Abstract
Objectives. We studied compliance with multiple-dose vaccine schedules, assessed factors associated with noncompliance, and examined timeliness of series completion among older children, adolescents, and adults.
Methods. We conducted a large, multisite, retrospective cohort study of older children, adolescents, and adults in the Vaccine Safety Datalink population from 1996 through 2004. We quantified the rates of completion of all required doses for varicella, hepatitis A, and hepatitis B vaccines according to their recommended schedules.
Results. Among those who received a first dose of varicella (n = 16 075), hepatitis A (n = 594 917), and hepatitis B (n = 590 445) vaccine, relatively few completed the series (55%–65% for hepatitis B vaccine and 40%–50% for hepatitis A and varicella vaccines in most age groups). Compliance was lowest among adolescents (35.9%) and Medicaid recipients (29.7%) who received varicella vaccine and among younger adult age groups who received hepatitis A vaccine (25%–35% across those age groups). Even among series completers, there was a relatively long interval of undervaccination between the first and last doses.
Conclusions. Compliance with multiple-dose vaccine series among older children, adolescents, and adults is suboptimal. Further evaluations of strategies to improve compliance in these populations are needed.
Many vaccines given to older children, adolescents, and adults require multiple doses for optimal immunogenicity and efficacy. Varicella vaccine is recommended in a 2-dose series for children 13 years or older without evidence of immunity.1 The hepatitis A2 and B3,4 vaccine series require 2 and 3 doses, respectively. Most recently, in 2007, vaccination with the 3-dose human papillomavirus vaccine series was recommended for adolescent and young adult women.5 Timely and complete vaccination with multiple-dose schedules is of public health importance, because an incomplete series may yield suboptimal disease protection.6–17 Comprehensive monitoring is conducted annually to evaluate compliance with vaccination schedules among infants and young children.18 However, there is little information on compliance with multiple-dose vaccine series in general populations of older children, adolescents, and adults.19–21
We conducted a large, population-based, retrospective cohort study of older children, adolescents, and adults in the Vaccine Safety Datalink population of approximately 8.8 million Medical Care Organization (MCO) enrollees, which represents about 3% of the US population. During the study period (1996–2004), we determined compliance with the 2- or 3-dose varicella, hepatitis A, or hepatitis B vaccine series in this population. We also assessed factors associated with noncompliance, and among those who completed the vaccination series, we examined the timeliness of completion to determine the excess duration of time that individuals remained undervaccinated.
METHODS
The study population was composed of enrollees of 7 of the 8 MCOs participating in the Vaccine Safety Datalink project: Group Health (Washington), Kaiser Permanente Northwest (Oregon), Kaiser Permanente Medical Care Program of Northern California, Southern California Kaiser Permanente Health Care Program, HealthPartners Research Foundation (Minnesota), Marshfield Clinic (Wisconsin), and Kaiser Permanente Colorado. Each MCO has administrative data systems that record information on demographics, enrollment, immunizations, and International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM)22 codes assigned to medical encounters.23 The study period for each MCO was based on the availability of data from the MCO's immunization registry. Most sites contributed data from January 1, 1997 to December 31, 2004. One site's immunization registry extended back to 1996, and a second site began contributing data in 2000. Four sites did not contribute data on adults 18 years and older.
Three cohorts of older children, adolescents, and adults were drawn from this population: (1) those who received a first dose of varicella vaccine on or after their 13th birthday, prior to their 46th birthday, and during the study period; (2) those who received a first dose of hepatitis A vaccine on or after their second birthday and during the study period; and (3) those who received a first dose of hepatitis B vaccine on or after their fifth birthday and during the study period. We chose lower age cutoffs for hepatitis A and B vaccines to exclude vaccines potentially received as part of the infant series and for varicella vaccine to include the population for which a 2-dose series has been recommended since vaccine licensure in 1996. To ensure complete identification of doses received as part of the multiple-dose series, participants were required to be continuously enrolled for at least 1 year prior to and at least 2 years following the date of the first dose of varicella, hepatitis A, or hepatitis B vaccination. Dates of first vaccination thus ranged from 1996 to 2002, with follow-up through 2004.
Vaccination Data
For each vaccine (varicella, hepatitis A, and hepatitis B), the date of the first and all subsequent doses of the same vaccine were identified. To ensure that we correctly identified the first vaccination recorded in the MCO immunization registry, we ascertained prior vaccination status from all available vaccination information in the registry—that is, not just in the 1 year prior if more than 1 year of data were available. If 2 doses of the same vaccine were recorded within 7 days, the second dose was assumed to be a duplicate record and was not counted as part of the vaccination series.
