessed through the WAB): 1) comprehension of sequential commands; and 2) speech fluency; and naming performance (measured by the PNT): 1) percentage of correctly named items. We assessed brain ROIs typically associated with speech and language, namely BA 22, 37, and 45.
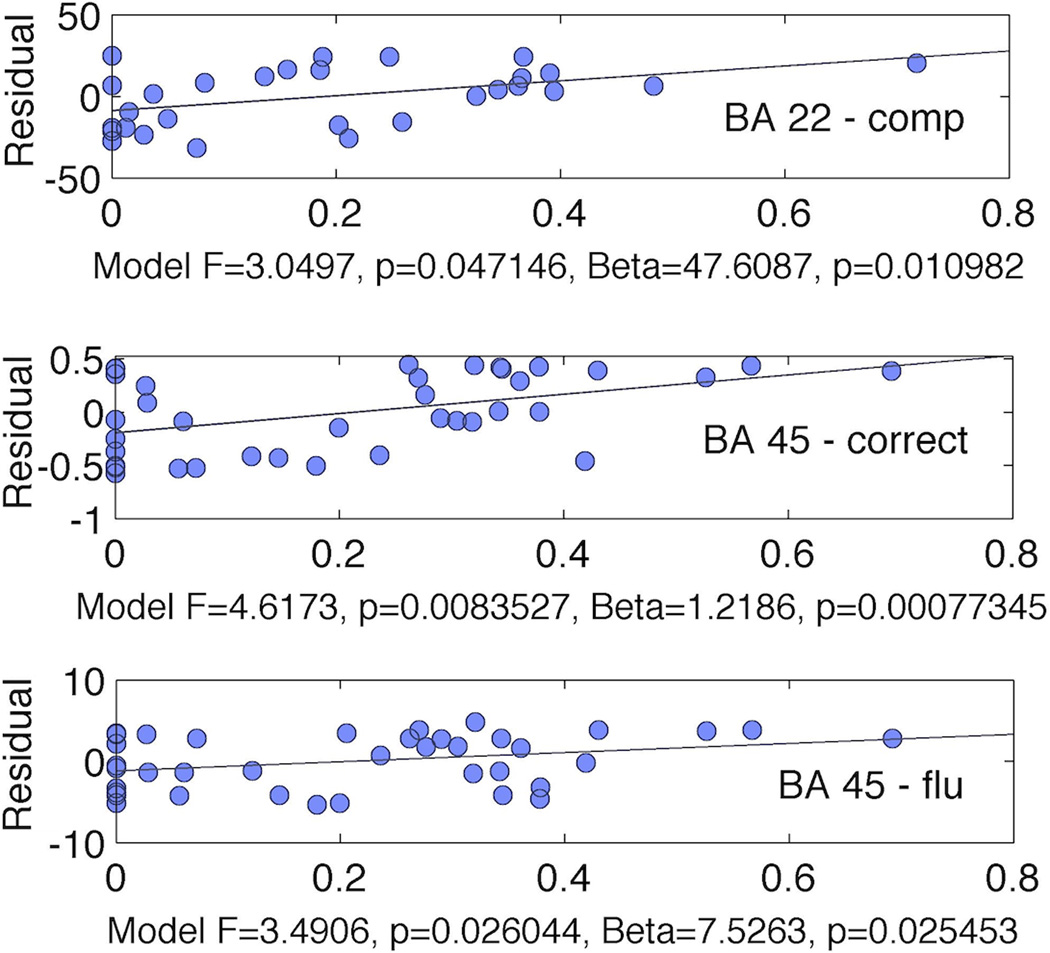
We observed a significant relationship between number of correctly named items on the PNT and a model composed of BA 45 necrotic damage and BA 45 disconnection (F=4.62, p<0.01; necrosis: β=0.43; p=0.03; disconnection β=1.21; p<0.001) (Figure 4). We also observed a significant relationship between comprehension and a model composed by BA 22 necrosis and BA 22 disconnection (F=3.05, p=0.047; necrosis: β=−0.35; p=0.43; disconnection β=47; p=0.01). However, in this model, necrosis was not independently associated with comprehension. Finally, we also observed significant relationship between fluency and a model composed by BA 45 necrosis and BA 45 disconnection F=4.62, p<0.01; necrosis: β=2.89; p=0.13; disconnection β=7.52; p=0.025). Similarly, in this model, necrosis alone was not an independent predictor of fluency (Figure 4).
