Abstract
Background
The Health and Safety Practices Survey of Healthcare Workers describes current practices used to minimize chemical exposures and barriers to using recommended personal protective equipment for the following: antineoplastic drugs, anesthetic gases, high level disinfectants, surgical smoke, aerosolized medications (pentamidine, ribavirin, and antibiotics), and chemical sterilants.
Methods
Twenty-one healthcare professional practice organizations collaborated with NIOSH to develop and implement the web-based survey.
Results
Twelve thousand twenty-eight respondents included professional, technical, and support occupations which routinely come in contact with the targeted hazardous chemicals. Chemical-specific safe handling training was lowest for aerosolized antibiotics (52%, n = 316), and surgical smoke (57%, n = 4,747). Reported employer procedures for minimizing exposure was lowest for surgical smoke (32%, n = 4,746) and anesthetic gases (56%, n = 3,604).
Conclusions
Training and having procedures in place to minimize exposure to these chemicals is one indication of employer and worker safety awareness. Safe handling practices for use of these chemicals will be reported in subsequent papers.
Keywords: web-based survey, cognitive testing, healthcare worker, training, employer safe handling procedures, self-report, professional practice organization
INTRODUCTION
Healthcare workers face a number of serious safety and health hazards on the job. In 2012, nonfatal injuries and illnesses in the Healthcare and Social Assistance (HCSA) industry sector accounted for one out of five incidents surpassing all other private industry sectors [BLS, 2013a]. Ergonomic hazards, same level falls, and workplace violence are major contributors to the high rate. Oftentimes overshadowed, chemical hazards also pose an established risk to the health of healthcare workers [McDiarmid, 2006; Condon et al., 2009; McDiarmid and Leone, 2009]. These include antineoplastic drugs for treating cancer, aerosolized medications used in respiratory therapy, high level disinfectants for reusable medical and dental devices, anesthetic gases, surgical smoke, chemical sterilants used in cold sterilization of medical equipment and supplies, chemicals for cleaning and disinfecting of hard non-porous surfaces, and laboratory chemicals [McDiarmid et al., 1993; Winstin, 1994; Rosenman et al., 2003; Rideout et al., 2005; NIOSH, 2007, 2012; OSHA, 2008, 2013; Condon et al., 2009; McDiarmid and Leone, 2009; Connor et al., 2010]. Because limited information is available on safe handling practices associated with the use of hazardous chemicals, coupled with the fact that the HCSA sector represents over 13% of the workforce with the largest projected growth of any industry sector [BLS, 2012, 2013b], the National Institute for Occupational Safety and Health (NIOSH) conducted the Health and Safety Practices Survey of Healthcare Workers. This hazard surveillance survey provides information on the extent and circumstances under which healthcare workers may be exposed to chemical agents. The survey presents a cross-section of current practices for reducing potential exposures and fills gaps in current knowledge about those practices which may guide interventions and future research. The survey focused on selected classes of chemical agents including antineoplastic drugs, anesthetic gases, aerosolized medications, chemical sterilants, high level disinfectants, and surgical smoke. Chemicals used by housekeeping/janitorial services workers to clean and disinfect hard surfaces and those used by laboratory workers were not included because we were unable to identify organizations through which we could contact these workers. Individual hazard modules were developed for each of the chemical hazards included in the survey. Hazard modules included questions on hazard-specific training, availability of facility specific safe handling guidelines, frequency and duration of chemical use, adherence to recommended safe handling guidelines, use of engineering controls and personal protective equipment (PPE), barriers to using PPE, and exposure monitoring and medical surveillance (if applicable). In addition to the hazard modules, a core module addressed cross-cutting issues and included demographic, occupation and employer characteristics.
This article describes methods used to develop and implement the web-based survey of healthcare workers. In addition, results are presented on training received in the safe use of the respective chemicals and whether the employer had procedures in place for minimizing exposure. Findings for each hazard module and core module will be presented separately.
MATERIALS AND METHODS
Survey Instrument Development
A public meeting with healthcare stakeholders representing professional practice organizations, industry, labor, and government was convened to seek comments on the content and conduct of the survey. These and other comments resulted in substantial revisions to the survey instrument. The revised instrument was subsequently reviewed by subject matter experts, including representatives of each of the participating professional practice organizations, and was further revised. The survey included seven hazard modules and a core module in addition to a screening module (Table I). The survey instrument underwent cognitive testing as well as pilot testing of the web instrument. These are described below.
TABLE I.
Specific Chemical Agents/Hazards by Module
| Module | Specific chemical agents/hazards/issues |
|---|---|
| Aerosolized medicationsa | Antibiotics amikacin, colistin and tobramycin; pentamidine; ribavirin |
| Antineoplastic drugs (compound) | Numerous specific chemotherapeutic agents |
| Antineoplastic drugs (administer) | Numerous specific chemotherapeutic agents |
| Chemical sterilants | Ethylene oxide, hydrogen peroxide gas plasma |
| High level disinfectants | Glutaraldehyde, orthophthaldehyde, peracetic acid, hydrogen peroxide |
| Surgical smokeb | Surgical smoke (generated by laser surgery or electrosurgeryc techniques) |
| Anesthetic gases | Desflurane, enflurane, halothane, sevoflurane, nitrous oxide |
| Core | Job-specificchemical, biological, physical agents; work-related injury, illness, exposure in past year; work organization (shift work, work hours, overtime, second job hours); physical job demands; hand hygiene practices; workplace violence and stress; seasonal influenza vaccination practices; health and safety perceptions; demographics |
Three submodules were developed, one each for aerosolized antibiotics, pentamidine, and ribavirin.
Two submodules were developed, one for laser surgery and one for electrosurgery.
Includes electrocautery, diathermy, and ultrasonic devices.
