Abstract
Device-related infections in recipients of left ventricular assist devices (LVAD) have been recognized as a major source of morbidity and mortality. They require a high level of diagnostic effort as part of the overall burden resulting from infectious complications in LVAD recipients. We present a multi-allergic patient who was treated for persistent sterile intrathoracic abscess formation and pericardial empyema following minimally invasive LVAD implantation including use of a sheet of e-polytetrafluoroethylene (ePTFE) membrane to restore pericardial integrity. Sterile abscess formation and pericardial empyema recurred after surgical removal until the ePTFE membrane was removed, suggesting that in disposed patients, ePTFE may be related to sterile abscess formation or sterile empyema.
Keywords: LVAD, ePTFE membrane, sterile abscess, pericardial empyema
Background
Device-related infections in LVAD recipients remain major complications associated with relevant morbidity and mortality. In the Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial, sepsis was the most common cause of death, accounting for 41 % of the fatal outcomes in the patient group who received LVADs [1]. Evaluation of infection in the framework of the ADVANCE Bridge to Transplant trial showed that driveline exit site infections occurred in 16.9 % and sepsis in 17.2 % of the study population, with fatal outcome in 17.5 % of those who developed sepsis [2].
The HVAD (HeartWare Inc., Massachusetts, USA) is a new generation continuous-flow LVAD that is small enough to fit entirely within the pericardium without need for creation of a pump pocket. When the device is implanted by a minimally invasive approach through a left anterolateral minithoracotomy, lateral closure of the pericardium to cover the device is usually not possible, and e-polytetrafluoroethylene (ePTFE) membrane or bovine pericardium may be used to prevent contact between the device protruding from the opening in the pericardial sac and the lung or the thoracic wall.
We present a case where a sterile intrathoracic abscess and intrapericardial empyema developed following closure of the pericardium using ePTFE membrane in a multi-allergic patient.
Case presentation
A 43 year old male with a history of multiple allergies including severe lactose intolerance presented with dilated cardiomyopathy (Intermacs level IV) secondary to the cardiotoxicity of polychemotherapy with doxorubicin, cyclophosphamide and vincristine for childhood laryngeal rhabdomyosarcoma. Implantation of an HVAD as a bridge to transplant was performed as an elective minimally invasive off-pump procedure through an L-shaped partial upper sternotomy through the third intercostal space and a left anterolateral minithoracotomy. An ePTFE membrane (Gore® Preclude® Pericardial Membrane, W. L. Gore & Associates, Inc. Flagstaff, USA) was used to restore pericardial integrity. The surgical procedure and the postoperative course were uneventful. Perioperative antimicrobial prophylaxis consisted of cefuroxime and vancomycin for 48 h.
After a hospital stay of 20 days, he was discharged. 3 weeks later, he was readmitted for suspected driveline infection with driveline exit swabs positive for Staphylococcus epidermidis. Computed tomography (CT) was performed but showed no signs of deep driveline infection. He was treated with intravenous linezolid (2 × 600 mg/d) and discharged on oral linezolid 10 days later. After 13 days, i.e. on post-implant day 65, he was readmitted for VAD-associated infection with significant quantities of spontaneous putrid discharge from the anterolateral thoracotomy wound. At the time of readmission, his clinical condition was not relevantly compromised apart from discomfort and tenderness at the thoracotomy site. LVAD parameters were within normal ranges. Anti-infective therapy was altered to intravenous rifampicin (3 × 300 mg i.v./d) and daptomycin (1 × 500 mg i.v./d), and he was immediately scheduled for surgical exploration.
After opening of the left anterolateral minithoracotomy wound, an accumulation of pus was found in the space between the pericardial sac containing the LVAD and the thoracic wall. The ePTFE membrane loosely covered the HVAD without adhering to the surrounding lung and/or thoracic wall. After removal of the pus and rinsing of the wound, vacuum therapy (VAC®, KCI, Texas, USA) was initiated. One day later, surgical revision was performed for ongoing bleeding from the wound. This time, an accumulation of liquid was palpable under the ePTFE membrane, and after incision of the membrane, a considerable quantity of viscous, putrid discharge drained from within the pericardial sac. An extensive series of swabs obtained during these surgical procedures all returned negative, as did several subsequent swabs taken from the driveline exit and the depth of the wound and a number of blood cultures.
