Abstract
Objectives. We sought to estimate the association between sedative hypnotic use and motor vehicle crash risk.
Methods. We conducted a new user cohort study of 409 171 adults in an integrated health care system. Health plan data were linked to driver license and collision records. Participants were aged 21 years or older, licensed to drive in Washington State, had at least 1 year of continuous enrollment between 2003 and 2008, and were followed until death, disenrollment, or study end. We used proportional hazards regression to estimate the risk of crash associated with 3 sedatives.
Results. We found 5.8% of patients received new sedative prescriptions, with 11 197 person-years of exposure. New users of sedatives were associated with an increased risk of crash relative to nonuse: temazepam hazard ratio (HR) = 1.27 (95% confidence interval [CI] = 0.85, 1.91), trazodone HR = 1.91 (95% CI = 1.62, 2.25), and zolpidem HR = 2.20 (95% CI = 1.64, 2.95). These risk estimates are equivalent to blood alcohol concentration levels between 0.06% and 0.11%.
Conclusions. New use of sedative hypnotics is associated with increased motor vehicle crash risk. Clinicians initiating sedative hypnotic treatment should consider length of treatment and counseling on driving risk.
Sedative hypnotic medications are commonly prescribed for treatment of insomnia.1–3 Residual sedation is common with sedatives, especially the class of short-acting GABA-agonists commonly referred to as “z-hypnotics” (zolpidem, zopiclone, and zaleplon),4,5 and sedation in itself is a causal factor for many motor vehicle crashes in the United States and abroad.6–8 Little is known about the impact of sedative medications on crash rates in the United States, with most research focused on elderly drivers or in simulation studies.9,10 A recent systematic review of pharmaceutical consumption and traffic safety concluded that larger studies were needed to evaluate the association between overall medication use and traffic crashes.11 Furthermore, the US Food and Drug Administration has issued multiple drug safety communications specifically for zolpidem over the past 2 years, pointedly making the case for patients and physicians to take great care in avoiding sedation while driving.12,13 There are recent changes in the Food and Drug Administration–approved labels for zolpidem products that suggest lower recommended dosing.12
The half-life of insomnia medications, including those with delayed-release dosage formulations, ranges from 1 to 11 hours.14 A longer half-life may promote continued sedation during the morning following a sleep induced by the drug.4 These delayed effects may produce slow reaction time and lack of appropriate judgment by someone operating a motor vehicle. Sedatives may increase the likelihood of crash by modifying 2 processes: (1) judgment governing when to operate a motor vehicle, and (2) increasing drowsiness or delaying reaction times.
Recent research has demonstrated that sedative hypnotic prescriptions may put people at a 3-fold increased risk of premature mortality, with more than 4-fold increased risk in people receiving 90 or more days of medication in the first year of treatment.15,16 A portion of this may be attributable to increased crash risk. Concern about increased crash risk led the Food and Drug Administration to recommend the identification of persons at risk for sedative-related crashes.17 No previous US study has compared the safety of sedatives with regard to crash risk at a population level. However, observational research from Canada, the European Union, and Taiwan suggest that sedative medications are associated with increased risk of motor vehicle crashes.9,18 We sought to investigate the risk of motor vehicle crash associated with sedative hypnotic use, and compared the crash risk associated with individual sedative medications among enrollees of a large health organization in Washington State.
METHODS
Group Health Cooperative (GH) is an integrated delivery system that covers approximately 600 000 individuals in Washington State. The GH enrollee population closely resembles the underlying community within Washington State with respect to age, race/ethnicity, and gender. Information on health plan enrollment, medical encounters, and pharmacy use are recorded and maintained in electronic medical records and automated databases. These data were linked by a unique consumer number assigned to each enrollee.19 The prescription drug formulary at GH included 3 sedative hypnotics during the study period: temazepam, trazodone, and zolpidem. Trazodone, an antidepressant medication, was included because of its near universal use at GH for insomnia rather than depression.
We conducted a retrospective population-based cohort study in the GH population. Eligibility criteria for the study were age 21 to 79 years during the study period (2003–2008), primary residence in Washington State with a Washington State driver’s license, and at least 18 months of continuous GH enrollment (defined as < 60-day lapse in membership) between January 1, 2003, and December 31, 2008, unless the participant died. We also required that each participant have a drug benefit through GH. Follow-up went through the earliest of end of the study period (December 31, 2008), disenrollment, 80 years of age, or death.
