Abstract
Here, we evaluated changes in autophagy after post-traumatic brain injury (TBI) followed by moderate hypothermia in rats. Adult male Sprague-Dawley rats were randomly divided into four groups: sham injury with normothermia group (37°C); sham injury with hypothermia group (32°C); TBI with normothermia group (TNG; 37°C); and TBI with hypothermia group (THG; 32°C). Injury was induced by a fluid percussion TBI device. Moderate hypothermia (32°C) was achieved by partial immersion in a water bath (0°C) under general anesthesia for 4 h. All rats were killed at 24 h after fluid percussion TBI. The ipsilateral hippocampus in all rats was analyzed with hematoxylin and eosin staining; terminal deoxynucleoitidyl transferase-mediated nick end labeling staining was used to determine cell death in ipsilateral hippocampus. Immunohistochemistry and western blotting of microtubule-associated protein light chain 3 (LC3), Beclin-1, as well as transmission electron microscopy performed to assess changes in autophagy. At 24 h after TBI, the cell death index was 27.90±2.36% in TNG and 14.90±1.52% in THG. Expression level of LC3 and Beclin-1 were significantly increased after TBI and were further up-regulated after post-TBI hypothermia. Further, ultrastructural observations showed that there was a marked increase of autophagosomes and autolysosomes in ipsilateral hippocampus after post-TBI hypothermia. Our data demonstrated that moderate hypothermia significantly attenuated cell death and increased autophagy in ipsilateral hippocampus after fluid percussion TBI. In conclusion, autophagy pathway may participate in the neuroprotective effect of post-TBI hypothermia.
Key words: : autophagy, cell death, hippocampus, moderate hypothermia, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major cause of morbidity and mortality. Hippocampal injury post-TBI might explain some of the morbidity observed in humans.1 There are two waves of neuronal cell death post-TBI. In the first wave, neuronal death results from the necrosis caused by membrane disruption, irreversible metabolic disturbance, and/or excitotoxicity immediately after mechanical trauma resulting from impact or penetration. During the second wave (6–48 h post-TBI), morphological features of apoptosis and necrosis occur in a more delayed fashion.2,3 In our previous study, we found that fluid percussion TBI induced significant cell death in ipsilateral hippocampus at 24 h post-TBI.3
Macroautophagy (hereafter referred to as autophagy) is a catabolic process in which cells degrade their own components by enveloping them in double-membrane vesicles referred to as autophagosomes that are then targeted for lysosomal degradation.4 Recently, a series of studies has reported the increase of autophagy in subarachnoid hemorrhage, intracerebral hemorrhage, hypoxia-ischemia, neurodegenerative disease, and TBI.5–10 However, the function of autophagy in TBI remains controversial. Some studies have proposed that activation of the autophagy pathway serves as a protective mechanism for maintaining cellular homeostasis post-TBI whereas other studies suggested that inhibition of the autophagy pathway may attenuate traumatic damage and functional outcome deficits.9–14
Several clinical and experimental studies have shown the neuroprotective effects of mild-to-moderate hypothermia, including inhibition of neurological injury, reduction of infarct size, and improvement of neurological outcome.15–18 In our previous studies, we found that post-traumatic moderate hypothermia significantly attenuated cell death within the hippocampus after fluid percussion TBI.3 However, precise mechanisms underlying this phenomenon are still unclear. In the present study, we sought to determine the changes in autophagy after post-TBI moderate hypothermia and the possible role of the autophagy pathway.
Methods
Animals
Adult male Sprague-Dawley rats (320–380 g) were randomly divided into four groups: sham injury with normothermia (SNG; 37°C, n=40); sham injury with hypothermia (SHG; 32°C, n=40); TBI with normothermia (TNG; 37°C, n=40); and TBI with hypothermia (THG; 32°C, n=40). All animal procedures were approved by the animal care and experimental committee of the School of Medicine at Shanghai Jiaotong University (Shanghai, China). Rats were housed in individual cages in a temperature- and humidity-controlled animal facility with 12-h light/dark cycle. Rats were housed in the animal facility for at least 7 days before surgery and were given free access to food and water during this period.
Surgical preparation
Rats were anesthetized by intraperitoneal (i.p.) injection of 10% chloral hydrate (3.3 mL/kg). Rats were mounted in a stereotaxic frame, an incision was made along the midline of the scalp, and a 4.8-mm diameter craniectomy was performed on the left parietal bone (midway between the bregma and lambda). A rigid plastic injury tube (modified Leur-loc needle hub, 2.6 mm inside diameter) was secured over the exposed, intact dura using cyanoacrylate adhesive. Two skull screws (2.1 mm diameter, 6.0 mm length) were placed in burr holes, 1 mm rostral to the bregma and 1 mm caudal to the lambda. The injury tube was secured to the skull with dental cement. Bone wax was used to cover the open needle hub connector after the dental cement hardened (5 min). The scalp was closed by sutures and animals were returned to their cages for recovery.
Lateral fluid percussion brain injury
A fluid percussion device (Virginia Commonwealth University Biomedical Engineering, Richmond, VA) was used to create TBI, as described in detail previously.19,20 Rats were subjected to TBI 24 h after the surgical procedure to minimize the possible confounding factors of surgery. In brief, the device is consisted of a Plexiglas cylindrical reservoir filled with 37°C isotonic saline. One end of the reservoir had a rubber-covered Plexiglas piston mounted on O-rings, and the opposite end had a pressure transducer housing with a 2.6-mm inside diameter male needle hub opening.
On the day of TBI, rats were anesthetized with 10% chloral hydrate (3.3 mL/kg, i.p.) and endotracheally intubated for mechanical ventilation. The resulting pressure pulse was measured in atmospheres (atm) using an extracranial transducer (Statham PA 85-100; Gloud, Oxnard, CA) and recorded on a storage oscilloscope (Tektronix 5111; Tektronix, Beaverton, OR). The suture was opened and bone wax was removed. Rats were disconnected from the ventilator, and the injury tube was connected to the fluid percussion cylinder. Then, a fluid pressure pulse was applied for 10 ms directly onto the exposed dura to produce moderate TBI (mTBI; 2.1–2.2 atm). The injury was delivered to within 10 sec after disconnecting from the ventilator. After the initial observation of apneic episodes, rats were ventilated with a 2:1 nitrous oxide/oxygen mixture without isoflurane and rectal and temporalis muscle temperature were recorded. Then, the needle hub, screws, and dental cement were removed from the skull and the scalp was sutured closed. Rats were extubated as soon as spontaneous breathing was observed. Sham TBI group rats were subjected to the same anesthetic and surgical procedures as those in the other group, but without being subjected to injury. Throughout the procedure, mean arterial blood pressure (MABP) was monitored continuously and blood gases were measured 15 min before and after fluid percussion injury (FPI).