Cognitive Interviews
Cognitive interviews are structured, open-ended interviews designed to gather detailed information about the thought processes respondents use to understand and answer survey questions, instructions, and other content. The purpose of these interviews was to identify and remove potential sources of response error. Participants for cognitive interviews were recruited from participating professional organizations and screened for assignment to the appropriate hazard module based on organizational affiliation. To be eligible to participate, the member must have used or been in contact with one or more of the targeted chemical hazards in the past 7 calendar days. Approximately 1 week before the interviews, eligible participants were mailed a paper survey questionnaire, monetary incentive, and instructions for completing and returning the questionnaire prior to scheduled interview.
The cognitive interviews on the seven hazard modules and core module were conducted in two rounds. A total of 24 interviewees (3 per module, including core module) participated in the first round, and 16 (2 per module) in the second. The interviewees represented occupations that would most likely complete a particular hazard module (e.g., respiratory therapist—aerosolized medications). Interviews were primarily conducted by phone and typically lasted about an hour. Revisions were made after each round of testing.
Development of the Web Survey Instrument
The instrument was subsequently programmed into a proprietary survey software application developed by Westat called SurveyBuilder™. The layout, format, and navigation of the survey questions were programmed using the latest web survey design principles [Couper, 2008; Dillman et al., 2008]. In addition to programming the survey itself, a survey home page (Fig. 1) and ancillary introductory pages were created. Hyperlinks to these pages contained background information about NIOSH; frequently asked questions; privacy and confidentiality statements; disclaimers, and contacts for questions.
FIGURE 1.
Survey home page.
Introductory pages (not shown) which followed the home page, included a page for participants to enter the Organization Key, a four letter code provided in the survey invitation email or web announcement. This code enabled grouping of respondent data by professional organization and also programming the first screener question presented to respondents.
The remaining introductory pages provided instructions for completing the survey including the importance of the screening questions, how to exit and re-enter survey, explanation of error messages when a question was skipped, and how a dynamic monthly calendar graphic highlighted days corresponding to the time period (described below) of certain questions.
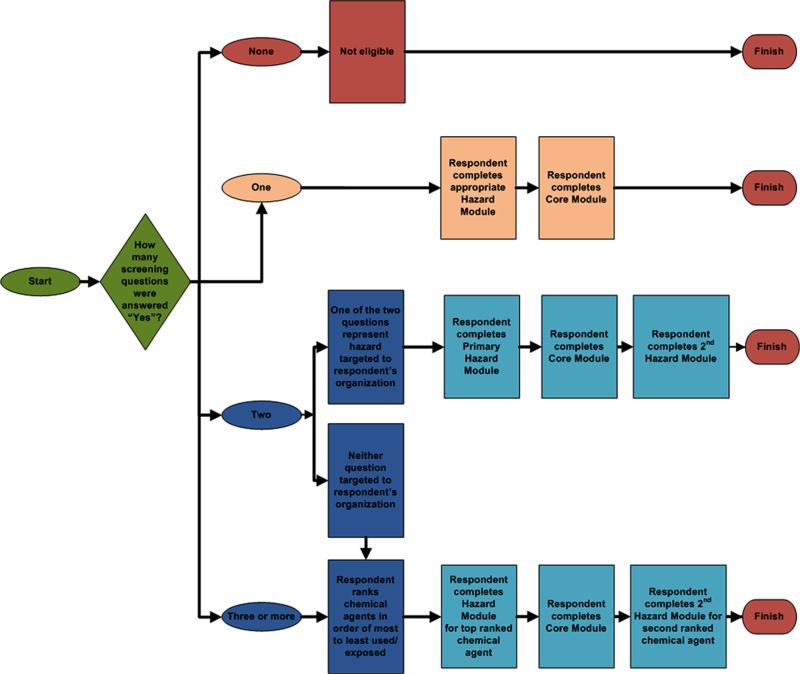
Following the introductory pages, the screening module was presented to determine whether respondents were eligible for the survey. Eligible participants included those who indicated that they used or came in contact with one or more of the targeted chemical hazards within the past 7 calendar days, or 30 calendar days for the aerosolized medication pentamidine which was infrequently administered. The first screener question corresponded to the primary hazard module for the respondent's professional organization (Table II), or in the case where there was no primary hazard module (e.g., Organization No. 21), the screener questions were randomly presented to respondents. The web survey was programmed to present only the top two hazard modules even when the respondent indicated they were exposed to more than two chemical hazards. The flow diagram in Figure 2 shows assignment of hazard modules based on responses to questions in the screening module.
TABLE II.
Primary Hazard Module and Occupation by Professional Organization
| Primary hazard module | Professional organization | Primary occupation of membership |
|---|---|---|
| Aerosolized medications | No. 1 | Respiratory therapist |
| Antineoplastic drugs (compound) | No. 2 | Pharmacy technician |
| No. 3 | Pharmacy technician | |
| No. 4 | Pharmacist | |
| Antineoplastic drugs (administer) | No. 5 | Hematology/oncology nurse |
| No. 6 | Infusion nurse | |
| No. 7 | Oncology nurse | |
| Chemical sterilants | No. 8 | Central supply technician/medical supply technician |
| High level disinfectants | No. 9 | Dental hygienist |
| No. 10 | Dental assistant | |
| No. 11 | Radiologic technologist | |
| No. 12 | Surgical technologist | |
| No. 13 | Gastroenterology nurse | |
| Surgical smoke | No. 14 | Perioperative nurse |
| No. 15 | Surgical assistant | |
| Anesthetic gases | No. 16 | Nurse anesthetist |
| No. 17 | Anesthesiologist assistant | |
| No. 18 | Dentist | |
| No. 19 | Anesthesiologist | |
| No. 20 | Perianesthesia nurse | |
| Nonea | No. 21 | Nurse |
Several of the hazard modules are relevant.
FIGURE 2.
Flow diagram showing assignment of hazard modules based on responses to screening questions.