The patient was admitted to the medical intensive care unit (ICU), and his heart transplant (HTX) listing status was upgraded to high urgency for mediastinitis and device infection. He remained hemodynamically stable without requiring inotropic or vasopressor support. After 8 days of vacuum therapy, the wound was therefore rinsed and closed.
When purulent discharge reoccurred 6 days after wound closure, CT showed an intrathoracic abscess extending up to the HVAD (Fig. 1a). CT-guided drainage of the abscess (Fig. 1b) and of a pleural effusion were performed, and antimicrobial therapy was extended to include piperacillin/tazobactam (3 × 4.5 g/d). 6 days later, repeat CT showed that the abscess had regained its previous size. The patient once again underwent surgery, this time comprising removal not only of the abscess and the intrapericardial empyema, but also of the ePTFE membrane, which had come to be suspected to be implicated in the complication. The foam dressing was changed 4 days later, and the wound was finally closed after 3 further days of vacuum therapy (VAC®, KCI, Texas, USA).
Fig. 1.
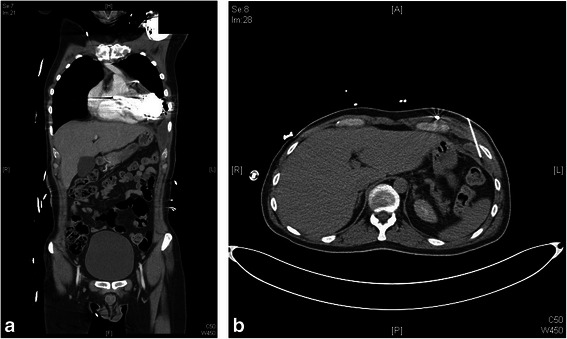
a CT showing abscess reaching up to and adjoining the HVAD. b CT showing CT-guided abscess drainage
Examination of a multitude of swabs and cultures obtained during all interventional and surgical procedures as well as PCR testing for bacteria and fungi of the ePTFE membrane failed to yield a causative organism. A swab that was taken from the chest drain several days after the initial surgical procedure returned positive for Pseudomonas aeruginosa grown on enriched medium. However, Pseudomonas aeruginosa was not cultured from any other site, leaving the diagnostic value of this particular swab doubtful. Nevertheless, antimicrobial therapy was altered to include levofloxacin (2 × 500 mg/d) one day after the first wound closure. From two swabs taken from the chest drains two days after the final wound closure, one remained negative while the other yielded Candida spp. sensitive to fluconazole. Contamination was suspected, however, as Candida albicans had not been grown from any of the intrathoracic swabs but was present in a large patch of axillary intertrigo that reached down to within a few centimeters from the thoracotomy wound and the chest drain exit site.
The anti-infective regimen, which had previously been altered to an empirical combination of rifampicin, cotrimoxazol and ciprofloxacin, was supplemented by fluconazole (1x200 mg/d) when Candida albicans was also cultured from the tip of a central venous catheter one week later, accompanied by a final spike in CRP on the same day, even though a blood culture obtained from the same catheter on the same day was negative and wound swabs and driveline exit swabs also kept returning negative. From then on, CRP kept dropping, and the WBC remained within normal ranges (Fig. 2)
Fig. 2.