Manipulation of temperature
Frontal cortex brain temperature was monitored with a digital electronic thermometer (model DP 80; Omega Engineering, Stamford, CT) and a 0.15-mm diameter temperature probe (model HYP-033-1-T-G-60-SMP-M; Omega Engineering) inserted 4.0 mm ventral to the surface of the skull. The probe was removed before FPI and replaced immediately after injury. Rectal temperatures were measured with an electronic thermometer with analog display (model 43 TE; YSI, Yellow Springs, OH) and a temperature probe (series 400; YSI). A brain temperature of 32°C was achieved by immersing the body of the anesthetized rat in ice-cold water.21 Skin and fur of all animals were protected from direct contact with water by placing the animal in a plastic bag (head exposed) before immersion. Animals were removed from the water bath when brain temperature was reduced to within 2°C of the target temperature. It took approximately 30 min to reach the target brain temperatures, which were maintained for 4 h under general anesthesia in room temperature by intermittent application of ice packs, as needed. A brain temperature of 37°C was achieved under general anesthesia with a heating blanket.
Hematoxylin and eosin staining
Rats were subjected to deep anesthesia by 10% chloral hydrate. At 24 h post-TBI, all rats were perfused transcardially by 4% paraformaldehyde in phosphate-buffered saline (PBS). Brains were removed, further fixed at 4°C overnight, and immersed in 30% sucrose/PBS at 4°C overnight. Specimens were mounted in optimum cutting temperature (OCT) compound. Serial sections were obtained in a cryostat and stained with toluidine blue for 30 min and then 2–3 drops of glacial acetic acid. Once the nucleus and granulation were clearly visible, sections were mounted in Permount or Histoclad.
Sections were cut in a microtome and adhered to glass slides with polylysine. Images of the ipsilateral hippocampus were captured at 100× by using a microscope (Nikon Labophot; Nikon USA, Melville, NY). Specimens were examined by two pathologist (blinded to group conditions) to identify cell death based on characteristic cellular morphological changes. There were eight rats in each of the four groups.
Terminal deoxynucleoitidyl transferase-mediated nick end labeling staining
Terminal deoxynucleoitidyl transferase-mediated nick end labeling (TUNEL) was used to detect cell death in the ipsilateral hippocampus. TUNEL assay was performed according to the manufacturer's directions by using the In Situ Cell Death Detection Kit (Roche, Indianapolis, IN). In brief, sections were washed for 30 min, 10 min each. Then, sections were incubated in 0.1% Triton-X and 0.1% sodium citrate and rinsed thrice with PBS for 10 min each time. Sections were incubated in 0.3% H2O2 in PBS/0.1% Tween-20 for 30 min to inhibit endogenous peroxidase activity and then rinsed with PBS. Sections were then incubated with 50 μL of TUNEL reaction mixture (In Situ Cell Death Detection Kit, Fluorescein; Roche) in a humidified atmosphere for 60 min at 37°C in the dark. Then, sections were rinsed thrice with PBS. Sections were then observed under a fluorescence microscope (Nikon TE300; Nikon, Tokyo, Japan). Negative control was carried out by incubating of sections in 50 μL of label solution without terminal transferase instead of TUNEL reaction mixture per well. At least 10 randomly selected microscopic fields were used for counting the TUNEL-positive cells (200× magnifications). Cell counting was conducted by an investigator blinded to group conditions. There were 8 rats in each of the four groups.
Detection of autophagy-related proteins microtubule-associated protein light chain 3 and Beclin-1
Immunohistochemical analysis was performed on 4-μm-thick formalin-fixed cryosections in the ipsilateral hippocampal injury region to determine the immunoreactivity of microtubule-associated protein light chain 3 (LC3) and Beclin-1.
Immunohistochemical analysis
The 4-μm-thick formalin-fixed, OCT-embedded sections were subjected to immunofluorescence analysis to determine immunoreactivity of LC3 and Beclin-1. Endogenous peroxidase was blocked with 3% H2O2 for 5 min, followed by a brief rinse in distilled water and a 15-min wash in PBS. Sections were cooled at room temperature for 20 min and rinsed in PBS. Nonspecific protein binding was blocked by incubation in 5% horse serum for 40 min. Sections were incubated with primary antibodies (Abs; anti-LC3 [CST3868], anti-beclin-1 [CST3738], and anti-IgG [immunoglobulin G], all diluted 1:200; Suzhou Ard Biological Co., Ltd., Shanghai, China) for 1 h at room temperature, followed by a 15-min wash in PBS. Sections were incubated with fluorescein isothiocyanate/cyanin 3–conjugated IgG (1:500 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 60 min at room temperature. For negative controls, sections were incubated in the absence of a primary Ab. Ten microscopic fields per each section were photographed randomly for counting the LC3 and Beclin-1-positive cells (200× magnifications, Nikon TE300; Nikon). An investigator blinded to group conditions collected all nonbiased stereological data for the study. There were 8 rats in each of the four groups.
