Abstract
Objectives. We estimated HIV prevalence among men who have sex with men (MSM) and transgender women in Bogotá, Colombia, and explored differences between HIV-positive individuals who are aware and unaware of their serostatus.
Methods. In this cross-sectional 2011 study, we used respondent-driven sampling (RDS) to recruit 1000 MSM and transgender women, who completed a computerized questionnaire and received an HIV test.
Results. The RDS-adjusted prevalence was 12.1% (95% confidence interval [CI] = 8.7, 15.8), comparable to a previous RDS-derived estimate. Among HIV-positive participants, 39.7% (95% CI = 25.0, 54.8) were aware of their serostatus and 60.3% (95% CI = 45.2, 75.5) were unaware before this study. HIV-positive–unaware individuals were more likely to report inadequate insurance coverage, exchange sex (i.e., sexual intercourse in exchange for money, goods, or services), and substance use than other participants. HIV-positive–aware participants were least likely to have had condomless anal intercourse in the previous 3 months. Regardless of awareness, HIV-positive participants reported more violence and forced relocation experiences than HIV-negative participants.
Conclusions. There is an urgent need to increase HIV detection among MSM and transgender women in Bogotá. HIV-positive–unaware group characteristics suggest an important role for structural, social, and individual interventions.
Colombia ranks second among countries in Latin America in HIV prevalence, with estimates ranging from 0.7% to 1.1% of the adult population.1 Men who have sex with men (MSM) represent the group most strongly affected, with prevalence of 18% to 20% based on venue-based convenience samples2,3 and 15% based on respondent-driven sampling (RDS).4 Colombia has a long history of armed conflict, and the pervasive conditions of violence, internal displacement, and poverty can be relevant to HIV transmission.5 “Social cleansing” by armed groups has been aimed at MSM and transgender women, as well as people living with HIV,5 and the stigma associated with homosexuality and HIV is widespread and inherent in structural inequalities in Colombia.6,7 Social epidemiological models posit that HIV is influenced by such structural (e.g., civil unrest, migration) and social factors (e.g., social networks, community attitudes), as well as individual characteristics (e.g., psychological characteristics, behavior).8
Public health efforts emphasize the importance of detecting and treating undiagnosed HIV as a means of reducing HIV incidence.9–11 In the United States, approximately 20% of HIV-positive individuals are thought to be unaware of their infection, but this group is estimated to be responsible for nearly half of new transmissions.12 There is limited research concerning awareness of serostatus in Latin America. Undiagnosed infection was found to be 89% among HIV-positive MSM sampled in Peru in 2011,13 and rates are likely to be high in Colombia because of low levels of testing,6 including among MSM.4,14 Recent studies of MSM in France, Peru, and the United States have found associations between undiagnosed infection and demographic characteristics such as age, income, and education13,15,16; risk behaviors14,17,18; family or intimate partner violence19; and health insurance coverage.20 We also examined awareness in relation to violence and forced relocation, conditions specific to the Colombian context.
Respondent-driven sampling was developed as a means of obtaining unbiased estimates from hidden populations,21–23 and it has been shown to capture a more diverse24,25 and hidden26 group of MSM than time–location or snowball sampling. Research has suggested, however, that biases can occur.27–29
Our current study and a study conducted by the United Nations Population Fund and the Colombian Ministry of Health and Social Protection (UNFPA/MSPS) were independently funded at approximately the same time to address the limited information about behavioral risk and HIV prevalence among Colombian MSM. Comparison of findings from the 2 studies provides evidence concerning reliability of the RDS-derived prevalence estimates. We estimated HIV prevalence among MSM and transgender women in Bogotá, Colombia, examined reliability of RDS-derived estimates in relation to the UNFPA/MSPS study,4 and investigated the role of the social and structural context of Colombia in both prevalence and awareness of positive serostatus.
METHODS
We present data from a larger study of MSM and transgender women in Bogotá, which included a formative qualitative phase (with key informant interviews, in-depth interviews, and focus groups), a quantitative pilot phase that resulted in revisions of a computerized questionnaire, and a full administration of the final version of the interview protocol, as well as rapid HIV testing. We chose Bogotá, the most populous city in Colombia, because it has a large community of MSM, many of whom migrated from other areas of the country, sometimes because of internal displacement or violence.
Participants
We recruited participants for the study (n = 1000) by RDS, after earlier pilot testing of the method and questionnaire with a sample of 100. RDS involves chain-referral sampling and treats sampling as a Markov process, using mathematical modeling to compensate for the nonrandom aspects of the approach.21,22 Participants recruit others into the study, and therefore it is necessary to account mathematically for biases arising from differing probability of selection (attributable to network size), as well as the tendency of individuals to recruit similar others (homophily). Recruitment continues until equilibrium is achieved—in other words, when the characteristics of the sample become independent of the characteristics of the initial participants (seeds) who were selected to begin the recruitment chains.
Eligibility criteria for the study included being between the ages of 18 and 49 years, being born as a biological male, living in Bogotá, and having had sexual intercourse with a man in the past 6 months. Therefore, male-to-female transgender individuals met eligibility requirements, and the sample of 1000 included 58 transgender women. We collected data in 2011, and obtained the target sample size in 10 months.
Procedure
Four individuals, selected to represent diverse socioeconomic backgrounds, served as initial seeds for RDS. Participants were reimbursed 70 000 Colombian pesos (about US $40). They could recruit up to 3 others to the study, and were paid 30 000 Colombian pesos (about US $17) for each participant whom they recruited. Recruitment coupons were distributed to participants until the target sample size was obtained. Unique serial numbers enabled the tracking of referral sources and chains. Potential participants called the project and underwent initial screening for eligibility. On the day of participation, participants presented their coupon, gave informed consent, completed the interview protocol, and then received pretest counseling, HIV testing, and posttest counseling.