Outcome Measures
For each vaccine, the primary outcome was completion of the series within 1 year of initiation (full compliance). In addition, to provide a more thorough assessment of compliance, we also identified those who completed the series more than 1 year but less than 2 years after receipt of the initial vaccination (late compliance) as well as those who completed the series more than 2 years after initiation (very late compliance). All of these definitions of compliance excluded the small fraction of persons who completed the vaccination series but received a second or third vaccination at less than the minimum recommended interval between doses (28 days between dose 1 and 2 for varicella vaccine, 180 days between dose 1 and 2 for hepatitis A vaccine, and 28 days between dose 1 and 2 and 56 days between dose 2 and 3 for hepatitis B vaccine). That group was defined as completing off-schedule. Last, persons who initiated but did not complete the vaccine series during the study period were defined as incomplete. To assess vaccine series completion, participants were followed for 2 to 9 years after the first vaccination, depending on the timing of the first vaccination during the study period and the duration of the study participant's enrollment in the MCO following vaccination.
Covariate Data
Characteristics of study participants, which were defined based on administrative data, included age in years at the time of the first dose of each vaccine (varicella, hepatitis A, and hepatitis B), gender, duration of continuous MCO enrollment prior to and after the first dose, calendar year of receipt of first dose, the number of visits (outpatient, emergency department, or hospital) in the year prior to the first dose, selected chronic conditions recorded during the year preceding the first dose, and Medicaid status in the year prior to the first dose. Participants were defined as having a chronic condition if, during an outpatient, emergency department, or hospital visit in the year prior to the receipt of first vaccine dose, they were assigned an ICD-9-CM code for any of the following conditions: diabetes, cancer, congestive heart failure, ischemic heart disease, renal disease, HIV, chronic obstructive pulmonary disease, liver disease, rheumatoid arthritis or systemic lupus erythematosus, dialysis recipient, or transplant recipient. A participant was defined as a Medicaid recipient if there was a record of receiving Medicaid benefits for at least 1 month in the year preceding the first dose. Analyses involving Medicaid information were restricted to the 4 sites for which this information was available.
Statistical Analysis
For each vaccine (varicella, hepatitis A, and hepatitis B), we computed the proportion of study participants who were full, late, and very late compliers, as well as the proportion who completed off-schedule or did not complete the series. To assess factors associated with being fully compliant with each vaccine series, we performed multivariable relative risk regression.24 Specifically, we modeled the probability of full compliance as a function of risk factors using a generalized linear model with a log link and binomial error distribution. When the model did not converge with the binomial error (< 5% of models), we used Gaussian error and computed model-robust (Huber–White) standard errors. We used this approach instead of logistic regression to estimate the relative risks because a large proportion of vaccine recipients (> 10%) are not fully compliant and the odds ratios obtained from logistic regression models would overestimate the desired relative risks except when the relative risk is zero. To further evaluate the timeliness of series completion, we plotted the cumulative proportion who completed each series (i.e., received all subsequent doses) over time by age groups among those who completed the vaccine series at any time after the minimum interval had elapsed (i.e., among full, late, and very late compliers).
RESULTS
A total of 16 075 participants received a first dose of varicella vaccine, 594 917 received a first dose of hepatitis A vaccine, and 590 445 received a first dose of hepatitis B (Table 1). Two sites contributed most (80%–90%) of the data. About half of all vaccine recipients were female, the mean duration of MCO enrollment was about 9 years, and most vaccines were received by children and adolescents. Because of the age study criteria, those receiving the hepatitis A vaccine tended to be younger and those receiving the varicella vaccine were older. Most first doses of hepatitis A were received later in the study period (2000–2002), more hepatitis B was received in the earlier study years (1996–2000), and the proportion of first varicella doses received was similar across all study years.
TABLE 1.
