Abstract
Peptides are labile toward proteolytic enzymes, and structural modifications are often required to prolong their metabolic half-life and increase resistance. One modification is the incorporation of non-α-amino acids into the peptide to deter recognition by hydrolytic enzymes. We previously reported the synthesis of chimeric α/δ-peptides from glutamic acids (Glu) and the sialic acid derivative Neu2en. Conformational analyses revealed these constructs adopt secondary structures in water and may serve as conformational surrogates of polysialic acid. Polysialic acid is a tumor-associated polysaccharide and is correlated with cancer metastasis. Soluble polysialic acid is rapidly cleared from the blood limiting its potential for vaccine development. One motivation in developing structural surrogates of polysialic acid was to create constructs with increased bioavailability. Here, we report plasma stability profiles of Glu/Neu2en α/δ-peptides. DOTA was conjugated at the peptide N-termini by solid phase peptide synthesis, radiolabeled with 111In, incubated in human blood plasma at 37 °C, and their degradation patterns monitored by cellulose acetate electrophoresis and radioactivity counting. Results indicate that these peptides exhibit a long half-life that is two- to three-orders of magnitude higher than natural α-peptides. These findings provide a viable platform for the synthesis of plasma stable, sialic acid-derived peptides that may find pharmaceutical application.
Keywords: cellulose acetate electrophoresis, chimeric α/δ-peptides, DOTA, glutamic acid, Neu2en, plasma stability, radiochelate, sialic acid
From the perspective of pharmaceutical development, the degradative action of proteases on proteins and peptides presents a significant hurdle and is a major impediment toward drug development (1). Structural modifications, such as the incorporation of non-α-, non-natural, and unnatural amino acids, are often required to prolong metabolic half-life and increase proteolytic resistance (2,3). One source of unnatural amino acid is sialic acid (Neu5Ac), a δ-sugar amino acid. We recently reported the solid phase peptide synthesis (SPPS) and conformational analysis of chimeric α/δ peptides (4) comprised of the Neu5Ac derivative Fmoc-Neu2en (1), Fmoc-L-Glu (2), and Fmoc-D-Glu (3) building blocks (Figure 1). This research endeavor was aimed at the designing and synthesizing of structural surrogates of polysialic acid (PSA) that are capable of eliciting a cross-reactive immune response to PSA. Polysialic acid is a polysaccharide comprised of up to 400 Neu5Ac residues joined in an α(2 → 8)-linkage (5) that is highly expressed on the tissue surface of adult human tumors and is correlated with tumor metastasis (6). It exists as a random coil in solution but adopts fluxional regions of helicity that are known to adopt three different conformations that are called G1+, G2+, and G3+ (7). These conformers differ in the number of residues per helical turn (n) and the chirality of their helix. An antibody against G3+ (right-handed helix, n = 9) has been identified and was found to be cross-reactive toward the polynucleotide poly (A). This antibody cross-recognition is rationalized by the similarities in their helical conformations and positioning of anionic functional groups. These findings presented a potential platform for vaccine development.
Figure 1.
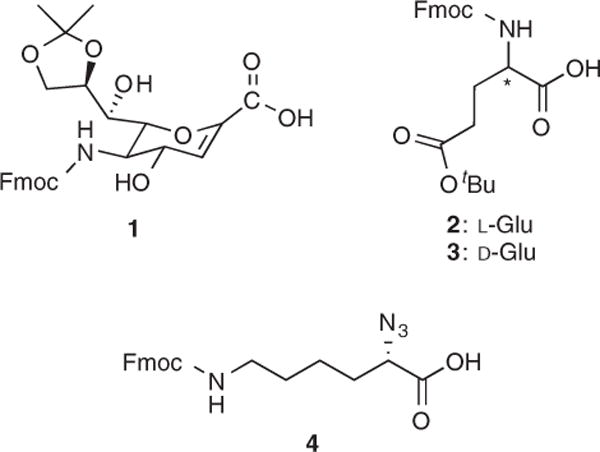
Amino acids used for solid phase peptide synthesis of chimeric α/δ-peptides.