Western blot analysis
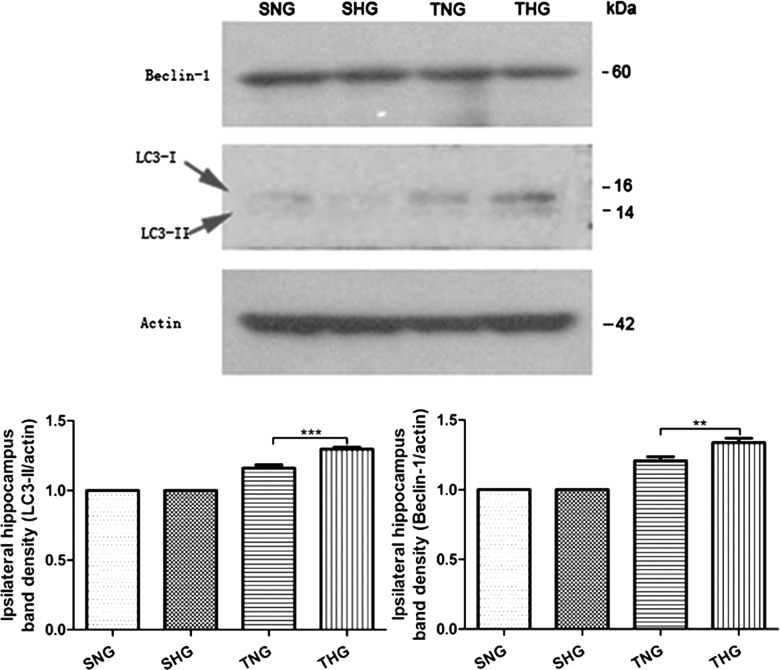
Four animals were run at each time point. Frozen brain samples were mechanically lysed in 20 mM of Tris (pH 7.6), containing 0.2% sodium dodecyl sulfate (SDS), 1% Triton X-100, 1% deoxycholate, 1 mM of phenylmethylsulphonyl fluoride, and 0.11 IU/Ml of aprotinin (all purchased from Sigma-Aldrich, St. Louis, Mo). Lysates were centrifuged at 12,000g for 20 min at 4°C. Protein concentration was estimated by Bradford's method. Samples (60 μg/lane) were separated by 12% SDS polyacrylamide gel electrophoresis and electrotransferred onto a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). The membrane was blocked with 5% skimmed milk for 1 h at room temperature and incubated with primary Abs against LC3 and Beclin-1 (1:1000 dilutes, respectively, both from CST, Inc., Danvers, MA) at 4°C overnight. Glyceraldehyde-3-phosphate dehydrogenase (diluted 1:10,000; Sigma-Aldrich) was used as the loading control. After the membrane was washed three times in Tris-buffered saline (TBS)+Tween-20 (TBST) for 10 min each, it was incubated in the appropriate horseradish-peroxidase–conjugated secondary Ab (diluted 1:10,000 in TBST) for 2 h. Then, the membrane was washed three times in TBST for 10 min each. Blotted protein bands were visualized by enhanced chemiluminescence Western blot detection reagents (Millipore, Kankakee, IL) and exposed to X-ray film. Developed films were digitized using an Epson Perfection 2480 scanner (Seiko Corp, Nagano, Japan). Results were quantified by Quantity One Software (Bio-Rad). Band density values were calculated as a ratio of LC3 and Beclin-1/β-actin, and values from SNG were used as 100%.There were 8 rats in each of the four groups.
Transmission electron microscopy
Samples for electron microscopy were fixed in phosphate-buffered glutaradehyde (2.5%) and osmium tetroxide (1%). Dehydration of the cortex was accomplished in acetone solutions at increasing concentrations. Tissue was embedded in an epoxy resin. Semithin (1-μm) sections through the sample were then made and stained with toluidine blue. Then, 600-Å-thin sections were made from a selected area of tissue defined by the semithin section, and these sections were stained with lead citrate and uranyl acetate. The ultrastructure of the brain was observed under a transmission electron microscope (JEM-1200X; JEOL, Peabody, MA). There were 8 rats in each of the four groups.
Statistical analysis
All data are presented as mean±standard error of the mean. SPSS software (17.0; SPSS, Inc., Chicago, IL) was used for statistical analysis of the data. All data were subjected to one-way analysis of variance. Post-hoc comparisons were made using Tukey's test. Statistical significance was inferred at p<0.05.
Results
Physiological data
Physiological parameters were assessed for all animals at 30 min before and 30 min and 4 h after the TBI or sham procedure (Table 1). Physiological parameters, with the exception of temperature, were within normal ranges for all groups. There was no statistical difference of physiological variables among groups, including MABP, pH, PO2, and PCO2. In contrast, brain and rectal temperature were significantly lower in the hypothermia groups than in the normothermia groups at 30 min and 4 h post-TBI (p<0.05).
Table 1.
Physiological Parameters
| Group | SNG | SHG | TNG | THG |
|---|---|---|---|---|
| Pretrauma (30 min before trauma) | ||||
| Temporalis temperature (°C) | 36.64±0.13 | 36.35±0.17 | 36.65±0.17 | 36.45±0.16 |
| Rectal temp erature (°C) | 36.85±0.14 | 37.13±0.16 | 36.65±0.15 | 37.13±0.19 |
| MABP(mm Hg) | 117.6±3.50 | 117.8±4.23 | 119.5±3.45 | 118.65±5.45 |
| pH | 7.43±0.08 | 7.44±0.03 | 7.43±0.09 | 7.45±0.06 |
| PO2(mm Hg) | 136.24±5.43 | 135.41±2.63 | 132.09±7.42 | 138.12±5.16 |
| PCO2(mm Hg) | 37.77±1.73 | 38.17±2.03 | 39.14±1.68 | 38.45±3.08 |
| Post-trauma (30 min after trauma) | ||||
| Temporalis temperature (°C) | 36.74±0.16 | 32.45±0.24* | 36.50±0.15 | 32.15±0.14† |
| Rectal temperature (°C) | 37.03±0.14 | 32.71±0.16* | 36.51±0.16 | 33.10±0.16† |
| MABP(mm Hg) | 115.30±3.21 | 116.8±3.58 | 120.5±3.57 | 119.6±4.45 |
| pH | 7.42±0.08 | 7.40±0.05 | 7.39±0.13 | 7.42±0.07 |
| PO2(mm Hg) | 131.84±7.12 | 134.76±4.23 | 136.42±3.27 | 135.87±2.65 |
| PCO2(mm Hg) | 38.54±2.08 | 39.18±3.27 | 38.45±1.73 | 38.73±2.19 |
| Post-trauma (4 h after trauma) | ||||
| Temporalis temperature (°C) | 36.82±0.19 | 32.51±0.15* | 36.65±0.12 | 32.52±0.13† |
| Rectal temperature (°C) | 37.22±0.21 | 32.91±0.18* | 37.31±0.17 | 32.86±0.15† |
| MABP (mm Hg) | 114.20±3.16 | 116.60±4.52 | 119.50±3.52 | 117.9±4.53 |
| pH | 7.45±0.07 | 7.42±0.06 | 7.41±0.08 | 7.44±0.09 |
| PO2 (mm Hg) | 134.56±7.17 | 132.56±3.45 | 137.25±4.18 | 135.62±2.78 |
| PCO2 (mm Hg) | 38.17±2.65 | 39.19±1.89 | 38.45±3.01 | 38.76±2.14 |
Data are presented with mean±standard error of the mean.
p<0.05 SHG versus SNG; †p<0.05 THG versus THG.
SNG, sham injury with normothermia group; SHG, sham injury with hypothermia group; TNG, TBI with normothermia group; THG, TBI with hypothermia group; MABP, mean arterial blood pressure.
