Abstract
Objectives. We estimated the costs and effectiveness of implementing a partner notification (PN) strategy for highly prevalent sexually transmitted diseases (STDs) within the Louisiana STD/HIV Program.
Methods. We carried out a telephone-based PN approach on an experimental basis in 2 public STD clinics in Louisiana from June 2010 to May 2012. We monitored data on the resources used for identifying, tracing, treating, and managing the infected cases and their partners to estimate the intervention costs.
Results. Our results indicated that implementation of telephone-based PN should not increase the STD control program’s expenses by more than 4.5%. This low-cost PN approach could successfully identify and treat 1 additional infected case at a cost of only $171. We found that the cost per disability-adjusted life year averted (a health outcome measure), because of the adoption of selective screening with partner tracing, was $4499. This was significantly lower than the gross domestic product per capita of the United States, a threshold used for defining highly cost-effective health interventions.
Conclusions. Adoption of PN for gonorrhea and chlamydia should be considered a national strategy for prevention and control of these diseases.
Although partner notification (PN) is considered one of the most effective means of controlling sexually transmitted diseases (STDs), a majority of states in the United States focus their PN resources on one STD only, namely, syphilis. The PN process generally involves interviewing infected persons to obtain information about exposed sex partners, and then locating and notifying partners for medical evaluation and treatment. Other STDs, like gonorrhea and chlamydia, are treated when people visit clinics. Most STD control programs in the United States do not make any attempt to identify the partners of gonorrhea and chlamydia cases.1 Although it is important to continue partner-tracing and notification for syphilis, the practice of not tracing the partner for other high-prevalent STDs needs careful evaluation. In 2012, the number of chlamydia and gonorrhea cases reported in the United States exceeded 1.4 million and 334 000, respectively, whereas syphilis cases were less than 50 000.2 Because of the high incidence of chlamydia and gonorrhea, it is assumed that intensive PN simply will not be affordable.3,4 We attempted to estimate the incremental cost and cost-effectiveness of tracing the partner in relatively high STD incident regions of the country. As part of this study, we implemented an experimental approach of partner-tracing and notification within the Louisiana STD control program.
PN is usually done by using 4 different methods—patient referral, provider referral, expedited partner therapy, and contract referral.1 Provider referral is the most commonly used PN strategy, and a number of studies have reported strong evidence for the high effectiveness of the method in increasing proportions of partners presenting for care.5–7 By implementing a simple telephone-based PN approach, we estimated the additional costs and effectiveness of the experimental strategy. The a priori hypothesis was that the telephone-based PN strategy would be highly cost-effective, and the additional resources needed for implementing the intervention would be relatively low. Quantification of costs and cost-effectiveness of a PN strategy might encourage re-evaluation of current practice of not doing any type of PN for gonorrhea and chlamydia cases.
METHODS
We classified individuals, symptomatic or asymptomatic, who were initially tested for an STD on the basis of risk history or by self-referral at the STD clinics, or as part of routine screening program at prenatal clinics and family planning clinics, as cases detected through selective screening. We classified individuals who were tested as part of an active case-finding operation as cases detected through PN. We selected the study population for gonorrhea and chlamydia from all chlamydia and gonorrhea cases detected at 2 STD clinics in Louisiana—one in New Orleans and the other in Shreveport. These 2 clinics were the top 2 clinics in Louisiana in detecting gonorrhea and chlamydia in 2009 and 2010, which is why we chose them for the PN experiment. We assigned every other eligible positive case to an interview by 1 of the 3 trained telephone interviewers, and these cases formed the study sample. The interviewers were carefully selected so that interviews could be conducted in English or in Spanish.
Rather than using the resource-intensive PN approach of the syphilis control program, we implemented a telephone-based strategy (i.e., contacting the cases and partners through telephone calls followed by letters mailed to contact addresses). The cost of contacting partners by telephone was considered to be much lower than contacting them through the Disease Intervention Specialists, as is done by the syphilis control program. We made at least 7 attempts to contact and interview each index case for obtaining detailed information on their sex partners, including their contact addresses and telephone numbers. If a patient could not be reached after first 2 attempts, the interviewers sent out letters to patients' addresses, requesting that he or she contact the interviewer.