There are inherent problems associated with using PSA as vaccine candidate. This includes the fact that PSA is a self-antigen, susceptible to enzymatic degradation, and is rapidly cleared from the blood. It was observed that over 90% of a sialic acid oligomer containing fifteen Neu5Ac units is removed from circulation within 30 min after injection into a mouse model (8). A critical vaccine design feature is to incorporate metabolically stable functionality that gives rise to stable conformational surrogates. From the four synthetic designs of our chimeric α/δ peptides (4), one compound (8) was found to assume a stable helix in aqueous medium that possesses a similar conformation and periodicity of carboxylate groups with G2+ (left-handed helix, n = 4), albeit with one-half the charge distribution. It is possible that antibodies could be elicited by peptide 8 that may be cross-reactive toward G2+ that could become a template for cancer vaccine development. As an important step toward that goal, we demonstrate in this report that peptides derived from Neu2en have remarkable human blood plasma stability.
To profile our chimeric α/δ-peptides and test their potential as pharmaceuticals, their 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetraacetic acid (DOTA) conjugates were subjected to in vitro human plasma stability studies to investigate their resistance toward proteolytic cleavage. in vitro plasma incubation closely reproduces the proteolytic activity in the blood (9) and provides a good estimation of in vivo metabolism. DOTA was conjugated at the free N-termini of peptides 5 and 8 to serve as a chelator for 111In to enable plasma stability monitoring using the radiochelate/cellulose acetate electrophoresis (CAE) method (10–13). The peptides Neu2en/D-Glu-6-DOTA (6) and L-Glu/Neu2en-6-DOTA (9) were prepared to represent two different synthetic designs (Figure 2): compound 6 contains unnatural D-Glu and is a random coil in aqueous solution while compound 9 has the natural L-Glu, reverse sequence from 6, and folds into a defined helical structure in aqueous solution (4). The azide-functionalized amino acid, Fmoc-α-azido-ε-aminocaproic acid (4), was incorporated into the constructs to serve a dual purpose: as a cap for the C-terminal amino acid to prevent fraying (14) and as a handle for future functionalization.
Figure 2.

Neu2en-derived chimeric α/δ-peptides and their DOTA and In3+-labeled conjugates.
Compounds 5 and 8 were prepared according to our established protocols (4), and DOTA was coupled to the peptides by SPPS using DOTA-NHS ester. The DOTA–peptide conjugates were cleaved from the resin using 30% TFA in CH2Cl2, precipitated using Et2O, dissolved in water, and lyophilized to give white fluffy solids of 6 and 9 in 83% and 79% yield, respectively (Supporting Information S1 and S2). The crude 6 and 9 were profiled using RP-C18 HPLC on a gradient of 5–50% MeOH in H2O with 0.1% TFA. Their chromatograms indicate that only one major compound was produced from the SPPS of these DOTA conjugates (Supporting Information S3 and S4). The peptide–DOTA conjugates 6 and 9 were characterized by Matrix Assisted Laser Desorption/Ionization-Time Of Flight (MALDI-TOF) mass spectrometry (Figure 3). These gave m/z values of 1638.5286 ([M+H]+) for 6 and 1638.5304 ([M+H]+) and 1660.5247 ([M+Na]+), which are consistent with the calculated molecular mass of 1638.6501 ([M+H]+) and 1660.6320 ([M+Na]+) for both compounds. The mass spectra of 6 and 9 showed the loss of one water molecule (m/z 1620.5261 and 1620.5321, respectively), while a loss of two water molecules (m/z 1602.5173) was also observed in 6.
Figure 3.
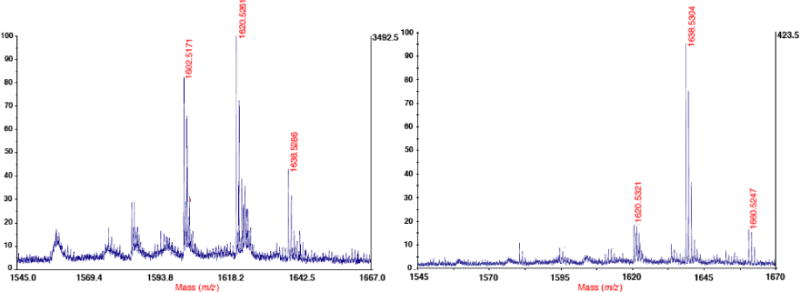
MALDI-TOF mass spectra of peptide–DOTA conjugates 6 and 9.
We have recorded the 1H NMR spectrum of 9 as additional support in the characterization of this DOTA conjugate and found it to be consistent with the structure. As compounds 6 and 9 were prepared from the same source as previously reported, fully characterized peptides (4), we deemed that further spectroscopic characterization was not necessary. Compounds 6 and 9 were tested for plasma stability using our established and validated protocols for monitoring protein/peptide stability (11–13,15). Radiometal labeling of peptide–DOTA conjugates was carried out using 111InCl3 in an aqueous ammonium citrate solution at pH 5.0–5.5. The reaction mixture was incubated at 37 °C for 6 and at 70 °C for 9 for 15 min; the excess radiometal sequestered by the addition of EDTA, and the peptide–DOTA–111In chelate was isolated by gel centrifugation using Sephadex G-50.