Histological examination of the ipsilateral hippocampus
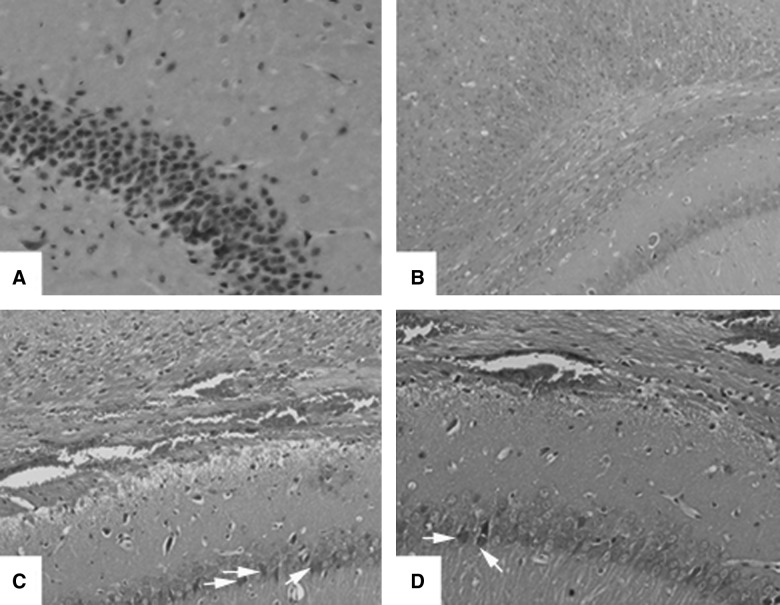
All sections from ipsilateral hippocampus were stained with hematoxylin and eosin (Fig. 1). There were extensive subcortical punctate hemorrhages and numerous dead neurons of typical pyramidal layered structure in the ipsilateral external capsule and the ipsilateral region between hippocampus and thalamus in TNG. However, in the THG group, subcortical punctuate hemorrhages were also observed in the peritrauma cortex, external capsule, and ipsilateral hippocampus. However, despite these changes, the structure within the peritraumatic domain was relatively normal. The sham injury group, including SNG and SHG, showed normal neuronal structure.
FIG. 1.
Hematoxylin and eosin staining of the ipsilateral hippocampus from SNG (A), SHG (B), TNG (C), and THG (D) at 24 h. These are representative images of coronal sections of ipsilateral hippocampus. Arrows indicate dead neurons. Magnification, 100×.
Terminal deoxynucleoitidyl transferase-mediated nick end labeling staining
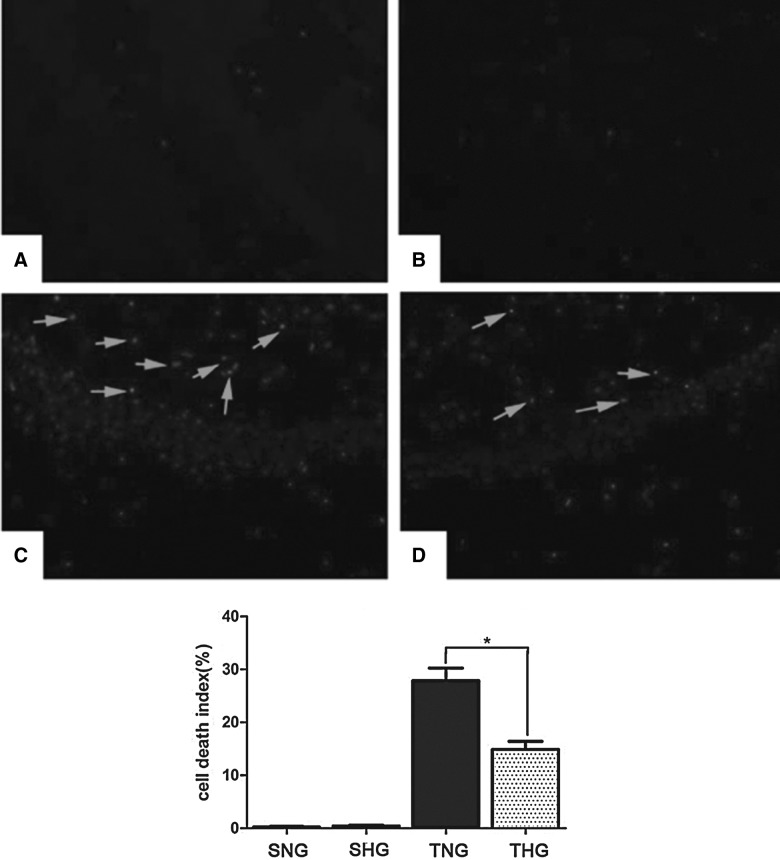
Few TUNEL-positive cells were found in brains of sham-injured rats, including SNG and SHG (Fig. 2A,B). At 24 h postinjury, the cell death index of the injured ipsilateral hippocampal CA1 neurons were markedly increased in TNG (27.9±2.36%; p<0.05; Fig. 2C), compared to sham-injured rats, including SNG and SHG. However, hypothermia treatment significantly decreased the cell death index, which was only 14.90±1.52% in THG (p<0.05; Fig. 2D). Few TUNEL-positive cells were found in brains of sham-injured rats, including SNG and SHG (Fig. 2A,B).
FIG. 2.
Terminal deoxynucleoitidyl transferase-mediated nick end labeling (TUNEL) immunohistochemistry staining of the ipsilateral hippocampal CA1 from (A), SHG (B), TNG (C), and THG (D) at 24 h. Arrows indicate TUNEL-positive cells. The average number of TUNEL-positive cells of total cell number in 10 microscopic fields per each section was evaluated as cell death index. *p<0.05 TNG versus THG. Magnification, 100×. SNG, sham injury with normothermia group; SHG, sham injury with hypothermia group; TNG, TBI with normothermia group; THG, TBI with hypothermia group.