Salient features of the web survey included: (i) programmed skip patterns—respondents were only presented with relevant follow-up questions based on their responses to key questions; (ii) ability to leave and return to survey at same location where they left off, provided it is within 48 hr (otherwise they would need to start from the beginning); (iii) prominently displayed error messages alerting respondent when no response was entered; and (iv) inclusion of calendar and photos depicting selected engineering controls and respirators/surgical masks to minimize response error.
Pilot Testing of the Web Survey Instrument
The primary purpose of the pilot test was to ensure that all aspects of the survey worked together by examining how it performed under “live conditions.” The entire web survey process was evaluated including contact procedures, respondent access to the web survey via a hyperlink from email, entering their Organization Key, web survey functionality and administration of the hazard modules, receipt control of completed surveys, and exporting of data files into statistical software.
Members of the professional practice organizations were recruited to participate in interviews in the same manner as for the cognitive interviews, and assigned appropriate hazard modules based on organizational affiliation. All interviews were conducted by telephone and concurrently via an internet conference hosting service. Interviewers observed respondents’ mouse movements and keystrokes remotely as respondents worked their way through the survey. Afterwards, interviewer administered a series of probes that addressed a variety of navigation, presentation, and other usability issues. Questions were also asked assessing respondents’ willingness to participate, potential participation motivators, overall impressions, suggestions for improvement, and any other issues from a healthcare worker's perspective. Each interview lasted less than 90 minutes. Participants were compensated for their time.
A total of 28 web survey pilot interviews were conducted (4 interviews per module with the core module evaluated in conjunction with the hazard modules). Problems identified during the pilot testing related to wordiness and imbalanced flow of introductory web pages, confusion with the Organization Key and Resume Survey Code, unclear meaning of calendar graphic, and confusion with varying timeframes in aerosolized medications submodules. Revisions were made to address concerns raised.
Study Population
The study population included members of 21 professional practice organizations representing healthcare occupations which routinely use or come in contact with one or more of the targeted chemical agents. A few hundred candidate professional organizations and several labor unions representing healthcare workers were initially contacted to determine level of interest and support for the survey. Many of these organizations were not eligible or considered for the survey because they: (i) did not maintain email addresses or lacked a centralized email list for their members; (ii) indicated that members either did not use or were not likely to use any of the chemical agents; (iii) decided not to participate; or (iv) did not respond.
Because survey recruitment for individual respondents was by email, the sampling frame represented a list of member email addresses maintained by each of the participating professional organizations. Each organization sent survey invitation and reminder emails to invited members (based on a NIOSH-written template) with an embedded survey link. Some organizations which could reliably filter known unexposed members (e.g., directors and retirees) further refined their email list. Either a random sample or a census of members was invited to participate in the survey, the former being used mainly by organizations with >3,000 member emails and the latter by organizations with <3,000 members. All but four organizations (Nos. 2, 15, 17, 21) selected a random sample to participate in the survey (Table II). Participants were not provided a monetary incentive to participate in the survey.
Survey Implementation
The survey was activated on January 28, 2011 and deactivated on March 29, 2011. The survey was accessible 24 hr a day, 7 days a week during this 8 week data collection period. Once respondents clicked on the link provided in email from their organization they were connected to the survey home page hosted on a secure server. A few weeks after the survey was launched, results showed relatively low numbers of participants for the following hazard modules: aerosolized medications, antineoplastic drugs (compounding), and chemical sterilants. In an effort to increase the number of participants in these and possibly other modules, the survey was opened to others, beyond those who were invited by the 21 partnering organizations, hereafter referred to as non-professional organization respondents. They could potentially be from a professional practice organization but were not specifically selected in our preliminary process. A survey announcement and link was posted in the following locations: NIOSH web pages including e-NEWS; web sites of professional occupational health and safety associations, labor unions, other federal agencies; and selected listservs. The announcement included a unique organization key which allowed us to identify non-professional organization respondents.
Data Analysis
Data were analyzed using SAS 9.3 [SAS, 2010]. Simple frequencies and prevalences are presented. In addition, prevalence of training received and reported standard procedures for minimizing exposure to hazardous chemicals were stratified by type of employer (ambulatory healthcare services or hospital as defined by the North American Industry Classification System Codes 621 and 622, respectively [U.S. Census Bureau, 2007]). Those working in other healthcare industries (nursing and residential care facilities, social assistance) or non-healthcare industries are excluded from the analysis due to small numbers. Chi square and P-value were calculated to determine if a relationship existed between (i) training received and (ii) reported standard procedures and the type of employer reported by the respondent. These were also calculated excluding non-professional organization respondents to determine if their exclusion changed prevalence or Chi square P-value.
Human Subjects Review Board
NIOSH Human Subjects Review Board determined that the activities in this project were surveillance and did not meet the criteria of research according to 45 CFR 46.1101(b) (2) and CDC Guidelines for Defining Public Health Research and Public Health Non-Research [CDC, 2010].
RESULTS
Numbers of Respondents
Overall, 12,028 survey respondents were eligible based on screening questions that established use/contact with the targeted chemical agents in the past 7 calendar days, or for pentamidine the past 30 calendar days, and went on to complete at least one hazard module. The numbers of respondents per module and submodule are presented in Table III. Overall, survey respondents completed more than 18,000 hazard modules/submodules and almost 11,000 completed the core module.
TABLE III.
Number of Respondents by Module
| Hazard module | Numbers of respondents |
|---|---|
| Aerosolized medications | 487 |
| Antibioticsa | 321 |
| Pentamidine | 227 |
| Ribavirin | 50 |
| Antineoplastic drugs (compound) | 457 |
| Antineoplastic drugs (administer) | 2,069 |
| Chemical sterilants | 428 |
| Ethylene oxide | 168 |
| Hydrogen peroxide gas | 347 |
| Plasma | |
| High level disinfectants | 4,657 |
| Surgical smoke | 4,752 |
| Laser surgery | 1,469 |
| Electrosurgeryb | 4,715 |
| Anesthetic gases | 3,610 |
| Core module | 10,912 |
| Total | 12,028 |
Includes tobramycin, amikacin, and colistin.
