Abstract
Objectives. We evaluated the effectiveness of a program that includes routine opt-out prenatal HIV screening, combination antiretroviral therapy (ART), and a multidisciplinary team in preventing perinatal HIV transmission.
Methods. A retrospective analysis was performed on HIV-infected pregnant women in northern Alberta, Canada, who delivered between January 1, 1999, and February 28, 2006.
Results. Ninety-eight women had 113 deliveries. Forty-three percent were diagnosed with HIV infection through prenatal screening. Approximately 60% of HIV-infected pregnant women were Aboriginal, with 45% reporting alcohol use and 42% illicit drug use during pregnancy. The use of combination ART during pregnancy increased throughout the study period; 89% or more received combination ART from 2004 through 2006. Only 1 of the 111 infants (0.9%) was confirmed to be HIV infected, and that infant was born to a woman with no prenatal care.
Conclusions. High rates of HIV testing using an opt-out approach, combined with efforts by a multidisciplinary team, resulted in a low rate of perinatal HIV transmission in our cohort. The added value of retesting high-risk women late in pregnancy or with rapid HIV tests at the time of delivery should be explored.
Combination antiretroviral therapy (ART), consisting of at least 3 antiretroviral drugs, is recommended during pregnancy to reduce perinatal transmission of HIV and to improve maternal health.1 Prevention strategies that include the use of combination ART during pregnancy have been associated with perinatal HIV transmission rates of 1% or less.2,3 However, lack of prenatal care, the absence of routine prenatal testing, and the sociodemographic characteristics of many HIV-infected pregnant women, including homelessness and addictions, contribute to higher rates of perinatal transmission in many parts of Canada and the United States.
Alberta is a Canadian province with a population of approximately 3 million people. HIV care is delivered by 2 separate programs in the northern and southern half of the province. From 1999 to 2006, between 800 and 1100 HIV-infected individuals were followed by the Northern Alberta HIV Program. The most common reported risk factors for HIV infection in this population included injection drug use (35%), heterosexual transmission (32%), and homosexual transmission (28%). Approximately one third of patients were female.
Prevention of perinatal HIV transmission is an important focus of the Northern Alberta HIV Program. For pregnant women, HIV care is delivered by a multidisciplinary team, including infectious disease physicians (adult and pediatric), obstetricians, virologists, social workers, pharmacists, nurses (clinical and public health), clinical psychologists and psychiatrists, and a dietician. Features of the perinatal program include a comprehensive perinatal protocol derived from published guidelines,1 interdisciplinary communication through monthly team meetings, close individualized social support for pregnant women with chaotic lifestyles, coordination of services for women giving birth in rural parts of Alberta, infant-feeding counseling and free infant formula for 1 year, and infant follow-up care. In addition, a perinatal public health designate nurse compiles patient information (e.g., estimated date of confinement, planned site of delivery, use of ART, and virological response) and communicates with other members of the team.4
Because prevention strategies depend on all pregnant women with HIV being identified, routine opt-out HIV screening for pregnant women has been recommended by the US Centers for Disease Control and Prevention (CDC) since 2003.5 Routine opt-out screening refers to HIV testing as part of the routine panel of tests for all pregnant women, with the provision that women are notified that the test is being carried out and have the opportunity to decline (opt out). In Alberta, routine prenatal opt-out HIV screening has been in place since September 1998. More than 95% of all pregnant women accessing antenatal care undergo HIV testing each year in Alberta, including the northern half of the province.6
We aimed to evaluate the effectiveness of the Northern Alberta HIV Program's perinatal HIV transmission program, which includes routine opt-out HIV screening, combination ART, and care delivered by a multidisciplinary team.
METHODS
We report a retrospective analysis of a cohort of HIV-positive pregnant women who gave birth in northern Alberta between January 1, 1999, and February 28, 2006. All women who were known to be HIV positive prior to pregnancy, as well as those diagnosed through prenatal screening or after delivery, were identified via the Northern Alberta HIV Program database, with infant data being derived from the Pediatric Infectious Disease Clinic database. This procedure should have captured all pregnant women known to have HIV in northern Alberta during this period, because all HIV testing for the province is performed at a single laboratory that communicates all relevant results to the Northern Alberta HIV Program. The program database includes demographic information, medical conditions, medications, new events (e.g., pregnancy), and laboratory values and is updated following each clinic visit.