Demographic, Enrollment, and Risk Factor Characteristics of Vaccine Recipients: Vaccine Safety Datalink, 1996–2004
| Varicella Vaccine Recipients (n = 16 075) | Hepatitis A Vaccine Recipients (n = 594 917) | Hepatitis B Vaccine Recipients (n = 590 445) | |
| Site, % | |||
| 1 | 6 | 6 | 6 |
| 2 | 51 | 55 | 45 |
| 3 | 6 | 4 | 6 |
| 4 | 3 | < 1 | 4 |
| 5 | 1 | < 1 | 3 |
| 6 | 1 | < 1 | 1 |
| 7 | 32 | 35 | 35 |
| Age, % | |||
| 2–4 y | 27 | ||
| 5–8 y | 15 | 15 | |
| 9–12 y | 19 | 44 | |
| 13–17 y | 73 | 17 | 26 |
| 18–29 y | 13 | 4 | 4 |
| 30–49 y | 14 | 8 | 7 |
| 50–64 y | 7 | 3 | |
| ≥ 65 y | 3 | 1 | |
| Male, % | 46 | 49 | 49 |
| Years of continuous enrollment during study period, mean (range) | |||
| Before first dose | 4.5 (1.0–11.9) | 4.8 (1.0–12.0) | 4.4 (1.0–12.0) |
| After first dose | 4.2 (2.0–9.2) | 3.8 (2.0–9.3) | 5.1 (2.0–9.3) |
| Total | 8.7 (3.0–14.1) | 8.6 (3.0–14.1) | 9.5 (3.0–14.1) |
| Year when first dose was received, % | |||
| 1996 | 13 | 2 | 15 |
| 1997 | 15 | 4 | 22 |
| 1998 | 17 | 5 | 22 |
| 1999 | 17 | 6 | 19 |
| 2000 | 14 | 28 | 10 |
| 2001 | 13 | 30 | 7 |
| 2002 | 11 | 25 | 5 |
| Any chronic condition, % | 7 | 9 | 7 |
| No. of medical visits in year prior to first dose | |||
| 0 | 55 | 49 | 57 |
| 1–10 | 42 | 48 | 41 |
| > 10 | 3 | 3 | 2 |
Compliance With the Varicella Vaccine Series
Among those aged 13 years and older who initiated the varicella vaccine series, only 36% to 56% received the second dose within 1 year, depending on age (Table 2). Over 40% of persons never received the second dose, and only a relatively small proportion completed the series more than 1 year after receipt of the first dose. Full compliance increased with age and was highest among adults aged 30 to 49 years (Table 2), a trend that was consistent across sites with adult data (see figure 2, available as a supplement to the online version of this article at http://www.ajph.org). Compliance also varied by site, particularly among adolescents: 24% to 60% for 13- to 17-year-olds, 48% to 60% for 18- to 29-year-olds, and 54% to 63% for 30- to 49-year-olds. Higher compliance was also observed for women, for those with 12 or more years of MCO enrollment, and in earlier study years (Table 3).
TABLE 2.
Percentage of Participants in Each Compliance Category, by Vaccine and Age Group: Vaccine Safety Datalink, 1996–2004
| Completed Series Within 1 Year of First Dose, % | Completed Series 1–2 Years After First Dose, % | Completed Series > 2 Years After First Dose, % | Did Not Complete Varicella or Hepatitis A Series Within Study Period, % | Did Not Complete Hepatitis B Series Within Study Period |
||||
| Age at First Dose, y | No. of Series Initiators | Completed Series Off-Schedule,a % | Received Only 1 Dose, % | Received Only 2 Doses, % | ||||
| Varicella vaccine | ||||||||
| 13–17 | 11 681 | 35.9 | 4.4 | 3.6 | 0.5 | 55.6 | ||
| 18–29 | 2 141 | 49.3 | 1.8 | 2.1 | 1.4 | 45.3 | ||
| 30–49 | 2 253 | 55.6 | 1.6 | 2.2 | 1.2 | 39.4 | ||
| Hepatitis A vaccine | ||||||||
| 2–4 | 159 916 | 50.1 | 30.8 | 9.5 | 1.7 | 7.9 | ||
| 5–8 | 91 352 | 39.3 | 22.3 | 17.1 | 1.7 | 19.7 | ||
| 9–12 | 115 765 | 48.4 | 22.5 | 13.1 | 2.0 | 14.0 | ||
| 13–17 | 101 226 | 40.3 | 19.7 | 9.2 | 1.9 | 28.8 | ||
| 18–29 | 21 351 | 25.0 | 6.9 | 7.3 | 3.0 | 57.8 | ||
| 30–49 | 48 219 | 35.2 | 7.1 | 8.7 | 4.2 | 44.9 | ||
| 50–64 | 38 700 | 43.6 | 7.0 | 8.9 | 5.5 | 35.0 | ||
| ≥ 65 | 18 388 | 43.6 | 6.3 | 6.9 | 6.3 | 37.0 | ||
| Hepatitis B vaccine | ||||||||
| 5–8 | 91 740 | 56.9 | 14.1 | 20.6 | 1.4 | 3.0 | 3.9 | |
| 9–12 | 259 549 | 63.4 | 15.2 | 10.5 | 1.4 | 3.8 | 5.7 | |
| 13–17 | 150 445 | 45.1 | 13.4 | 10.2 | 1.3 | 13.9 | 16.1 | |
| 18–29 | 23 179 | 41.4 | 4.9 | 4.6 | 2.1 | 25.7 | 21.2 | |
| 30–49 | 41 359 | 55.7 | 4.0 | 3.1 | 2.7 | 18.1 | 16.4 | |
| 50–64 | 18 776 | 62.2 | 3.3 | 2.8 | 3.0 | 15.1 | 13.7 | |
| ≥ 65 | 5 397 | 61.1 | 2.8 | 2.0 | 3.3 | 18.3 | 12.5 | |
Off-schedule completers completed all required doses but received a second or third vaccination at less than the minimum recommended interval between doses.