Interview protocol.
The interview protocol included both previously used questions and new items. Existing items had already been translated and back-translated. We developed specific questions designed for Colombian participants (e.g., experiences of violence, socioeconomic status [SES]) in Spanish. To ensure clarity and language consistent with Colombian Spanish, we conducted 2 focus groups in Colombia with participants of diverse educational levels. On the basis of findings from the pilot administration, we revised the questionnaire.
We used audio computer-assisted self-interviewing on touch-screen laptops to administer the questionnaire—a procedure that facilitated participation of individuals with low literacy. Questions included date of birth, gender identity, sexual orientation, education, income, and current relationship status (main partner or not). To assess SES further we used strata, a government designation given to neighborhoods, buildings, and houses. This classification ranges from 1 (the poorest) to 6 (the wealthiest), and strata are highly correlated with SES30 and frequently used as a proxy in Colombia.
We also inquired about history of HIV testing (ever, most recent, and year) and HIV status (positive, negative, or unknown). We used this information in combination with the actual HIV test results to determine awareness of serostatus. Among HIV-positive participants, we classified those who had reported on the questionnaire that they were negative or did not know their serostatus as HIV-positive–unaware. We classified those who had reported being positive on the questionnaire as HIV-positive–aware. We classified participants who tested HIV-negative as such, regardless of whether they had reported negative or unknown serostatus in the questionnaire.
We also assessed history of sexually transmitted infections (STIs) and exchange sex (i.e., sexual intercourse in exchange for money, goods, or services), as well as alcohol use, drug use, and condomless anal intercourse in the past 3 months. We asked about type of health insurance. Possible responses included contributory (the person pays for insurance), subsidized (the government pays private insurers to provide coverage), linked (the government provides emergency treatment in government-supported public hospitals but there is no other care), none, or unknown. We grouped together those with linked care, those with no insurance, and those who reported that they did not know their insurance so that we could have comparable categories to those used by the UNFPA/MSPS study.4
We included 2 variables as indicators of the broader social context in Colombia. Colombia’s ambient violence was evident in the qualitative phase of our larger project: participants described having experienced various types of attacks.5 Therefore, in the questionnaire we assessed experiences of violence with 5 questions regarding how frequently participants had been severely beaten, attacked with a knife, shot, raped, and tortured. Response options included 0 for never; 1 for 1 or 2 times; 2 for several times; 3 for many times. We used the mean as the scale score. In addition, we assessed forced relocation by asking if participants had ever been forced to leave the place where they were living, with response options of 0 for no and 1 for yes.
HIV testing.
We administered the OraQuick Advance Rapid HIV 1/2 Antibody Test (Orasure Technologies, Bethlehem, PA). Participants who had a reactive test received additional counseling and had blood drawn by a nurse for confirmatory tests, which were then conducted with the enzyme-linked immunosorbant assay (MP Diagnostics, Singapore) and the western blot assay (Ray Biotech, Norcross, GA). Newly identified cases were referred to medical providers.
Statistical analyses.
We obtained RDS-derived indicators of prevalence, homophily, and equilibrium by using the RDS Analysis Tool (RDSAT) 7.1 (Heckathorn, Ithaca, NY). We used the χ2 test and 1-way analyses of variance to examine differences among HIV-positive–aware, HIV-positive–unaware, and HIV-negative groups. We also examined recruitment patterns with the χ2 test.
RESULTS
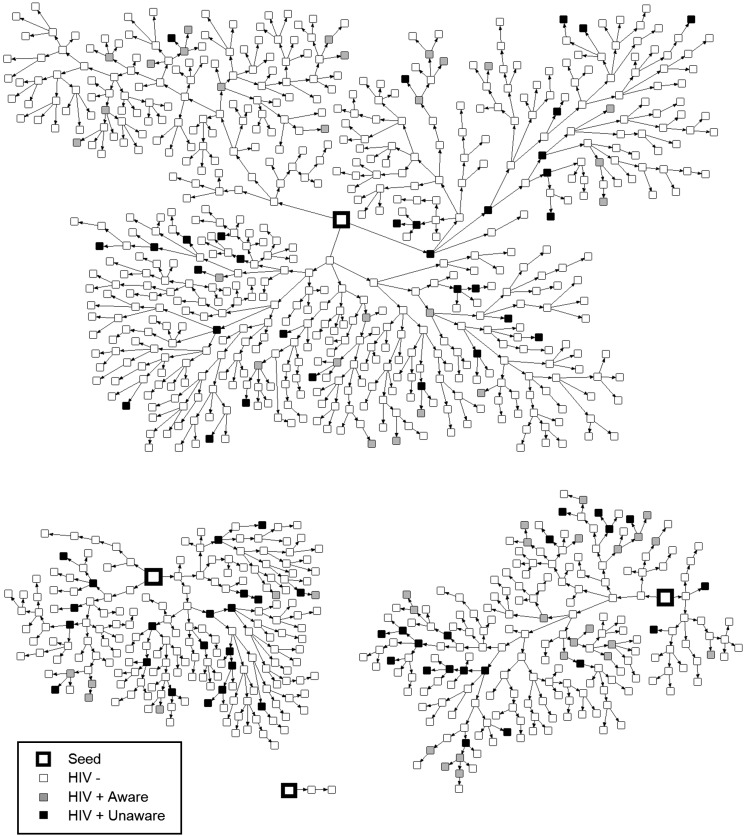
The recruitment chains generated by the 4 seeds can be seen in Figure 1, which shows that 3 of the seeds produced almost the entire sample through 11 or 12 waves each. The fourth seed’s chain ended in 2 waves and resulted in only 2 participants. Participants typically reported that their recruiter was a friend (85%); less common were a partner (9%), work colleague (2%), relative (0.5%), or other type of relationship (3%).