Data
Among the eligible population, we developed a data set linking GH administrative, medical encounter, and pharmacy records with Washington State Department of Licensing driving license records and Department of Transportation motor vehicle crash records. We extracted demographic characteristics, medical encounters, and prescription records for each study participant. We calculated the Charlson Comorbidity Index for each participant by using an adapted algorithm based on International Classification of Diseases, Ninth Revision, Clinical Modification codes from the administrative database.20 We used the algorithm described by Gallian (using complete name and date of birth) to calculate driver’s license numbers for study participants.21 All police-reported crashes during the study period in which the primary driver was listed as matching a study driver’s license number were returned by the Department of Transportation.
We extracted dispensings of sedative hypnotics on the GH formulary (trazodone [Desyrel], temazepam [Restoril], and zolpidem [Ambien, Ambien CR]) during the study period from the GH pharmacy database. Dispensing data included the drug name, date of dispensing, strength, intended days supplied, and National Drug Code.
We defined duration of exposure by collapsing individual dispenses into periods of continuous use (episodes). We defined exposure to sedative hypnotics as a time-varying covariate with the start date defined as the date of dispensing of the first sedative prescription. We assumed an 80% compliance factor (1.25) multiplied by the days supplied for each dispensing to determine the run-out date of the dispensing.22,23 We defined the period of continuous use as within the compliance-adjusted run-out date of one dispensing and fill date of the subsequent dispensing. We defined the end date of a continuous episode as the run-out date of the last prescription in the continuous episode.23 We evaluated only the initial continuous exposure episode for each participant to maintain a new-user study design and avoid healthy user bias. We matched motor vehicle crash records to the periods of exposure and nonexposure.
We implemented a new-user study design by using the first 3 months of eligibility for each participant to identify current sedative use and exclude those users from the study.24 We then followed the nonuser group to identify new sedative use in the remaining years of the study. We also stratified exposure by the length (days) of continuous sedative prescriptions (1–30, 31–120, 121–240, 241–360, and ≥ 361 concurrent days).
Statistical Analyses
We used Cox proportional hazards regression to estimate the multivariable adjusted hazard ratio (HR) and 95% confidence interval (CI) for sedative medication use with motor vehicle crash as the outcome. This approach applied a nonparametric time-to-event model, which assumed that the instantaneous rate of crash is constantly proportional between the exposed and unexposed groups over time. By employing robust sandwich estimator standard errors in the model, we allowed for nonlinearity of HRs between the exposed and unexposed groups.25,26 We tested the proportional hazards assumption with visual assessment of plotted cumulative sums of the Martingale residuals as well as the Kolmogorov-type supremum test using 1000 simulations.27
We first defined all 3 sedatives of interest as a composite exposure to estimate the overall association of sedatives with crash risk. We then individually estimated the association for each medication, with linear hypothesis tests for the equality of the estimates. We adjusted all multivariate models for age (quadratic term), gender, calendar year of exposure (categorical), prescription opioid dispensings, and Charlson Comorbidity Index at study entry (categorized into 0, 1, 2, and 3 or greater with 0 serving as the reference group). We considered other prescription medications (antiseizure, antidementia, antidepressant) and medical diagnoses (neurological and cardiovascular disorders) as possible confounders on the basis of a recent National Highway Traffic Safety report28 but none were found to be associated with both the exposure and outcome and we therefore omitted them from the final models.
We also translated the model-based HR estimates into blood alcohol concentration (BAC) equivalents by using data from Peck et al.29 To do so, we matched the HRs found in our analyses with the relative risk values in Peck et al. for participants aged 21 to 55 or more years of age and extracted the BAC equivalents that were associated with that relative risk. Although it is not an exact match, this translation provides the relative context necessary for interpreting the risk of crash.