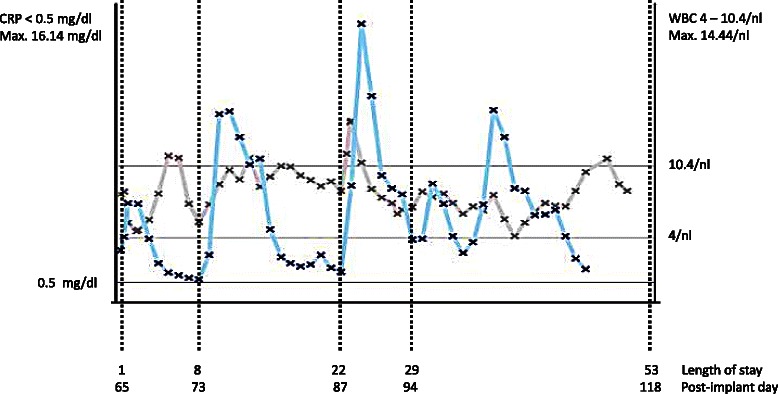
Infection markers (CRP and WBC count) during the 53 day hospital stay. Day 1: surgical exploration and initiation of underpressure therapy; day 8: wound closure; day 22: extensive surgical re-exploration; day 29: wound closure
A final CT scan showed that abscess formation had not recurred. As the patient’s clinical condition, his surgical wounds, the driveline exit site as well as his infection markers continued to be inconspicuous, his HTX urgency status was downgraded, and he was transferred to the general ward and discharged to self-care on post-implant day 118 after a total hospital stay of 53 days including 46 days in the medical ICU/IMC. His antibiotic regimen at discharge consisted of fluconazole (1 × 200 mg/d), ciprofloxacin (2 × 500 mg/d), sulfomethaxozole/trimethoprim(2 × 960 mg/d) and rifampicin (2 × 450 mg) to be continued for a total of 14 days.
Follow-up CT on post-implant day 135 showed no recurrence of abscess formation. His driveline exit site and thoracotomy wound were inconspicuous. Blood and urine cultures, as well as routine screening swabs and a swab from the driveline exit site returned negative for bacteria and fungi. During his most recent outpatient clinic visit on post-implant day 359, i.e. 265 days after final closure of the anterolateral thoracotomy wound, his clinical condition and infection markers were still inconspicuous.
Discussion
Management of infections in VAD recipients remains a challenging task, with the infections specific to or requiring particular considerations in VAD recipients ranging from superficial driveline exit site infection to devastating entities such as pump and/or cannula infection, endocarditis and sepsis. The range of treatment options correspondingly covers the entire spectrum from local treatment to major cardiac surgery including device removal with or without temporary mechanical circulatory support, VAD-to-VAD exchange or urgent heart transplantation [3–6].
LVAD exchange for infection was found to be associated with relevant morbidity and mortality, as well as a considerable risk of recurring infection [7–10]. The cases of VAD exchange for infection reported to date, however, mostly concerned the HeartMate II. Our patient, in contrast, received an HVAD, which renders the situation somewhat different in that the pump housing is attached directly to the heart and the entire system is completely contained within the pericardial sac. Regardless of the implantation characteristics, however, the risks of retaining the VAD and those of timely VAD exchange under controlled conditions before development of frank mediastinitis and sepsis need to be carefully weighed against each other. At the same time, the likelihood of availability of a donor organ for any given patient must be considered before proceeding with one or the other approach.
Studies of the microbiology of VAD-associated infection showed the majority of infections to be caused by staphylococcal organisms [11–16]. The share of culture-negative infections reported is low, with inability to identify the causative organism, like in other non-valvular cardiovascular device related infections, being frequently attributed to previous exposure to antibiotics diminishing the sensitivity of subsequent microbiological studies [15, 17].
Upon admission for suspected purulent device infection, antimicrobial treatment in our patient was escalated to a combination of daptomycin and rifampicin in the absence of recent microbiological findings guiding anti-infective therapy. Staphylococcus epidermidis, after all, was not cultured from any site after driveline exit site swabs had briefly been positive during the previous hospital stay, and the infectious complication had developed while the patient was on anti-infective treatment with linezolid.
Daptomycin was chosen as it had previously proven effective in the treatment of patients presenting with multi-drug resistant organisms after VAD implantation and for its unique ability to penetrate into biofilms on cardiovascular devices such as LVADS [18–20]. Rifampicin was added for its synergistic effect with daptomycin [21]. Subsequently, anti-infective therapy was altered to different broad combinations of antibiotics that did not, however, comprise antifungals until the last few days of the hospital stay.
