Abstract
Most estimates of US deaths associated with influenza circulation have been similar despite the use of different approaches. However, a recently published estimate suggested that previous estimates substantially overestimated deaths associated with influenza, and concluded that substantial numbers of deaths during a future pandemic could be prevented because of improvements in medical care.
We reviewed the data sources and methods used to estimate influenza-associated deaths. We suggest that discrepancies between the recent estimate and previous estimates of the number of influenza-associated deaths are attributable primarily to the use of different outcomes and methods. We also believe that secondary bacterial infections will likely result in substantial morbidity and mortality during a future influenza pandemic, despite medical progress.
ANNUAL OR SEASONAL INFLUenza epidemics are caused by influenza viruses that incorporate slight antigenic differences from recently circulating viruses (i.e., antigenic drift).1 By contrast, a pandemic is caused by influenza A viruses that contain a new hemagglutinin for which there is little or no preexisting human immunity (i.e., an antigenic shift).1 Among the four pandemics in the past 120 years, the 1918 pandemic caused the greatest morbidity and mortality.2 Data from previous influenza pandemics and epidemics provide useful information for preparing for future influenza pandemics. For example, understanding age-specific mortality rates from past pandemics has aided the US government in planning for the distribution of vaccines and antivirals during future pandemics.3–5 Such data, when used to inform policy decisions, are most useful when evaluated carefully in light of their strengths and limitations.
The Centers for Disease Control and Prevention (CDC) has estimated deaths associated with influenza epidemics for more than four decades. Estimates from a recent study that used data from the 1990–1991 through 1998–1999 respiratory illness seasons have been cited widely.6 That study used deaths coded for respiratory and circulatory causes and influenza virus surveillance data to estimate that approximately 36 000 influenza-associated deaths occurred annually in the United States during this period. Approximately 90% of these deaths occurred among people aged 65 years or older. These estimates are consistent with estimates made by several other groups,7,8 but are higher than estimates reported by Doshi and others.9,10
CAUSE-OF-DEATH CODING
In his recent study, Doshi examined trends in annual influenza-associated mortality and questioned whether higher mortality rates were associated with influenza pandemics rather than with seasonal epidemics.9 His two chief conclusions were that mortality associated with the next pandemic is unlikely to be greater than that associated with seasonal influenza and that previous influenza-modeling estimates overestimated mortality associated with influenza. His conclusions were based on a presentation of US mortality records for which only the specific code for influenza was used as the cause of death. For example, for the International Classification of Diseases, 10th Revision (ICD-10),11 this would have been code J10 or J11.
For several reasons, the number of influenza-related deaths cannot be determined solely by reports of influenza-coded deaths. First, most adult patients with symptoms consistent with influenza infection are not tested for influenza. Those who are generally receive rapid tests of only modest sensitivity. In addition, many influenza-associated deaths occur one or two weeks after the initial infection (when viral shedding has ended), either because of secondary bacterial infections12–14 or because the influenza has exacerbated chronic illnesses (e.g., congestive heart failure or chronic obstructive pulmonary disease).15 Even when influenza infection is confirmed by laboratory testing, those results are rarely reported on death certificates.16 For these reasons, we believe that Doshi's conclusion that influenza is associated with many fewer deaths than previously estimated is not supported by the weight of the available data.
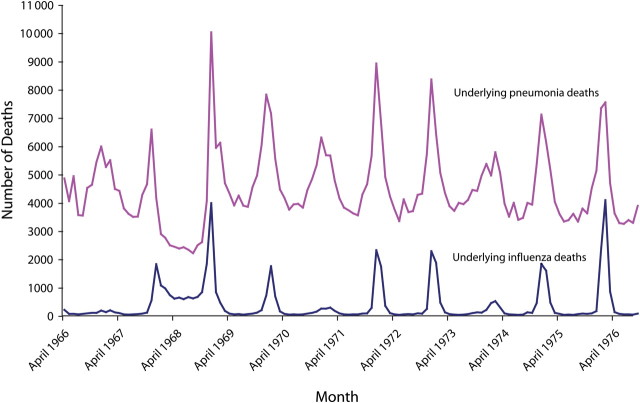
The inability of death certificates to reliably and consistently attribute death to influenza has been understood for many decades, and this understanding led to the development of statistical models to better estimate influenza-associated deaths. This point is illustrated by a graph of the monthly number of deaths from 1962 through 1976 for which a pneumonia- or influenza-specific ICD code was listed as the underlying cause of death (Figure 1).