Includes electrocautery, diathermy, and ultrasonic procedures.
Of those who responded to the screener, 488 were eligible but did not continue with the survey or were later determined ineligible. Another 10,169 responded to the screener but had not been exposed to any chemicals in the time period specified in the questionnaire, so were ineligible to participate. Nine percent of respondents were eligible and completed one hazard module only; they did not continue on to complete the core module and therefore lack demographic information.
Most Prevalent Occupations of Survey Respondents
Table IV presents the occupational groups of respondents who completed the core module. Nurses accounted for 55% of the 10,781 respondents who provided their occupation, followed by technologists/technicians (14%), physicians (12%), dentists/other dental professionals (9%), therapists (7%), pharmacists/other pharmacy professionals (2%). Over three-quarters of the nurses were represented by five specialty occupations: nurse anesthetist, oncology nurse, operating room nurse, hematology/oncology nurse and gastroenterology/endoscopy nurse. Physicians and therapists were almost exclusively anesthesiologists and respiratory therapists, respectively. Dentists/other dental professionals were primarily: dental hygienists, dentists, and dental assistants. Pharmacists and other pharmacy professionals were pharmacists and pharmacy technicians. Most technologists/technicians were surgical technologists, central supply technicians, and to a lesser extent sterilization technicians and radiologic technologists. The majority of respondents in the “other” category were surgical assistants and anesthesiologist assistants; medical assistants were also included in this category.
TABLE IV.
Distribution of Core Module Respondents by Major Occupational Group and Most Prevalent Detailed Occupation(s) in These Groups
| Major occupational group and most prevalent detailed occupations (n = 10,781) | % of total | % for each detailed occupation |
|---|---|---|
| Nurse | 55 | |
| Nurse anesthetist | 32 | |
| Oncology nurse | 14 | |
| OR nurse | 14 | |
| Hematology/oncology nurse | 11 | |
| Gastroenterology/endoscopy nurse | 10 | |
| Other | 19 | |
| Technologists/technicians | 14 | |
| Surgical technologist | 58 | |
| Central supply/processing technician | 20 | |
| Sterilization technician | 7 | |
| Radiologic technologist | 7 | |
| Other | 8 | |
| Physicians | 12 | |
| Anesthesiologist | 99 | |
| Other | 1 | |
| Dentists/other dental professionals | 9 | |
| Dental hygienist | 44 | |
| General dentist | 40 | |
| Dental assistant | 13 | |
| Other | 3 | |
| Therapists | 7 | |
| Respiratory therapist | 99 | |
| Other | 1 | |
| Pharmacists/other pharmacy professionals | 2 | |
| Pharmacist | 59 | |
| Pharmacy technician | 40 | |
| Other | 1 | |
| Other healthcare professionals | 2 | |
| Surgical assistant | 52 | |
| Anesthesiologist assistant | 39 | |
| Other | 9 |
Demographic, Occupational, and Employer Characteristics
Table V presents occupational and employer characteristics of core module respondents. Respondents were predominately female (72%), white (91%), 41–55 years of age (46%), and had at least a bachelor's degree (61%). Core module respondents spent most of their time in direct patient care, over a decade in their current occupation (65%), and lacked affiliation with a labor union (89%). Respondents were predominately employed by large, non-public hospitals (57% with >249 employees, 88% non-public, 70% hospitals). All geographic regions of the U.S were included.
TABLE V.
Demographic and Occupation Characteristics of Core Module Respondents
| Characteristica | (n) % |
|---|---|
| Genderb | (10,566) |
| Male | 28 |
| Female | 72 |
| Racec | (10,420) |
| White | 91 |
| Black | 4 |
| Asian | 5 |
| Other | 2 |
| Ethnicity | (10,527) |
| Hispanic | 3 |
| Ageb | (10,373) |
| 18–25 years | 1 |
| 26–40 years | 18 |
| 41–55 years | 46 |
| 56–70 years | 34 |
| >70 years | 1 |
| Educationb | (10,509) |
| Grade 12 or less | 3 |
| Vocational certificate | 7 |
| Associate's degree | 22 |
| Bachelor's degree | 28 |
| Master's degree | 22 |
| Doctoral degree/plus | 17 |
| Percent of time spent in direct patient care (n)b | (10,749) |
| 76–100% | 70 |
| 51–75% | 13 |
| 26–50% | 6 |
| 1–25% | 7 |
| No direct patient care | 5 |
| Time in current occupation (n)b | (10,722) |
| <1 year | 3 |
| 1–5 years | 17 |
| 6–10 years | 15 |
| 11–20 years | 25 |
| >20 years | 40 |
| Time with current employer (n)b | (10,753) |
| <1 year | 7 |
| 1–5 years | 28 |
| 6–10 years | 20 |
| 11–20 years | 22 |
| >20 years | 23 |
| Employer industry category (n)b | (10,773) |
| Ambulatory healthcare services | 27 |
| Hospital | 70 |
| Nursing and residential care | 1 |
| Social assistance/services | 2 |
| Size of employer (n)b | (10,712) |
| 1–9 | 12 |
| 10–99 | 22 |
| 100–249 | 9 |
| 250–1,000 | 24 |
| >1,000 | 33 |
| Employer ownership type (n)b | (10,591) |
| For profit | 44 |
| Non-profit | 44 |
| City, county, district, state, fed gov't | 9 |
| Other | 3 |
Percents based on number of respondents to individual questions (i.e., number of eligible respondents less number who chose not to answer question).
Percents may not add up to exactly 100% due to rounding.
Percents may add to more than 100% because respondents could select more than one answer.