We generated all analyses with SAS software for Windows, version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
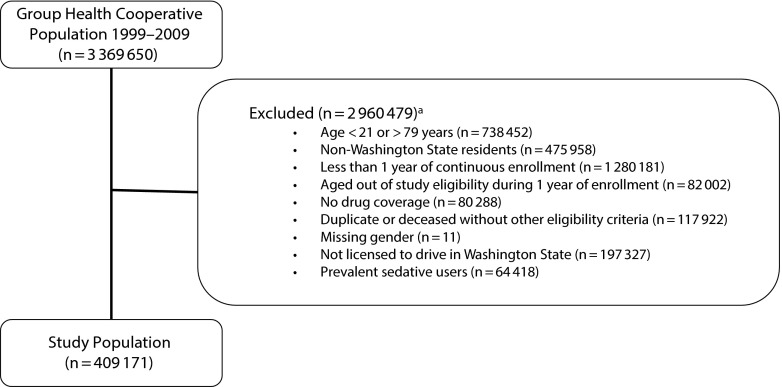
The final cohort comprised 409 171 participants (Figure 1). Selected baseline demographic characteristics, medication exposures, and frequency counts of motor vehicle crashes for the study cohort are reported in Table 1. The median age of the study population was 42 years. Slightly more than half (54%) were female, and most (94%) were relatively healthy (Charlson Comorbidity Index score of 0). Among our sample, 5.8% of patients (11 197 person-years; n = 23 803) had incident sedative hypnotic use. Non–sedative users contributed 1.5 million person-years of unexposed time to the study. Among the cohort, the police-reported crash rate was 27 per 1000 person-years, 10% of whom had experienced more than 1 crash during the study period.
FIGURE 1—
Study enrollment flow: adults in Washington State Group Health Cooperative with a Washington State driver’s license, 2003–2008.
aExcluded subjects may have met more than 1 exclusion criteria
TABLE 1—
Selected Characteristics of Study Cohort at Baseline, Exposures and Outcomes: Sedative Hypnotic Use and Risk of Motor Vehicle Crash Among Adult Group Health Cooperative Enrollees, Washington State, 2003–2008
| Characteristic | New User Cohort (n = 409 171) |
| Male gender, no. (%) | 190 810 (46.6) |
| Age, y, median (IQR) | 42.0 (30–54) |
| Charlson Comorbidity Index, % | |
| 0 | 94.8 |
| 1 | 3.5 |
| 2 | 1.1 |
| ≥ 3 | 0.6 |
| Medication exposure time (person-years) | |
| Temazepam | 1 657 |
| Trazodone | 7 725 |
| Zolpidem | 1 816 |
| Motor vehicle crashes, no. (%) | |
| None | 385 880 (94.3) |
| ≥ 1 | 23 291 (5.7) |
Note. IQR = interquartile range.
Trazodone was the most commonly prescribed sedative (56%), followed by temazepam (22%) and zolpidem (22%). The model coefficients for the 4 Cox regressions of crash risk associated with new sedative use are reported in Table 2. Incident use of any sedative was associated with an increased risk of crashes (HR = 1.90; 95% CI = 1.63, 2.20) compared with nonuse. New prescriptions dispensed for each sedative were associated with an increased risk of crash relative to nonuse: temazepam (HR = 1.27; 95% CI = 0.85, 1.91), followed by trazodone (HR = 1.91; 95% CI = 1.62, 2.25) and zolpidem (HR = 2.20; 95% CI = 1.64, 2.95). The difference between trazodone and temazepam was not statistically significant (P = .081), but the differences between trazodone and zolpidem, and temazepam and zolpidem were (P = .013 and P = .001, respectively). The Kolmogorov-type supremum test indicated that the proportional hazards assumption was not violated (P = .23).
TABLE 2—
Incident Sedative Users Model Estimates: Adult Group Health Cooperative Enrollees, Washington State, 2003–2008
| Medication Exposure | HR (95% CI) | P for Equivalence With Zolpidem |
| All sedatives | 1.90 (1.63, 2.20) | |
| Trazodone | 1.91 (1.62, 2.25) | .013 |
| Temazepam | 1.27 (0.85, 1.91) | .001 |
| Zolpidem | 2.20 (1.64, 2.95) | Ref |
Note. CI = confidence interval; HR = hazard ratio. All models adjusted for gender, age, calendar year, Charlson Comorbidity Index at study entry, and coprescription of opioids.