FIGURE 1.
Reported underlying pneumonia and influenza deaths, by month: United States, 1966–1976.
In the spring of 1968, the number of deaths for which pneumonia was listed as the underlying cause sharply decreased concurrently with a sharp increase in the number of deaths for which influenza was listed as the underlying cause. These changes in mortality coding practices occurred after the emergence of the new pandemic strain was detected in Asia and publicly announced but before the 1968 pandemic influenza A(H3N2) virus actually arrived in the United States. This change in coding practices continued through the summer of 1968 and into the peak of the 1968–1969 pandemic. During the summer of 1968 (June–August), when no significant circulation of influenza would be expected, the number of deaths coded as influenza was 1584% higher than in the summer before and the summer after the 1968 pandemic. Similarly, the number of deaths coded as pneumonia during the summer of 1968 was 40% lower than in the summers of 1967 and 1969.
We suggest that because physicians and medical examiners were aware that an influenza pandemic had begun worldwide, they were more likely to code deaths clinically compatible with pneumonia and influenza as being caused by influenza. However, it is simply not plausible that the 1968–1969 pandemic caused a decrease in the number of pneumonia deaths compared with the seasons before and after the pandemic. Rather, knowledge that a pandemic was under way resulted in diagnostic substitution of an influenza-specific ICD code for the nonspecific ICD codes for pneumonia used during nonpandemic years.
During the 1975–1976 influenza epidemic, a similar change occurred in the proportions of influenza and pneumonia ICD codes selected as the underlying cause of death. However, during this period, concern that a pandemic might emerge led to an increase in the use of the influenza-specific ICD code on death certificates. During the swine influenza vaccine campaign, the total number of deaths for which the underlying cause was listed as influenza was actually similar (7417 deaths) to the number reported during the 1968–1969 pandemic (7433 deaths). To avoid the effect of such artifacts on annual estimates of the influenza disease burden, it is common practice to combine pneumonia and influenza deaths and apply statistical methods to estimate the burden of influenza.17 The CDC's estimate of the number of deaths associated with the influenza epidemic for the 1975–1976 season (24 600 deaths) was close to the expected annual average for the 1972–1973 through 1991–1992 seasons (21 300 deaths).18 That these estimates would be of similar magnitude is expected, of course, because a pandemic did not occur during the 1975–1976 influenza season.
STATISTICAL MODELING
Because sensitive and specific influenza testing is seldom conducted prior to the death of individuals with influenza-compatible illnesses and because of the changes over time in the use of influenza-specific ICD codes, simply counting deaths for which influenza has been coded as the underlying cause on death certificates can lead to both over- and underestimates of the magnitude of influenza-associated mortality. Thus, it has been standard practice for decades in the United States and other developed countries with temperate climates to use statistical modeling techniques to estimate annual deaths associated with influenza.6,18–23
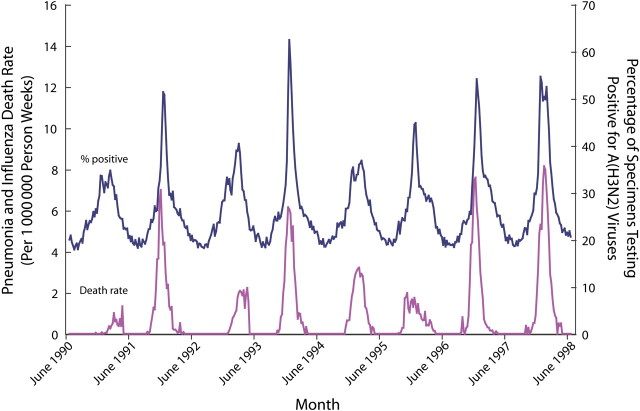
Figure 2 illustrates why statistical modeling techniques are commonly accepted for estimating influenza-associated deaths. The dotted line in this figure represents the percentage of respiratory specimens submitted to World Health Organization collaborating laboratories in the United States that tested positive for influenza A(H3N2). The solid line plots rates of deaths for which the underlying cause was attributed to pneumonia or influenza. Peaks in influenza A(H3N2) activity are clearly and consistently associated with peaks in rates of pneumonia and influenza deaths. Figure 3 displays the observed number of pneumonia and influenza deaths and the estimated numbers of influenza-associated deaths by influenza virus type and subtype. This graph shows that the CDC's statistical model only attributes deaths to influenza when influenza viruses are circulating and that it attributes deaths separately by type and subtype.
