Abstract
Background
The incidence of diverticulitis has been associated with geographic and seasonal variation. Low circulating vitamin D is associated with diverticulitis. We investigated the association between ultraviolet (UV) light, and diverticulitis.
Methods
We identified non-elective diverticulitis admissions in the Nationwide Inpatient Sample and linked hospital locations to UV data. We examined UV exposure in relation to risk of admission for diverticulitis.
Results
We identified geographic and seasonal trends among 226,522 non-elective admissions for diverticulitis. Compared to high UV areas, low UV areas had a higher rate of diverticulitis (751 versus 668 per 100,000 admissions, p <0.0001), diverticular abscess (12.0 versus 9.7%, p <0.0001), and colectomy (13.5% versus 11.5%, p<0.0001). We also observed significant seasonal variation with a lower rate of diverticulitis in the winter months (645 per 100,000) compared with summer (748 per 100,000), (p <0.0001). The summer increase was more evident in areas with the greatest UV fluctuation versus areas with the least UV fluctuation (120 versus 70 per 100,000, p =0.01).
Conclusions
Low UV is associated with an increased rate of diverticulitis admissions and greater seasonal variation. Because UV exposure largely determines vitamin D status, these findings support a role for vitamin D in the pathogenesis of diverticulitis.
Keywords: Diverticulitis, ultraviolet light, vitamin D, epidemiology
Introduction
Diverticulitis is a common disease with an incompletely understood pathophysiology. Diverticulitis results in 200,000 hospital admissions a year [1] and its sequelae generate substantial morbidity. Diverticulosis is ubiquitous in older American adults [2], but the trigger that leads to diverticulitis is unknown.
Geographic [3] and seasonal [4] variation in diverticulitis has been previously shown, with higher incidence in Western countries and in the summertime . Recent studies have questioned traditional theoretical triggers for diverticulitis, such as nut and seed impaction in diverticula,[5] and demonstrated novel associations with obesity [6], physical activity [7], and NSAID use [8].
Recently, we observed an association between low vitamin D levels and the development of diverticulitis [9]. Vitamin D synthesis depends on ultraviolet (UV) light, which varies according to latitude, with greater annual fluctuation occurring farther from the equator. Associations exist between UV exposure, vitamin D, and several diseases. In the colon, low levels of vitamin D are associated with Crohn's disease [10], colorectal cancer [11], and decreased cancer-specific survival [12]. Residence in higher latitudes is associated with inflammatory bowel disease [13]. In this study, we investigate a potential link between UV exposure as a determinant of vitamin D levels and diverticulitis.
Methods
We identified non-elective admissions for diverticulitis in the Nationwide Inpatient Sample (NIS), 2001-2005. We included patients >18 years old with a non-elective admission associated with a billing code for diverticulitis (ICD-9 562.11 or 562.13). Among those with diverticulitis we identified those with intra-abdominal abscess (569.5 or 567.2×) or requiring left-sided colorectal resection (45.75, 45.76, 48.62, 48.63). We also identified a cohort of patients with diverticular hemorrhage (562.12)
We linked NIS hospital zip codes to vitamin D action spectrum UV irradiance to estimate the UV exposure experienced by an individual in the year prior to admission. We used a uniform measure of sun exposure based on the estimated number of minutes needed to achieve the UV equivalent of a 1000IU oral dose of vitamin D (25-hydroxyvitamin D). This measurement has been previously described [14] and was calculated using spectrophotometer-validated data. We categorized this measure into quartiles based on exposure times for Fitzpatrick skin type I [15]. Time needed to achieve this dose in summer is nearly uniform across the United States: 2.7-5.7 minutes. Variation is greatest in winter, 6.7-103 minutes. Therefore, locations with the lowest winter irradiance experience the greatest seasonal fluctuation. Univariable analysis was performed with two tailed t-tests. We used multivariate logistic regression to estimate the influence of season and UV exposure on admission for diverticulitis adjusting for age, gender, race, and hospital location. Statistical analysis performed in SAS Software, version 9.2.
Results
From 2001 to 2005, we identified 32.4 million adult inpatient admissions within the National Inpatient Sample. Of these, 226,522 (0.7%), were non-elective admissions with a code for diverticulitis, yielding an overall rate of 699.8 per 100,000 total admissions. Baseline characteristics were similar between patients admitted to hospitals within different UV quartiles (eTable One). We observed higher rates of diverticulitis hospitalization among white patients, patients over age 60, and rural Americans (Table One).
Table 1.