Stratification by the length of the continuous use among incident users revealed a range of HRs with varying peaks in risk during the first year of therapy that decreased as the length of exposure increased. The overall peak of crash risk occurred between 121 and 240 days of exposure (HR = 4.14; 95% CI = 2.96, 5.78), with this primarily driven by the risk of crash from trazodone during this time period (HR = 4.20; 95% CI = 2.91, 6.05). Yet, the peak for zolpidem occurred earlier, between 31 and 120 days (HR = 5.69; 95% CI = 3.14, 10.29). After 1 or more years of continuous therapy, there was a significant ongoing increased crash risk among those prescribed trazodone (HR = 1.27; 95% CI = 1.02, 1.59) or zolpidem (HR = 1.76; 95% CI = 1.21, 2.55), and overall across all sedatives (HR = 1.35; 95% CI = 1.11, 1.65; Table 3).
TABLE 3—
Incident Sedative Use by Exposure Length Model Estimates and Blood Alcohol Concentration Equivalents: Adult Group Health Cooperative Enrollees, Washington State, 2003–2008
| Medication Exposure | HR (95% CI) | BAC Equivalents Crash Risk Range, % |
| All sedatives | ||
| 0–30 d | 1.98 (1.44, 2.72) | 0.07–0.10 |
| 31–120 d | 3.47 (2.45, 4.91) | 0.10–0.13 |
| 121–240 d | 4.14 (2.96, 5.78) | 0.11–0.14 |
| 241–360 d | 3.17 (2.24, 4.48) | 0.09–0.13 |
| > 360 d | 1.35 (1.11, 1.65) | 0.05–0.08 |
| Trazodone | ||
| 0–30 d | 2.00 (1.38, 2.91) | 0.07–0.11 |
| 31–120 d | 3.52 (2.42, 5.14) | 0.10–0.13 |
| 121–240 d | 4.20 (2.91, 6.05) | 0.11–0.14 |
| 241–360 d | 3.85 (2.71, 5.47) | 0.10–0.13 |
| > 360 d | 1.27 (1.02, 1.59) | 0.04–0.08 |
| Temazepam | ||
| 0–30 d | 1.65 (0.86, 3.15) | 0.00–0.11 |
| 31–120 d | 1.62 (0.57, 4.59) | 0.00–0.13 |
| 121–240 d | 1.47 (0.44, 4.85) | 0.00–0.13 |
| 241–360 d | 2.69 (1.24, 5.84) | 0.06–0.14 |
| > 360 d | 0.89 (0.52, 1.53) | 0.00–0.08 |
| Zolpidem | ||
| 0–30 d | 1.61 (0.73, 3.53) | 0.00–0.12 |
| 31–120 d | 5.69 (3.14, 10.29) | 0.11–0.16 |
| 121–240 d | 4.26 (2.18, 8.32) | 0.09–0.15 |
| 241–360 d | 2.23 (0.99, 5.02) | 0.04–0.13 |
| > 360 d | 1.76 (1.21, 2.55) | 0.05–0.10 |
Note. BAC = blood alcohol concentration; CI = confidence interval; HR = hazard ratio. All models adjusted for gender, age, calendar year, Charlson Comorbidity Index at study entry, and coprescription of opioids.
We mapped the incident sedative hypnotic exposure estimates from fully saturated models to BAC level risks (Table 3). Incident sedative use for less than 1 year was associated with BAC levels of between 0.07% and 0.14%.
DISCUSSION
This study suggests that filled prescriptions for 3 different types of sedative hypnotic agents were associated with increased risk of motor vehicle crash for new users of these medications. Sedative hypnotic exposure nearly doubled the risk of crash in new users. Of those evaluated, temazepam appears to be the sedative with the lowest risk of motor vehicle crashes. Translating these results to BAC equivalents suggests that the risk of motor vehicle crash in sedative hypnotic users is similar to that found in alcohol intoxication, exceeding the US legal limit to operate a motor vehicle.30
When we examined the length of continuous exposure to sedative medications, there was a trend toward increased risk over time, with a peak between 1 and 4 months for zolpidem but at 4 to 8 months for trazodone and 8 to 12 months for temazepam. The risk of crash decreased over time for all 3 medications, yet there remained an increased risk of crash even after 1 year of prescription fills for both trazodone and zolpidem. The decreasing risk over the length of the study may result from tolerance to the sedative properties of the medications or, alternatively, it may reflect adjustment of driving behaviors on the basis of perception of risky behaviors.