FIGURE 2.
Rate of underlying pneumonia and influenza deaths and percentage of World Health Organization specimen testing positive for influenza A(H3N2) viruses, per week: United States, 1990–1991 through 1997–1998 respiratory illness seasons.
FIGURE 3.
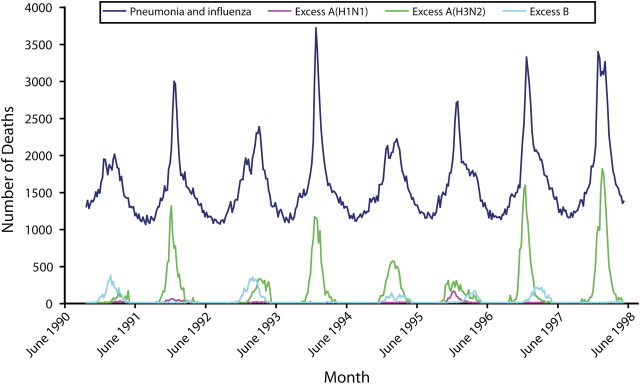
Numbers of influenza and pneumonia deaths and estimated influenza-associated deaths by influenza type and subtype, per week: United States, 1990–1991 through 1997–1998 respiratory illness seasons.
Finally, World Health Organization surveillance data reveals that when influenza viral activity begins early or late in the respiratory illness season, peaks in the rates of pneumonia and influenza deaths, respiratory and circulatory deaths, and all-cause deaths coincide with the peaks in influenza virus circulation. For example, when influenza viral activity peaked early in the 2003–2004 season, pneumonia and influenza deaths also peaked early. In three seasons when influenza activity peaked very late in the season (1982–1983, 1992–1993, 2001–2002), peaks in pneumonia and influenza deaths also occurred within two weeks of the peaks in influenza viral activity.
Contrary to Doshi's suggestion that estimates of influenza-associated deaths have varied dramatically in various publications,9 seasonal influenza-related death estimates are actually markedly similar, especially considering that they use a variety of statistical models.6–8 Of course, when different outcomes are modeled, somewhat different estimates of influenza-associated deaths are obtained. For example, the death estimates cited by Doshi from Thompson et al.6 were derived by modeling underlying pneumonia and influenza deaths, whereas the estimates by Dushoff et al.8 were based on modeling deaths for which pneumonia or influenza was listed anywhere on the death certificate. The Dushoff et al. estimates were therefore higher. Most importantly, from a policy perspective, the majority of influenza-associated death estimates made for the United States are in the same range (i.e., tens of thousands per year), demonstrating the importance of influenza prevention and control strategies.
PANDEMIC VARIATIONS
Even when consistent methods are used to estimate their effects, influenza pandemics and seasonal epidemics vary greatly in severity, primarily because of varying degrees of preexisting immunity to specific influenza viruses at the population level and the relative virulence of those viruses.24 For example, the 1957–1958 and 1968–1969 pandemics resulted in far fewer deaths than did the 1918–1919 pandemic,4,25 likely because of the specific HA, NA, and PB1 genes found in the 1918 virus.26,27 During several influenza epidemics that occurred after the 1920s, the rates of influenza-associated deaths exceeded the rates of deaths associated with the 1968–1969 pandemic and in some cases with the 1957–1958 pandemic.25,28–30
Another example of this phenomenon is that the estimated numbers of deaths associated with the 1968–1969 influenza pandemic (between 34 000 and 56 00025,31–33) are similar to recent annual estimates of deaths associated with seasonal influenza epidemics (from 33 000–51 0006,8). The similarity between death estimates for the 1968 pandemic and more recent seasonal epidemics may be explained partly by increases in the average age of the US population during the past several decades; most influenza deaths occur in older individuals. However, it is not known what other host factors may be involved. These findings reinforce the fact that year-to-year variability in annual estimates of influenza-associated deaths must be evaluated with accepted and consistent statistical methods.