In retrospect, a considerable diagnostic effort (Table 1) was dedicated to identifying a causative organism. This is reflected by a total of 18 superficial, deep, intrathoracic and intrapericardial wound swabs including swabs from the LVAD surface subjected to microbiological examination during a hospital stay of 53 days. PCR testing for bacteria and fungi was additionally performed on fluid obtained by CT guided aspiration from the intrathoracic abscess and on a specimen from the ePTFE membrane removed during surgical exploration. Results of PCR testing, all intrathoracic and intrapericardial swabs including swabs from HVAD surface, as well as all swabs taken from the driveline exit site were negative. The only swabs that returned positive were taken by undefined methods and under non-standardized conditions from closed chest drainage systems and must be considered of doubtful diagnostic value.
Table 1.
Microbiological testing including routine screening for multi-resistant organisms performed during hospital stay of 53 days
| Summary of microbiological testing | ||
|---|---|---|
| Test | No. | Result |
| Superficial, deep, intrathoracic and intrapericardial wound swabs | 18 | Negative |
| Driveline exit site swabs | 6 | Negative |
| Swabs from chest drains | 3 | 1 positive for Pseudomonas aeruginosaa |
| 1 positive for Candida spp.a | ||
| Culture from abscess fluid | 1 | Negative |
| Culture from pleural effusion | 1 | Negative |
| Blood culture | 3 | Negative |
| Microbiological examination of ePTFE membrane | 1 | Negative |
| PCR for bacteria and fungi of ePTFE membrane | 1 | Negative |
| PCR for bacteria and fungi of fluid aspirated from abscess | 1 | Negative |
| PCR for bacteria and fungi of pleural effusion | 1 | Negative |
| Nose swabs | 6 | Negative |
| Rectal swabs | 6 | 1 positive for Enterobacter cloacae |
| 1 positive for E. coli | ||
| Anal swab | 1 | Positive for E. coli, Klebsiella pneumonia, Candida albicans and Candida utilis |
| Stool culture | 1 | Positive for Candida albicans and Candida non-albicans |
| Urine culture | 7 | Negative |
| Tip of central venous catheter | 3 | 1 positive for Candida albicans |
| Axillary skin swab | 1 | Positive for Candida albicans |
aSuspected contamination
When microbiological studies failed to identify a causative organism, the presence of the ePTFE membrane, in combination with the patient’s severe allergic disposition, came to be suspected of being involved in the complication.
ePTFE is a material widely used in cardiac surgery and appreciated for its chemical inertness and biocompatibility. Whether or not it is superior to a number of alternatives available in preventing adhesions and providing a clear plane of dissection at redo-surgery or, in the case of patients receiving LVADs as bridge to recovery or transplant, at LVAD explantation or heart transplantation is a subject of ongoing debate [22–26]. Permanent presence in the mediastinum of non-resorbable foreign material additionally poses questions with regard to its implications in terms of infection. However, over the past two decades, different studies concluded that ePTFE membrane can safely be used in cardiac surgery to limit adhesions without increasing the risk of infection [27–30].
As infection is a particular issue with VAD recipients, questions were also raised with regard to the material to be chosen for covering the components of the various devices during the implantation procedure. A number of studies were dedicated to the use of ePTFE membrane in the context of VAD implantation and found that it significantly facilitated explantation [31–34].
Leprince et al. [34], studying 23 patients in whom ePTFE membranes had been used to cover LVADs or total artificial hearts, found that infection had occurred in only one case. They concluded that ePTFE membranes can be used to limit adhesion between tissues and device surfaces without increasing the risk of infection. Safety of ePTFE membrane was similarly emphasized by Vitali et al. [33], who used ePTFE pericardial membrane in 20 patients implanted with a Novacor assist device. In contrast, Holman et al. [32] reported membranes in two of seven patients to have become infected with Staphylococcus aureus but nevertheless advocated the use of ePTFE membrane.
In our patients, who routinely undergo minimally invasive HVAD implantation, ePTFE membrane or, more recently, bovine pericardium are used to close the lateral gap in the pericardial sac to prevent direct contact between device and lung or thoracic wall rather than to achieve pericardial closure and facilitate re-sternotomy after full median sternotomy. When the anterolateral thoracotomy wound in our patient was reopened on post-implant days 65 and 87, neither adhesion to surrounding structures nor any changes to the membrane itself were observed, but an accumulation of viscous purulent fluid beneath the membrane, in addition to intrathoracic abscess formation, represented a highly alarming finding.