FIGURE 1—
Recruitment chains for the 4 seeds in respondent-driven sampling of men who have sex with men and transgender women: Bogotá, Colombia, 2011.
We used RDSAT to examine waves to equilibrium for 4 variables: HIV status, age, SES, and type of insurance coverage. Although the full sample was 1000, RDS-related statistics are based on a sample of 996 because 4 participants had missing coupon information, but none of these had recruited anyone else and all 4 were HIV-negative. Equilibrium was reached in 2 waves for HIV status and insurance type, 5 waves for age, and 3 waves for SES. Thus, the number of waves to obtain the full sample was well in excess of that needed to achieve equilibrium. In estimates of homophily (possible range −1 to 1), deviations from zero were relatively small for HIV status (0.12–0.13) and insurance type (0.04–0.10), thus indicating only a slight tendency to recruitment of similar others. Homophily was slightly higher for SES (0.18–0.25) and age (0.11–0.31), with greater in-group recruitment among younger participants.
There were 124 participants or 12.4% who were HIV-positive. The RDS-adjusted prevalence was 12.1% (95% confidence interval [CI] = 8.7, 15.8). In comparison, the UNFPA/MSPS study reported an RDS-adjusted prevalence of 15% (95% CI = 10.9, 19.9) in Bogotá.4 Although HIV prevalence in the current study was slightly lower than that found in the UNFPA/MSPS study, both values fell within the confidence interval of the other study.
Table 1 shows RDS-adjusted HIV prevalence for selected demographic and personal characteristics of the sample. For variables with corresponding groupings to those of the UNFPA/MSPS study,4 we provided comparison estimates. Eligibility criteria were similar in the 2 studies, except UNFPA/MSPS did not set an upper age limit and required sexual intercourse with a man in the past 12 months.
TABLE 1—
Respondent-Driven Sampling–Adjusted HIV Prevalence by Demographic Characteristics Among Men Who Have Sex With Men and Transgender Women: Bogotá, Colombia, 2011
| Current Study |
UNFPA/MSPS Study |
|||||
| Demographic Characteristic | Total No. | HIV-Positive, No. | Adjusted % (95% CI) | Total No. | HIV Positive, No. | Adjusted % (95% CI) |
| Age, y | ||||||
| 18–24 | 640 | 48 | 7.5 (4.2, 10.9) | 248 | 19 | 7.4 (3.7, 11.6) |
| 25–34 | 274 | 47 | 19.1 (11.2, 28.0) | 138 | 27 | 15.9 (8.8, 24.1) |
| 35–44 | 61 | 25 | 32.7 (17.3, 49.9) | 53 | 21 | 40.5 (21.1, 56.1) |
| 45–49 or over 44a | 21 | 4 | 18.5 (0.7, 45.4) | 46 | 12 | 21.8 (6.7, 40.6) |
| SES | ||||||
| Lowb | 823 | 100 | 11.6 (8.2, 15.4) | 400 | 64 | 14.5 (10.2, 19.8) |
| Highc | 173 | 24 | 14.7 (6.1, 25.9) | 79 | 14 | 18.0 (6.0, 29.6) |
| Insurance type | ||||||
| Contributory | 301 | 46 | 15.5 (8.0, 24.2) | 241 | 39 | 14.0 (8.7, 20.6) |
| Subsidized | 325 | 38 | 13.3 (7.2, 19.8) | 129 | 26 | 18.5 (10.7, 28.8) |
| Linked, none, or unknown | 370 | 40 | 8.8 (5.6, 12.6) | 108 | 14 | 13.6 (5.7, 24.2) |
| Education | ||||||
| Less than high school | 165 | 30 | 20.5 (11.3, 31.2) | |||
| Finished high school | 209 | 31 | 13.5 (6.2, 22.0) | |||
| Some university | 471 | 43 | 8.2 (4.6, 12.7) | |||
| Finished university | 151 | 20 | 16.6 (6.0, 27.1) | |||
| Weekly income, Colombian pesos | ||||||
| 0–50 000 | 354 | 24 | 6.7 (3.5, 10.3) | |||
| 51 000–100 000 | 213 | 33 | 18.0 (9.6, 27.3) | |||
| 101 000–200 000 | 222 | 35 | 14.9 (6.7, 23.6) | |||
| > 200 000 | 207 | 32 | 14.2 (6.1, 22.3) | |||
| Gender identity | ||||||
| Transgender woman | 58 | 8 | 18.0 (3.7, 35.5) | |||
| Man | 938 | 116 | 11.6 (8.3, 15.5) | |||
| Forced relocation | ||||||
| Yes | 112 | 27 | 18.6 (8.9, 30.5) | |||
| No | 884 | 97 | 11.2 (7.6, 15.0) | |||
| Main partner | ||||||
| Yes | 486 | 57 | 10.8 (6.8, 15.5) | |||
| No | 510 | 67 | 13.1 (8.4, 18.8) | |||
| Exchange sexd | ||||||
| Yes | 282 | 44 | 18.2 (10.9, 26.6) | |||
| No | 714 | 80 | 9.8 (6.4, 13.7) | |||
Note. SES = socioeconomic status; UNFPA/MSPS = United Nations Population Fund and the Colombian Ministry of Health and Social Protection. Variables without comparison data either involved different response categories or were not included in the UNFPA/MSPS Study.