Rate of non-elective admission for diverticulitis, 2001-2005
| Rate of Admissiona | ORb | 95% CIb | p-valueb | |
|---|---|---|---|---|
| Sex | ||||
| Female | 689.7 (80.2) | 1 | ||
| Male | 709.5 (75.1) | 1.02 | 1.01-1.03 | <0.001 |
| Race | ||||
| Black | 428.2 (102.3) | 1 | ||
| White | 809.6 (64.6) | 1.73 | 1.70-1.76 | <.001 |
| Age | ||||
| <60 years | 522.4 (82.9) | 1 | ||
| ≥ 60 years | 883.2 (83.4) | 1.51 | 1.49-1.52 | <0.001 |
| UV Quartile | ||||
| UV1 | 751.8 (66.7) | 0.94 | 0.92-0.95 | 0.05 |
| UV2 | 746.8 (56.1) | 0.82 | 0.81-0.83 | <0.001 |
| UV3 | 651.8(42.4) | 1.04 | 1.03-1.06 | <0.001 |
| UV4 | 668.1 (36.9) | 1.0 | ||
| Hospital Location | ||||
| Rural | 929.7 (119.5) | 1 | ||
| Urban | 644.1 (92.8) | 0.77 | 0.75-0.78 | <0.001 |
Rate of non-elective admission for diverticulitis per 100,000 total adult admissions. Values are mean (standard deviation).
Ajusted for age, gender, race, hospital location, quartiles of UV exposure, and admission year. As many states in the NIS do not report race or report it inconsistently, only patients coded as white or black were included in the multivariate analysis.
Rates of diverticulitis admissions were significantly higher in areas of low UV light (UV1) compared with areas of high UV light (UV4) (751.8 versus 668.1/100K admissions, p <0.0001). In subgroup analysis, this association was especially evident in patients under age 60 (n=88,477) (eTable Two). Patients <60 in UV1 had 106 greater diverticulitis admissions per 100,000 total admissions compared to those in UV4. In contrast, patients over 60 in UV1 had only 37 greater diverticulitis admissions per 100,000 total admissions compared those in UV4 (pheterogeneity <0.0001).
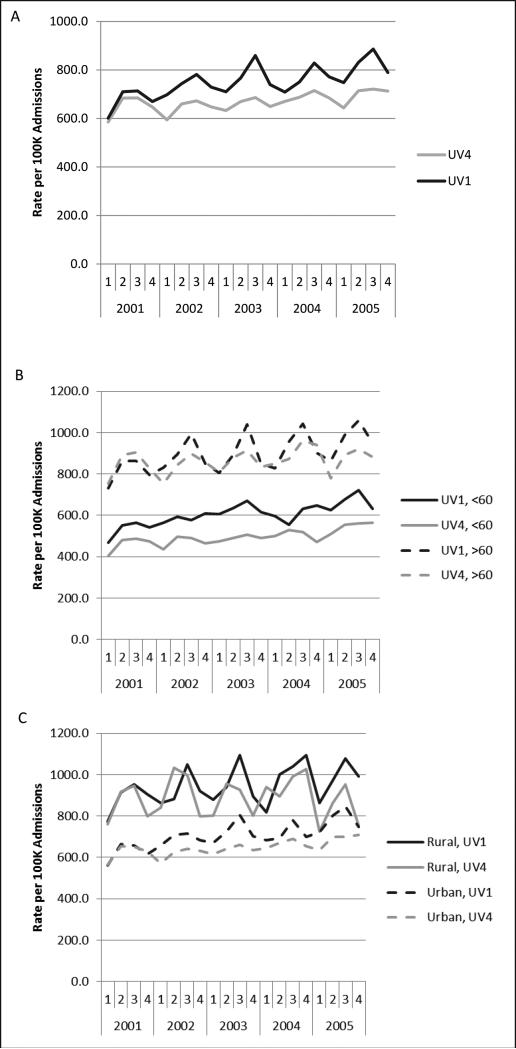
We also observed that rates of diverticulitis were highest in the third discharge quarter (June, July, August) and lowest in the first (January, February, March) (eTable Three). The incidence of diverticulitis increased about 16% in the third quarter. Although seasonal variation was observed in all UV groups, we observed significantly greater variation in areas with lower winter UV irradiance, and therefore greater annual fluctuation (eTable Three, Figure 1A). Admissions rose 17% in the summer in UV1 (darkest) areas versus 11% in UV4 (sunniest) areas, p = 0.01. Compared with individuals in UV1, the multivariate OR for admission was 0.94 (95% CI, 0.92-0.95) in UV4 and 0.82 (95% CI, 0.81-083) in UV3. Test of interaction between discharge quarter and UV exposure quartile demonstrated a greater effect of season on admissions in UV1 as compared to UV4 (p = 0.047).