The higher incubation temperature for 9 was used because 111In labeling did not proceed at 37 °C. Heating to effect DOTA radiolabeling is not unusual as it is known that the DOTA–M3+ complex formation rate is typically slow (16), and temperatures as high as 80–95 °C have been used to speed up the chelation process and achieve high labeling efficiency (17–19). What is more significant in this observation is the distinct difference in the thermal requirements between 6 and 9, which provides insight into how these molecules behave in aqueous solution. One possible explanation for their differential chelation rates may be conformational. Compound 6 is known to exist as a random coil (4) in which case the DOTA conjugated at the N-terminus is likely to be freely exposed for easier 111In coordination. Compound 9 forms a right-handed helix (4) which may render the tethered DOTA less accessible requiring heating to partially unwind the helix to give access for the radiometal to coordinate. Another possible explanation is the nature of the amino acid that is directly bonded to the chelator. In compound 6, DOTA is amide linked to an acyclic D-Glu while in 9, it is connected to the amine at C-5 of the N-terminal Neu2en. The conjugation to Neu2en of 8 renders the DOTA vicinal to the sugar pyran ring and the glycerol side chain at C-6, which is visualized in the proposed model (Figure 4) obtained from the minimum energy structures of 9. A similar conformation of DOTA was observed in the X-ray crystal structure of In-DOTA-p-aminoanilide wherein the aromatic ring of aniline is positioned on the same face as the chelating functional groups (20). The pyran ring and the side chain could potentially reduce the accessibility of DOTA for metal coordination. The observed slow radiolabeling of 9 at low temperature could be a consequence of both the peptide conformation and the nature of the N-terminal amino acid.
Figure 4.

View of a truncated model of the possible conformation of DOTA at the N-terminal Neu2en of 9 showing the proximal ring and glycerol side chain of Neu2en. (C-atoms colored in green, N-atoms colored in blue, and O atoms colored in red; residues 2–7 were truncated, and all H-atoms were omitted for clarity).
The radiolabeled compounds 7 and 10 were incubated in human blood plasma at 37 °C for up to 72 h. Samples (10 μL) were drawn at 0, 4, 24, and 72 h, analyzed by CAE, and followed by band radioactivity visualization using a strip scanner (11,13). Figures 5 and 6 show the plots of the scanned electropherograms of 7 and 10, respectively, as a function of distance of the band from origin (x-axis) versus intensity of radioactivity (y-axis). These plots show that 59% and 88% of 6 and 9, respectively, remained intact after 72 h of incubation in plasma. An illustrative comparison of the orientations of poly-L-Ala helical segment of T4 lysozyme (pdb: 1l64) and 7 and 10 (Figure 7) on the enzyme active site following Schechter and Berger’s nomenclature (21) shows significant spatial and linear differences. The side chains of peptides 7 and 10 do not perfectly fit in the enzyme subsites. These findings are consistent with Schechter and Berger’s model (21) that requires appropriate groove fitting, side chain orientation, and hydrophobic, electrostatic, and/or H-bonding interactions for effective binding and enzymatic bond cleavage. Furthermore, the pitch of 10 is 11.8 Å, a value that is more than double from those observed in natural L-α-peptides. Based on these structural differences, it is believed that the chimeric α/δ-peptides do not fit in the enzyme active sites and the required mediation by side chain functionalities does not occur, hence their resistance toward degradation. These structural differences with natural α-peptides were also used to explain the stability of Seebach’s unnatural β- and γ-peptides against peptidases (22).
Figure 5.

Plot of the scanned electropherograms of 7 at 0 h = blue, 4 h = green, 24 h = pink, and 72 h = brown; the plot in cyan is 7 in PBS buffer without plasma.
Figure 6.

Plot of the scanned electropherograms of 10 at 0 h = blue, 4 h = green, 24 h = pink, and 72 h = cyan.
Figure 7.

An illustrative comparison of the orientations of poly-L-Ala helical segment of T4 lysozyme (pdb: 1l64) and 7 and 10 on the enzyme active site following Schechter and Berger’s nomenclature showing that the side chains of peptides 7 and 10 do not perfectly fit in the enzyme subsites.