The current study used the age range of 45–49 years, while the UNFPA/MSPS study used age > 44 years.
Strata 1–3.
Strata 4–6.
Sexual intercourse in exchange for money, goods, or services.
Comparisons showed that the sample in the current study was younger than that of UNFPA/MSPS study: 64% versus 51% of our participants were aged 24 years or younger, and 8% versus 20% of our participants were aged at least 35 years. HIV prevalence estimates in the different age groups, however, were quite similar across studies and within the confidence intervals of both studies. The distribution of participants in higher or lower SES was nearly equivalent, but HIV prevalence estimates associated with SES groups, though comparable, were slightly lower in the current study. Insurance type was less consistent across samples, with more participants reporting contributory insurance in the UNFPA/MSPS study. HIV prevalence among participants with different types of insurance, however, was quite similar.
HIV testing before this study was reported by 54% of the participants. Of these, 60% had been tested within the 2 most recent years; 22% had been tested within 3 or 4 years before the study; and the remaining 18% before that. Of the 124 HIV-positive participants, only 53 had previously known their serostatus and, of these, 91% were receiving antiretroviral treatment. Seventy-one other participants who tested positive were unaware that they were living with HIV. The RDS-adjusted estimates indicated that 39.7% (95% CI = 25.0%, 54.8%) of the HIV-positive participants were aware of their serostatus, and 60.3% (95% CI = 45.2%, 75.5%) were unaware before the HIV test administered in this study.
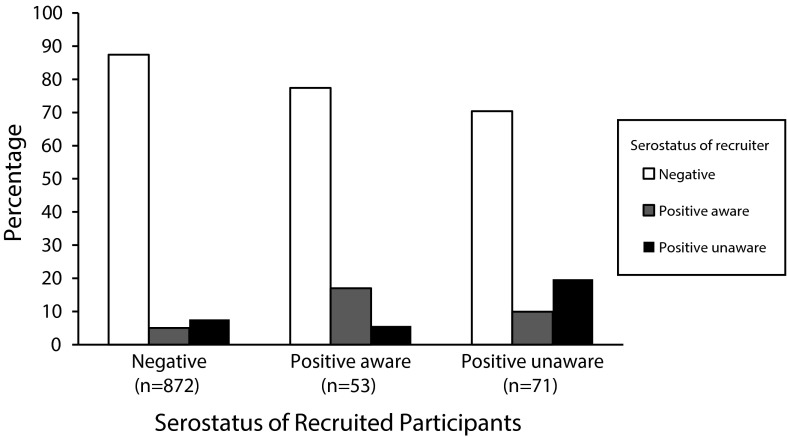
Although indicators of homophily did not suggest a strong bias toward in-group recruitment based on positive or negative HIV status, we explored this issue further by examining recruitment patterns among the 3 groups (HIV-positive–aware, HIV-positive–unaware, and HIV-negative). For each HIV-status group, Figure 2 shows the unadjusted percentages of individuals who were recruited by participants who were HIV-negative, HIV-positive–aware, and HIV-positive–unaware. The HIV-negative participants recruited participants of each HIV-status group in proportions nearly equivalent to those of the total sample. By contrast, there was a bias for HIV-positive–aware participants to recruit HIV-positive–aware individuals and, similarly, for HIV-positive–unaware participants to recruit HIV-positive–unaware others. Moreover, the HIV-positive–unaware group recruited disproportionately more HIV-positive–aware participants than would be expected: in total, 30% of the participants recruited by HIV-positive–unaware participants were HIV-positive. Chi-square testing confirmed that the pattern of recruitment differed in the 3 groups (χ2 (4) = 29.05; P < .001). The network maps in Figure 1 also provide a graphic illustration of the increased likelihood of HIV-positive participants’ recruitment of other positive individuals. In addition, Figure 1 shows that HIV-positive–unaware individuals were often located at the end of recruitment branches.
FIGURE 2—
HIV status of recruited participants by serostatus of the recruiter in respondent-driven sampling of men who have sex with men and transgender women: Bogotá, Colombia, 2011.
To further explore ways in which the HIV-positive–unaware group differed from HIV-positive–aware and HIV-negative individuals, we examined bivariate associations of serostatus group with demographic characteristics, risk-related behaviors, and social context variables. Table 2 shows results of the χ2 test and 1-way analyses of variance. HIV-positive–aware participants were older and had better insurance coverage than either of the other 2 groups. We found no significant differences in SES or transgender identity, but the finding for transgender participants should be interpreted cautiously because of small numbers: only 1 of 53 HIV-positive–aware individuals (1.9%), in contrast with 7 of 71 HIV-positive–unaware (9.9%) and 50 of 876 HIV-negative (5.7%) were transgender women.