TABLE 3.
Relative Risks (RRs) for Vaccine Series Completion Within 1 Year of Initiation: Vaccine Safety Datalink, 1996–2004
| Varicella Vaccine Recipients, RR (95% CI) | Hepatitis A Vaccine Recipients, RR (95% CI) | Hepatitis B Vaccine Recipients, RR (95% CI) | |
| Site | |||
| 1 | Reference | Reference | Reference |
| 2 | 0.86 (0.81, 0.92) | 1.03 (1.02, 1.04) | 1.17 (1.16, 1.18) |
| 3 | 0.69 (0.62, 0.76) | 1.19 (1.17, 1.22) | 1.12 (1.10, 1.13) |
| 4 | 0.80 (0.70, 0.91) | 0.88 (0.81, 0.94) | 1.41 (1.39, 1.43) |
| 5 | 0.87 (0.72, 1.05) | 0.89 (0.83, 0.95) | 1.34 (1.32, 1.36) |
| 6 | 1.08 (0.89, 1.32) | 1.19 (1.01, 1.40) | 1.74 (1.72, 1.77) |
| 7 | 0.46 (0.42, 0.50) | 1.06 (1.04, 1.07) | 1.08 (1.06, 1.09) |
| Age group, y | |||
| 2–4 | Reference | ||
| 5–8 | 0.78 (0.77, 0.79) | Reference | |
| 9–12 | 0.93 (0.92, 0.94) | 1.11 (1.10, 1.11) | |
| 13–17 | Reference | 0.77 (0.76, 0.78) | 0.82 (0.82, 0.83) |
| 18–29 | 1.06 (1.00, 1.12) | 0.51 (0.49, 0.52) | 0.75 (0.73, 0.76) |
| 30–49 | 1.19 (1.13, 1.25) | 0.74 (0.73, 0.75) | 1.00 (0.99, 1.01) |
| 50–64 | 0.91 (0.89, 0.92) | 1.10 (1.08, 1.11) | |
| ≥ 65 | 0.90 (0.89, 0.92) | 1.07 (1.05, 1.10) | |
| Male | 0.93 (0.90, 0.96) | 0.98 (0.97, 0.99) | 0.97 (0.96, 0.97) |
| Enrollment duration, y | |||
| 3–6 | Reference | Reference | Reference |
| 7–9 | 1.02 (0.97, 1.08) | 0.97 (0.96, 0.98) | 1.05 (1.04, 1.06) |
| 10–11 | 1.04 (0.98, 1.11) | 1.01 (1.00, 1.02) | 1.11 (1.10, 1.12) |
| ≥ 12 | 1.07 (1.01, 1.14) | 1.11 (1.09, 1.12) | 1.22 (1.21, 1.23) |
| Year of first dose | |||
| 1996 | Reference | Reference | Reference |
| 1997 | 0.88 (0.83, 0.93) | 1.13 (1.10, 1.17) | 1.04 (1.03, 1.04) |
| 1998 | 0.84 (0.79, 0.89) | 1.17 (1.14, 1.20) | 1.09 (1.08, 1.10) |
| 1999 | 0.83 (0.78, 0.88) | 1.21 (1.18, 1.25) | 1.08 (1.07, 1.09) |
| 2000 | 0.78 (0.73, 0.83) | 1.32 (1.29, 1.35) | 1.04 (1.03, 1.05) |
| 2001 | 0.77 (0.72, 0.83) | 1.32 (1.29, 1.35) | 1.08 (1.07, 1.09) |
| 2002 | 0.77 (0.72, 0.82) | 1.29 (1.26, 1.32) | 1.10 (1.09, 1.11) |
| Any chronic condition | 0.96 (0.90, 1.03) | 1.00 (0.99, 1.00) | 0.99 (0.98, 1.00) |
| No. of medical visits in year prior to dose 1 | |||
| 0 | Reference | Reference | Reference |
| 1–10 | 1.02 (0.98, 1.07) | 1.05 (1.04, 1.06) | 1.01 (1.00, 1.02) |
| > 10 | 1.04 (0.94, 1.14) | 1.15 (1.13, 1.16) | 1.05 (1.04, 1.06) |
Note. CI = confidence interval. RRs are estimated from multivariable models that adjust for all covariates. For varicella vaccine recipients, n = 16 075. For hepatitis A vaccine recipients, n = 594 917. For hepatitis B vaccine recipients, n = 590 445.