Prevalence of Training and Employer Standard Procedures
Respondents administering aerosolized antibiotics were the least likely to have received training on their safe use (48% reported they were never trained), followed closely by those exposed to surgical smoke (43% reported they were never trained) (Table VI). Workers most likely to have received training were those who administered antineoplastic drugs (95%) and those who used hydrogen peroxide gas plasma as a chemical sterilant (92%). Of those who administered anesthetic gases, most respondents reported that their training occurred more than 12 months earlier (66%). For aerosolized antibiotics, pentamidine, and surgical smoke, more respondents were trained over 12 months ago than within the past 12 months. Training received within the past 12 months was most prevalent for those who administered antineoplastic drugs (61%).
TABLE VI.
Training and Awareness of Employer Procedures for Working With Hazardous Chemicals
| Trained on safe handling/minimizing exposure to these chemicals | n | ≤12 months ago % | >12 months ago % | Never % |
|---|---|---|---|---|
| Aerosolized medications | ||||
| Antibiotics: tobramycin, amikacin, colistin | 316 | 22 | 30 | 48 |
| Pentamidine | 209 | 26 | 49 | 25 |
| Ribavirin | 49 | 49 | 35 | 16 |
| Antineoplastic drugs | ||||
| Compounding | 455 | 45 | 42 | 13 |
| Administration | 2,061 | 61 | 34 | 5 |
| Chemical sterilants | ||||
| Ethylene oxide (EtO) | 167 | 54 | 35 | 11 |
| Hydrogen peroxide gas plasma | 343 | 51 | 41 | 8 |
| High level disinfectants | 4,572 | 48 | 35 | 17 |
| Surgical smoke | 4,747 | 25 | 32 | 43 |
| Anesthetic gases | 3,599 | 17 | 66 | 17 |
| Employer has standard procedures for handling/minimizing exposure to these chemicals | n | Yes % | No % | Doesn't know % |
|---|---|---|---|---|
| Aerosolized medications | ||||
| Antibiotics: tobramycin, amikacin, colistin | 306 | 55 | 25 | 20 |
| Pentamidine | 210 | 82 | 5 | 13 |
| Ribavirin | 48 | 81 | 8 | 10 |
| Antineoplastic drugs | ||||
| Compounding | 456 | 83 | 7 | 10 |
| Administration | 2,060 | 94 | 3 | 3 |
| Chemical sterilants | ||||
| Ethylene oxide (EtO) | 167 | 92 | 2 | 6 |
| Hydrogen peroxide gas plasma | 342 | 90 | 3 | 7 |
| High level disinfectants | 4,566 | 81 | 5 | 14 |
| Surgical smoke | 4,746 | 32 | 28 | 40 |
| Anesthetic gases | 3,604 | 56 | 18 | 25 |
The proportion of respondents who reported that their employer had standard procedures for minimizing exposure to hazardous chemicals ranged from 32% for those exposed to surgical smoke to 94% for those who reported administering antineoplastic drugs (Table VI). For anesthetic gases and aerosolized antibiotics, the proportion reporting having procedures was slightly over half (56% and55%, respectively). A significant proportion also reported that they did not know if their employers had standard procedures; for surgical smoke 40% did not know, for anesthetic gases 25% did not know. Those who administered antineoplastic drugs were least likely to report that they did not know whether their employer had procedures for minimizing employees’ exposure (3%).
Examining hazard-specific training by type of employer, workers compounding antineoplastic drugs, handling high level disinfectants, and administering anesthetic gases in hospitals were less likely to have been trained than those who used these chemicals and worked in ambulatory healthcare services (Table VII). Those who worked with ethylene oxide (EtO) were more likely to be trained if they worked in a hospital than in ambulatory healthcare services. Few respondents who administered aerosolized medications worked for an employer they classified as ambulatory healthcare services, and therefore no comparison was done. Removing non-professional organization respondents did not alter the percents or P-values for these comparisons.
TABLE VII.
Training and Awareness of Employer Procedures for Working With Hazardous Chemicals by Type of Employer: Ambulatory Healthcare Services Or Hospital
| Trained on safe handling/minimizing exposure to these chemicals | Ambulatory (n) | Ambulatory, never trained (%) | Hospital (n) | Hospital, never trained (%) | P-value for X2 |
|---|---|---|---|---|---|
| Aerosolized medications | |||||
| Antibiotics: tobramycin, amikacin, colistin | 11 | — a | 257 | 48 | — |
| Pentamidine | 15 | — | 171 | 23 | — |
| Ribavirin | 3 | — | 37 | 14 | — |
| Antineoplastic drugs | |||||
| Compounding | 250 | 7 | 172 | 16 | <0.01 |
| Administration | 716 | 5 | 1,181 | 5 | 0.77 |
| Chemical sterilants | |||||
| Ethylene oxide (EtO) | 23 | 39 | 131 | 5 | <0.01 |
| Hydrogen peroxide gas plasma | 31 | 3 | 289 | 9 | 0.29 |
| High level disinfectants | 1,339 | 14 | 2,423 | 17 | 0.02 |
| Surgical smoke | 767 | 43 | 3,817 | 44 | 0.49 |
| Anesthetic gases | 824 | 14 | 2,524 | 18 | <0.01 |
| Employer has standard procedures for handling/minimizing exposure to these chemicals | Ambulatory (n) | Ambulatory, no procedures/unaware (%) | Hospital (n) | Hospital, no procedures/unaware (%) | P-value for X2 |
|---|---|---|---|---|---|
| Aerosolized medications | |||||
| Antibiotics: tobramycin, amikacin, colistin | 11 | — | 255 | 45 | — |
| Pentamidine | 16 | — | 171 | 15 | — |
| Ribavirin | 3 | — | 37 | 16 | — |
| Antineoplastic drugs | |||||
| Compounding | 251 | 20 | 172 | 13 | 0.07 |
| Administration | 717 | 7 | 1,177 | 5 | 0.02 |
| Chemical sterilants | |||||
| Ethylene oxide (EtO) | 23 | 30 | 131 | 4 | <0.01 |
| Hydrogen peroxide gas plasma | 31 | 10 | 288 | 9 | 0.96 |
| High level disinfectants | 1,342 | 22 | 2,422 | 15 | <0.01 |
| Surgical smoke | 766 | 69 | 3,818 | 68 | 0.97 |
| Anesthetic gases | 827 | 37 | 2,525 | 45 | <0.01 |
Dash indicates that due to small numbers no statistic was calculated.