Figure 4.
Damage and disconnection of regions in the left hemisphere were independent predictors of naming performance. The x-axis represents the percentage of fibers in the left hemisphere BA compared with the homologous right hemisphere BA. The y-axis represents the residual error from the correlation between the percent of cortical damage to the left BA and the behavioral performance. The upper plot demonstrates the relationship between BA 22 and auditory comprehension of sequential commands (“comp”); the middle plot, between BA 45 and number of items correctly named on the PNT (“correct”) and the lower plot between BA 45 and fluency. Below each plot, we demonstrate the statistical results for the multiple linear regression model when BA cortical damage and BA cortical disconnection were defined as independent variables and the behavioral measure as the dependent variable. The model F is shown along with beta and p values associated with cortical disconnection.
Interestingly, aphasia severity (AQ), was not associated with a model composed by necrosis and disconnection in BA 45 (p=0.34), BA 22 (p=0.8) or BA 37 (p=0.84), suggesting that specific language impairments are distinctly associated with regional necrosis and disconnection.
Discussion
In this study, we evaluated the relationships between cortical necrosis and cortical disconnection with global aphasia measures and naming performance. We introduced new imaging methodology to assess connectome abnormalities in patients with chronic stroke, and we demonstrated that cortical disconnection and cortical necrosis are coexisting phenomena. We observed that correct naming is dependent upon the preservation of cortical integrity and the preservation of cortical connectivity of BA 45. It is important to note that this finding does not suggest that disconnection and necrosis in other cortical regions does not contribute to naming impairment. Rather, our study focused on a small set of cortical regions, including BA 45, to clearly demonstrate how disconnection contributes to behavioral impairment.
These findings are relevant to the understanding of the mechanisms underlying stroke related impairments because they underscore two important factors: 1) cortical disconnection is an independent form of damage, which may not be readily appreciated by measurement of cortical necrosis; and 2) cortical disconnection is a complementary factor that explains behavioral deficits such as naming impairment.
1) Cortical disconnection as an “invisible” form of damage
The full extent of structural injury is usually appreciated only weeks or months after the ischemic event through structural MRI22. Even though it is possible to define the extension of the necrotic lesions into subcortical regions, it is difficult to quantify, based on visual inspection alone, the magnitude of white matter reduction, particularly as it relates to fibers that connect cortical regions remote from the stroke site.
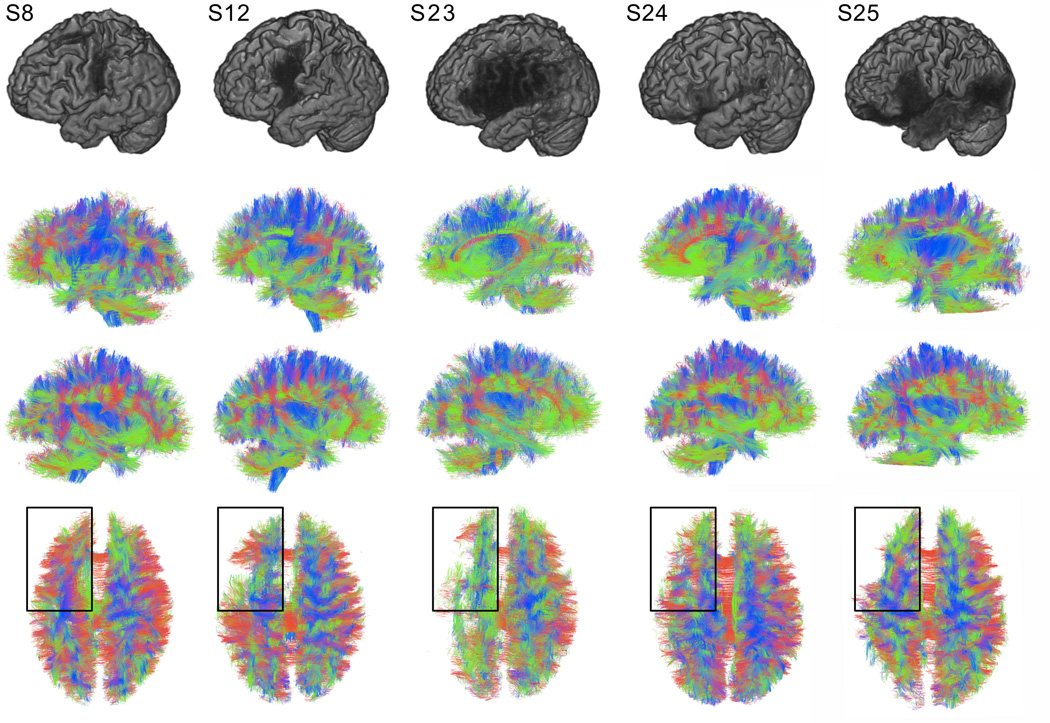
In this study, we demonstrated that cortical regions in the hemisphere affected by the stroke exhibit a reduction in connectivity compared with their homologue counterparts in the non-affected hemisphere. This observation suggests that cortical areas that are apparently intact during visual inspection of MRI images exhibit a reduction in structural connectivity. The degree of connectivity reduction cannot be inferred based on the location of the necrosis alone. This fact is perhaps best explained in Figure 5 where preservation of connectivity of the inferior frontal gyrus may occur for some subjects with anterior supra-Sylvian necrosis, but is absent for other subjects with a relatively similar extent of damage.