The largest body of evidence regarding the risk of motor vehicle collisions and sedative hypnotics comes from Scandinavia and The Netherlands.31–33 However, only 1 study from that region has been able to compare commonly used hypnotic sleep medications.34 The largest studies, a registry-based cohort from Norway, evaluated almost 13 000 collisions among the 3.1 million residents in 2004 to 2005 and examined the association with exposure to several drug classes. The first study found a standardized incidence ratio (SIR) of crash for benzodiazepine hypnotics of 3.3 (95% CI = 2.1, 4.7). For comparison, the SIR for calcium receptor antagonists was 0.9 (95% CI = 0.5, 1.5).29 Gustavsen et al. followed this same national cohort from 2004 to 2006, and compared the risks associated with 2 z-hypnotics (zopiclone and zolpidem) and 2 benzodiazepines (flurnitrazepam and nitrazepam).34 That study found the highest risk of crash in users of flurnitrazepam with an SIR of 4.0 (95% CI = 2.4, 6.4), with zolpidem of lower risk at an SIR of 2.2 (95% CI = 1.4, 3.4). One recent additional study from Taiwan examined 5183 participants with a diagnosis code of a motor vehicle accident in a matched case–control methodology and found that 1 month of use of benzodiazepines, hypnotics, or z-hypnotics was associated with odds ratios for crash of 1.68 (95% CI = 1.50, 1.88), 1.80 (95% CI = 1.43, 2.26), and 1.63 (95% CI = 1.32, 2.01), respectively.18
Other investigators have examined simulated driving behavior, which can be informative, but differs from real driving behavior. Eighteen healthy elderly participants were studied by Leufkens and Vermeeren comparing temazepam, zopiclone, and placebo treatment 10 to 11 hours before a standardized driving test. In this study, zopiclone was found to affect driving performance, but temazepam’s effect was similar to placebo.32 Partinen et al. undertook a 3-arm, double-blind, placebo-controlled crossover study of zolpidem and temazepam, studying the effect of taking these drugs after midnight on driving simulator ability the following morning. This was a small study of female participants (n = 18) and the primary outcome of time to simulated crash did not differ between the groups. There was a statistically significant difference in lane position deviation: zolpidem produced greater deviations than either temazepam (0.135 meters of deviation; P = .05) or placebo (0.117 meters of deviation; P = .025).35 Therefore, although an association between the sedative hypnotics and motor vehicle crashes likely exists, previous research has not evaluated crash risk for different sedative medications. Furthermore, the largest cohort studies have not been replicated to examine the validity or generalizability of its results beyond the Norwegian population.
Limitations
This study has a number of important limitations. As with all observational research, there is the possibility for confounding by indication. Medical conditions being treated by the drug exposures may be independently associated with crash and such confounding may bias our model estimates. Yet these conditions are not typically acute or rapid onset, thus allowing participants to contribute both exposed and unexposed time, which should mitigate this bias at the individual patient level.36,37 Trazodone is indicated for use to treat major depressive disorder but is used off-label for insomnia; thus, the underlying conditions for which study participants were undergoing treatment may have varied compared with the other sedatives.
Second, our ascertainment of exposure in the retrospective analysis was based on prescription dispensing records at GH and may not represent actual ingestion of medications in the period preceding a crash.
Third, we are unable to ascertain the actual driving status of the study participants; thus, we do not know if systematic differences exist between the exposed and nonexposed participants with regard to actual driving behaviors, experience, and total miles driven, though we restricted study participation to those individuals who were licensed to drive during the study period.
Fourth, we were unable to evaluate alcohol or illicit drug use as potential confounders. We only had access to alcohol status for participants who crashed and these data were incomplete (22% unknown or missing). Concomitant use of alcohol with sedatives is discouraged because of synergistic effects and could be an important area for future research.
Fifth, the relative sedative effects of the 3 chemicals we studied are not well understood and cannot be proportionately adjusted for. Finally, we evaluated people aged 21 to 79 years from a single large health plan in Washington State, which could affect generalizability beyond GH.