Detection of microtubule-associated protein light chain 3 and Beclin-1
Immunofluorescence
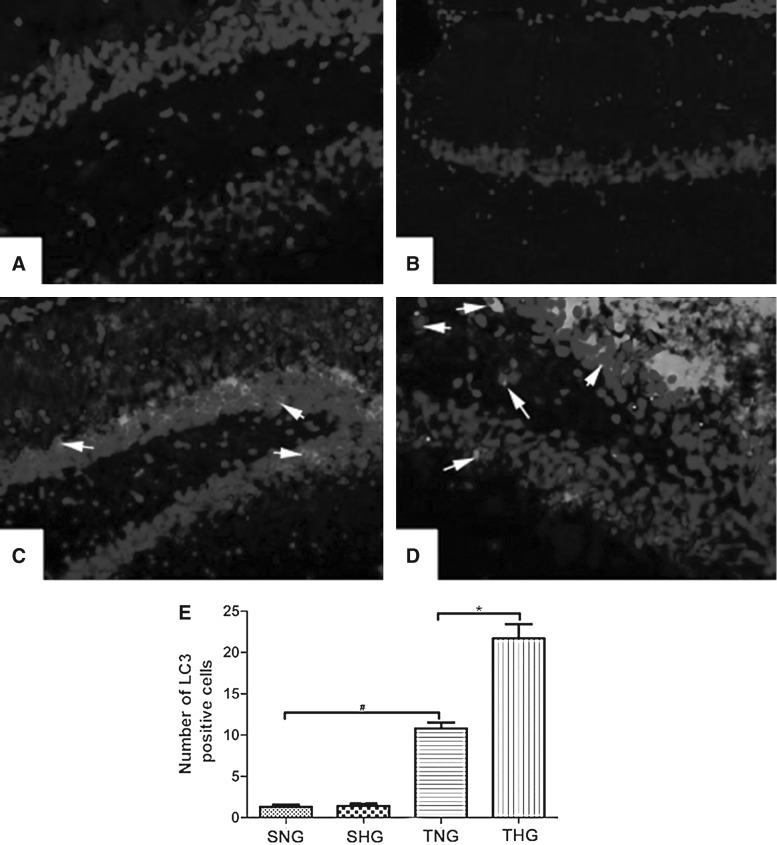
Increased LC3-positive cells were found in brain samples in TNG and much more in THG (Fig. 3C,D). Only a few LC3-positive cells were observed in SNG and SHG (Fig. 3A,B). LC3 immunoreactivity scores are shown with quantitative analysis (p<0.05 TNG vs. SNG; p<0.05 TNG vs. THG; Fig. 3E).
FIG. 3.
Immunofluorescent analysis of LC3 expression (A: SNG; B: SHG; C: TNG; D: THG). The average number of LC3-positive cells of total cell number in 10 microscopic fields per each section was evaluated. There was no difference between SNG and SHG. Arrows indicate LC3-positive cells. #p<0.05 TNG vs. SNG; *p<0.05 TNG vs. THG. Magnification, 100×. LC3, microtubule-associated protein light chain 3; SNG, sham injury with normothermia group; SHG, sham injury with hypothermia group; TNG, TBI with normothermia group; THG, TBI with hypothermia group.
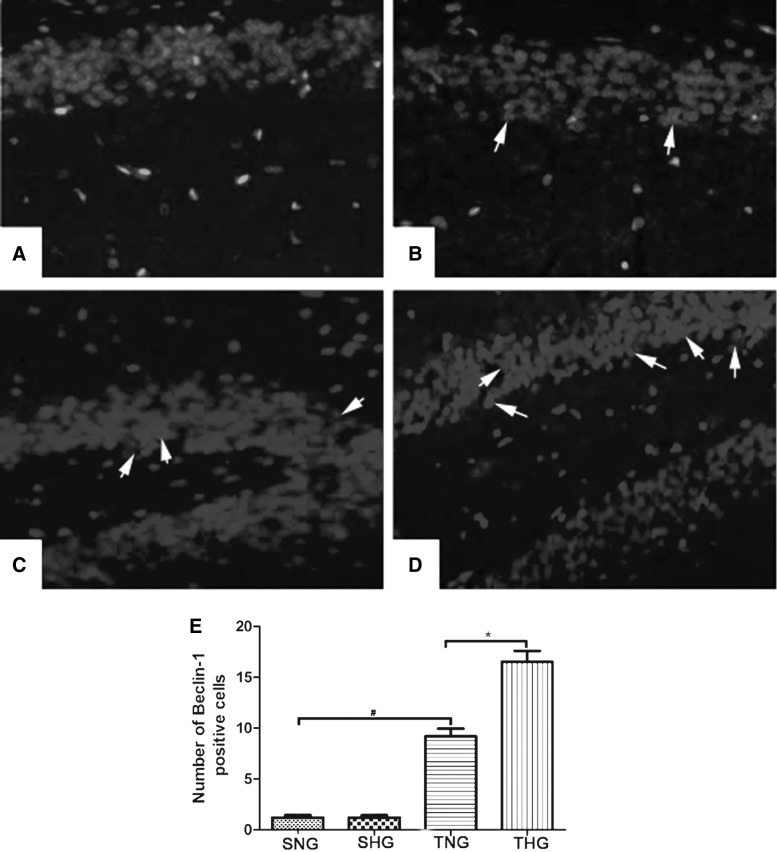
Increased Beclin-1 positive cells were found in brain samples in TNG and further increased in THG (Fig. 4C,D). Only a few LC3/Beclin-1-positive cells were observed in SNG and SHG (Fig. 4A.B). Beclin-1 immunoreactivity scores are shown with quantitative analysis (p<0.05 TNG vs. SNG; p<0.05 TNG vs. THG; Fig. 4E).
FIG. 4.
Immunofluorescent analysis of Beclin-1 expression (A: SNG; B: SHG; C: TNG; D: THG). The average number of Beclin-1-positive cells of total cell number in 10 microscopic fields per each section was evaluated. There was no difference between SNG and SHG. Arrows indicate Beclin-1-positive cells. #p<0.05 TNG vs. SNG; *p<0.05 TNG vs. THG. Magnification, 100×. SNG, sham injury with normothermia group; SHG, sham injury with hypothermia group; TNG, TBI with normothermia group; THG, TBI with hypothermia group.
Western blotting analysis
Western blotting analysis showed low levels of LC3 and Beclin-1 in SNG and SHG. Expression levels of LC3 and Beclin-1 were significantly increased post-TBI and further increased after moderate hypothermia treatment (Fig. 5).
FIG. 5.
Immunoblots of LC3-I, LC3-II, and Beclin-1. β-Actin was used as load control. LC3, microtubule-associated protein light chain 3; SNG, sham injury with normothermia group; SHG, sham injury with hypothermia group; TNG, TBI with normothermia group; THG, TBI with hypothermia group.