The plasma half-life of each radiolabeled conjugate was calculated by the least squares analysis of the integration peaks versus time from Figures 4 and 5 following pseudo-first-order kinetics (23,24). Figure 8 shows that 7 has t1/2 = 98 h while 10 has t1/2 = 388 h. These results indicate that peptides 7 and 10 survive the cocktail of proteolytic enzymes found in blood plasma (2) and are two- to three-orders of magnitude more stable than natural a-peptides. The blood plasma stability of 7 and 10 is significantly longer than what has been observed in vivo in mice for PSA (8) and for hybrid α/β-peptide mimics of Bcl-xL with t1/2 ranging from approximately 9 to >1200 min in isolated enzymes and 820 to >2200 min in 50% fetal bovine serum (25). Over the past decade, investigations into the proteolytic resistance of unnatural peptides have typically employed isolated enzymes (22,25–29) or fetal bovine serum (25), and only one very recent report utilized human serum (30). The study reported herein is the first account of human blood plasma stability of unnatural, chimeric peptides.
Figure 8.
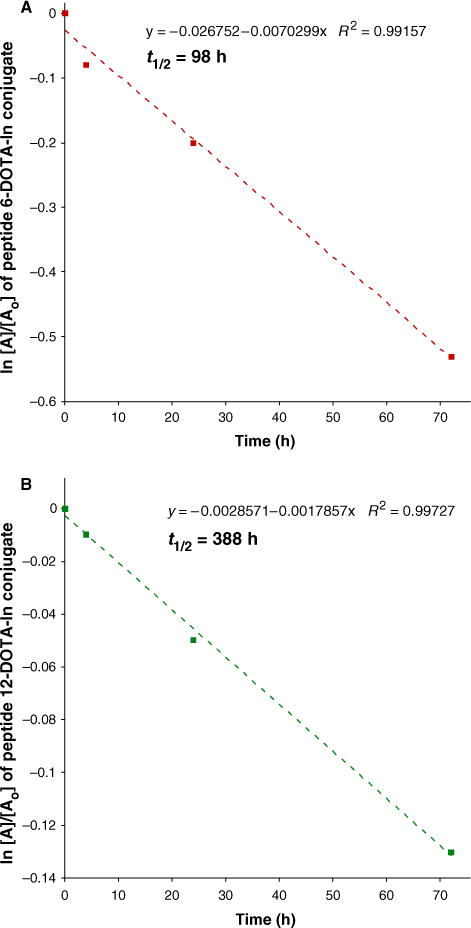
Pseudo first order rate plots of the stability profiles of (A) 7 and (B) 10 in human blood plasma showing their long t1/2 of 98 h and 388 h, respectively.
This study reports the conjugation of DOTA to chimeric α/δ-hybrid peptides by SPPS, radiometal labeling, and human plasma stability studies of 7 and 10. The conjugates showed different thermal requirements for effective radiolabeling, which is correlated with the differences in peptide sequence and conformational stability in aqueous solution. Both peptides were shown to be human plasma stable with t1/2 of 98 and 388 h for 7 and 10, respectively. The long plasma half-life is two- to three-orders of magnitude higher than natural α-peptides, confirming our hypothesis that these chimeric peptides may show favorable bioavailability profiles. The findings in this study further establish the potential of 8 as a structural surrogate for PSA. Furthermore, this is the first account of conjugation of DOTA to the δ-amino of sialic acid or its derivative, the synthesis of sialic acid-based peptide–DOTA conjugate, and the investigation into plasma stability of this novel class of compounds. Our future plans include the determination of the immunogenic properties of this PSA surrogate.
Supplementary Material
Figure S1. SPPS of 6 (Neu2en/D-Glu-6-DOTA conjugate).
Figure S2. SPPS of 9 (L-Glu/Neu2en-6-DOTA conjugate).
Figure S3. Reverse phase HPLC chromatogram of crude 6.
Figure S4. Reverse phase HPLC chromatogram of crude 9.
Acknowledgments
M. Siebert is acknowledged for his help in molecular modeling. The coordinates for In-DOTA-3AM-A was obtained through the generosity of Prof. M. Colvin (UC Merced). This research was supported by the National Science Foundation (Grant CHE-0518010).