Figure One.
Incidence of non-elective diverticulitis hospitalizations by discharge year and quarter for sunniest (UV4) and darkest (UV1) areas. A: All patients. B: Patients older than 60 versus patients younger than 60 years. C: Rural versus urban patients
Certain subgroups had greater seasonal variation. White patients, patients over 60 years old, and rural patients demonstrated significantly higher rates of admission in the summer compared to their respective referent groups (eTable Three, Figures 1). Admissions among patients over age 60 increased 20.7% in the summer as compared to 13.3% among patients <60 (p <0.0001).
Rates of abscess and surgery among diverticulitis patients were modestly but significantly higher in low UV areas. The incidence of diverticular abscess was significantly higher in UV1 (12.0%) compared to UV4 (9.7%), p <0.0001. Similarly, rates of surgery were significantly higher in UV1 (13.5%) compared to UV4 (11.5%), p<0.0001. We did not find seasonal variation in rates of surgery or abscess. No significant difference was found in diverticular hemorrhage admissions between UV1and UV4 (282.0 versus 277.6 per 100,000, p =0.80) and no seasonal variation was appreciated.
On multivariate analysis, increasing age, female gender, white race, and rural residence were significantly associated with admission for diverticulitis (eTable Three). Compared to the third discharge quarter, all other quarters were inversely associated with admission (OR 0.87, 95% CI 0.84-0.89 for first quarter).
Discussion
A large national database linked with detailed UV data reveals significantly higher overall rates and greater seasonal variation in diverticulitis admissions in areas with low winter vitamin D spectrum UV irradiance. Multivariate analysis supports greater influence of season in low UV areas, with a significant summer increase in admissions. Taken together, these data suggest that greater seasonal flux in UV irradiance is correlated with greater seasonal variation in diverticulitis admissions.
Previously, we have shown that low serum vitamin D levels are associated with subsequent diverticulitis. The underlying mechanism between UV irradiance and diverticulitis may be variation in vitamin D. In animal models, vitamin D deficiency worsens colitis [16] and Salmonella infection [17]. Vitamin D has been shown to reduce pro-inflammatory cytokines [18,19] and deficiency is associated with inflammation and neoplasia. Thus, the low levels of vitamin D may explain the increased rate of diverticulitis admissions, abscess, and colectomy observed in darker areas.
Our observed association between higher admission rates in summer months may appear inconsistent with the vitamin D hypothesis. However, an association between vitamin D deficiency and diverticulitis may require a prolonged latency. The association between low vitamin D and diverticulitis may occur several months prior to clinical presentation. A similar pattern of low winter vitamin D levels followed by summer peaks of disease has been described for tuberculosis [20].
We observed that there was greater geographic yet lower seasonal variation among younger patients. This may suggest that overall vitamin D deficiency is more important in younger patients, while flux has a greater impact on older patients. Seasonal variation was more pronounced among white than among black patients, which could reflect greater annual fluctuation in vitamin D levels among white patients or may reflect differences in genetics, dietary patterns, or lifestyle habits that may mitigate the influence UV light exposure on vitamin D levels [21]. We also observed greater overall rates of admission and seasonality among rural hospitals . This may reflect greater variation in circulating vitamin D exposure in rural areas due to more time spent outdoors .
Alternative explanations for the seasonal and geographic variation seen in this study could include an infectious etiology. Variation in the prevalence of diverticulosis could contribute to variation in diverticulitis, although we did not obsevere similar seasonal or geographic variation in diverticular hemorrhage. Variation in diet, or other factors associated with diverticulitis, such as obesity, NSAID use, and physical activity could also potentially contribute to the observed trends. We controlled for race and age in our multivariate model, but other population-level differences could also influence our results.
The strengths of this study include use of a large national database, linkages with detailed UV irradiance data, and use of multivariate analyses to examine the interaction between season, geography, and diverticulitis. There are several limitations to this study. First, billing codes may misclassify some diverticulitis patients. However, this proportion is unlikely to vary with geography or season. Second, our analysis only included inpatients excluding the diverticulitis cases managed in the emergency room or outpatient setting. Third, important individual-level variables such as diet, supplement use, lifestyle habits such as sun protection, and socioeconomic status are not well-captured by the NIS.
In summary, our study demonstrates that low winter UV is associated with higher rates of diverticulitis, and that areas with greater annual UV fluctuation have larger seasonal variation. Because UV irradiance is associated with vitamin D levels, this study supports the hypothesis that low vitamin D levels and greater seasonal fluctuation in vitamin D may contribute to the pathophysiology of diverticulitis.