Compliance With the Hepatitis A Vaccine Series
Among those who received a first dose of hepatitis A vaccine, the proportion who received the second dose within 1 year ranged from 40% to 50% in most age groups (Table 2). However, younger adults were less compliant, with only 25% of 18- to 29-year-olds and 35% of 30- to 49-year-olds completing the series within 1 year, and this effect was consistent across adult sites (see figure 2, available online). In contrast to what was found with varicella vaccine, most adolescents who did not complete the series within 1 year eventually received the second dose during the study period. As with varicella vaccine, most adults who did not receive a second dose within 1 year never received the dose within the study period. Full compliance with the hepatitis A series also varied by site (Table 3; see also figure 2, available online); however, sites with the lowest hepatitis A vaccine compliance were not the same as those with the lowest varicella vaccine compliance. Full compliance also increased with duration of MCO enrollment, number of medical visits in the year prior to the first dose, and calendar year of the first dose (Table 3).
Compliance With the Hepatitis B Vaccine Series
Rates of full compliance were generally higher with hepatitis B than with the other vaccines, with close to 60% of series initiators receiving all 3 doses within 1 year in most age groups (Table 2). Full compliance rates were lower among adolescents (45%) and 18- to 29-year-olds (41%), and this pattern was consistent across sites (see figure 2, available online). Among those not fully compliant, children aged 12 years or younger tended to receive subsequent vaccine doses late, adults tended not to complete the series, and adolescents contributed to both late and incomplete compliance categories in relatively similar proportions. Full compliance also varied by site and increased with duration of MCO enrollment (Table 3).
Compliance Among Medicaid Recipients
A total of 71%, 94%, and 61% of the varicella, hepatitis A, and hepatitis B cohort members at 4 sites had information in their administrative record about their Medicaid status, which represented 11 399, 560 073, and 361 577 first vaccinations, respectively (see table 4, available as a supplement to the online version of this article at http://www.ajph.org). Among these individuals, 3% were Medicaid recipients; compared with nonrecipients of Medicaid, recipients had relative risks of being fully compliant with the varicella, hepatitis A, and hepatitis B vaccine series of 0.80 (95% confidence interval [CI] = 0.68, 0.94), 0.91 (95% CI = 0.90, 0.93), and 0.81 (95% CI = 0.80, 0.83), respectively, as estimated from multivariable models. This suggests that those who receive Medicaid are less likely to complete multiple-dose vaccine series than those who do not receive Medicaid.
First to Last Dose Interval Among Vaccine Series Completers
Even among persons who completed the vaccine series at any time during the study period after the minimum recommended interval had elapsed, there was often a relatively long interval between receipt of the first and last vaccinations in the series, and children and adolescents were generally slower to complete all multiple-dose series than adults (Figure 1). Among the approximately 50% who completed the varicella vaccine series, 35% of adolescents and about 20% of adults had not received the second dose within 3 months of the first dose, and nearly 20% of adolescents and 10% of adults still had not completed the series after 1 year. Among the 75% of persons who completed the hepatitis A vaccine series, 40% to 50% of older children and adolescents and 25% to 40% of adults had not received a second dose within 1 year of the first, and by 2 years about 20% in all age groups still had not completed the series. Among the 80% of those who received all 3 doses of hepatitis B vaccine, 30% to 40% of children and adolescents and 10% to 20% of adults were not fully compliant after 1 year, and even after 2 years, 15% to 25% of children and adolescents had not completed the 3-dose series.