Doshi states that the majority of deaths during the 1918–1919 pandemic was attributable to secondary bacterial complications and suggests that most of these complications are now preventable because of improvements in health care. Published data suggest that a substantial number of secondary bacterial infections occurred during the 1918–1919 pandemic,12,34–37 and some of these infections are now likely preventable. For example, pneumococcal conjugate vaccine is 18% to 30% effective in preventing pneumonia in children38 and therefore could reduce mortality associated with secondary pneumococcal pneumonia in a future pandemic. However, this vaccine is not licensed for use in adults, and even if it were used widely, it would be unlikely to prevent substantial mortality in the United States, because the seven serotypes included in the vaccine cause less than 20% of invasive pneumococcal disease among adults.39
Pneumococcal polysaccharide vaccine, which is recommended and licensed for use in all adults aged 65 years and older, is considerably less effective against invasive pneumococcal disease among persons with chronic illnesses, a population that is substantially larger today than it was during any of the 20th-century pandemics.40 There is little evidence that this vaccine is effective in reducing all-cause pneumonia and mortality.41 In addition, improvements in care of the critically ill have not been shown to reduce mortality from pneumococcal disease.42
Staphylococcus aureus, another cause of secondary bacterial pneumonia during pandemics36,43 and interpandemic periods,44,45 presents two important medical challenges. First, there is no vaccine for prevention of S. aureus pneumonia. Second, antimicrobial resistance has developed in S. aureus over the past 40 years, limiting treatment options. Multiple reports of community-associated methicillin-resistant S. aureus have described especially severe clinical presentations, including necrotizing pneumonia, septic shock, and death within 24 to 48 hours of illness onset, despite intensive antimicrobial therapy and supportive care.46–48 Thus, although many of the deaths that occurred during the 1918–1919 pandemic may have been associated with secondary bacterial infections, available data do not provide sufficient reassurance that antibiotics, intensive care units, and pneumococcal vaccines will lead to a substantial reduction in mortality during the next pandemic.
CONCLUSIONS
It has long been recognized that influenza is associated with substantial mortality during both epidemics and pandemics. Modeling estimates from the CDC and several other groups build on several decades of work devoted to best understanding how to use US vital statistics data to assess the health burden of influenza. Doshi's estimates of influenza deaths are lower than estimates produced by the CDC and other groups primarily because he used a narrow outcome and did not use statistical models developed to more accurately estimate the total number of influenza-associated deaths. These differences lead to substantially different conclusions regarding the effect of influenza on deaths in the United States during both seasonal epidemics and the three pandemics of the 20th century.
No one can predict the severity of any influenza season, including a pandemic season. On June 11, 2009, the World Health Organization (WHO) declared the start of the 2009 influenza pandemic. As of July 31, 2009, 168 countries and territories had reported at least one laboratory-confirmed case of pandemic H1N1 2009, and all continents had been affected by the pandemic. Over 162 000 cases were reported to the WHO, and the actual number of infected persons was certainly greater. By the time this piece is published, circulation of this virus will be reestablished in the Northern hemisphere. In order to plan to reduce the health effects of this and further pandemics in the United States and elsewhere, we must be ready to make the best estimates of influenza's impact using rigorous and sound methods and understand how mortality estimates for a pandemic compare with those for seasonal influenza. Progress is continuing to be made by the engaged scientific community in improving the methods used to make these estimates.
Acknowledgments
This study was funded by the Centers for Disease Control and Prevention.