Conclusions
The 3 sedative hypnotics included in our study appear to be associated with increased risk of motor vehicle crash, with the risk depending on the sedative being used and the length of continuous exposure. The potential for residual confounding by underlying sleep disorders cannot be ignored. Future research is needed to investigate various sleep disorders and risk of crash to identify whether the medications or the sleep disorders are the causal factor in this association.
Additional important areas for future research include the latency of medication ingestion in relation to crashes, sedative dose, tolerance to these medications, concurrent consumption of other substances, and the link between sedatives and fatal crashes, as well as further investigation into the factors that influence crash risk related to the length of prescription exposure. Identifying high-risk prescriptions may improve public health by educating the prescribers and users of these medications about the particular types of exposures that could increase an individual’s likelihood of crashing.
Depending on an individual’s need to drive regularly combined with a medical indication for sedative use, the choice of a particular sedative may affect the risk of crashing. Prescribers, pharmacists, and patients should discuss this potential risk and consider the implications of this analysis when selecting a sedative hypnotic medication. Physicians may also wish to consider nonsedating approaches to encouraging healthy sleep. In the interest of the public safety on the roads and highways in the United States, individuals who have been prescribed sedative hypnotic medications should be counseled about driving risk and alternative transportation strategies addressed when under the influence of these medications. Future research should evaluate whether this association extends to crash-associated mortality.
Acknowledgments
We wish to acknowledge Thomas D. Koepsell, MD, MPH, for his contributions to this research.
Human Participant Protection
This study was approved by the Group Health Research Institute institutional review board.
References
- 1.Zammit G. Comparative tolerability of newer agents for insomnia. Drug Saf. 2009;32(9):735–748. doi: 10.2165/11312920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Botteman M. Health economics of insomnia therapy: implications for policy. Sleep Med. 2009;10(suppl 1):S22–S25. doi: 10.1016/j.sleep.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. 2009;13(3):205–214. doi: 10.1016/j.smrv.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Gunja N. The clinical and forensic toxicology of z-drugs. J Med Toxicol. 2013;9(2):155–162. doi: 10.1007/s13181-013-0292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul MA, Gray G, MacLellan M et al. Sleep-inducing pharmaceuticals: a comparison of melatonin, zaleplon, zopiclone, and temazepam. Aviat Space Environ Med. 2004;75(6):512–519. [PubMed] [Google Scholar]
- 6.Cummings P, Koepsell TD, Moffat JM, Rivara FP. Drowsiness, counter-measures to drowsiness, and the risk of a motor vehicle crash. Inj Prev. 2001;7(3):194–199. doi: 10.1136/ip.7.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlm K, Bjornstigb U, Ostrom M. Alcohol and drugs in fatally and non-fatally injured motor vehicle drivers in northern Sweden. Accid Anal Prev. 2009;41(1):129–136. doi: 10.1016/j.aap.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Borkenstein RF, Crowther RP, Shumate RP, Ziel HB, Zylman R. Bloomington, IN: Department of Police Administration, Indiana University; 1964. The role of the drinking driver in traffic accidents. [Google Scholar]
- 9.Ray WA, Fought RL, Decker MD. Psychoactive drugs and the risk of injurious motor vehicle crashes in elderly drivers. Am J Epidemiol. 1992;136(7):873–883. doi: 10.1093/aje/136.7.873. [DOI] [PubMed] [Google Scholar]
- 10.Hetland A, Carr DB. Medications and impaired driving. Ann Pharmacother. 2014;48(4):494–506. doi: 10.1177/1060028014520882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orriols L, Salmi LR, Philip P et al. The impact of medicinal drugs on traffic safety: a systematic review of epidemiological studies. Pharmacoepidemiol Drug Saf. 2009;18(8):647–658. doi: 10.1002/pds.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDA Drug Safety Communication. Risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist) 2013. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm334033.htm. Accessed September 9, 2014.
- 13.FDA Drug Safety Communication. FDA approves new label changes and dosing for zolpidem products and a recommendation to avoid driving the day after using Ambien CR. 2013. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm352085.htm. Accessed September 9, 2014.
- 14.Micromedex 2.0. Available at: http://www.thomsonhc.com/micromedex2. Accessed May 3, 2010.