Finally, however, we succeeded neither in identifying a causative organism nor in proving involvement of the ePTFE membrane in the development of the complication with absolute certainty. But given the patient’s severe allergic disposition, the fact that neither pericardial empyema nor intrathoracic abscess formation recurred after removal of the ePTFE membrane may be interpreted as suggestive of involvement of the membrane in the complication.
The level of diagnostic effort undertaken in this particular patient was motivated by a high degree of awareness of the potentially detrimental consequences of VAD infection. Even though the complication resolved without major cardiac surgery being required, the patient had a hospital stay of 53 days most of which he spent at the medical ICU/IMC, thus illustrating that infection in a growing population of VAD recipients represents a veritable burden and relevant economic factor the impact of which grows with the severity of infection and extent of diagnostics and therapeutic interventions required.
Conclusions
In disposed patients, ePTFE may be related to sterile abscess formation or sterile empyema. This case, together with another case where infection developed in a patient in whom ePTFE membrane was used to cover an HVAD, motivated us to abandon the use of ePTFE membrane. We now use bovine pericardium for restitution of pericardial integrity after minimal invasive HVAD implantation through an anterolateral minithoracotomy.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Acknowledgments
We thank the physicians of the intensive care unit for the care they provided for the patient.
Funding
None of the authors have received any funding for the preparation of this paper.
Abbreviations
- CRP
C-reactive protein
- CT
computed tomography
- ePTFE
extruded polytetrafluoroethylene
- HTX
heart transplantation
- HVAD
ventricular assist device of HeartWare Inc.
- ICU
intensive care unit
- IMC
intermediate care unit
- LVAD
left ventricular assist device
- PCR
polymerase chain reaction
- VAD
ventricular assist device
- WBC
white blood cells
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AK conceived the design of the project, collected and interpreted the data and drafted the manuscript. WV has contributed to collecting and interpreting the data and drafting the manuscript. KM has been involved in the surgical treatment of the patient, has critically revised the manuscript and contributed to interpreting the data. TP has been involved in the surgical treatment of the patient, has critically revised the manuscript and contributed to interpreting the data. AB has critically revised the manuscript and contributed to interpreting the data. MA has critically revised the manuscript, contributed to interpreting the data and given final approval of the version to be published. BFA has contributed to design and coordination of the project, interpreted the data and critically revised the manuscript, and given final approval of the version to be published. SUA has supervised design and coordination of the project, interpreted the data, critically revised the manuscript and given final approval of the version to be published. All authors have read and approved the final manuscript.
Contributor Information
A. Kornberger, Phone: 0049-69-6301-6112, Email: angela.kornberger@kgu.de
V. Walter, Email: Veronika.Walter@kgu.de
M. Khalil, Email: Mahmud.Khalil@kgu.de
P. Therapidis, Email: Panagiotis.Therapidis@kgu.de
B. Assmus, Email: Birgit.Assmus@kgu.de
A. Moritz, Email: Anton.Moritz@kgu.de
A. Beiras-Fernandez, Email: Andres.Beiras@kgu.de
U. A. Stock, Email: Ulrich.Stock@kgu.de
References
- 1.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–43. [DOI] [PubMed]