FIGURE 1.
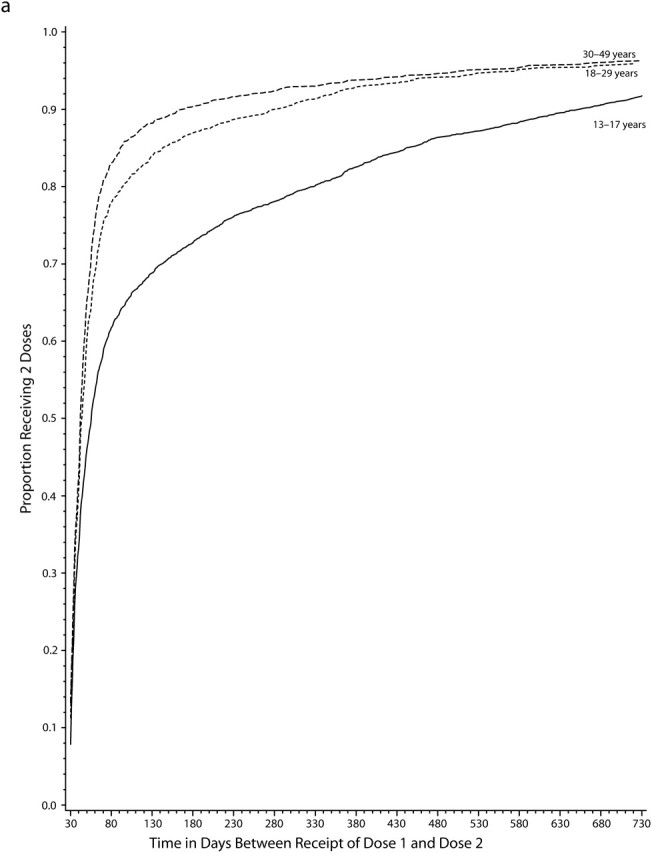
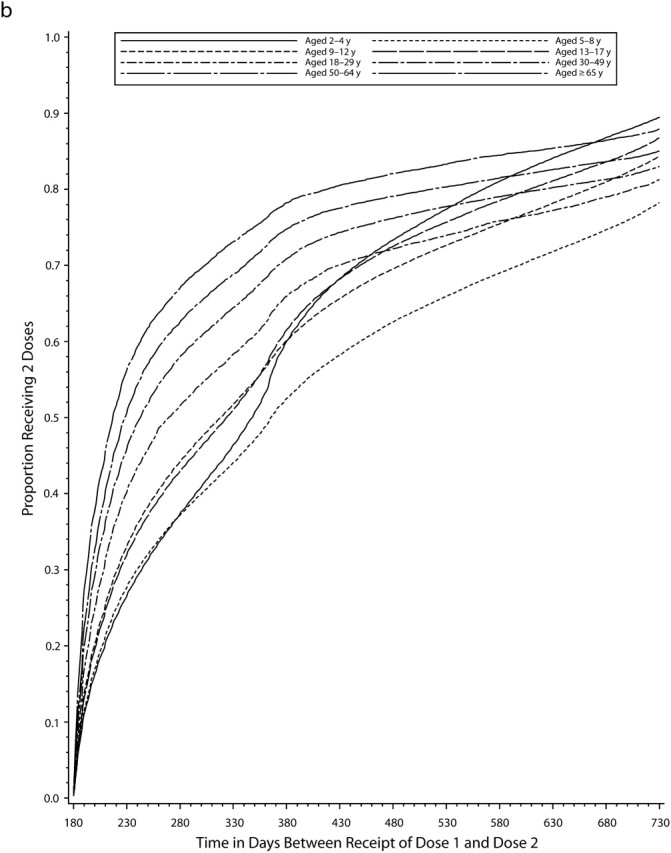
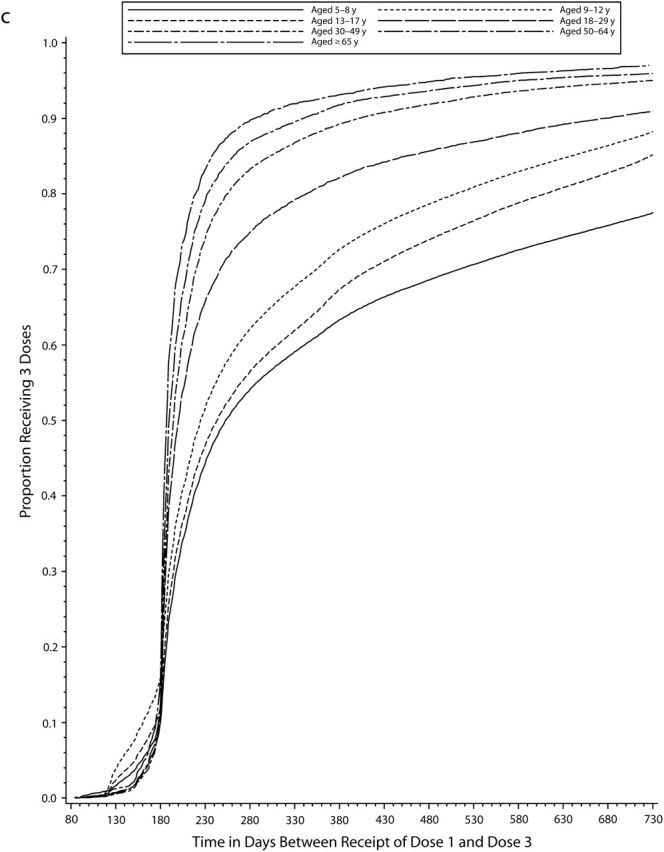
Cumulative proportion of individuals who completed the vaccine series, by time (in days) since first dose for (a) the varicella vaccine, (b) the hepatitis A vaccine, and (c) the hepatitis B vaccine vaccine: Vaccine Safety Datalink, 1996–2004.
DISCUSSION
In this large, population-based study of older children, adolescents, and adults, we found that compliance with multiple-dose varicella, hepatitis A, and hepatitis B vaccine series was low, particularly among adolescents, young adults, and those of low socioeconomic status as defined by receipt of Medicaid benefits. Rates of completion of all required vaccine doses within 1 year of the first dose were highest for hepatitis B vaccine (55%–65% in most age groups), whereas hepatitis A and varicella vaccine compliance was lower (40%–50% for most age groups), even though those vaccination series require only 2 doses. Even among the relatively modest proportion of individuals who ultimately received the recommended number of doses, the interval between series initiation and completion was long (1–2 years or more) for many persons, especially children and adolescents, leaving many undervaccinated and thus at higher risk for disease for extended time periods. These results, which indicate poor compliance in our study population of insured individuals enrolled in MCOs, are especially concerning because rates could be even lower among those with more limited access to health care.
In addition to age and socioeconomic status, full compliance with multiple-dose vaccine series among older children, adolescents, and adults increased modestly with duration of MCO enrollment and the number of medical visits in the year prior to the first dose, and it varied by MCO site, although the trends in compliance rates across sites differed depending on the vaccine, with some sites demonstrating high levels of compliance for 1 vaccine series but not for others. The association between calendar year and compliance also varied by vaccine; compliance rates decreased over time for varicella and increased for hepatitis A and B vaccines. Other measured factors, including gender and the presence of a chronic condition, were not consistently associated with multiple-dose compliance for the vaccines we studied.
The lowest rates of completion of multiple-dose vaccine series within 1 year (25%–35%) were observed among adolescents and Medicaid recipients for varicella vaccine and among younger adults for the hepatitis A vaccine series. These rates were unexpectedly low, and the reasons for such low compliance were unclear and therefore warrant further investigation. In particular, that over half of adolescents who initiated the varicella vaccine series never received a second dose is concerning. In light of the low levels of compliance in these subgroups and in our study population in general, further efforts that focus on increasing completion rates among those who initiate the series should be made. Possible approaches include improving immunization tracking systems and developing methods for following up on undervaccinated individuals.