Figure 5.
This diagram demonstrates surface brain and white matter fiber anatomies for five representative patients to illustrate the variability in cortical lesion location and the degree of naming impairment. Subjects are demonstrated per column. The first row shows the reconstruction of the cortical surface from T1 weighted images (the stroke lesion is apparent as the area of liquefactive necrosis). The second, third and fourth rows demonstrate reconstructed whole brain deterministic white matter tracts (viewed from the left side, right side and above, respectively). Note that that subjects 8 and 12 (S8 and S12) demonstrated lesions encompassing the inferior frontal gyrus, however their performance in confrontation naming was the relatively good (the percentage of correctly named items on the PNT was respectively 91% [AQ=96], and 90% [AQ=21]). In turn, subjects 23, 24 and 25 (S23, S24 and S 25) exhibited profound difficulty in naming (4% [AQ=23], 5% [AQ=31], 5%[AQ=71]), albeit with variable necrotic lesion anatomy. Importantly, left inferior frontal fibers (highlighted by the dark rectangle) were relatively preserved in patients with good naming performance (S8 and S12), but largely absent in patients with poor performance (S23, 24 and 25) suggesting a common mechanism underlying naming and structural integrity.
To assess the extent of disconnection in different cortical regions, we relied on diffusion MRI tractography. Although MRI tractography has the advantage of providing in-vivo information about structural brain integrity, it is not without technical limitations23. In fact, structural connectome mapping is a novel methodological approach in neuroscience and neuroimaging, with growing popularity and applicability11, 12. In this study, we introduce new technical improvements to enable connectome mapping in subjects with stroke. Nevertheless, the results should be interpreted in the context of limitations of MRI tractography.
2) Cortical disconnection and naming performance
We demonstrated that naming performance is associated with preservation of cortical integrity and cortical connectivity of BA 45. Importantly, cortical connectivity predicts naming performance when controlling for the volume of necrosis.
Our group has demonstrated that loss of white matter fibers supporting the dominant inferior frontal gyrus can lead to severe aphasia, even if the region appears to have been spared by the stroke24. Our current study provides further evidence suggesting that preserved cortical connectivity is an independent predictor of accurate naming performance in subjects with chronic aphasia. Furthermore, disconnection may play a role in aphasia recovery. Our group has demonstrated that naming recovery as a result of therapy is associated with functional modulation of the inferior frontal cortex25, leading to an increase in the number of correctly named items. However, functional modulation of the frontal region does not occur in all subjects with a partially preserved frontal cortex. Subjects who do not achieve functional recruitment fail to improve and a possible reason underlying suboptimal frontal recruitment is the lack of structural connectivity. This theory was not tested in the study, but it could be addressed by future research.