For pregnant women, the following information was recorded from the database or medical charts: sociodemographic characteristics, date of HIV diagnosis, mode of HIV acquisition, HCV status, HIV viral load, CD4 cell count closest to delivery, and time of initiation and type of ART. Information on smoking, alcohol, and illicit drug use during pregnancy, which was obtained from medical charts, was based on patients’ self-reports. Data on the delivery and neonatal characteristics were also collected, including mode of delivery, gender, birthweight, and gestational age. For women with multiple pregnancies, data were collected from each pregnancy. Infants from multiple births were recorded individually.
Applying the CDC definition, we considered infants to be infected with HIV if 2 HIV polymerase chain reactions, performed on separate blood samples at least 4 weeks apart, were positive.7 Infants were considered to be uninfected if they seroreverted (i.e., if passively transferred antibodies were cleared from their systems) or if 2 HIV polymerase chain reactions—the first at an infant age of at least 1 month and the second at an infant age of at least 4 months—were negative. Those with incomplete testing were deemed to have “indeterminate status.” Premature delivery was defined as delivery before 37 weeks of gestation. Gestational age was based on ultrasonography. Elective cesarean delivery was defined as cesarean delivery before the rupture of membranes and onset of labor.
All data were entered into a FOXPRO database (Microsoft Corp, Redmond, WA) and analyzed with SPSS version 13 for Windows (SPSS Inc, Chicago, IL).
RESULTS
From January 1, 1999, through February 28, 2006, 115 HIV-infected women in the study had 155 pregnancies. Of these pregnancies, 13 (8%) resulted in spontaneous abortion, 20 (13%) were terminated, and 9 (6%) had expected delivery dates beyond the study period. A total of 113 pregnancies (73%) in 98 women resulted in deliveries (including 4 sets of twins); 111 infants were included in the analyses and 6 were excluded (4 infants were born out of the province, 1 infant's chart could not be located, and 1 twin was stillborn).
The demographic and clinical characteristics of the 98 women who gave birth are outlined in Table 1. Forty-three percent of these women were first diagnosed with HIV at the time of prenatal screening; most of the women who gave birth (62%) were of Aboriginal ethnicity (First Nations, Inuit, or Metis), 15% were White, 17% were Black, and the remainder were of other or unknown ethnicity. The mode of HIV acquisition was heterosexual activity in 55% of the women, injection drug use in 38%, and other or unknown in 7%. Sixty-five women (66%) reported smoking during pregnancy; alcohol and illicit drug use during pregnancy was reported for 44 (45%) and 41 (42%) of the women, respectively.
TABLE 1.
Demographic and Clinical Characteristics of HIV-Infected Pregnant Women (N = 98): Northern Alberta, 1999–2006
| Mean (Range) or No. (%) | |
| Age at first delivery, y | 26 (18–42) |
| Race/ethnicity | |
| White | 15 (15.3) |
| Aboriginal | 61 (62.2) |
| Black | 17 (17.3) |
| Other or unknown | 5 (5.1) |
| Mode of acquisition of HIV | |
| Heterosexual | 54 (55.1) |
| Injection drug use | 37 (37.8) |
| Other | 2 (2.0) |
| Unknown | 5 (5.1) |
| Positive for HCV antibody | |
| Yes | 37 (37.8) |
| No | 59 (60.2) |
| Not known | 2 (2.0) |
| Time of first positive HIV test | |
| Before first pregnancy | 52 (53.0) |
| Prenatal screening | 42 (42.9) |
| At delivery | 2 (2.0) |
| Following delivery | 2 (2.0) |
| CD4 cell count at delivery, cells/μL | 394 (196)a |
SD, not percentage.