Our study followed the national guidelines for contacting patients and their partners as described in the Louisiana Disease Intervention Specialists Manual,8 which is based on Centers for Disease Control and Prevention (CDC) trainings. The national guidelines require that the study interviewers start the conversation by making a general statement like “This is John Doe calling from Louisiana Department of Health and Hospitals. May I talk to YY?” If the person is identified as the intended person, then the interviewer verifies the date of birth and address. The interviewer also verifies the date of the recent visit to the health care provider. The caller never mentions the STD clinic during the conversation. When contacting individuals by letter, the letter asks the patient to contact the Louisiana Department of Health and Hospitals for a health-related matter. The information of the interviewer, along with telephone number, is provided in the letter. Once the person calls the interviewer, then they go through the previously described steps to confirm the identity of the person and to maintain confidentiality. Following the CDC guidelines, information on sex partners was limited to those within 60 days of date of specimen collection. If an index case did not have any sex partner within 60 days of specimen collection, then information on the most recent sex partner was obtained.1,9
Study Population
A total of 18 765 persons were tested for gonorrhea and chlamydia at the 2 designated sites—Delgado STD Clinic in New Orleans and Caddo Parish Health Unit in Shreveport—from June 2010 to May 2012. Among those tested, 322 were tested as part of PN. Therefore, total of 18 443 individuals were screened as part of the selective screening, of whom 5005 tested positive for either chlamydia or gonorrhea, or both. Chlamydia positivity was 17.1%, and gonorrhea positivity was 9.9%. For ensuring compliance with the institutional review board application, 685 cases were excluded from the study for not meeting the age criteria (individuals younger than 19 years of age), resulting in a total of 4320 infected cases as the study population. The institutional review board guidelines for patients younger than 19 years of age are very difficult to implement if telephone-based PN system is adopted.
For the experimental implementation of PN strategy, we decided to randomly sample 50% of eligible gonorrhea and chlamydia cases. Because the total eligible infected cases identified in the 2 clinics were 4320 during the project period, we selected 2160 chlamydia and gonorrhea cases, and we assigned the case files to the project research assistants for conducting the interviews. Three part-time field interviewers or research assistants were appointed by the project to conduct the telephone interviews from the offices of the Louisiana STD control program. Of the 2160 cases sampled for case management and PN, 1259 were infected with chlamydia, 571 with gonorrhea, and 330 were infected with both chlamydia and gonorrhea. Interviewers were trained on protocols for interviewing STD cases and their partners, including confidentiality issues related to patient and partner interviews.
Measuring Costs and Effectiveness
The cost analysis was done from the perspective of the Louisiana STD control program, and all resources used in detecting 1 case of chlamydia or 1 case of gonorrhea through selective screening alone and through selective screening combined with PN were valued in monetary terms. We adopted a micro-costing approach10 so that all recurrent resources used for the purpose of identifying and treating the cases and partners could be quantified. In this study, we did not measure the indirect costs and the fixed costs of the STD control program. To derive a measure of total cost, we assumed that the overhead cost was 30% of the recurrent costs of selective screening and PN. This was the mid-value of the indirect cost rates approved by the Department of Health and Human Services for the Louisiana Department of Health and Hospitals (the approved rates varied between 25% and 35% depending on service type).
Our principal objectives were to estimate (1) the total cost of selective screening plus PN for chlamydia and gonorrhea, (2) the incremental cost-effectiveness of the experimental PN approach adopted by our study per infected case treated, and (3) the cost-effectiveness of selective screening and PN for chlamydia and gonorrhea per disability-adjusted life years (DALYs) averted. Expressing the cost-effectiveness ratio in terms of DALYs averted allowed us to compare the specific interventions with the threshold level of cost-effectiveness to determine the acceptability of the interventions from a societal point of view.
The primary measure of effectiveness was the number of new chlamydia and gonorrhea cases detected and treated through selective screening and PN. The cases were converted into DALYs lost using the World Health Organization’s estimates of DALYs lost per case because of chlamydia and gonorrhea in the United States in 2004. In 2004, the United States lost 20 DALYs per 100 000 population from these 2 STDs, and because the rate of these 2 STDs was 528 per 100 000 population, DALYs lost per case became 0.038 per year.11 We used this parameter to estimate the total DALYs lost for the cases treated among the study population. Although cause-specific incidence rates and disease burdens have not been updated for the United States since 2004, it was unlikely that disease burden per gonorrhea and chlamydia case changed significantly over the years.