References
- 1.Fiore AE, Shay DK, Haber P, et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007;56(RR-6):1–54. [PubMed] [Google Scholar]
- 2.Glezen WP. Emerging infections: pandemic influenza. Epidemiol Rev. 1996;18(1):64–76. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. HHS Pandemic Influenza Plan. 2005. Available at: http://www.dhhs.gov/pandemicflu/plan/pdf/HHSPandemicInfluenzaPlan.pdf. Accessed April 28, 2009.
- 4.Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998;178(1):53–60. [DOI] [PubMed] [Google Scholar]
- 5.Luk J, Gross P, Thompson WW. Observations on mortality during the 1918 influenza pandemic. Clin Infect Dis. 2001;33(8):1375–1378. [DOI] [PubMed] [Google Scholar]
- 6.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. [DOI] [PubMed] [Google Scholar]
- 7.Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005;165(3):265–272. [DOI] [PubMed] [Google Scholar]
- 8.Dushoff J, Plotkin JB, Viboud C, Earn DJ, Simonsen L. Mortality due to influenza in the United States–an annualized regression approach using multiple-cause mortality data. Am J Epidemiol. 2006;163:181–187. [DOI] [PubMed] [Google Scholar]
- 9.Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health. 2008;98(5):939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doshi P. Influenza vaccination: policy versus evidence: policy is in the lead. BMJ. 2006;333(7576):1020–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Classification of Diseases, 10th Revision. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 12.Soper GA. The influenza pneumonia pandemic in the American army camps during September and October. 1918. Science. 1918;48(1245):451–456. [DOI] [PubMed] [Google Scholar]
- 13.Robertson L, Caley JP, Moore J. Importance of Staphylococcus aureus in pneumonia in the 1957 epidemic of influenza A. Lancet. 1958;2(7040):233–236. [DOI] [PubMed] [Google Scholar]
- 14.Bisno AL, Griffin JP, Van Epps KA, Niell HB, Rytel MW. Pneumonia and Hong Kong influenza: a prospective study of the 1968–1969 epidemic. Am J Med Sci. 1971;261(5):251–263. [DOI] [PubMed] [Google Scholar]
- 15.Eickhoff TC, Sherman IL, Serfling RE. Observations on excess mortality associated with epidemic influenza. JAMA. 1961;176:776–782. [DOI] [PubMed] [Google Scholar]
- 16.Wiselka M. Influenza: diagnosis, management, and prophylaxis. BMJ. 1994;308(6940):1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noymer A. Influenza analysis should include pneumonia. Am J Public Health. 2008;98(11):1927–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997;87(12):1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serfling RE. Methods for current statistical analysis of excess pneumonia-influenza deaths. Public Health Rep. 1963;78(6):494–505. [PMC free article] [PubMed] [Google Scholar]
- 20.Housworth J, Langmuir AD. Excess mortality from epidemic influenza, 1957–1966. Am J Epidemiol. 1974;100(1):40–48. [DOI] [PubMed] [Google Scholar]
- 21.Tillett HE, Smith JW, Clifford RE. Excess morbidity and mortality associated with influenza in England and Wales. Lancet. 1980;315(8172):793–795. [DOI] [PubMed] [Google Scholar]
- 22.Lui KJ, Kendal AP. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health. 1987;77(6):712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson KG. Impact of influenza and respiratory syncytial virus on mortality in England and Wales from January 1975 to December 1990. Epidemiol Infect. 1996;116(1):51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox NJ, Subbarao K. Influenza. Lancet. 1999;354(9186):1277–1282. [DOI] [PubMed] [Google Scholar]
- 25.Noble GR. Epidemiogical and Clinical Aspects of Influenza. Basic and Applied Influenza Research. Boca Raton, FL: CRC Press; 1982:11–50. [Google Scholar]
- 26.Pappas C, Aguilar PV, Basler CF, et al. Single gene reassortants identify a critical role for PB1, HA, and NA in the high virulence of the 1918 pandemic influenza virus. Proc Natl Acad Sci USA. 2008;105(8):3064–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tumpey TM, Basler CF, Aguilar PV, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310(5745):77–80. [DOI] [PubMed] [Google Scholar]