Supplementary Material
Acknowledgement
Funding acknowledgements:
This work was supported by a Clinical Research Award from the American College of Gastroenterology and K24 DK 098311. ATC is a Damon Runyon Clinical Investigator. The funding organizations had no input into the design or content of the study or manuscript.
Footnotes
Conflict of interest disclosure:
LHM: None.
MS: None.
LS: None
EG: None
A.T.C. previously served as a consultant to Bayer Healthcare, Millennium Pharmaceuticals, Pfizer Inc. and Pozen Inc. for work unrelated to the topic of this manuscript.
Author Contributions
Design and conduct of the study: Maguire, Giovannucci, Strate, Chan
Collection and management of data: Maguire, Song
Analysis and interpretation of the data: Maguire, Song, Giovannucci, Strate, Chan
Preparation of manuscript: Maguire, Song, Chan
Review and approval of the manuscript: Maguire, Song, Giovannucci, Strate, Chan
Dr. Chan had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation: Lecture presentation, Digestive Diseases Week, Chicago, IL, May 5/2-5/6/2014
References
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012 Nov. 143(5):1179–1187. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almy TP, Howell DA. Medical progress. Diverticular disease of the colon. N Engl J Med. 1980 Feb 7;302(6):324–31. doi: 10.1056/NEJM198002073020605. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen GC, Sam J, Anand N. Epidemiological trends and geographic variation in hospital admissions for diverticulitis in the United States. World J Gastroenterol. 2011 Mar 28;17(12):1600–5. doi: 10.3748/wjg.v17.i12.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricciardi R, Roberts PL, Read TE, et al. Cyclical increase in diverticulitis during the summer months. Arch Surg. 2011 Mar;146(3):319–23. doi: 10.1001/archsurg.2011.27. [DOI] [PubMed] [Google Scholar]
- 5.Strate LL, Liu YL, Syngal S, Aldoori WH, Giovannucci EL. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008 Aug 27;300(8):907–1. doi: 10.1001/jama.300.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strate LL, Liu YL, Aldoori WH, Syngal S, Giovannucci EL. Obesity increases the risks of diverticulitis and diverticular bleeding. Gastroenterology. 2009 Jan;136(1):115–122. doi: 10.1053/j.gastro.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strate LL, Liu YL, Aldoori WH, Giovannucci EL. Physical activity decreases diverticular complications. Am J Gastroenterol. 2009 May;104(5):1221–30. doi: 10.1038/ajg.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strate LL, Liu YL, Huang ES, Giovannucci EL, Chan AT. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011 May;140(5):1427–33. doi: 10.1053/j.gastro.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maguire LH, Song M, Strate LE, Giovannucci EL, Chan AT. Higher Serum Levels of Vitamin D are Associated with Reduced Risk of Diverticulitis. Clin Gastroenterol Hepatol. 2013 Dec;11(12):1631–5. doi: 10.1016/j.cgh.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology. 2012 Mar;142(3):482–9. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians' Health Study and a meta-analysis of prospective studies. Cancer Prev Res. 2011 May;4(5):735–43. doi: 10.1158/1940-6207.CAPR-10-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedirko V, Riboli E, Tjønneland A, et al. Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in western European populations. Cancer Epidemiol Biomarkers Prev. 2012 Apr;21(4):582–93. doi: 10.1158/1055-9965.EPI-11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalili H, Huang ES, Ananthakrishnan AN, et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012 Dec;61(12):1686–92. doi: 10.1136/gutjnl-2011-301574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fioletov VE, McArthur LJ, Mathews TW, Marrett L. Estimated ultraviolet exposure levels for a sufficient vitamin D status in North America. J Photochem Photobiol. 2010 Aug 2;100(2):57–66. doi: 10.1016/j.jphotobiol.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988 Jun;124(6):869–71. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Zhang H, Wu H, et al. Protective role of 1,25(OH)2vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012 May 30;12:57. doi: 10.1186/1471-230X-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu S, Liao AP, Xia Y, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010 Aug;177(2):686–97. doi: 10.2353/ajpath.2010.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006 Apr;83(4):754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012 Mar 1;188(5):2127–35. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh GC, Hawthorne G, Turner AM, Kunst H, Dedicoat M. Tuberculosis incidence correlates with sunshine: an ecological 28-year time series study. PLoS One. 2013;8(3):e57752. doi: 10.1371/journal.pone.0057752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013 Nov 21;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.