The cost of selective screening included the cost of testing and contacting the infected patients to notify them about test results, diagnosis, and treatment (if not treated based on syndromic assessments while being tested). The total cost of PN was derived by combining costs associated with serology, surveillance, case management, and contacting patients and their partners. Among all the assigned cases, the interviewers were successful in contacting 1185 individuals (54.9%) through telephone or letters. The field interviewers identified 495 partners from the interviewed cases, and once the partners were contacted, they were requested to come to the clinics for testing and treatment.
Estimating the Cost of Intervention
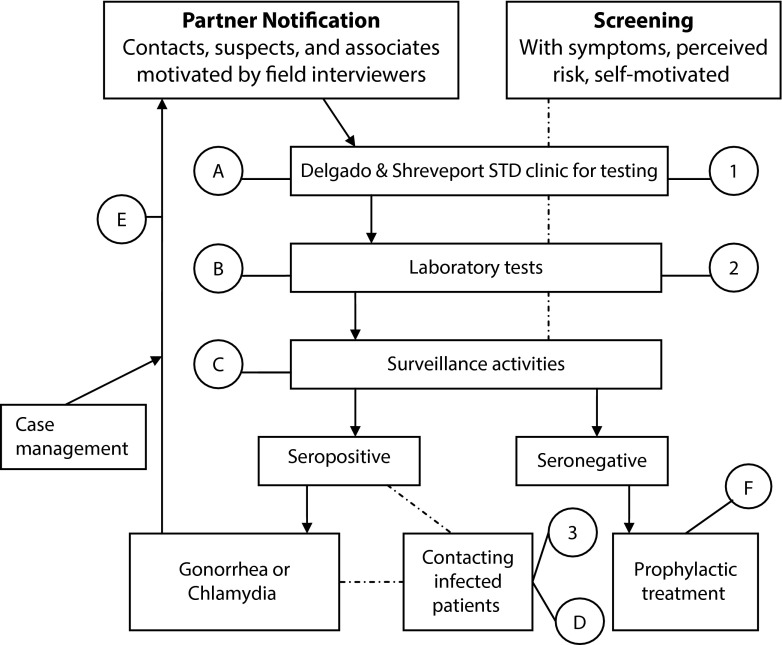
We used a schematic diagram12 to identify the components of costs or resource use for selective screening and PN (Figure 1). The components of selective screening are labeled by the numbers 1, 2, and 3, whereas the components of partner tracing and notifications are shown by the letters A, B, C, D, E, and F. All the resources used at each of the steps identified in Figure 1 were quantified and evaluated in monetary terms. To estimate the monetary values of personnel cost, we used the Louisiana Department of Civil Service pay scale for 2009 plus 28% adjustment for fringe benefits. The wage represented the average for all individuals functioning in the designated roles in the Louisiana STD control program. The hourly wages, including fringe benefits, were as follows: clerical personnel $17.95; phlebotomist $32.79; nurse $41.97; epidemiologist $31.78; and field interviewers $12.50. We obtained the time required to complete the activities related to screening, tracing, treatment, etc., through a survey of the involved personnel. A total of 8 nurses, 4 registration clerks, 2 phlebotomists, 3 telephone interviewers, and an epidemiologist were surveyed to estimate the time required for the tasks performed.
FIGURE 1—
Cost components of selective screenings and partner notifications: Louisiana, 2010–2012.
Note. STD = sexually transmitted disease. “A” stands for the cost of personnel for phlebotomy (i.e., nurse, clerks, and supplies related to drawing of blood); “B,” the cost of work time unit for tests performed; “C,” the personnel for surveillance activities; “D,” the personnel to contact infected patients (field interviewers), plus phone call and letter-related supplies and cost; “E,” the cost of personnel for case management; and “F,” the cost of prophylactic treatment of partners. Cost components of selective screening: (1) personnel for phlebotomy, clerical work, and supplies related to task; (2) cost of work time unit for tests performed; and (3) personnel to contact infected patients (field interviewers) plus phone call and letter-related supplies and costs.