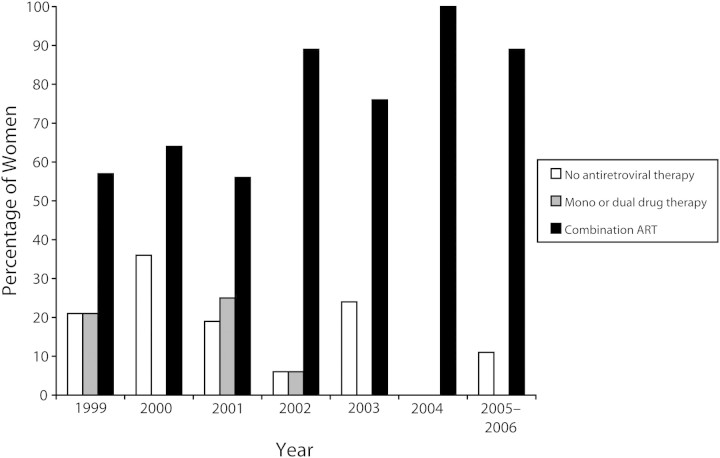
Figure 1 shows trends in antiretroviral drug use during the study period. Overall, the proportion of women who received combination ART during pregnancy increased over time. For 18 of the 113 deliveries (16%), ART was not received during pregnancy. Reasons for absence of ART included viral load of less than 400 copies/mL without therapy (4 patients) and HIV diagnosis in the immediate postpartum period (4 patients); the reason was not documented for the other 10 patients. Of the 95 pregnancies in which ART was given, 5 women (5%) were on antiretroviral drugs prior to the pregnancy and 73 (77%) had suppressed viral loads (i.e., < 400 copies/mL) at delivery or within 30 days prior to delivery.
FIGURE 1.
Trends in antenatal antiretroviral therapy (ART): northern Alberta, 1999–2006.
Note. “Combination ART” means 3 drugs or more.
Overall, HIV viral load closest to delivery was suppressed in 72% of the 113 pregnancies. Viral load was between 400 and 1000 copies/mL in 7% of pregnancies, between 1001 and 10 000 copies/mL in 4%, more than 10 000 copies/mL in 11%, and unknown in 6%. Of the 25 women in whom viral load was more than 400 copies/mL, 10 of them had started ART less than 2 months prior to delivery, 9 did not receive ART during pregnancy, 1 had documented resistance mutations, and 5 had undocumented reasons for nonsuppression. In 17 of these 25 women, the viral load was more than 1000 copies/mL; 4 of these 17 women had elective cesarean deliveries. Intravenous zidovudine (GlaxoSmithKline, Mississauga, Ontario) was administered during labor in 88 of the 113 deliveries (78%), and a dose of oral nevirapine (Boehringer Ingelheim, Burlington, Ontario) during labor was administered in 6 (5%). In 13 women (12%), there was insufficient documentation to determine whether intravenous zidovudine had been administered. For the 12 women in whom intravenous zidovudine was not given during labor, 1 mother delivered prematurely in prison, 7 women did not disclose their HIV status at the time of delivery, and 4 women were diagnosed postnatally.
Of the 111 infants included in the analyses, 82 (74%) were born at full term and 28 (25%) were preterm (3 were delivered at < 32 weeks of gestation and 25 at 32–36 weeks of gestation). Data were unavailable for 1 infant. Two infants did not receive any ART following delivery, 79 (71%) received 1 antiretroviral drug (zidovudine in all but 1 case), and 30 (27%) received 2 or more antiretroviral drugs. Eighty-three infants (75%) were below or equal to the 50th percentile for birthweight according to gestational age.8 One infant was HIV positive, 101 infants were HIV negative, and 9 infants had an indeterminate status. Reasons for incomplete testing for these 9 infants included young age at the time of reporting (n = 1), moved out of region or lost to follow-up (n = 5), and death (n = 3).
The 3 deaths included a boy with Down's syndrome who died unexpectedly at age 3 months while in a rural hospital with a respiratory illness (HIV viral load was undetectable the week of death), a previously well girl who died at home at 5 weeks of age of apparent sudden infant death syndrome (HIV viral load was undetectable at age 14 days), and a boy born at 27 weeks of gestation who died at 5 weeks of sepsis while still in the neonatal intensive care unit (HIV viral load was undetectable at 4 weeks of age). In the case of the single HIV-infected infant, the mother was seronegative during her first pregnancy 1 year earlier. She did not seek prenatal care during her second pregnancy, lived in a remote community, and was not identified as a high-risk individual at hospital intake during labor. Blood was drawn for HIV serology at delivery in a rural hospital but was not tested until 4 days postpartum, at which point it was found to be positive. The infant's HIV viral load was 37 000 copies/mL on day 17 of life.