- 28.Collins SD, Lehmann J. Trends and epidemics of influenza and pneumonia: 1918–1951. Public Health Rep. 1951;66(46):1487–1516. [PMC free article] [PubMed] [Google Scholar]
- 29.Viboud C, Tam T, Fleming D, Handel A, Miller MA, Simonsen L. Transmissibility and mortality impact of epidemic and pandemic influenza, with emphasis on the unusually deadly 1951 epidemic. Vaccine. 2006;24(44–46):6701–6707. [DOI] [PubMed] [Google Scholar]
- 30.Viboud C, Tam T, Fleming D, Miller MA, Simonsen L. 1951 influenza epidemic, England and Wales, Canada, and the United States. Emerg Infect Dis. 2006;12(4):661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi K, Thacker SB. Mortality during influenza epidemics in the United States, 1967–1978. Am J Public Health. 1982;72(11):1280–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stroup DF, Thacker SB, Herndon JL. Application of multiple time series analysis to the estimation of pneumonia and influenza mortality by age 1962–1983. Stat Med. 1988;7(10):1045–1059. [DOI] [PubMed] [Google Scholar]
- 33.Alling DW, Blackwelder WC, Stuart-Harris CH. A study of excess mortality during influenza epidemics in the United States, 1968-1976. Am J Epidemiol. 1981;113(1):30–43. [DOI] [PubMed] [Google Scholar]
- 34.Nuzum JW, Pilot I, Stangl FH, Bonar BE. 1918 pandemic influenza and pneumonia in a large civil hospital. IMJ Ill Med J. 1976;150(6):612–616. [PubMed] [Google Scholar]
- 35.Abrahams A, Hallows N, French H. A further investigation into influenza pneumococcal and influenza-streptococcal septicaemia: epidemic influenza “pneumonia” of highly fatal type and its relation to “purulent bronchitits.” Lancet. 1919;193:1–11. [Google Scholar]
- 36.Chickering HT, Park JH. Staphylococcus aureus pneumonia. JAMA. 1919;72:617–623. [Google Scholar]
- 37.Brundage JF, Shanks GD. Deaths from bacterial pneumonia during the 1918–19 influenza pandemic. Emerg Infect Dis. 2008;14(8):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen J, Black S, Shinefield H, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J. 2006;25(9):779–781. [DOI] [PubMed] [Google Scholar]
- 39.Moore MR, Gertz RE, Woodbury RL, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197(7):1016–1027. [DOI] [PubMed] [Google Scholar]
- 40.Wu S, Green A. Projection of Chronic Illness Prevalence and Cost Inflation. Santa Monica, CA: RAND Corporation; 2000. [Google Scholar]
- 41.Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2008;(1):CD000422. [DOI] [PubMed] [Google Scholar]
- 42.Hook EW, Horton CA, Schaberg DR. Failure of intensive care unit support to influence mortality from pneumococcal bacteremia. JAMA. 1983;249(8):1055–1057. [PubMed] [Google Scholar]
- 43.Schwarzmann SW, Adler JL, Sullivan RJ, Marine WM. Bacterial pneumonia during the Hong Kong influenza epidemic of 1968–1969. Arch Intern Med. 1971;127(6):1037–1041. [PubMed] [Google Scholar]
- 44.Hageman JC, Uyeki TM, Francis JS, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis. 2006;12(6):894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40(1):100–107. [DOI] [PubMed] [Google Scholar]
- 46.Ito T, Iijima M, Fukushima T, et al. Pediatric pneumonia death caused by community-acquired methicillin-resistant Staphylococcus aureus, Japan. Emerg Infect Dis. 2008;14(8):1312–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(suppl 5):S378–S385. [DOI] [PubMed] [Google Scholar]
- 48.Tong SY, Anstey NM, Lum GD, Lilliebridge RA, Stephens DP, Currie BJ. Fatal community-associated methicillin-resistant Staphylococcus aureus pneumonia after influenza. Med J Aust. 2008;188(1):61. [DOI] [PubMed] [Google Scholar]
- 49.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans. New Engl J Med. Published ahead of print May 7, 2009. 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 50.Influenza A. H1N1. Update 12. Geneva, Switzerland: World Health Organization, 2009. Available at: http://www.who.int/csr/don/2009_05_03a/en/index.html. Accessed May 12, 2009. [Google Scholar]