The results described in this manuscript highlight the importance of subcortical lesions in patients with aphasia. Kreisler et al demonstrated that basal nuclei and subcortical lesions were frequently associated with almost all forms of language impairment26. Cortical disconnection as a result of specific subcortical lesions may play a significant role in aphasia27. In fact, unmeasured disconnection can account for discrepancies regarding the crucial anatomy supporting naming. Specifically, since lesion studies rely on the manual delineation of areas of frank necrosis, they may underappreciate damage as a result of remote cortical disconnection. Expressive deficits are commonly observed after strokes affecting the dominant inferior frontal gyrus (involving Brodmann areas [BA] 44 and 45)28 However, naming involves a network of peri-Sylvian structures and naming impairment may occur as a result of lesions affecting several aspects of the cortical language network. Hillis and colleagues demonstrated that several regions are essential for distinct processes underlying naming29. Specifically, anomia may arise from combined dysfunction involving the left anterior, inferior and posterior middle/superior temporal cortex, posterior inferior frontal and inferior parietal cortex3, 29. Dronkers and colleagues demonstrated that damage to the left mid-posterior middle temporal gyrus (MTG) prevents the retrieval of names associated with objects (i.e. lexical-semantic retrieval)30. These findings complement observations from Schwartz et al. demonstrating that semantic errors during naming are associated with damage to the left anterior and mid MTG31, 32, while phonological errors are associated with lack of integrity of the left supramarginal gyrus and inferior frontal cortex33. However, note that the results form Dronkers and Schwartz and colleagues do not completely agree with the findings from Hillis and her group. Importantly, disagreement among studies may be related to two issues. First, naming involves multiple cognitive domains such as visual/auditory perception and processing, semantic decision, lexical retrieval, phonological encoding, and speech articulation3. Second, it does not appear that factors such as apraxia of speech (AOS) can account for our findings as it is typically associated with damage to BA 44 or premotor cortex and not BA 4534–36. Finally, the location of damage or cortical dysfunction may be underestimated by current methods used to define the frank lesion. As such, more pervasive network damage can underlie a more salient lesion but be underappreciated by brain mapping techniques, and localization fails to define a crucial region or network.
In summary, we suggest that correct naming in subjects with chronic aphasia is dependent upon the preservation of cortical integrity and the preservation of cortical connectivity of BA 45. We also suggest that structural evaluation of brain damage in relationship with language impairment after stroke can be improved by measuring the subcortical injury and its remote ramifications into distant cortical disconnection. This connectivity-based approach can improve the understanding of the mechanisms leading to language and behavioral impairments after stroke. It may also provide evidence for degree of structural integrity supporting recovery.
Supplementary Material
Footnotes
Disclosure: The authors report no financial or nonfinancial conflicts of interest associated with this study.
References
- 1.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 2.Hillis AE. Aphasia: Progress in the last quarter of a century. Neurology. 2007;69:200–213. doi: 10.1212/01.wnl.0000265600.69385.6f. [DOI] [PubMed] [Google Scholar]
- 3.Newhart M, Ken L, Kleinman JT, Heidler-Gary J, Hillis AE. Neural networks essential for naming and word comprehension. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 2007;20:25–30. doi: 10.1097/WNN.0b013e31802dc4a7. [DOI] [PubMed] [Google Scholar]
- 4.Thomalla G, Glauche V, Weiller C, Rother J. Time course of wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2005;76:266–268. doi: 10.1136/jnnp.2004.046375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herve D, Molko N, Pappata S, Buffon F, LeBihan D, Bousser MG, et al. Longitudinal thalamic diffusion changes after middle cerebral artery infarcts. J Neurol Neurosurg Psychiatry. 2005;76:200–205. doi: 10.1136/jnnp.2004.041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lama S, Qiao M, Kirton A, Sun S, Cheng E, Foniok T, et al. Imaging corticospinal degeneration in neonatal rats with unilateral cerebral infarction. Experimental neurology. 2011;228:192–199. doi: 10.1016/j.expneurol.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Wade DT, Hewer RL, David RM, Enderby PM. Aphasia after stroke: Natural history and associated deficits. J Neurol Neurosurg Psychiatry. 1986;49:11–16. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damasio AR. Aphasia. The New England journal of medicine. 1992;326:531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- 9.Croquelois A, Bogousslavsky J. Stroke aphasia: 1,500 consecutive cases. Cerebrovascular diseases. 2011;31:392–399. doi: 10.1159/000323217. [DOI] [PubMed] [Google Scholar]