DISCUSSION
To our knowledge, we are the first to describe HIV transmission following implementation of routine opt-out testing in which a high proportion of pregnant women were tested (i.e., < 5% of women opted out). In this cohort of HIV-infected women in the era of combination ART, the rate of definite perinatal transmission of HIV was low (1 of 111 infants). More than 90% of HIV-exposed infants were monitored until a definitive diagnosis could be made. The only definite infection occurred in an infant born to a woman with unrecognized HIV infection and no prenatal care. The rate of transmission we report, which covers January 1, 1999 to February 28, 2006, is much lower than that previously reported in northern Alberta.9 In that earlier study, which reported on a cohort of infants perinatally exposed to HIV between January 1, 1988, and December 31, 1999, 15 of 71 infants were HIV infected (21%), and for the subset of women whose HIV infection was not diagnosed until after delivery, the rate of perinatal HIV transmission was 7 of 18 children (39%).9 During both time periods, antiretroviral drugs were provincially funded in Alberta.
The decreased rate of vertical transmission of HIV in northern Alberta in more recent years is likely multifactorial. A high proportion (more than 95%) of all pregnant women accessing antenatal care undergo HIV testing each year in Alberta.6 Almost half of the women in this study were first recognized to be HIV infected through routine opt-out prenatal screening. Some had no obvious risk factors and likely would not have been screened if the opt-out program had not been implemented. Interestingly, a seroprevalence study of an opt-out program in Alberta, conducted between 2002 and 2004, identified 3 HIV-positive mothers who accessed antenatal care but were not tested for HIV during that pregnancy; because of the anonymous nature of that study, the pregnancy and infant outcomes were not reported.10
Increased use of ART would have also contributed to the decreased rate of vertical transmission of HIV. As in our study, the Canadian Perinatal Surveillance Program has noted a steady increase in the proportion of HIV-infected pregnant women receiving ART, with a high of 89% in 2006.11 This has coincided with a decrease in the incidence of confirmed HIV-infected infants nationally, from 45 of 114 exposed infants in 1994 (39%) to 5 of 192 (3%) in 2006.11 It is acknowledged, however, that the numbers reported nationally do not reflect all infants perinatally exposed to HIV infection, because not all pregnant women are aware of their HIV status and not all HIV-infected infants may be reported through this voluntary reporting system.
Although harder to quantify, the expansion of the multidisciplinary team caring for HIV-infected pregnant women in northern Alberta over the past several years has also likely resulted in decreased rates of perinatal HIV transmission. All positive HIV tests identified through prenatal screening are reported to a public health designate nurse. The nurse maintains an ongoing list of pregnant HIV-infected women in the region, and information is communicated at regular multidisciplinary team rounds. Despite universal access to health care services in Canada, an estimated 5% of pregnant women in Alberta do not routinely access antenatal care for social or geographic reasons. All pregnant HIV-positive women are closely followed and encouraged by the team to improve their clinic attendance and medication adherence; team members also coordinate medication supply at the planned site of delivery. In addition, women are counseled to avoid breastfeeding, and provincially funded infant formula is provided for all babies born to HIV-infected mothers.7 Contraception advice and care is also provided postpartum.
Despite numerous barriers to positive infant outcomes (e.g., maternal substance use, unsuppressed viral load in about one third of patients at delivery), no transmission occurred in women who were known to be HIV infected at the time of delivery. This suggests there is value in prenatal diagnosis and repeated attempts to initiate combination ART even if full adherence and viral suppression cannot always be achieved. Reasons for this apparent success are that (1) ART reduces transmission despite unsuppressed viral loads and (2) even partial implementation of the perinatal protocol is beneficial (e.g., intrapartum zidovudine or nevirapine given to the mother and infant).12,13
Forty-three percent of HIV-infected pregnant women were unaware of their HIV status prior to prenatal screening, suggesting that other voluntary counseling and testing strategies miss opportunities for earlier diagnosis. Aboriginal women (including First Nations, Inuit, and Metis) represented more than half of HIV-infected pregnant women in northern Alberta, although individuals identified as Aboriginal constitute only 5.8% of the population of Alberta.14 Further research is required to determine optimum strategies for accessing this hard-to-reach population and offering HIV testing to them, including testing before pregnancy for Aboriginal women of child-bearing potential. Another intervention that may be effective is repeat HIV testing of high-risk women, either at the end of pregnancy or with rapid HIV tests during labor. The single case of pediatric HIV infection that occurred in our study may have been prevented if the mother had been screened during labor and appropriate antiretroviral drugs had been available.