Source. Adapted from Reynolds et al.12
During selective screenings, patients were registered first, and then they were sent to the nurses for history taking and physical examination. Nurses also completed the “reproductive health exam form” during this encounter. After that, either the phlebotomist or the nurse drew blood or collected samples for testing. During the registration process, the clerk obtained the telephone number and address of the patient. This contact information was used later by the interviewers for PN.
Table A1 (available as a supplement to the online version of this article at http://www.ajph.org) shows the average time spent by clinic personnel and the monetary value of time spent on STD cases. After the initial visit and specimen collection, the test results were available within a week. Nurses in the clinic then called the patients by phone or sent letters. Table 1 lists all the cost components, with costs incurred for the selective screening activities. Total cost of selective screenings for chlamydia and gonorrhea during the project period was $711 269.
TABLE 1—
Cost of Selective Screening for Chlamydia and Gonorrhea in 2 Sexually Transmitted Disease Clinics: Louisiana, 2010–2012
| Cost Components of Selective Screenings | Cost, $ |
| Patient registration clerk | 55 175 |
| Phlebotomist’s time | 92 425 |
| Nurse visit time | 304 590 |
| Cost of test, at $13.20/test | 243 448 |
| Phone call, at $0.25/call | 1 251 |
| Time spent on phone call by nurse | 7 002 |
| Letter, at $0.45/postage and $0.15/envelope | 601 |
| Time spent on the letter by nurse | 2 801 |
| Epidemiologist’s time | 3 976 |
| Total cost of selective screening | 711 269 |
Total cost of PN was derived by combining costs associated with serology, surveillance and case management, and contacting patients and their partners. During the study period, 909 (76.7%) of the contacted cases were fully interviewed, 231(19.5%) were partially interviewed, 34 (2.9%) refused interview, and 11 cases (< 1%) could not be interviewed because of language barriers (3 cases) or patients being out of the area. Four hundred ninety-five partners were elicited from the 1140 interviewed cases. The interviewers then contacted the partners identified, and requested them to come to the clinics for medical evaluation and treatment. Among the 495 partners, 322 came to the study clinics for further evaluation; 218 (67.7%) of the partners who sought evaluation were diagnosed as a newly infected case, and 88 (27.3%) were not infected. All these partners were treated when they came to the clinics. The average time spent on these partner cases were assumed to be similar to the time spent on the index case, except that the partners were not followed-up on further for new partner tracing.
RESULTS
Table 2 summarizes the total cost of partner tracing and notification, including prophylactic treatment of partners both for the study population and for the state of Louisiana. Total cost of PN was $28 669 for a 50% sample of all the eligible positive cases identified in the study clinics. The cost of selective screening for gonorrhea and chlamydia was $711 269 for the study population, and the cost of selective screening followed by PN was $(711 269 + 28 669) = $739 938.
TABLE 2—
Cost of Partner Notification of Index Cases of Chlamydia and Gonorrhea in 2 Sexually Transmitted Disease Clinics: Louisiana, 2010–2012
| Cost Components for Partner Notification | Cases in Study Population | Cost for Study Cases/Population, $ | Cases Expected in Louisiana in 2013 | Cost Estimates for Louisiana in 2013, $ |
| Clerk’s time | 322 | 963 | 4 266 | 12 758 |
| Phlebotomist’s time | 322 | 1 614 | 4 266 | 21 383 |
| Time value of nurse | 322 | 5 318 | 4 266 | 70 455 |
| Cost of laboratory tests | 322 | 4 250 | 4 266 | 56 306 |
| Time of the epidemiologist | 2 160 | 900 | 28 617 | 11 924 |
| Case management, including eliciting partners | 218 | 5 212 | 2 888 | 69 047 |
| Prophylactic treatment of partners | 88 | 601 | 1 167 | 7 970 |
| Phone call and letter cost to contact assigned cases | 2 160 | 3 302 | 28 617 | 43 747 |
| Time cost to contact assigned cases | 2 160 | 5 093 | 28 617 | 67 475 |
| Phone call and letter cost to contact partners | 445 | 556 | 5 896 | 7 367 |
| Time cost to contact partners | 445 | 860 | 5 896 | 11 395 |
| Total partner notification cost | 28 669 | 379 827 |