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Manning MC, Patel K, Borchardt RT. Stability of protein pharmacauticals. Pharm Res. 1989;6:903–918. doi: 10.1023/a:1015929109894. [DOI] [PubMed] [Google Scholar]
- 2.Werle M, Bernkop-Schnurch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30:351–367. doi: 10.1007/s00726-005-0289-3. [DOI] [PubMed] [Google Scholar]
- 3.Adessi C, Soto C. Converting a peptide into a drug: strategies to improve stability and bioavailability. Curr Med Chem. 2002;9:963–978. doi: 10.2174/0929867024606731. [DOI] [PubMed] [Google Scholar]
- 4.Saludes JP, Ames JB, Gervay-Hague J. Synthesis and structural characterization of sialic acid-glutamic acid hybrid foldamers as conformational surrogates of alpha-2,8-linked polysialic acid. J Am Chem Soc. 2009;131:5495–5505. doi: 10.1021/ja808286x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakata D, Troy FA. Degree of polymerization (DP) of polysialic acid (PolySia) on neural cell adhesion molecules (N-CAMs) – Development and application of a new strategy to accurately determine the DP of polySia chains on N-CAMs. J Biol Chem. 2005;280:38305–38316. doi: 10.1074/jbc.M508762200. [DOI] [PubMed] [Google Scholar]
- 6.Ledermann JA, Pasini F, Olabiran Y, Pelosi G. Detection of the neural cell-adhesion molecule (NCAM) in serum of patients with small-cell lung cancer (SCLC) with limited or extensive disease, and bone marrow infiltration. Int J Cancer Suppl. 1994;8:49–52. doi: 10.1002/ijc.2910570710. [DOI] [PubMed] [Google Scholar]
- 7.Brisson JR, Baumann H, Imberty A, Perez S, Jennings HJ. Helical epitope of the group-B meningococcal alpha(2–8)-linked sialic-acid polysaccharide. Biochemistry. 1992;31:4996–5004. doi: 10.1021/bi00136a012. [DOI] [PubMed] [Google Scholar]
- 8.Gregoriadis G, McCormack B, Wang Z, Lifely R. Polysialic acids – potential in drug delivery. FEBS Lett. 1993;315:271–276. doi: 10.1016/0014-5793(93)81177-2. [DOI] [PubMed] [Google Scholar]
- 9.Falciani C, Lozzi L, Pini A, Corti F, Fabbrini M, Bernini A, Lelli B, Niccolai N, Bracci L. Molecular basis of branched peptides resistance to enzyme proteolysis. Chem Biol Drug Des. 2007;69:216–221. doi: 10.1111/j.1747-0285.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Meares CF. Synthesis, metal chelate stability studies, and enzyme digestion of a peptide-linked DOTA derivative and its corresponding radiolabeled immunoconjugates. Bioconjug Chem. 1993;4:275–283. doi: 10.1021/bc00022a005. [DOI] [PubMed] [Google Scholar]
- 11.Cole WC, DeNardo SJ, Meares CF, McCall MJ, DeNardo GL, Epstein AL, et al. Comparative serum stability of radiochelates for antibody radiopharmaceuticals. J Nucl Med. 1987;28:83–90. [PubMed] [Google Scholar]
- 12.Li M, Meares CF, Zhong GR, Miers L, Xiong CY, DeNardo SJ. Labeling monoclonal-antibodies with Yttrium-90-DOTA and Indium-111-DOTA chelates – a simple and efficient method. Bioconjug Chem. 1994;5:101–104. doi: 10.1021/bc00026a001. [DOI] [PubMed] [Google Scholar]
- 13.DeNardo SJ, Peng JSB, DeNardo GL, Mills SL, Epstein AL. Immunochemical aspects of monoclonal-antibodies important for radiopharmaceutical development. Nucl Med Biol. 1986;13:303–310. doi: 10.1016/0883-2897(86)90002-4. [DOI] [PubMed] [Google Scholar]
- 14.Gregar TQ, Gervay-Hague J. Synthesis of oligomers derived from amide-linked neuraminic acid analogues. J Org Chem. 2004;69:1001–1009. doi: 10.1021/jo035312+. [DOI] [PubMed] [Google Scholar]
- 15.Natarajan A, Kumaresan PR, DeNardo SJ, DeNardo GL, Mi-rick G, Lam KS. Development of TNKase specific cleavable peptide linked radioimmunoconjugates for radioimmunotherapy. Bioorg Med Chem Lett. 2008;18:4802–4805. doi: 10.1016/j.bmcl.2008.07.097. [DOI] [PubMed] [Google Scholar]
- 16.De Leon-Rodriguez LM, Kovacs Z. The synthesis and chelation chemistry of DOTA-peptide conjugates. Bioconjug Chem. 2008;19:391–402. doi: 10.1021/bc700328s. [DOI] [PubMed] [Google Scholar]