Limitations of this study include its relatively small sample size and the retrospective design. The low rate of perinatal transmission cannot be directly attributed to the effects of opt-out prenatal testing or the multidisciplinary team, although both of these are probably important factors. In addition, the self-reported rates of alcohol and illicit drug use may not entirely capture the social indicators of the chaotic lifestyles of these HIV-infected pregnant women, who benefit from a multidisciplinary team approach and close follow-up. The fact that a high proportion of women in our cohort were initially diagnosed with HIV at prenatal screening, as well as the known benefits of antiretroviral therapy to prevent perinatal HIV transmission, both point to the importance of routinely testing all pregnant women for HIV.
The use of combination ART during pregnancy for HIV-infected women and the implementation of routine prenatal screening has resulted in remarkably low rates of perinatal transmission of HIV in northern Alberta. The provision of care by a multidisciplinary team is also important for ongoing follow-up and support for those who have substance use and other complex social issues. Innovative strategies are needed to decrease the rate of HIV infection among Aboriginal women of child-bearing potential. Because a small proportion of women may not present for antenatal care or may seroconvert during pregnancy, the value of repeat HIV testing of high-risk women late in pregnancy or rapid HIV testing at the onset of labor should be explored.
Acknowledgments
We acknowledge the outstanding care provided to HIV-positive pregnant women and their families by all care providers in the Northern Alberta HIV Program multidisciplinary team, the public health staff of the regional health authorities, and community agencies.
Human Participant Protection
This study was approved by the Health Research Ethics Board of the University of Alberta.
References
- 1.AIDSinfo, US Department of Health and Human Services. Public Health Service Task Force Recommendations For Use of Antiretroviral Drugs in Pregnant HIV-1 Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States. Available at: http://www.aidsinfo.nih.gov/Guidelines/GuidelineDetail.aspx?MenuItem&=Guidelines&Search=Off&GuidelineID=9&ClassID=2. Updated November 2, 2007. Accessed May 15, 2008.
- 2.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. [DOI] [PubMed] [Google Scholar]
- 3.European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:458–465. [DOI] [PubMed] [Google Scholar]
- 4.Stadnyk M, Twiddy T, Foisy M, et al. Seamless perinatal care in Capital Health and northern Alberta: the success of a team approach. Can J Infect Dis Med Microbiol. 2007;18(suppl B):83B. [Google Scholar]
- 5.Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006;55(no. RR-14):1–17. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. HIV Testing Among Pregnant Women—United States and Canada, 1998–2001. MMWR Morb Mortal Wkly Rep. 2002;51(45):1013–1016. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Guidelines for national human immunodeficiency virus case surveillance, including monitoring for human immunodeficiency virus infection and acquired immune deficiency syndrome. MMWR Recomm Rep. 1999;48(no. RR-13):1–28. [PubMed] [Google Scholar]
- 8.Robertson CM, Svenson LW, Kyle JM. Birth weight by gestational age for Albertan liveborn infants, 1985 through 1998. J Obstet Gynaecol Can. 2002;24:138–148. [DOI] [PubMed] [Google Scholar]
- 9.Robinson JL, Lee BE. Prevention of perinatal transmission of HIV infection. CMAJ. 2000;163:831–832. [PMC free article] [PubMed] [Google Scholar]
- 10.Plitt SS, Singh AE, Lee BE, Preiksaitis JK. HIV Seroprevalence among women opting-out of prenatal HIV screening in Alberta, Canada: 2002–2004. Clin Infect Dis. 2007;45:1640–1643. [DOI] [PubMed] [Google Scholar]
- 11.Public Health Agency of Canada. HIV/AIDS Epi updates, November 2007. Available at: http://www.phac-aspc.gc.ca/aids-sida/publication/epi/pdf/epi2007_e.pdf.Q43. Accessed October 31, 2008.
- 12.Wade NA, Birkhead GS, Warren BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med. 1998;339:1409–1414. [DOI] [PubMed] [Google Scholar]
- 13.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. [DOI] [PubMed] [Google Scholar]
- 14.Statistics Canada. 2006 Census of Canada. Alberta Aboriginal population profile. Available at: http://www12.statcan.ca/english/census06/data/profiles/aboriginal/Index.cfm?Lang=E. Accessed October 31, 2008.