Table 2 also shows the cost projections for the state of Louisiana if the telephone-based PN was introduced to identify and treat the partners of gonorrhea and chlamydia cases. Using each of the cost categories listed in Table 2, the total cost of implementing PN was estimated for the state. It should be noted that total number of gonorrhea and chlamydia cases in the state was much higher than the cases identified in the parish health units of Louisiana. In 2013, the total number of gonorrhea and chlamydia cases identified in Louisiana was 37 408, including 8791 cases in individuals younger than age 19 years, but only 11 857 were identified at the parish health units (private facilities reported a significant proportion of all cases). Louisiana Sanitary Code requires all providers (public or private) to report syphilis, chlamydia, and gonorrhea cases to the Louisiana STD control program within 5 business days of diagnosis. Because PN is standard for syphilis, all syphilis cases, irrespective of their initial identification clinic, go through the Louisiana STD program’s PN process. If the new PN approach is adopted for chlamydia and gonorrhea, it will become standard practice for all eligible cases. In that case, the telephone-based program would have interviewed 28 617 gonorrhea and chlamydia patients in 2013 (excluding those who were younger than age 19 years), generating approximately 5896 contacts and 4055 treated cases. (The CDC treatment guideline requires that every partner of active cases who show up at the clinic are treated pending results of the blood test.9) Based on the infection rate among treated partners in the study, the estimated number of infected partners identified through the telephone-based program should be 2888 for the state. Therefore, the total cost of telephone-based PN for the state would have been $379 827, or approximately 4% of the 2013 budget of the Louisiana STD control program ($9.185 million).
The measure of effectiveness used in this study was the number of chlamydia and gonorrhea cases treated. We found a total of 5005 gonorrhea or chlamydia cases through selective screening. Among the detected cases, only 3088 got treated. Partner tracing and notification for 50% of eligible cases generated 495 partners, but only 306 of them were treated. We could not locate 96 partners because of nonresponses, insufficient information, or not living in the area. Another 71 partners already received treatment by the time the program contacted them. The remaining partners refused examination. Of the 306 partners treated by the PN system, 218 were found to be infected, whereas the remaining 88 were not infected according to the test results. These 88 partners were prophylactically treated. It was possible that some of these prophylactically treated partners would have developed STDs if not treated, but no estimates were available on the number of potential cases averted through prophylactic treatment. To obtain a conservative estimate of cost-effectiveness, we assumed that none of the prophylactically treated individuals represented a case of prevented infection. Because PN was simply an added component with selective screening, the cases identified through selective screening and PN should be (5005 + 218), or 5223, cases. If we considered the number of infections treated, the effectiveness measure would be 3306.
Using the average DALYs lost per case of chlamydia and gonorrhea treated (as derived from World Health Organization estimates for the United States), the effectiveness measures for selective screening and selective screening plus PN were 117.3 and 125.6 DALYs averted, respectively. Table 3 reports the cost-effectiveness ratios of selective screening, selective screening with PN, and the incremental cost-effectiveness ratio for PN over and above selective screening. The cost-effectiveness ratios were expressed using the number of gonorrhea and chlamydia infections treated and the DALYs averted because of the treatment of infected cases.
TABLE 3—
Cost-Effectiveness of Selective Screening and Partner Notification for Chlamydia and Gonorrhea in 2 Sexually Transmitted Disease Clinics: Louisiana, 2010–2012
| Interventions | Cost, Including 30% Overhead | Effectiveness | Cost-Effectiveness |
| Selective screening for chlamydia and gonorrhea | $924 650 | 3 088 infected cases treated | $299/case |
| 117.3 DALYs averted | $7 880/DALY averted | ||
| Selective screening plus telephone-based partner notification | $961 919 | 3 306 infected cases treated | $291/case |
| 125.6 DALYs averted | $7 657/DALY averted | ||
| Partner notification plus selective screening compared with selective screening alone | $37 269 | 218 infected cases treated | $171/additional case treated |
| 8.3 DALYs averted | $4 499/additional DALY averted |
Note. DALY = disability-adjusted life year.