Differences existed between respondents who worked for hospitals versus ambulatory healthcare services as to whether their employer had standard procedures for minimizing exposure to chemical hazards. Those who administered antineoplastic drugs, had contact with high level disinfectants, or used EtO and who worked in ambulatory healthcare services were less likely to report their employer had standard procedures or they were aware that their employer had procedures for minimizing exposure to hazardous chemicals compared to hospital employees. Respondents working for hospitals who administered anesthetic gases were more likely to report their employer did not have procedures or they were unaware of whether their employer had procedures for minimizing their exposure compared to those who worked in ambulatory healthcare settings.
DISCUSSION
The Health and Safety Practices Survey of Healthcare Workers represents the largest federally sponsored survey of healthcare workers that addresses safety and health practices and use of hazardous chemicals. The content of the survey was based on input from a diverse group of healthcare stakeholders representing industry, labor, academia, and NIOSH researchers. Queried practices included hazard-specific training, employer safe handling procedures, use of recommended safe handling guidelines such as engineering controls and PPE, and barriers to using PPE. This web-based survey presents a new method for surveillance of current occupational practices that captures a wide cross-section of workers and workplaces. This type of surveillance can be used to fill in gaps and guide interventions and future research to understand whether authoritative guidelines to minimize exposure have been adopted in a cross-section of workplaces and insight into why they have not. Since the survey was targeted through professional organizations to workers who were likely to use these chemicals, we could ask specific questions about recommended practices that were relevant to them and obtain a robust response from workplaces diverse in size, type, geographic location, and other characteristics. Although our sample was not strictly representative of workplace practices across the country, it provides evidence that recommended practices have not been universally adopted and workers continue to be vulnerable to health consequences. This paper focuses on training and awareness of employer safe handling procedures for hazardous chemicals widely used in healthcare.
A report by the NORA Healthcare and Social Assistance Sector Council titled: State of the Sector: Healthcare and Social Assistance states that “Recognition and anticipation of potential hazards is the first step in preventing work-related illnesses” [Condon et al., 2009]. Some of the tools that workers need to recognize and anticipate chemical hazards include training and standard procedures for handling them safely. Table VIII provides a partial listing of safety and health guidelines developed by government agencies and professional organizations. For each class of chemical agent, we asked whether the worker had training, the time frame the training took place, and whether their employer had standard procedures for handling the chemicals safely.
TABLE VIII.
Health and Safety Guidelines for Selected Chemical Hazards
| Chemical agent | Health and safety guidelines |
|---|---|
| Aerosolized medications | OSHA Technical Manual (OTM) Section VI: Chapter 2: Controlling occupational exposure to hazardous drugs https://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html |
| NIOSH alert on preventing occupational exposures to antineoplastic and other hazardous drugs. www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf | |
| Antineoplastic drugs | American Society of Health-System Pharmacists (ASHP) guidelines for handling hazardous drugs. www.ashp.org/doclibrary/bestpractices/prepgdlhazdrugs.aspx |
| NIOSH alert on preventing occupational exposures to antineoplastic and other hazardous drugs. http://www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf | |
| OSHA Technical Manual (OTM) Section VI: Chapter 2: Controlling occupational exposure to hazardous drugs https://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html | |
| Occupational Safety and Health Administration (OSHA) safety and health topic page: Hazardous drugs. https://www.osha.gov/SLTC/index.html | |
| Chemical sterilants | OSHA Standard 1910.1047 ethylene oxide. http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=10070 |
| OSHA. Ethylene Oxide (EtO): Understanding OSHA's exposure monitoring requirements. https://www.osha.gov/Publications/OSHA_ethylene_oxide.pdf | |
| NIOSH. Current intelligence bulletin no. 52: Ethylene oxide sterilizers health care facilities-engineering controls and work practices. http://www.cdc.gov/niosh/docs/89-115/ | |
| High level disinfectants | NIOSH. Glutaraldehyde: Occupational hazards in hospitals. http://www.cdc.gov/niosh/docs/2001-115/ |
| OSHA. Best practices for the safe use of glutaraldehyde in health care. https://www.osha.gov/Publications/3258-08N-2006-English.html | |
| NIOSH Evaluation of worker exposures to peracetic acid-based sterilant during endoscope reprocessing. www.cdc.gov/niosh/hhe/reports/pdfs/2006-0298-3090.pdf | |
| Surgical smoke | NIOSH hazard controls: Control of smoke from laser/electric surgical procedures http://www.cdc.gov/niosh/docs/hazardcontrol/hc11.html |
| OSHA surgical suite eTool. https://www.osha.gov/SLTC/etools/hospital/surgical/surgical.html | |
| AORN management of surgical smoke tool kit. http://www.aorn.org/smoketoolkit/ | |
| Information alert: Laser plume in surgical procedures. http://www.ccohs.ca/otherhsinfo/alerts/alert61.txt | |
| OSHA safety and health topics: Laser/electrosurgery plume. https://www.osha.gov/SLTC/laserelectrosurgeryplume/ | |
| Information Alert: Laser plume in surgical procedures. http://www.ccohs.ca/otherhsinfo/alerts/alert61.txt | |
| Anesthetic gases | OSHA Technical Manual (OTM) Section VI: Chapter 1: Hospital investigations: Health hazards. https://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_1.html |
| OSHA. Anesthetic gases: Guidelines for workplace exposures. 2000. https://www.osha.gov/dts/osta/anestheticgases/index.html | |
| NIOSH. Controlling exposures to nitrous oxide during anesthetic administration. http://www.cdc.gov/niosh/docs/94-100/ | |
| NIOSH. Control of nitrous oxide in dental operatories. http://www.cdc.gov/niosh/docs/hazardcontrol/hc3.html | |
| NIOSH. Waste anesthetic gases—Occupational hazards in hospitals. http://www.cdc.gov/niosh/docs/2007-151/pdfs/2007-151.pdf |
Although all workers who use or have potential exposure to hazardous chemicals should be trained and have procedures for their safe use, these administrative controls were not universal for all chemical agents included in this study, most notably for aerosolized antibiotics and surgical smoke. Stakeholders, including respiratory therapists, expressed concern over aerosolized antibiotics tobramycin, amikacin, and colistin as well as ribavirin and pentamidine as potential respiratory irritants/asthmagens. Ribavirin and pentamidine are classified as hazardous drugs and have therefore received more attention from government agencies and professional practice organizations including release of precautionary guidelines [OSHA, 1999; NIOSH, 2004; ASHP, 2006]. The aerosolized antibiotics included in this study have not been classified as hazardous drugs at this time. This may be one explanation for why so many respondents who administered aerosolized antibiotics were not trained and lacked safe handling procedures. For surgical smoke, we expected many more respondents to have received training and have procedures for minimizing exposure since exposure control guidelines have been available for many years [NIOSH, 1996; OSHA, 2008].