TABLE 2—
Comparisons of HIV Status Groups on Demographic and Risk-Related Characteristics Among Men Who Have Sex With Men and Transgender Women Recruited Through Respondent-Driven Sampling (n = 1000): Bogotá, Colombia, 2011
| HIV Status Group |
||||
| Characteristic | Negative (n = 876), % or Mean (95% CI) | Positive-Aware (n = 53), % or Mean (95% CI) | Positive-Unaware (n = 71), % or Mean (95% CI) | Test Statistic, χ2(2) or F (2, 997) |
| Transgender | 5.7 (4.2, 7.2) | 1.9 (0.0, 5.6) | 9.9 (2.9, 16.8) | 3.64 |
| Low SESa | 83.0 (80.5, 85.5) | 77.4 (66.1, 88.6) | 83.1 (74.4, 91.8) | 1.11 |
| Insurance group | ||||
| Contributory | 29.3 (26.3, 32.4)x | 56.6 (43.3, 70.0)y | 22.5 (12.8, 32.3)x | 19.8*** |
| Subsidized | 32.8 (29.7, 36.0) | 24.5 (12.9, 36.1) | 35.2 (24.1, 46.3) | 1.80 |
| Linked, none, or unknown | 37.9 (34.7, 41.1)x | 18.9 (8.3, 29.4)y | 42.3 (30.8, 53.7)x | 8.58* |
| Ever had STI | 28.0 (24.3, 30.3)x | 67.9 (55.4, 80.5)y | 47.9 (36.3, 59.5)z | 46.5*** |
| Ever did exchange sexb | 27.2 (24.2, 30.1)x | 17.0 (6.9, 27.8)x | 49.3 (37.7, 60.9)y | 19.4*** |
| Binge drinking in past 3 mo | 14.6 (12.3, 17.0)x | 9.4 (1.6, 17.3)x | 28.2 (29.4, 52.3)y | 10.8** |
| Drug use in past 3 mo | 26.4 (23.5, 29.4)x | 22.6 (14.6, 38.3)x | 40.9 (29.4, 52.3)y | 7.5* |
| Condomless anal intercourse in past 3 mo | 61.6 (58.4, 64.9)x | 37.7 (24.7, 50.8)y | 66.2 (55.2, 77.2)x | 12.9** |
| Forced relocation | 9.9 (7.9, 11.8)x | 26.4 (14.6, 38.3)y | 18.3 (9.3, 27.3)y | 17.5*** |
| Mean agec | 24.2 (23.8, 24.6)x | 33.5 (32.2, 35.9)y | 26.4 (24.8, 28.0)z | 62.4*** |
| Mean experiences of violencec | 0.22 (0.20, 0.24)x | 0.31 (0.21, 0.42)y | 0.33 (0.26, 0.41)y | 7.7*** |
Note. CI = confidence interval; SES = socioeconomic status; STI = sexually transmitted infection. Entries marked with “x, y, or z” are significantly different from each other.
Strata 1–3.
Sexual intercourse in exchange for money, goods, or services.
The corresponding test statistic is F(2, 997).
*P < .05; **P < .01; ***P < .001.
Several indicators of past or current risk behaviors showed different patterns for the 3 groups. The HIV-positive–aware group was most likely to have been diagnosed with an STI at some time, but HIV-positive–unaware individuals were also more likely to have had an STI than HIV-negative individuals. An indicator of recent sexual risk—condomless anal intercourse in the past 3 months—was least likely among respondents who were HIV-positive and aware of their status, and about equally common among HIV-positive–unaware and HIV-negative individuals. Nearly half of the HIV-positive–unaware participants reported having participated in exchange sex, by contrast with lower proportions of the other 2 groups. Similarly, binge drinking and drug use in the previous 3 months were also reported more by HIV-positive–unaware individuals. Among the social context variables, HIV-positive individuals—regardless of awareness of serostatus—reported more experiences of violence and greater likelihood of forced relocation than HIV-negative individuals.
DISCUSSION
Consistent with the social epidemiological model,8 individual, social, and structural factors were associated with HIV prevalence and awareness of serostatus. The large number of undiagnosed cases found was alarming but understandable given the low rates of HIV testing in Colombia,6 including among MSM.4,14 Our finding that HIV-positive–aware individuals were older and had better insurance coverage than HIV-positive–unaware individuals was consistent with the literature,13,16,20 including our previous mixed-methods paper on testing among MSM in Bogotá that noted barriers created by the structure of the health care system.14
The impact of the structural context was also evident in the finding that HIV-positive individuals had more violent experiences and forced relocation. In Colombia violent encounters are not uncommon, and sexual minorities and people living with HIV are sometimes the victims of hate crimes because of the stigma associated with their identities.5 Qualitative interviews in our study had pointed to HIV-positive status as a reason for targeted killings and internal displacement in certain parts of Colombia.5 It is also possible, however, that dislocation and violent encounters are indicative of greater marginalization associated with more generalized social vulnerability (e.g., poverty, limited education, limited access to health care, and possibly high viral load in the partner pool because of untreated infections). In such a context, lack of knowledge concerning HIV and of ways to prevent transmission, as well as a lower sense of agency and ability to control the conditions of one’s life, could lead to greater likelihood of becoming infected.
Social factors were clearly associated with recruitment patterns. Despite low homophily relative to positive or negative serostatus, RDS-referral patterns suggested that social networks of HIV-positive–aware and HIV-positive–unaware participants include the presence of similar others. For those who are aware of their positive serostatus, this finding is consistent with literature indicating that HIV-positive individuals tend to develop, as one of their many identities, an identity as a person living with HIV,31 which could have influenced the composition of their social networks and, subsequently, their recruitment patterns. For HIV-positive–unaware individuals, however, knowledge of serostatus would not be a source of identity development. Rather, it seems likely that the presence of other HIV-positive–unaware people within the social network would be the result of shared behavioral patterns and values, such as the alcohol and drug use found in this and other studies.13,16 Previous research has found evidence of in-group recruiting among HIV-positive drug users.32 Norms encouraging substance use within a social network that includes individuals who are unaware of their positive serostatus and therefore likely to have high viral loads because of lack of treatment, could contribute to increased risk of HIV transmission.