Table 3 shows that both the selective screening and selective screening with PN were highly cost-effective interventions. The incremental cost of identifying an additional case through partner tracing and notification was quite small, and even for highly prevalent STDs, the additional resources needed would not be very high. More importantly, cost per DALY averted implied that selective screening with PN was highly cost-effective. Any intervention with a cost-effectiveness ratio lower than the gross domestic product per capita of the country per DALY averted was categorized as highly cost-effective, and in this case, the cost-effectiveness ratio was approximately 15% to 16% of GDP per capita of the United States.
DISCUSSION
In the United States, most of the STD control programs are not tracing partners for gonorrhea and chlamydia because of the perceived high cost of implementing the partner-tracing mechanism. Although the partner-tracing approach recommended for syphilis is quite expensive, low-cost alternative strategies could be designed. We implemented a low-cost approach to PN and were able to identify and treat a significant number of additional cases, not including the partners who sought treatment without being contacted by the PN system. It was likely that most of these individuals treated by the clinics would have remained untreated in the absence of the PN system. The telephone-based PN approach for gonorrhea and chlamydia, if implemented in the state of Louisiana, would have increased the cost of the STD control program by approximately $380 000 per year. This represented only an approximate 4.1% increase from the current level of expenditure on STD control. Therefore, adding telephone-based PN with selective screening of chlamydia and gonorrhea should be affordable for most of the STD control programs of the country. The cost of identifying and treating 1 additional infected case through telephone-based PN was found to be $171, a fraction of the cost of dealing with 1 complication of untreated gonorrhea or chlamydia. The complications of untreated gonorrhea or chlamydia include but are not limited to infertility in men and women; vertical transmission of infection, which causes blindness or blood infection in newborns; and a significantly higher risk of HIV infection and transmission. The cost-effectiveness of selective screening with telephone-based PN was $4499 per DALY averted, which indicated that the intervention would be highly cost-effective from society’s point of view.
Although the experimental PN system we adopted was based on public clinic patients, using the study parameters should provide reasonable estimates of outcomes and costs for private clinic patients as well. Racial and gender composition of public and private clinic STD patients are very similar, and because of the private sector reimbursement system, it is unlikely that PN would be implemented by the private sector clinics. Currently, partner tracing of all syphilis cases are conducted by the Louisiana STD control program, and PN for gonorrhea and chlamydia would also remain a public sector activity. The cost of identifying partners might differ by socioeconomic and educational status of persons with these diseases, and this might create some bias in the estimation of cost for contacting partners of private patients. The treatment and evaluation costs, however, should remain the same, irrespective of patient characteristics. Therefore, in general, cost and outcome estimates derived from public sector patients could be used to extrapolate state-level outcomes and costs.
Limitations
A number of limitations of the study should be noted. In our study, the time spent by health care providers in various STD-related activities was obtained by interviews with the personnel involved in the provision of services. The subjective reporting of time allocation often resulted in lumping of time and the possibility of overestimation of time spent in each of these tasks. Valuation of time in monetary terms was done by using average wage levels of different personnel types rather than time value of each of the personnel involved. Drugs used for treating patients were valued at Louisiana STD control program price levels, which might have underestimated the true economic value of drugs. Another significant limitation was the exclusion of individuals who were younger than 19 years of age. For a telephone-based PN system, obtaining partner information for this group was problematic because of the requirement for obtaining parental consent. Finally, our calculated effectiveness measures assumed that the individuals prophylactically treated would not have contracted STD if not treated. This was clearly not a valid assumption. For syphilis, published estimates indicate that between 7% and 45% (average of 19%) of prophylactically treated cases may become true STD cases if untreated.13–15 Therefore, the number of infections treated would be higher than the number assumed in the estimation of cost-effectiveness for the PN strategy. Not considering the potential infection cases among the individuals who were prophylactically treated made the cost-effectiveness ratio higher than what it would be. Finally, the PN system could not interview 3 partners because of language barriers. The experimental design assumed that the partners would be either English or Spanish speakers based on the experience of the syphilis PN system in the Louisiana.