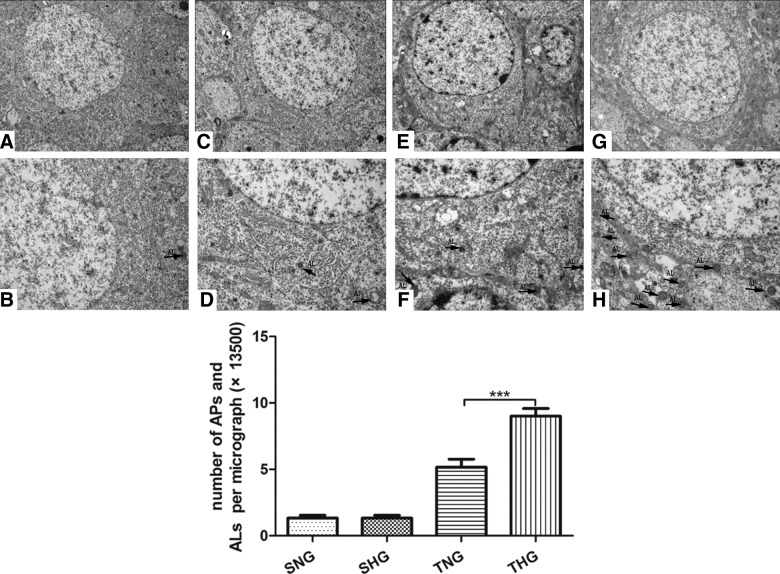
Transmission electron microscopy
Neurons and glial cells in SNG and SHG appeared healthy with normal endoplasmic reticulum, mitochondria, lysosomes, and nucleus, indicating that moderate hypothermia had little effect on autophagy-related structures. Numerous neurons displayed multiple vacuole-related structures containing electron-dense materials or double membranous material; properties of autophagosomes (APs) and autolysosomes (ALs) in TNG as well as number of APs and ALs were further up-regulated after post-TBI hypothermia (p<0.05; Fig. 6).
FIG. 6.
Electron micrographs of autophagy-related vesicular compartments (A and B: SNG; C and D: SHG; E and F: TNG; G and H: THG). Scale bar was 5 μm (upper half of the figure) and 2 μm (lower half of the figure). Arrows indicate the autophagosomes and autolysosomes. APs, autophagosomes; ALs, autolysosomes; SNG, sham injury with normothermia group; SHG, sham injury with hypothermia group; TNG, TBI with normothermia group; THG, TBI with hypothermia group.
Discussion
In the present study, we found that mTBI causes ipsilateral hippocampal cell death at 24 h after fluid percussion TBI and that moderate hypothermia could attenuate hippocampal cell death; we also found that mTBI induced the increase of autophagy in hippocampus at 24 h post-TBI. Further, we found that moderate hypothermia significantly increased autophagy in the ipsilateral hippocampus after fluid percussion TBI. The results suggest that activation of the autophagy pathway by post-TBI moderate hypothermia may be a neuroprotective mechanism.
Apoptosis post-TBI is an important intracellular pathway leading to cell death and early brain injury. Using the lateral fluid percussion TBI model in rats, TUNEL-positive cells and activation of proapoptotic molecules, such as caspases, were found to peak 12–72 h post-trauma.3,22 This activation of the caspases was shown to be followed by activation of up- and downstream apoptotic pathways, such as p53, c-Jun, c-Myc and c-Fos, and apoptosis-inducing factor, together with activation of antiapoptotic molecules, such as B-cell lymphoma 2 (Bcl-2).23–25 In accord with our previous research, fluid percussion TBI could induce significant ipsilateral hippocampal cell death, evaluated with TUNEL assay.
Autophagy can also be induced post-TBI.9–14,26,27 The first study investigating the association between autophagy and TBI was conducted by Diskin and colleagues.11 In that study, it was demonstrated that Beclin-1 level dramatically increased near the site of injury in the closed-head injury model in mice. Various animal models and experimental animals are used to observe the changes of autophagy post-TBI. For instance, Liu and colleagues used a fluid percussion TBI model to observe the changes of autophagy in Sprague-Dawley rats and found that both autophagosomes and autolysosomes were markedly accumulated in neurons from 4 h onward post-TBI.9 Zhang and colleagues studied changes of autophagy post-TBI in the controlled cortical injury system and demonstrated that autophagy was elevated in the early stage post-TBI and lasted for at least 32 days thereafter.12 Viscomi and colleagues used rapamycin, which could enhance autophagy by inactivating mammalian target of rapamycin and demonstrated that autophagy serves as a protective mechanism for maintaining cellular homeostasis after TBI.26,28 However, there are also counterviews on autophagy post-TBI. Whereas it is doubtless that autophagy increases after experimental TBI, however, several studies have concluded that the increased autophagy may contribute to the overall neuropathology and functional outcome deficits.10,13,14
Whereas many studies have focused on the modulation of autophagy by apoptotic-signaling pathways, it is also clear that autophagy can regulate apoptosis.4 In the present study, we have found that, post-TBI, moderate hypothermia could increase expression of autophagy and attenuate hippocampal cell death, which may suggest that the autophagy pathway may play an important role in the neuroprotective function of moderate hypothermia.
Despite that clinical studies conducted to date have not shown convincing evidence of benefit, a large number of animal and experimental models of TBI demonstrated a beneficial effect of hypothermia. Mechanisms of preventing or minimizing secondary insults by moderate hypothermia are complex. Earlier work showed that moderate hypothermia after brain injury is associated with various pathological mechanisms, including reduction of cerebral metabolic rate, limitation of apoptosis, mitochondrial dysfunction and disruptions to calcium homeostasis, attenuation of inflammatory and immune response, suppression of free radicals, reduction of blood–brain barrier disruption, vascular permeability, and epileptic activity.29–31 But the accurate mechanisms of cerebral protection provided by hypothermia after brain injury have not been fully addressed.
Autophagy is a highly regulated process involving bulk degradation of cytoplasmic macromolecules and organelles in mammalian cells through the lysosomal system. Induced autophagy is essential for maintenance of cellular homeostasis and cell survival.32 Beclin-1 and LC3 are biomarkers for detecting changes in autophagy. Beclin-1, a mammalian ortholog of yeast Atg6, which could regulate Vps-34 and promote formation of Beclin-1-Vps-34-Vps-15 core complexes, plays a central role in autophagy, and a previous study has shown its involvement in the regulation of autophagy.13 LC3, an autophagosomal ortholog of yeast Atg8, is one of the most reliable markers in the study of autophagy induction.33 LC3 is synthesized as a pro-LC3, which is then cleaved by ATG4 protease to form the 16- to 18-kD molecule, LC3-I. On activation of autophagy, LC3-I is conjugated with phosphatidylethanolamine and converted into LC-3-II.34
The cytoprotective function of autophagy is mediated, in many circumstances, by negative modulation of apoptosis. Possible mechanisms include interactions between Beclin-1 and Bcl-2/Bcl-xL (B-cell lymphoma-extra large). Beclin-1 is a novel Bcl-2-homology (BH)-3 domain-only protein,35 mainly located in the cytoplasmic structures, including the endoplasmic reticulum, mitochondria, and the perinuclear membrane. Under nutrient-sufficient conditions, Beclin-1 is bound by Bcl-2 or Bcl-xL, inhibiting its ability to initiate autophagy. Antiapoptotic Bcl-2 family members interact with the BH-3 domain of Beclin-1.36 During starvation or other stress conditions, however, Bcl-2 and Bcl-xL must be displaced from Beclin-1 to permit autophagy. Previous studies have explored the possible mechanisms of dislocation of Bcl-2 and Beclin-1, which is necessary for induction of autophagy. Possible mechanisms include competitive displacement of Beclin-1 BH3 domain by other Bcl-2 family proteins or translocation of the nuclear protein high-mobility group box 1 to the cytosol, c-Jun N-terminal kinase–mediated phosphorylation of Bcl-2, death-associated protein kinase (DAPK)-mediated phosphorylation of Beclin-1, nutrient-deprivation factor 1 dysfunction, Beclin-1 self-interaction, and tumor necrosis factor receptor–associated factor 6–mediated ubiquitination of Beclin-1.36–43 Besides, brain trauma conditions have been found to regulate interaction between endogenous Bcl-2 and Beclin-1 by altering their total protein levels.27 Collectively, autophagy negatively modulates neuronal cell death in a complex manner, in order to attenuate hippocampal cell death after post-TBI hypothermia.