- 2.John R, Aaronson KD, Pae WE, Acker MA, Hathaway DR, Najarian KB, et al. Drive-line infections and sepsis in patients receiving the HVAD system as a left ventricular assist device. J Heart Lung Transplant. 2014;33(10):1066–73. [DOI] [PubMed]
- 3.Hannan MM, Husain S, Mattner F, Danziger-Isakov L, Drew RJ, Corey GR, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant. 2011;30(4):375–84. [DOI] [PubMed]
- 4.Califano S, Pagani FD, Malani PN. Left ventricular assist device-associated infections. Infect Dis Clin North Am. 2012;26(1):77–87. doi: 10.1016/j.idc.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Nienaber JJ, Kusne S, Riaz T, Walker RC, Baddour LM, Wright AJ, et al. Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis. 2013;57(10):1438–48. [DOI] [PMC free article] [PubMed]
- 6.Nienaber J, Wilhelm MP, Sohail MR. Current concepts in the diagnosis and management of left ventricular assist device infections. Expert Rev Anti Infect Ther. 2013;11(2):201–210. doi: 10.1586/eri.12.163. [DOI] [PubMed] [Google Scholar]
- 7.Dembitsky WP, Tector AJ, Park S, Moskowitz AJ, Gelijns AC, Ronan NS, et al. Left ventricular assist device performance with long-term circulatory support: lessons from the REMATCH trial. Ann Thorac Surg. 2004;78(6):2123–9. [DOI] [PubMed]
- 8.Levy DT, Guo Y, Simkins J, Puius YA, Muggia VA, Goldstein DJ, et al. Left ventricular assist device exchange for persisting infection: a case series and review of the literature. Transpl Infect Dis. 2014;16(3):453–60. [DOI] [PubMed]
- 9.Chamogeorgakis T, Doval CE, Smedira NG, Starling RC, Gonzalez-Stawinski GV. Outcomes associated with surgical management of infections related to the HeartMate II left ventricular assist device: implications for destination therapy patients. J Heart Lung Transplant. 2012;31(8):904–906. doi: 10.1016/j.healun.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Adamson RM, Dembitsky WP, Baradarian S, Chammas J, Jaski B, Hoagland P, et al. HeartMate left ventricular assist system exchange: results and technical considerations. ASAIO J. 2009;55(6):14–9. [DOI] [PubMed]
- 11.Gordon RJ, Weinberg AD, Pagani FD, Slaughter MS, Pappas PS, Naka Y, et al. Prospective, multicenter study of ventricular assist device infections. Circulation. 2013;127(6):691–702. [DOI] [PMC free article] [PubMed]
- 12.Holman WL, Park SJ, Long JW, Weinberg A, Gupta L, Tierney AR, et al. Infection in permanent circulatory support: experience from the REMATCH Trial. J Heart Lung Transpl. 2004;23(12):1359–65. [DOI] [PubMed]
- 13.Maniar S, Kondareddy S, Topkara VK. Left ventricular assist device infections: past, present and future. Expert Rev Med Devices. 2011;8(5):627–634. doi: 10.1586/erd.11.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topkara VK, Kondareddy S, Malik F, Wang IW, Mann DL, Ewald GA, et al. Infectious complications in patients with left ventricular assist device: etiology and outcomes in the continuous-flow era. Ann Thorac Surg. 2010;90(4):1270–7. [DOI] [PubMed]
- 15.Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device infections. Lancet Infect Dis. 2006;6(7):426–437. doi: 10.1016/S1473-3099(06)70522-9. [DOI] [PubMed] [Google Scholar]
- 16.Simon D, Fischer S, Grossman A, Downer C, Hota B, Heroux A, et al. Left ventricular assist device-related infection: treatment and outcome. Clin Infect Dis. 2005;40(8):1108–15. [DOI] [PubMed]
- 17.Baddour LM, Bettmann MA, Bolger AF, Epstein AE, Ferrieri P, Gerber MA, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015–31. [DOI] [PubMed]
- 18.Walter V, Stock UA, Soriano-Romero M, Schnitzbauer A, Moritz A, Beiras-Fernandez A. Eradication of a chronic wound and driveline infection after redo-LVAD implantation. J Cardiothorac Surg. 2014;9:63. doi: 10.1186/1749-8090-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beiras-Fernandez A, Kur F, Kiefer S, Sodian R, Schmoeckel M, Weis M, et al. Multidrug-resistant gram-positive infections in patients with ventricular assist devices: the role of daptomycin. Transplant Proc. 2009;41(6):2589–91. [DOI] [PubMed]