Concern over health and safety practices in outpatient work settings have been a concern for those handling antineoplastics in the past [Valanis et al., 1992; Martin and Larson, 2003]. We looked at employer type (hospital versus ambulatory healthcare services) to see if there were differences in whether workers had been trained or had procedures for safe handling of the chemicals. In our sample, hospital workers were less likely than those working in ambulatory health care settings to be trained on safety practices for compounding antineoplastic drugs, using high level disinfectants, and administering anesthetic gases. In addition, hospital workers were also less likely to report having procedures for handling anesthetic gases than those in ambulatory healthcare settings. A study of workers who handled antineoplastic drugs by Valanis et al. [1992] reported that, based on PPE use, hospital staff are better protected than workers in outpatient facilities. Our survey found, based on employers having safe handling procedures, hospital workers administering antineoplastic drugs are still better protected. In addition, hospital workers who administered antineoplastic drugs were more likely to report their employer had safe handling procedures than those who worked for ambulatory health care settings. Health and safety practices in different types of healthcare work settings should be examined further.
This survey has both strengths and limitations. A major strength is that it represents one of the largest cross-sectional surveys addressing a wide variety of occupational health and safety hazards in a targeted yet diverse healthcare worker population. Best practices such as cognitive and pilot testing of the survey instrument were used in the development and implementation of the survey. Over 12,000 unique respondents, representing a wide range of professional, technical and support occupations, completed the survey (i.e., completed at least one hazard module). These respondents completed over 18,000 total hazard modules/submodules. Survey findings serve as a valuable resource for surveillance of hazards, potential exposures, exposure controls and barriers to their use. The data are also expected to be useful for priority setting, assessing knowledge gaps, and health and safety promotion.
Several limitations need to be considered. The study population was targeted to professional practice organizations whose members are likely to use or come in contact with the chemical agents under investigation, but because the invitation was by email and the survey was only available via the internet, participants were limited. Survey participants who have resources to belong to a professional organization may be more likely to be further along in their career, better paid, more educated, and more aware of health and safety issues and thus may not represent all healthcare workers. Response rate cannot be calculated because classes of chemical agents under study were specified in the invitation email and eligibility was based on whether or not invitees used specific hazardous chemicals on the job; it is unknown who decided not to participate because they did not use any of the chemicals versus those who used them but decided not to participate for other reasons. Survey findings reflect the experiences and practices of the respondents and are not generalizable to all healthcare workers or to all members of each of the professional organizations. Demographic information was not available for respondents who participated in one hazard module but not the core module. Survey data are self-reported by healthcare workers; responses were not confirmed via observation, records or other means.
In conclusion, this web-based survey served as a cost-effective surveillance tool to gauge current health and safety practices in a population of healthcare workers who would have been more difficult to reach by other means. Although the survey data are not generalizable to all healthcare workers, the data nevertheless provide valuable insight on the availability of training and procedures for minimizing exposure risk as well as other topics.
ACKNOWLEDGMENTS
The authors thank Martha Stapleton and her colleagues from Westat, Inc., for their collaboration in developing, testing and conducting the survey. We also thank Dr. Mick Couper, Institute for Social Research, University of Michigan, for his insightful comments relating to the development of the web survey instrument. The authors are grateful to the professional practice organizations and members who participated in the survey. This project was supported by the National Institute for Occupational Safety and Health.
Contract grant sponsor: National Institute for Occupational Safety and Health.
Institution at which the work was performed: Division of Surveillance, Hazard Evaluations and Field Studies, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Cincinnati, Ohio.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of company names or products does not constitute end or sement by the National Institute for Occupational Safety and Health.
Footnotes
Disclosure Statement: The authors report no conflicts of interests.
REFERENCES
- American Society of Health-System Pharmacists (ASHP) ASHP guidelines on handling hazardous drugs. Am J Health Syst Pharm. 2006;63:1172–1193. [Google Scholar]
- Bureau of Labor Statistics (BLS) Industry employment. [November 21, 2013];Occup Outlook Q. 2012 55(4):33–39. at http://bls.gov/opub/ooq/2011/winter/industry.htm. [Google Scholar]
- Bureau of Labor Statistics (BLS) [November 21, 2013];Table II. Numbers of nonfatal occupational injuries and illnesses by industry and case types, 2012. 2013a at http://www.bls.gov/iif/oshwc/osh/os/ostb3583.pdf.
- Bureau of Labor Statistics (BLS) Labor force statistics from the current population survey. Household data annual averages. Table 18. [November 21, 2013];Employed persons by detailed industry, sex, race and Hispanic or Latino ethnicity, 2012. 2013b at http://www.bls.gov/cps/cpsaat18.htm.