- 15.Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2(1):e000850. doi: 10.1136/bmjopen-2012-000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weich S, Pearce HL, Croft P et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ. 2014;348:g1996. doi: 10.1136/bmj.g1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farkas RH, Unger EF, Temple R. Zolpidem and driving impairment—identifying persons at risk. N Engl J Med. 2013;369(8):689–691. doi: 10.1056/NEJMp1307972. [DOI] [PubMed] [Google Scholar]
- 18.Chang CM, Wu EC, Chen CY et al. Psychotropic drugs and risk of motor vehicle accidents: a population-based case–control study. Br J Clin Pharmacol. 2013;75(4):1125–1133. doi: 10.1111/j.1365-2125.2012.04410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders KW, Davis RL, Stergachis A. Group health cooperative. In: Strom BL, editor. Pharmacoepidemiology. 4th ed. West Sussex, England: John Wiley and Sons; 2005. pp. 223–239. [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.Gallian JA. Assigning driver’s license numbers. Math Mag. 1991;64(1):13–22. [Google Scholar]
- 22.Boudreau DM, Yu O, Chubak J et al. Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res Treat. 2014;144(2):405–416. doi: 10.1007/s10549-014-2870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Psaty BM, Heckbert SR, Koepsell TD et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274(8):620–625. [PubMed] [Google Scholar]
- 24.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 25.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 26.Barlow WE. Robust variance estimation for the case–cohort design. Biometrics. 1994;50(4):1064–1072. [PubMed] [Google Scholar]
- 27.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 28.LeRoy AA, Morse ML. Washington, DC: National Highway Traffic Safety Administration; 2008. Multiple medications and vehicle crashes: analysis of databases. DOT HS 810 858. [Google Scholar]
- 29.Peck RC, Gebers MA, Voas RB, Romano E. The relationship between blood alcohol concentration (BAC), age, and crash risk. J Safety Res. 2008;39(3):311–319. doi: 10.1016/j.jsr.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 30.DUI/DWI laws March 2012. Insurance Institute for Highway Safety. Available at: http://www.iihs.org/laws/dui.aspx. Accessed March 3, 2012.
- 31.Engeland A, Skurtveit S, Morland J. Risk of road traffic accidents associated with the prescription of drugs: a registry-based cohort study. Ann Epidemiol. 2007;17(8):597–602. doi: 10.1016/j.annepidem.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Leufkens TR, Vermeeren A. Highway driving in the elderly the morning after bedtime use of hypnotics: a comparison between temazepam 20 mg, zopiclone 7.5 mg, and placebo. J Clin Psychopharmacol. 2009;29(5):432–438. doi: 10.1097/JCP.0b013e3181b57b43. [DOI] [PubMed] [Google Scholar]
- 33.Bezemer KDB, Smink BE, van Maanen R, Verschraagen M, de Gier JJ. Prevalence of medicinal drugs in suspected impaired drivers and a comparison with the use in the general Dutch population. Forensic Sci Int. 2014;241:203–211. doi: 10.1016/j.forsciint.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Gustavsen I, Bramness JG, Skurtveit S, Engeland A, Neutel I, Morland J. Road traffic accident risk related to prescriptions of the hypnotics zopiclone, zolpidem, flunitrazepam and nitrazepam. Sleep Med. 2008;9(8):818–822. doi: 10.1016/j.sleep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Partinen M, Hirvonen K, Hublin C, Halavaara M, Hiltunen H. Effects of after-midnight intake of zolpidem and temazepam on driving ability in women with non-organic insomnia. Sleep Med. 2003;4(6):553–561. doi: 10.1016/j.sleep.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Suissa S. Statistical methods in pharmacoepidemiology: advances and challenges. Stat Methods Med Res. 2009;18(1):3–6. doi: 10.1177/0962280208099879. [DOI] [PubMed] [Google Scholar]
- 37.Signorello LB, McLaughlin JK, Lipworth L, Friis S, Sorensen HT, Blot WJ. Confounding by indication in epidemiologic studies of commonly used analgesics. Am J Ther. 2002;9(3):199–205. doi: 10.1097/00045391-200205000-00005. [DOI] [PubMed] [Google Scholar]