- 20.Weis F, Beiras-Fernandez A, Kaczmarek I, Sodian R, Vicol C, Reichart B, et al. Daptomycin for eradication of a systemic infection with a methicillin-resistant Staphylococcus aureus in a biventricular assist device recipient. Ann Thorac Surg. 2007;84(1):269–70. [DOI] [PubMed]
- 21.Steenbergen JN, Mohr JF, Thorne GM. Effects of daptomycin in combination with other antimicrobial agents: a review of in vitro and animal model studies. J Antimicrob Chemother. 2009;64(6):1130–1138. doi: 10.1093/jac/dkp346. [DOI] [PubMed] [Google Scholar]
- 22.Meus PJ, Wernly JA, Campbell CD, Takanashi Y, Pick RL, Zhao-Kun Q, et al. Long-term evaluation of pericardial substitutes. J Thorac Cardiovasc Surg. 1983;85(1):54–8. [PubMed]
- 23.Bunton RW, Abregas AA, Miller AP. Pericardial closure after cardiac operations: an animal study to assess currently available materials with particular reference to their suitability for use after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1990;100(1):99–107. [PubMed] [Google Scholar]
- 24.Heydorn WH, Ferraris BA, Berry WR. Pericardial substitutes: a survey. Ann Thorac Surg. 1988;46(5):567–569. doi: 10.1016/S0003-4975(10)64699-1. [DOI] [PubMed] [Google Scholar]
- 25.Boyd WD, Tyberg JV, Cox JL. A review of the current status of pericardial closure following cardiac surgery. Expert Rev Cardiovasc Ther. 2012;10(9):1109–1118. doi: 10.1586/erc.12.87. [DOI] [PubMed] [Google Scholar]
- 26.Cannata A, Petrella D, Russo CF, Bruschi G, Fratto P, Gambacorta M, et al. Postsurgical intrapericardial adhesions: mechanisms of formation and prevention. Ann Thorac Surg. 2013;95(5):1818–26. [DOI] [PubMed]
- 27.Loebe M, Alexi-Meskhishvili V, Weng Y, Hausdorf G, Hetzer R. Use of polytetrafluoroethylene surgical membrane as a pericardial substitute in the correction of congenital heart defects. Tex Heart Inst J. 1993;20(3):213–217. [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs JP, Iyer RS, Weston JS, Amato JJ, Elliott MJ, deLeval MR, et al. Expanded PTFE membrane to prevent cardiac injury during resternotomy for congenital heart disease. Ann Thorac Surg. 1996;62(6):1778–82. [DOI] [PubMed]
- 29.Bhatnagar G, Fremes SE, Christakis GT, Goldman BS. Early results using an ePTFE membrane for pericardial closure following coronary bypass grafting. J Card Surg. 1998;13(3):190–193. doi: 10.1111/j.1540-8191.1998.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 30.Kaijwara H, Hamada T, Ichikawa Y, Ishi M, Yamazaki I. Experience with expanded polytetrafluoroethylene (ePTFE Gore-Tex) surgical membrane for coronary artery bypass grafting: does ePTFE surgical membrane predispose to postoperative mediastinitis? Artif Organs. 2004;28(9):840–845. doi: 10.1111/j.1525-1594.2004.07298.x. [DOI] [PubMed] [Google Scholar]
- 31.Copeland JG, Arabia FA, Smith RG, Covington D. Synthetic membrane neo-pericardium facilitates total artificial heart explantation. J Heart Lung Transpl. 2001;20(6):654–656. doi: 10.1016/S1053-2498(01)00248-0. [DOI] [PubMed] [Google Scholar]
- 32.Holman WL, Bourge RC, Zorn GL, Brantley LH, Kirklin JK. Use of expanded polytetrafluoroethylene pericardial substitute with ventricular assist devices. Ann Thorac Surg. 1993;55(1):181–183. doi: 10.1016/0003-4975(93)90506-D. [DOI] [PubMed] [Google Scholar]
- 33.Vitali E, Russo C, Tiziano C, Lanfranconi M, Bruschi G. Modified pericardial closure technique in patients with ventricular assist device. Ann Thorac Surg. 2000;69(4):1278–1279. doi: 10.1016/S0003-4975(99)01554-4. [DOI] [PubMed] [Google Scholar]
- 34.Leprince P, Rahmati M, Bonnet N, Bors V, Rama A, Léger P, et al. Expanded polytetrafluoroethylene membranes to wrap surfaces of circulatory support devices in patients undergoing bridge to heart transplantation. Eur J Cardiothorac Surg. 2001;19(3):302–6. [DOI] [PubMed]