In the present study, we observed that fluid percussion TBI could induce increased autophagy in the ipsilateral hippocampus, and moderate hypothermia could accelerate this process. Autophagy is the main mechanism for the bulk elimination of aberrant cell components and could interact with other cell death mechanisms, and we found that moderate hypothermia could attenuate cell death in the ipsilateral hippocampus. Therefore, we propose that neuroprotective effects of moderate hypothermia may be related to interaction between autophagy and other cell death mechanisms. Further studies are required to demonstrate the possible mechanisms of the promotion of autophagy by moderate hypothermia.
Acknowledgments
This work was supported by grants from a National Science and Nature grant (no. 81271381) and the National Key project (no. 2012CB518100).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Qu C., Mahmood A., Lu D., Goussev A., Xiong Y., and Chopp M. (2008). Treatment of traumatic brain injury in mice with marrow stromal cells. Brain Res. 1208, 234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenzlinger P.M., Morganti-Kossmann M.C., Laurer H.L., and McIntosh T.K. (2001). The duality of the inflammatory response to traumatic brain injury. Mol. Neurobiol. 24, 169–181 [DOI] [PubMed] [Google Scholar]
- 3.Jia F., Mao Q., Liang Y.M., and Jiang J.Y. (2009). Effect of post-traumatic mild hypothermia on hippocampal cell death after traumatic brain injury in rats. J. Neurotrauma 26, 243–252 [DOI] [PubMed] [Google Scholar]
- 4.Gordy C., and He Y.W. (2012). The crosstalk between autophagy and apoptosis: where does this lead? Protein Cell 3, 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jing C.H., Wang L., Liu P.P., Wu C., Ruan D., and Chen G. (2012). Autophagy activation is associated with neuroprotection against apoptosis via a mitochondrial pathway in a rat model of subarachnoid hemorrhage. Neuroscience 213, 144–153 [DOI] [PubMed] [Google Scholar]
- 6.He Y., Wan S., Hua Y., Keep R.F., and Xi G. (2008). Autophagy after experimental intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 28, 897–905 [DOI] [PubMed] [Google Scholar]
- 7.Balduini W., Carloni S., and Buonocore G. (2012). Autophagy in hypoxia-ischemia induced brain injury. J. Matern. Fetal Neonatal Med. 25 Suppl 1, 30–34 [DOI] [PubMed] [Google Scholar]
- 8.Son J.H., Shim J.H., Kim K.H., Ha J.Y., and Han J.Y. (2012). Neuronal autophagy and neurodegenerative diseases. Exp. Mol. Med. 44, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C.L., Chen S., Dietrich D., and Hu B.R. (2008). Changes in autophagy after traumatic brain injury. J. Cereb. Blood Flow Metab. 28, 674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo C.L., Li B.X., Li Q.Q., Chen X.P., Sun Y.X., Bao H.J., Dai D.K., Shen Y.W., Xu H.F., Ni H., Wan L., Qin Z.H., Tao L.Y., and Zhao Z.Q. (2011). Autophagy is involved in traumatic brain injury-induced cell death and contributes to functional outcome deficits in mice. Neuroscience 184, 54–63 [DOI] [PubMed] [Google Scholar]
- 11.Diskin T., Tal-Or P., Erlich S., Mizrachy L., Alexandrovich A., Shohami E., and Pinkas-Kramarski R. (2005). Closed head injury induces upregulation of Beclin 1 at the cortical site of injury. J. Neurotrauma 22, 750–762 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y.B., Li S.X., Chen X.P., Yang L., Zhang Y.G., Liu R., and Tao L.Y. (2008). Autophagy is activated and might protect neurons from degeneration after traumatic brain injury. Neurosci. Bull. 24, 143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark R.S., Bayir H., Chu C.T., Alber S.M., Kochanek P.M., and Watkins S.C. (2008). Autophagy is increased in mice after traumatic brain injury and is detectable in human brain after trauma and critical illness. Autophagy 4, 88–90 [DOI] [PubMed] [Google Scholar]
- 14.Lai Y., Hickey R.W., Chen Y., Bayir H., Sullivan M.L., Chu C.T., Kochanek P.M., Dixon C.E., Jenkins L.W., Graham S.H., Watkins S.C., and Clark R.S. (2008). Autophagy is increased after traumatic brain injury in mice and is partially inhibited by the antioxidant gamma-glutamylcysteinyl ethyl ester. J. Cereb. Blood Flow Metab. 28, 540–550 [DOI] [PubMed] [Google Scholar]
- 15.Jiang J.Y. (2009). Clinical study of mild hypothermia treatment for severe traumatic brain injury. J. Neurotrauma 26, 399–406 [DOI] [PubMed] [Google Scholar]
- 16.Dixon C.E., Markgraf C.G., Angileri F., Pike B.R., Wolfson B., Newcomb J.K., Bismar M.M., Blanco A.J., Clifton G.L., and Hayes R.L. (1998). Protective effects of moderate hypothermia on behavioral deficits but not necrotic cavitation following cortical impact injury in the rat. J. Neurotrauma 15, 95–103 [DOI] [PubMed] [Google Scholar]
- 17.Celik S.E., Ozturk H., and Tolunay S. (2006). Therapeutic effect of hypothermia and dizocilpine maleate on traumatic brain injury in neonatal rats. J. Neurotrauma 23, 1355–1365 [DOI] [PubMed] [Google Scholar]
- 18.Bramlett H.M., Green E.J., Dietrich W.D., Busto R., Globus M.Y., and Ginsberg M.D. (1995). Posttraumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits. J. Neurotrauma 12, 289–298 [DOI] [PubMed] [Google Scholar]
- 19.Dixon C.E., Lyeth B.G., Povlishock J.T., Findling R.L., Hamm R.J., Marmarou A., Young H.F., and Hayes R.L. (1987). A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67, 110–119 [DOI] [PubMed] [Google Scholar]