- 17.Cooper MS, Sabbah E, Mather SJ. Conjugation of che-lating agents to proteins and radiolabeling with trivalent metallic isotopes. Nat Protoc. 2006;1:314–317. doi: 10.1038/nprot.2006.49. [DOI] [PubMed] [Google Scholar]
- 18.Wild D, Schmitt JS, Ginj M, Macke HR, Bernard BF, Krenning E, et al. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging. 2003;30:1338–1347. doi: 10.1007/s00259-003-1255-5. [DOI] [PubMed] [Google Scholar]
- 19.Sosabowski JK, Mather SJ. Conjugation of DOTA-like chelating agents to peptides and radiolabeling with trivalent metallic isotopes. Nat Protoc. 2006;1:972–976. doi: 10.1038/nprot.2006.175. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, He ZJ, Hsieh WY, Fanwick PE. Synthesis, characterization, and X-ray crystal structure of In(DOTA-AA) (AA = p-aminoanilide): a model for In-111-labeled DOTA-biomolecule conjugates. Inorg Chem. 2003;42:8831–8837. doi: 10.1021/ic0349914. [DOI] [PubMed] [Google Scholar]
- 21.Schechter I, Berger A. On size of active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 22.Frackenpohl J, Arvidsson PI, Schreiber JV, Seebach D. The outstanding biological stability of beta- and gamma-peptides toward proteolytic enzymes: an in vitro investigation with fifteen peptidases. Chembiochem. 2001;2:445–455. doi: 10.1002/1439-7633(20010601)2:6<445::aid-cbic445>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Powell MF, Stewart T, Otvos L, Urge L, Gaeta FCA, Sette A, et al. Peptide stability in drug development.2. Effect of single amino acid substitution and glycosylation on peptide reactivity in human serum. Pharm Res. 1993;10:1268–1273. doi: 10.1023/a:1018953309913. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes AI, Gregoriadis G. Polysialylated asparagin-ase: preparation, activity and pharmacokinetics. Biochim Biophys Acta. 1997;1341:26–34. doi: 10.1016/s0167-4838(97)00056-3. [DOI] [PubMed] [Google Scholar]
- 25.Sadowsky JD, Murray JK, Tomita Y, Gellman SH. Exploration of backbone space in foldamers containing alpha-and beta-amino acid residues: developing protease-resistant oligomers that bind tightly to the BH3-recognition cleft of Bcl-x(L) Chembiochem. 2007;8:903–916. doi: 10.1002/cbic.200600546. [DOI] [PubMed] [Google Scholar]
- 26.Disney MD, Hook DF, Namoto K, Seeberger PH, Seebach D. N-linked glycosylated beta-peptides are resistant to degradation by glycoamidase A. Chem Biodivers. 2005;2:1624–1634. doi: 10.1002/cbdv.200590132. [DOI] [PubMed] [Google Scholar]
- 27.Seebach D, Abele S, Schreiber JV, Martinoni B, Nussbaum AK, Schild H, et al. Biological and pharmacokinetic studies with beta-peptides. Chimia. 1998;52:734–739. [Google Scholar]
- 28.Hook DF, Bindschadler P, Mahajan YR, Sebesta R, Kast P, Seebach D. The proteolytic stability of ‘designed’ beta-peptides containing alpha-peptide-bond mimics and of mixed alpha,beta-peptides: application to the construction of MHC-binding peptides. Chem Biodivers. 2005;2:591–632. doi: 10.1002/cbdv.200590039. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt MA, Weisblum B, Gellman SH. Interplay among folding, sequence, and lipophilicity in the antibacterial and hemolytic activities of alpha/beta-peptides. J Am Chem Soc. 2007;129:417–428. doi: 10.1021/ja0666553. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed S, Kaur K. The proteolytic stability and cytotoxicity studies of L-aspartic acid and L-diaminopropionic acid derived beta-peptides and a mixed alpha/beta-peptide. Chem Biol Drug Des. 2009;73:545–552. doi: 10.1111/j.1747-0285.2009.00803.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. SPPS of 6 (Neu2en/D-Glu-6-DOTA conjugate).
Figure S2. SPPS of 9 (L-Glu/Neu2en-6-DOTA conjugate).
Figure S3. Reverse phase HPLC chromatogram of crude 6.
Figure S4. Reverse phase HPLC chromatogram of crude 9.