Conclusions
Our results indicated that implementing a PN strategy for STDs like gonorrhea and chlamydia was highly cost-effective and should be considered for adoption. If the health care system does not have resources to implement the intensive partner-tracing program, telephone-based PN is a viable alternative. A telephone-based approach is very low cost, and additional resources needed for adopting the intervention should not increase the STD program cost by more than 4.5%. Because of the number of additional cases this PN system could identify, the intervention becomes highly cost-effective in addition to being affordable.
Acknowledgments
This study was supported by a grant (STD Control & Prevention in Louisiana: incremental cost-effectiveness of partner notification and optimal intensity of partner notification, RWJF 67618) from the Robert Wood Johnson Foundation.
We thank the Louisiana STD/HIV Program for providing data and assisting in implementing this research. Detailed comments and suggestions received from three anonymous reviewers of the article are gratefully acknowledged. We also thank Lizheng Shi, PhD, of Tulane University for his assistance in administrative management of the project during the last few months of the study.
Human Participant Protection
This study was approved by the Tulane University human research protection program (institutional review board ref # 10-166798).
References
- 1.Centers for Disease Control and Prevention. Program Operations Guidelines for STD Prevention, 2010. Partner Services. Atlanta, GA: US Department of Health and Human Services; Available at: http://www.cdc.gov/std/program/partners.pdf. Accessed August 18, 2014. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2012. Atlanta, GA: US Department of Health and Human Services; 2013. [Google Scholar]
- 3.Golden MR, Hogben M, Handsfield HH, St. Lawrence JS, Potterat JJ, Holmes KK. Partner notification for HIV and STD in the United States: low coverage for gonorrhea, chlamydial infection, and HIV. Sex Transm Dis. 2003;30(6):490–496. doi: 10.1097/00007435-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Partner notification for preventing human immunodeficiency virus (HIV) infection—Colorado, Idaho, South Carolina, Virginia. MMWR Morb Mortal Wkly Rep. 1988;37(25):393–396. 401–402. [PubMed] [Google Scholar]
- 5.Ferreira A, Young T, Mathews C, Zuna M, Low N. Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev. 2013;10:CD002843. doi: 10.1002/14651858.CD002843.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathews C, Coetzee N, Zwarenstein M et al. A systematic review of strategies for partner notification for sexually transmitted diseases, including HIV/AIDS. Int J STD AIDS. 2002;13(5):285–300. doi: 10.1258/0956462021925081. [DOI] [PubMed] [Google Scholar]
- 7.Macke BA, Maher JE. Partner notification in the United States: an evidence-based review. Am J Prev Med. 1999;17(3):230–242. doi: 10.1016/s0749-3797(99)00076-8. [DOI] [PubMed] [Google Scholar]
- 8.Louisiana Office of Public Health–STD/HIV Program. Disease Intervention Specialist Reference Manual 2010. New Orleans: Louisiana Department of Health and Hospitals; 2010. [Google Scholar]
- 9.Workowski KA, Berman S Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110. Correction in MMWR Recomm Rep. 2011;60(1):18. [PubMed] [Google Scholar]
- 10.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. Oxford, UK: Oxford University Press; 2005. pp. 55–72. [Google Scholar]
- 11.World Health Organization. Death and DALY Estimates for 2004 by cause for countries: persons, all ages data by country. Available at: http://www.who.int/healthinfo/global_burden_disease/estimates_country/en. Accessed May 13, 2014.
- 12.Reynolds SL, Kapadia A, Leonard L, Ross M. Examining the direct costs and effectiveness of syphilis detection by screening and partner notification. J Public Health Med. 2001;23(4):339–345. doi: 10.1093/pubmed/23.4.339. [DOI] [PubMed] [Google Scholar]
- 13.Schober PC, Gabriel G, White P, Felton WF, Thin RN. How infectious is syphilis? Br J Vener Dis. 1983;59:217–219. doi: 10.1136/sti.59.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hook EW, III, Marra CM. Acquired syphilis in adults. N Engl J Med. 1992;326:1060–1069. doi: 10.1056/NEJM199204163261606. [DOI] [PubMed] [Google Scholar]
- 15.Schroeter AL, Turner RH, Lucas JB, Brown WJ. Therapy for incubating syphilis. Effectiveness of gonorrhea treatment. JAMA. 1971;218(5):711–713. [PubMed] [Google Scholar]