- 20.McIntosh T.K., Vink R., Noble L., Yamakami I., Fernyak S., Soares H., and Faden A.L. (1989). Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244 [DOI] [PubMed] [Google Scholar]
- 21.Jiang J.Y., Lyeth B.G., Kapasi M.Z., Jenkins L.W., and Povlishock J.T. (1992). Moderate hypothermia reduces blood-brain barrier disruption following traumatic brain injury in the rat. Acta Neuropathol. 84, 495–500 [DOI] [PubMed] [Google Scholar]
- 22.Tashlykov V., Katz Y., Gazit V., Zohar O., Schreiber S., and Pick C.G. (2007). Apoptotic changes in the cortex and hippocampus following minimal brain trauma in mice. Brain Res. 1130, 197–205 [DOI] [PubMed] [Google Scholar]
- 23.Lu J., Moochhala S., Kaur C., and Ling E. (2000). Changes in apoptosis-related protein (p53, Bax, Bcl-2 and Fos) expression with DNA fragmentation in the central nervous system in rats after closed head injury. Neurosci. Lett. 290, 89–92 [DOI] [PubMed] [Google Scholar]
- 24.Raghupathi R., Conti A.C., Graham D.I., Krajewski S., Reed J.C., Grady M.S., Trojanowski J.Q., and McIntosh T.K. (2002). Mild traumatic brain injury induces apoptotic cell death in the cortex that is preceded by decreases in cellular Bcl-2 immunoreactivity. Neuroscience 110, 605–616 [DOI] [PubMed] [Google Scholar]
- 25.Slemmer J.E., Zhu C., Landshamer S., Trabold R., Grohm J., Ardeshiri A., Wagner E., Sweeney M.I., Blomgren K., Culmsee C., Weber J.T., and Plesnila N. (2008). Causal role of apoptosis-inducing factor for neuronal cell death following traumatic brain injury. Am. J. Pathol. 173, 1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viscomi M.T., D'Amelio M., Cavallucci V., Latini L., Bisicchia E., Nazio F., Fanelli F., Maccarrone M., Moreno S., Cecconi F., and Molinari M. (2012). Stimulation of autophagy by rapamycin protects neurons from remote degeneration after acute focal brain damage. Autophagy 8, 222–235 [DOI] [PubMed] [Google Scholar]
- 27.Sadasivan S., Dunn W.A., Jr., Hayes R.L., and Wang K.K. (2008). Changes in autophagy proteins in a rat model of controlled cortical impact induced brain injury. Biochem. Biophys. Res. Commun. 373, 478–481 [DOI] [PubMed] [Google Scholar]
- 28.Meijer A.J., and Codogno P. (2004). Regulation and role of autophagy in mammalian cells. Int. J. Biochem. Cell Biol. 36, 2445–2462 [DOI] [PubMed] [Google Scholar]
- 29.Polderman K.H. (2009). Mechanisms of action, physiological effects, and complications of hypothermia. Crit. Care Med. 37, 7 Suppl., S186–S202 [DOI] [PubMed] [Google Scholar]
- 30.Moore E.M., Nichol A.D., Bernard S.A., and Bellomo R. (2011). Therapeutic hypothermia: benefits, mechanisms and potential clinical applications in neurological, cardiac and kidney injury. Injury 42, 843–854 [DOI] [PubMed] [Google Scholar]
- 31.Urbano L.A., and Oddo M. (2012). Therapeutic hypothermia for traumatic brain injury. Curr. Neurol. Neurosci. Rep. 12, 580–591 [DOI] [PubMed] [Google Scholar]
- 32.Uchiyama Y., Shibata M., Koike M., Yoshimura K., and Sasaki M. (2008). Autophagy-physiology and pathophysiology. Histochem. Cell Biol. 129, 407–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maiuri M.C., Criollo A., Tasdemir E., Vicencio J.M., Tajeddine N., Hickman J.A., Geneste O., and Kroemer G. (2007). BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L). Autophagy 3, 374–376 [DOI] [PubMed] [Google Scholar]
- 34.Kabeya Y., Mizushima N., Yamamoto A., Oshitani-Okamoto S., Ohsumi Y., and Yoshimori T. (2004). LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J. Cell Sci. 117, 2805–2812 [DOI] [PubMed] [Google Scholar]
- 35.Oberstein A., Jeffrey P.D., and Shi Y. (2007). Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J. Biol. Chem. 282, 13123–13132 [DOI] [PubMed] [Google Scholar]
- 36.Kang R., Zeh H.J., Lotze M.T., and Tang D. (2011). The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 18, 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo S., and Rubinsztein D.C. (2007). Atg5 and Bcl-2 provide novel insights into the interplay between apoptosis and autophagy. Cell Death Differ. 14, 1247–1250 [DOI] [PubMed] [Google Scholar]
- 38.Maiuri M.C., Le Toumelin G., Criollo A., Rain J.C., Gautier F., Juin P., Tasdemir E., Pierron G., Troulinaki K., Tavernarakis N., Hickman J.A., Geneste O., and Kroemer G. (2007). Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 26, 2527–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei Y., Pattingre S., Sinha S., Bassik M., and Levine B. (2008). JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 30, 678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zalckvar E., Berissi H., Eisenstein M., and Kimchi A. (2009). Phosphorylation of Beclin 1 by DAP-kinase promotes autophagy by weakening its interactions with Bcl-2 and Bcl-XL. Autophagy 5, 720–722 [DOI] [PubMed] [Google Scholar]
- 41.Zalckvar E., Berissi H., Mizrachy L., Idelchuk Y., Koren I., Eisenstein M., Sabanay H., Pinkas-Kramarski R., and Kimchi A. (2009). DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 10, 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang R., Livesey K.M., Zeh H.J., Loze M.T., and Tang D. (2010). HMGB1: a novel Beclin 1-binding protein active in autophagy. Autophagy 6, 1209–1211 [DOI] [PubMed] [Google Scholar]
- 43.Tang D., Kang R., Livesey K.M., Cheh C.W., Farkas A., Loughran P., Hoppe G., Bianchi M.E., Tracey K.J., Zeh H.J., 3rd and Lotze M.T. (2010). Endogenous HMGB1 regulates autophagy. J. Cell Biol. 190, 881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]