- 10.Daducci A, Gerhard S, Griffa A, Lemkaddem A, Cammoun L, Gigandet X, et al. The connectome mapper: An open-source processing pipeline to map connectomes with mri. PloS one. 2012;7:e48121. doi: 10.1371/journal.pone.0048121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagmann P, Cammoun L, Gigandet X, Gerhard S, Grant PE, Wedeen V, et al. Mr connectomics: Principles and challenges. Journal of neuroscience methods. 2010;194:34–45. doi: 10.1016/j.jneumeth.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS biology. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sporns O. The human connectome: Origins and challenges. Neuroimage. 2013;80:53–61. doi: 10.1016/j.neuroimage.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Sporns O. The human connectome: A complex network. Ann N Y Acad Sci. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- 15.Sporns O, Tononi G, Kotter R. The human connectome: A structural description of the human brain. PLoS computational biology. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kertesz A. The western aphasia battery - revised. New York: Grune & Stratton; 2007. [Google Scholar]
- 17.Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The philadelphia naming test: Scoring and rationale. Clinical Aphasiology. 1996;24:121–133. [Google Scholar]
- 18.Francis WN, Kučera H, Mackie AW. Frequency analysis of english usage : Lexicon and grammar. Boston: Houghton Mifflin; 1982. [Google Scholar]
- 19.Dabul B. Apraxia battery for adults. Examiner's manual. Austin, Tex: Pro-Ed; 2000. [Google Scholar]
- 20.Bonilha L, Nesland T, Rorden C, Fridriksson J. Asymmetry of the structural brain connectome in healthy older adults. Frontiers in psychiatry. 2014;4:186. doi: 10.3389/fpsyt.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller SS, Crow T, Foundas A, Amunts K, Roberts N. Broca's area: Nomenclature, anatomy, typology and asymmetry. Brain Lang. 2009;109:29–48. doi: 10.1016/j.bandl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Caplan LR. Caplan's stroke a clinical approach. Philadelphia: Elsevier/Saunders; 2009. [Google Scholar]
- 23.Gigandet X, Griffa A, Kober T, Daducci A, Gilbert G, Connelly A, et al. A connectome-based comparison of diffusion mri schemes. PloS one. 2013;8:e75061. doi: 10.1371/journal.pone.0075061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fridriksson J, Bonilha L, Rorden C. Severe broca's aphasia without broca's area damage. Behav Neurol. 2007;18:237–238. doi: 10.1155/2007/785280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fridriksson J, Richardson JD, Fillmore P, Cai B. Left hemisphere plasticity and aphasia recovery. Neuroimage. 2012;60:854–863. doi: 10.1016/j.neuroimage.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreisler A, Godefroy O, Delmaire C, Debachy B, Leclercq M, Pruvo JP, et al. The anatomy of aphasia revisited. Neurology. 2000;54:1117–1123. doi: 10.1212/wnl.54.5.1117. [DOI] [PubMed] [Google Scholar]
- 27.Bonilha L, Fridriksson J. Subcortical damage and white matter disconnection associated with non-fluent speech. Brain. 2009;132:e108. doi: 10.1093/brain/awn200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul broca's historic cases: High resolution mr imaging of the brains of leborgne and lelong. Brain. 2007;130:1432–1441. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- 29.DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- 30.Baldo JV, Arevalo A, Patterson JP, Dronkers NF. Grey and white matter correlates of picture naming: Evidence from a voxel-based lesion analysis of the boston naming test. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49:658–667. doi: 10.1016/j.cortex.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker GM, Schwartz MF, Kimberg DY, Faseyitan O, Brecher A, Dell GS, et al. Support for anterior temporal involvement in semantic error production in aphasia: New evidence from vlsm. Brain Lang. 2011;117:110–122. doi: 10.1016/j.bandl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, et al. Anterior temporal involvement in semantic word retrieval: Voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132:3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135:3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitwell JL, Duffy JR, Strand EA, Xia R, Mandrekar J, Machulda MM, et al. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: An mri and fdg-pet study. Brain Lang. 2013;125:245–252. doi: 10.1016/j.bandl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain. 2012;135:1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson JD, Fillmore P, Rorden C, Lapointe LL, Fridriksson J. Re-establishing broca's initial findings. Brain Lang. 2012;123:125–130. doi: 10.1016/j.bandl.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.