It is important to note that HIV-positive–unaware participants often ended recruitment branches; thus, the group’s disproportionate referral of positive individuals is even more striking. Reasons for failing to refer others to the study could stem from the fact that they had just learned of their seropositive status, so it is likely that they had greater concerns than recruitment. It is also possible that the stigma associated with HIV may have inhibited them, because efforts to recruit others could lead to conversations about test results.
Individual characteristics were also linked to awareness of serostatus. As has been previously reported,13,16 we found that the HIV-positive–unaware group was more likely to have engaged in potentially risky behaviors such as drug use, binge drinking, and sex work. In terms of condomless anal intercourse, however, the HIV-positive–unaware participants were similar to the HIV-negative participants: both were more likely to report unprotected sexual intercourse than were HIV-positive–aware individuals. This finding supports previous conclusions that knowledge of positive serostatus tends to lead to decreases in risky sexual behavior.13,17,18,33
Our prevalence findings corroborate those reported by the UNFPA/MSPS study.4 Although slightly lower in the current study, estimated HIV prevalence was quite similar, including when broken down by age group, SES, and type of insurance coverage. The minor differences in prevalence may have been attributable to our sample’s greater proportion of younger participants, who have had a shorter period of sexual activity in which exposure and transmission could have occurred. Although the general correspondence between findings of the 2 studies provides evidence of their reliability, the RDS-generated prevalence estimates were lower than those found with venue-based convenience samples.2,3 This discrepancy may be attributable to differing patterns of risk behavior among individuals obtained via recruitment in gay venues or gay-oriented Internet sites,34–37 in contrast with sampling methods such as RDS that reach broader representation of the gay community.24–26
Young people were overrepresented in the sample for this study. In Bogotá in 2011, young men aged between 18 and 24 years constituted 25% of the population of men aged 18 to 49 years.30 By contrast, this age category (18–24 years) accounted for almost two thirds of our sample (and more than half of the UNFPA/MSPS sample)—a pattern that suggests that prevalence estimates may be artificially low. Given changing societal attitudes, however, the age distribution of MSM may not be equivalent to that of the general population of men, but rather reflect a tendency toward greater representation of younger ages. Therefore, the extent of the possible bias and the way to correct for that bias are not clear, which constitutes a limitation of the current study.
Biases in RDS-derived samples can be the result of nonrandom distribution or acceptance of recruitment coupons.38 Young Colombian MSM or transgender women may be more engaged in the LGBT community than their older counterparts and, therefore, more likely to have social network contacts who could offer them a coupon. Moreover, younger MSM and transgender women might be more willing to accept a coupon, because they might have both the motivation and the time to participate in a study that included remuneration.
This study included both MSM and transgender women. HIV prevalence estimates for the 2 groups indicated higher rates among the transgender group, but the small number of transgender participants restricted feasibility of doing separate analyses on associated variables. Future research should investigate factors related to serostatus and awareness in this group.
Despite limitations, this study makes an important contribution by highlighting the widespread problem of undiagnosed infection among Colombian MSM and transgender women. Lack of awareness of positive serostatus has negative consequences for the infected individuals, their sexual partners, and public health efforts to decrease transmission. Although public policy dictates that individuals can receive free testing twice a year,39 effective implementation would require changes in protocols and practices of health care systems and their providers.14 Findings suggest that existing interventions focusing on social networks,40 popular opinion leaders,41–45 and empowerment of individuals and communities46–48 could be adapted to increase detection and treatment and to decrease behaviors associated with lack of awareness. However, any interventions in Colombia should also address structural issues related to violence, dislocation, and the health care system.
Acknowledgments
The project described was supported by the National Institute of Child Health and Human Development (award R01HD057785). During the preparation of this article, authors also received support from the District of Columbia Developmental Center for AIDS Research (P30AI087714).
We thank our Colombian colleagues for their invaluable contributions to this project. Specifically, we are grateful to Fabian Betancourt; Marcela Aguilar-Pardo; Jorge Pacheco; Elvia Vargas Trujillo, PhD; and Profamilia Colombia. We are also grateful to the participants for giving of their time, information, and perspectives.
Note. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institute of Child Health and Human Development or National Institutes of Health.
Human Participant Protection
The study was reviewed by the George Washington University institutional review board and the Profamilia Board of Ethics. The researchers complied with the Principles of the Ethical Practice of Public Health.
References
- 1.Joint United Nations Programme on HIV/AIDS. Report on the Global AIDS Epidemic 2013. 2013. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. Accessed October 21, 2014. [PubMed]
- 2.Montano SM, Sanchez JL, Laguna-Torres A et al. Prevalences, genotypes, and risk factors for HIV transmission in South America. J Acquir Immune Defic Syndr. 2005;40(1):57–64. doi: 10.1097/01.qai.0000159667.72584.8b. [DOI] [PubMed] [Google Scholar]
- 3.Vallejo F, Leal L, Alzate M Prevalence and risk factors for HIV-1 among MSM and FCSW in Bogota, Colombia. Oral presentation at: XIV International AIDS Conference; July 2, 2002; Washington, DC.
- 4. United Nations Population Fund and Colombian Ministry of Health and Social Protection. Sexual behavior and HIV prevalence among men who have sex with men in 7 cities in Colombia (Bogotá, Medellín, Cali, Barranquilla, Cúcuta, Pereira, and Cartagena) [in Spanish]. Bogotá, Colombia: Ministerio de Salud y Protección; 2011: No. convenio 168.