- CDC [November 6, 2013];Distinguishing public health research and public health nonresearch. 2010 at http://www.cdc.gov/od/science/integrity/docs/cdc-policy-distinguishing-public-health-research-nonresearch.pdf. [Google Scholar]
- Condon M, Chen L, Weissman D. Chapter 15. Chemical and other hazardous exposures. In: National Institute for Occupational Safety and Health (NIOSH), editor. State of the sector—healthcare and social assistance: Identification of research opportunities for the next decade of NORA. NIOSH; Cincinnati: 2009. [July 12, 2013]. (DHHS (NIOSH) Pub. No. 2009-139) at http://www.cdc.gov/niosh/docs/2009-139/ [Google Scholar]
- Connor TH, DeBord DG, Pretty JR, Oliver MS, Roth TS, Lees PS, Krieg EF, Rogers B, Escalante CP, Toennis CA, et al. Evaluation of antineoplastic drug exposure of health care workers at three university-based U.S. cancer centers. J Occup Environ Med. 2010;52(10):1019–1027. doi: 10.1097/JOM.0b013e3181f72b63. [DOI] [PubMed] [Google Scholar]
- Couper MP. Designing effective web surveys. Cambridge University Press; New York: 2008. p. 387. [Google Scholar]
- Dillman DA, Smyth JD, Christian LM. Internet, mail, and mixed-mode surveys: The tailored design method. 3rd edition John Wiley and Sons; New York:: 2008. p. 512. [Google Scholar]
- Martin S, Larson E. Chemotherapy-handling practices of outpatient and office-based oncology nurses. Oncol Nurs Forum. 2003;30:575–581. doi: 10.1188/03.ONF.575-581. [DOI] [PubMed] [Google Scholar]
- McDiarmid MA. Chemical hazards in health care—High hazard, high risk, but low protection. Ann NY Acad Sci. 2006;1076:601–606. doi: 10.1196/annals.1371.032. [DOI] [PubMed] [Google Scholar]
- McDiarmid MA, Leone M. Chapter 14. Hazardous drugs. In: National Institute for Occupational Safety and Health (NIOSH), editor. State of the sector—healthcare and social assistance: Identification of research opportunities for the next decade of NORA. NIOSH; Cincinnati: 2009. [July 12, 2013]. (DHHS (NIOSH) Pub. No. 2009-139) at http://www.cdc.gov/niosh/docs/2009-139/ [Google Scholar]
- McDiarmid MA, Fujikawa J, Schaefer J, Weinmann G, Chaisson RE, Hudson CA. Health effects and exposure assessment of aerosol pentamidine handlers. Chest. 1993;104:382–385. doi: 10.1378/chest.104.2.382. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) [August 31, 2013];Control of smoke from laser/electric surgical procedures. 1996 at http://www.cdc.gov/niosh/docs/hazardcontrol/hc11.html.
- National Institute for Occupational Safety and Health (NIOSH) NIOSH alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. NIOSH; Cincinnati: 2004. [August 26, 2013]. (DHHS (NIOSH) Pub. No. 2004-165) at http://www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf. [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) Waste anesthetic gases—Occupational hazards in hospitals. NIOSH; Cincinnati: 2007. [June 18, 2013]. (DHHS (NIOSH) Pub. No. 2007-151) at http://www.cdc.gov/niosh/docs/2007-151/pdfs/2007-151.pdf. [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) NIOSH list of antineoplastic and other hazardous drugs in healthcare settings 2012. NIOSH; Cincinnati: 2012. [July 12, 2013]. (DHHS (NIOSH) Pub. No. 2012-150) at http://www.cdc.gov/niosh/docs/2012-150/pdfs/2012-150.pdf. [Google Scholar]
- Occupational Safety and Health Administration (OSHA) Section VI: Chapter 2 Controlling occupational exposure to hazardous drugs. [February 7, 2014];OSHA Technical Manual (OTM), TED1-0.15A. 1999 at https://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html.
- Occupational Safety and Health Administration (OSHA) [February 6, 2013];Lasers/ electrosurgery plume. 2008 at http://www.osha.gov/SLTC/laserelectrosurgeryplume/index.html.
- Occupational Safety and Health Administration, (OSHA) [February 6, 2013];Hospital eTool laboratory. 2013 at http://www.osha.gov/SLTC/etools/hospital/lab/lab.html.
- Rideout K, Teschke K, Dimich-Ward H, Kennedy SM. Considering risks to healthcare workers from glutaraldehyde alternatives in high-level disinfection. J Hosp Infect. 2005;59:4–11. doi: 10.1016/j.jhin.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Rosenman KD, Reilly MJ, Schill DP, Valiante D, Flattery J, Harrison R, Reinisch F, Pechter E, Davis L, Tumpowsky CM, et al. Cleaning products and work-related asthma. J Occup Environ Med. 2003;45:556–563. doi: 10.1097/01.jom.0000058347.05741.f9. [DOI] [PubMed] [Google Scholar]
- SAS. SAS version 9.3. SAS Institute, Inc.; Cary: 2010. [Google Scholar]
- U.S. Census Bureau. [January 13, 2014];North American Industry Classification System (NAICS) 2007 at http://www.census.gov/cgi-bin/sssd/naics/naicsrch?chart=2007.
- Valanis B, Vollmer WM, Labuhn K, Glass A, Corelle C. Antineo-plastic drug handling protection after OSHA guidelines: Comparison by profession, handling activity, and work site. J Occup Med. 1992;34:149–155. doi: 10.1097/00043764-199202000-00014. [DOI] [PubMed] [Google Scholar]
- Winstin C. The effects of smoke plume generated during laser and electrosurgical procedures. Minim Invasive Surg Nurs. 1994;8:99–102. [PubMed] [Google Scholar]