- 5.Zea MC, Reisen C, Bianchi F et al. Armed conflict, homonegativity and forced internal displacement: implications for HIV among Colombian gay, bisexual and transgender individuals. Cult Health Sex. 2013;15(7):788–803. doi: 10.1080/13691058.2013.779028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrivillaga M, Hoyos PA, Tovar LM, Varela MT, Correa D, Zapata H. HIV testing and counselling in Colombia: evidence from a national health survey and recommendations for health-care services. Int J STD AIDS. 2012;23(11):815–821. doi: 10.1258/ijsa.2012.011468. [DOI] [PubMed] [Google Scholar]
- 7.Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Soc Sci Med. 2003;57(1):13–24. doi: 10.1016/s0277-9536(02)00304-0. [DOI] [PubMed] [Google Scholar]
- 8.Poundstone KE, Strathdee SA, Celentano DD. The social epidemiology of human immunodeficiency virus/acquired immunodeficiency syndrome. Epidemiol Rev. 2004;26:22–35. doi: 10.1093/epirev/mxh005. [DOI] [PubMed] [Google Scholar]
- 9.Cowan SA, Gerstoft J, Haff J, Christiansen AH, Nielsen J, Obel N. Stable incidence of HIV diagnoses among Danish MSM despite increased engagement in unsafe sex. J Acquir Immune Defic Syndr. 2012;61(1):106–111. doi: 10.1097/QAI.0b013e31825af890. [DOI] [PubMed] [Google Scholar]
- 10.Das M, Chu PL, Santos G Success of test and treat in San Francisco? Reduced time to virologic suppression, decreased community viral load, and fewer new HIV infections, 2004 to 2009. Poster presentation at: Conference on Retroviruses and Opportunistic Infections; February 28, 2011; Boston, MA.
- 11.Montaner J. Treatment as prevention within the framework of current guidelines. J Int AIDS Soc. 2010;13(suppl 4):O12. [Google Scholar]
- 12.Hall HI, Holtgrave DR, Tang T, Rhodes P. HIV transmission in the United States: considerations of viral load, risk behavior, and health disparities. AIDS Behav. 2013;17(5):1632–1636. doi: 10.1007/s10461-013-0426-z. [DOI] [PubMed] [Google Scholar]
- 13.Vagenas P, Ludford KT, Gonzales P et al. Being unaware of being HIV-infected is associated with alcohol use disorders and high-risk sexual behaviors among men who have sex with men in Peru. AIDS Behav. 2014;18(1):120–127. doi: 10.1007/s10461-013-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisen CA, Zea MC, Bianchi FT et al. HIV testing among MSM in Bogotá, Colombia: the role of structural and individual characteristics. AIDS Educ Prev. 2014;26(4):328–344. doi: 10.1521/aeap.2014.26.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. HIV Surveillance—United States, 1981–2008. MMWR Morb Mortal Wkly Rep. 2011;60(21):689–693. [PubMed] [Google Scholar]
- 16.Velter A, Barin F, Bouyssou A et al. HIV prevalence and sexual risk behaviors associated with awareness of HIV status among men who have sex with men in Paris, France. AIDS Behav. 2013;17(4):1266–1278. doi: 10.1007/s10461-012-0303-1. [DOI] [PubMed] [Google Scholar]
- 17.Lauby JL, Millett GA, LaPollo AB, Bond L, Murrill CS, Marks G. Sexual risk behaviors of HIV-positive, HIV-negative, and serostatus-unknown Black men who have sex with men and women. Arch Sex Behav. 2008;37(5):708–719. doi: 10.1007/s10508-008-9365-6. [DOI] [PubMed] [Google Scholar]
- 18.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39(4):446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 19.Koblin BA, Torian L, Xu G et al. Violence and HIV-related risk among young men who have sex with men. AIDS Care. 2006;18(8):961–967. doi: 10.1080/09540120500467182. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Prevalence and awareness of HIV infection among men who have sex with men—21 Cities, United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(37):1201–1207. [PubMed] [Google Scholar]
- 21.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–199. [Google Scholar]
- 22.Heckathorn DD. Respondent-driven sampling II: deriving valid population estimates from chain-referral samples. Soc Probl. 2002;49(1):11–34. [Google Scholar]
- 23.Ramirez-Valles J, Heckathorn DD, Vázquez R, Diaz RM, Campbell RT. From networks to populations: the development and application of respondent-driven sampling among IDUs and Latino gay men. AIDS Behav. 2005;9(4):387–402. doi: 10.1007/s10461-005-9012-3. [DOI] [PubMed] [Google Scholar]
- 24.Carballo-Diéguez A, Balan I, Marone R et al. Use of respondent driven sampling (RDS) generates a very diverse sample of men who have sex with men (MSM) in Buenos Aires, Argentina. PLoS ONE. 2011;6(11):e27447. doi: 10.1371/journal.pone.0027447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendall C, Kerr LRFS, Gondim RC et al. An empirical comparison of respondent-driven sampling, time location sampling, and snowball sampling for behavioral surveillance in men who have sex with men, Fortaleza, Brazil. AIDS Behav. 2008;12(4, suppl):S97–S104. doi: 10.1007/s10461-008-9390-4. [DOI] [PubMed] [Google Scholar]
- 26.Paz-Bailey G, Miller W, Shiraishi RW, Jacobson JO, Abimbola TO, Chen SY. Reaching men who have sex with men: a comparison of respondent-driven sampling and time-location sampling in Guatemala City. AIDS Behav. 2013;17(9):3081–3090. doi: 10.1007/s10461-013-0589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goel S, Salganik MJ. Assessing respondent-driven sampling. Proc Natl Acad Sci USA. 2010;107(15):6743–6747. doi: 10.1073/pnas.1000261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu X, Bengtsson L, Britton T et al. The sensitivity of respondent-driven sampling. J R Stat Soc Ser A. 2012;175(1):191–216. [Google Scholar]
- 29.Yamanis TJ, Merli MG, Neely WW et al. An empirical analysis of the impact of recruitment patterns on RDS estimates among a socially ordered population of female sex workers in China. Sociol Methods Res. 2013;42(3):392–345. doi: 10.1177/0049124113494576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Departamento Administrativo Nacional de Estadística. Post-Census studies 2005–2020 [in Spanish] 2009. Available at: http://www.dane.gov.co/files/investigaciones/poblacion/proyepobla06_20/7Proyecciones_poblacion.pdf. Accessed October 21, 2014.
- 31.Baumgartner LM, David KN. Accepting being poz: the incorporation of the HIV identity into the self. Qual Health Res. 2009;19(12):1730–1743. doi: 10.1177/1049732309352907. [DOI] [PubMed] [Google Scholar]
- 32.Abramovitz D, Volz EM, Strathdee SA, Patterson TL, Vera A, Frost SD. Proyecto ElCuete. Using respondent-driven sampling in a hidden population at risk of HIV infection: who do HIV-positive recruiters recruit? Sex Transm Dis. 2009;36(12):750–756. doi: 10.1097/OLQ.0b013e3181b0f311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colfax GN, Buchbinder SP, Cornelisse PGA, Vittinghoff E, Mayer K, Celum C. Sexual risk behaviors and implications for secondary HIV transmission during and after HIV seroconversion. AIDS. 2002;16(11):1529–1535. doi: 10.1097/00002030-200207260-00010. [DOI] [PubMed] [Google Scholar]
- 34.O’Leary A, Horvath KJ, Rosser S. Associations between partner-venue specific personal responsibility beliefs and transmission risk behavior by HIV-positive men who have sex with men (MSM) AIDS Behav. 2013;17(5):1855–1861. doi: 10.1007/s10461-012-0291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollock JA, Halkitis PN. Environmental factors in relation to unprotected sexual behavior among gay, bisexual, and other MSM. AIDS Educ Prev. 2009;21(4):340–355. doi: 10.1521/aeap.2009.21.4.340. [DOI] [PubMed] [Google Scholar]
- 36.Raymond HF, Rebchook G, Curotto A et al. Comparing Internet-based and venue-based methods to sample MSM in the San Francisco Bay Area. AIDS Behav. 2010;14(1):218–224. doi: 10.1007/s10461-009-9521-6. [DOI] [PubMed] [Google Scholar]
- 37.Tsui HY, Lau JTF. Comparison of risk behaviors and socio-cultural profile of men who have sex with men survey respondents recruited via venues and the Internet. BMC Public Health. 2010;10:232. doi: 10.1186/1471-2458-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCreesh N, Copas A, Seeley J et al. Respondent driven sampling: determinants of recruitment and a method to improve point estimation. PLoS ONE. 2013;8(10):e78402. doi: 10.1371/journal.pone.0078402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ministerio de Protección Social. Circular no. 0063. 2006. Available at: http://www.minsalud.gov.co/salud/Documents/observatorio_vih/documentos/normas_decretos_marco_nacional_VIH/circular 063 2006.pdf. Accessed October 21, 2014.
- 40.Latkin CA, Davey-Rothwell MA, Knowlton AR, Alexander KA, Williams CT, Boodram B. Social network approaches to recruitment, HIV prevention, medical care, and medication adherence. J Acquir Immune Defic Syndr. 2013;63(suppl 1):S54–S58. doi: 10.1097/QAI.0b013e3182928e2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caceres C, Solomon S, Woelk G et al. Formative study conducted in five countries to adapt the community popular opinion leader intervention. AIDS. 2007;21(suppl 2):S91–S98. doi: 10.1097/01.aids.0000266461.33891.d0. [DOI] [PubMed] [Google Scholar]
- 42.Jones KT, Gray P, Whiteside YO et al. Evaluation of an HIV prevention intervention adapted for Black men who have sex with men. Am J Public Health. 2008;98(6):1043–1050. doi: 10.2105/AJPH.2007.120337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly JA, St Lawrence JS, Diaz YE et al. HIV risk behavior reduction following intervention with key opinion leaders of population: an experimental analysis. Am J Public Health. 1991;81(2):168–171. doi: 10.2105/ajph.81.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly JA, Murphy DA, Sikkema KJ et al. Randomised, controlled, community-level HIV-prevention intervention for sexual-risk behaviour among homosexual men in US cities. Lancet. 1997;350(9090):1500–1505. doi: 10.1016/s0140-6736(97)07439-4. [DOI] [PubMed] [Google Scholar]
- 45.Kelly JA, St Lawrence JS, Stevenson LY et al. Community AIDS/HIV risk reduction: the effects of endorsements by popular people in three cities. Am J Public Health. 1992;82(11):1483–1489. doi: 10.2105/ajph.82.11.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kegeles SM, Hays RB, Coates TJ. The Mpowerment Project: a community-level HIV prevention intervention for young gay men. Am J Public Health. 1996;86(8):1129–1136. doi: 10.2105/ajph.86.8_pt_1.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maiorana A, Kegeles S, Fernandez P et al. Implementation and evaluation of an HIV/STD intervention in Peru. Eval Program Plann. 2007;30:82–93. doi: 10.1016/j.evalprogplan.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rebchook GM, Kegeles SM, Huebner D. the TRIP Research Team. Translating Research Into Practice: the dissemination and initial implementation of an evidence-based HIV prevention program. AIDS Educ Prev. 2006;18(suppl A):119–136. doi: 10.1521/aeap.2006.18.supp.119. [DOI] [PubMed] [Google Scholar]