Comparisons to evidence from other settings
Unlike multiple-dose vaccine series coverage among infants aged 19 to 35 months, which is estimated annually by the Centers for Disease Control and Prevention using provider-reported data from the National Immunization Survey,18 relatively little data are available on compliance with multiple-dose series among older children, adolescents, and adults. Although existing national vaccine surveys among adolescents19 and adults20 estimate coverage with the 3-dose hepatitis B series and with at least 1 dose of varicella vaccine among adolescents with no prior history of varicella disease, they do not report on series completion rates among varicella, hepatitis A, or hepatitis B vaccine series initiators. Existing studies in the literature on completion of hepatitis A and B vaccine series among adults generally have been limited to highly restricted populations, such as those with high-risk sexual behaviors or intravenous drug users.25–27
The limited data that are available, however, are consistent with our main finding that compliance is suboptimal in these age groups. Several studies that collected survey data with provider-verified immunization histories have found that the rates of 2-dose hepatitis A vaccine completion among older children and adolescents who initiate the series is modest (63%–69%),28–30 even in the context of an aggressive community-based vaccination intervention.30 Estimated rates of hepatitis B series completion among adolescents have varied from 45% to 73%, depending on the population and study design,31,32 with some of the highest rates achieved only after allowing longer follow-up, sending postcard reminders, and offering a reimbursement incentive.32
In contrast to the low compliance with multiple-dose vaccine series observed in our study and by previous studies with older children, adolescents, and adults, compliance with infant vaccination series is markedly higher.20 Factors probably facilitating compliance for infant vaccinations include routine well-child visits, a good understanding of universally recommended vaccination schedules by pediatric medical providers, high parental awareness of the importance of vaccination, and external requirements such as school entry laws and Healthcare Effectiveness Data and Information Set (HEDIS) measurements. Conversely, potential compliance barriers among older children, adolescents, and adults include lack of routine medical visits, lack of prioritization of vaccination relative to other medical care, population mobility, lack of a medical home, a relative lack of familiarity by patients and medical staff with the recommended schedules (since these vaccinations are often not given as part of a universal schedule), lack of tracking by health systems and, with the exception of hepatitis B vaccine for adolescents, lack of inclusion of such vaccinations as part of HEDIS measures or other quality assurance activities.30,33–36
Strengths and Limitations
The main strengths of our study include the geographically diverse, population-based sample of participants, the quality of the immunization data available from the MCO systems,37 and the large sample size, which provided statistical power to examine a wide variety of potential factors associated with noncompliance. Our population may not represent those with less access to health care, however, and thus it is possible that our data overestimate compliance rates in the general population. Another limitation of our study is that we only evaluated potential predictors of compliance that were available in administrative data sources. A more comprehensive assessment of predictors of and barriers to compliance beyond these factors, such as better markers of socioeconomic status, would be useful. Last, we did not validate the vaccination data from the administrative immunization registries by reviewing medical charts, which could introduce some error into our analyses. However, data from the Vaccine Safety Datalink immunization registries are well established, are monitored regularly for quality, and have been shown to have high agreement with vaccine data obtained via medical chart abstraction.37
Conclusions
Our population-based study of compliance with multiple-dose vaccination schedules among older children, adolescents, and adults provides a compelling assessment of compliance rates in practice. The low rates of compliance that we observed offer an opportunity to raise awareness of existing gaps in compliance, indicate that system changes may be needed to improve vaccination practices, and may also inform decisions regarding vaccine formulations. If, for example, approximately equivalent immunogenicity, effectiveness, and duration of protection could be achieved by use of either a single dose of a vaccine with a higher antigen content or a multiple-dose series of a combination vaccine, clinical effectiveness might be maximized by vaccine options that involve fewer doses. Such changes could ultimately lead to improved vaccine compliance, which could in turn reduce both individual and community risk for serious vaccine-preventable diseases.
Acknowledgments
The Centers for Disease Control and Prevention (grant 200-2002-00732) funded the Vaccine Safety Datalink contract, which was administered by America's Health Insurance Plans.
We thank Vaccine Safety Datalink staff members for their valuable data management, programming, and project management contributions, which enabled the assembly of the Vaccine Safety Datalink files. We also acknowledge the support of the staff at America's Health Insurance Plans.
Note. Prior to journal submission, the manuscript was reviewed and approved as part of the clearance process of the Centers for Disease Control and Prevention. The results and conclusions of this article, however, are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
N. P. Klein received grant support from Merck and GlaxoSmithKline. S. J. Jacobsen received grant funding from and served as an unpaid consultant to Merck Research Labs. L. Jackson received research funding from and has served as a consultant to vaccine manufacturers, including Sanofi Pasteur, Novartis, Wyeth, and GlaxoSmithKline.
Human Participant Protection
The study received institutional review board approval from all participating sites.
References
- 1.Centers for Disease Control and Prevention. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45(RR-11):1–36. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45(RR-15):1–30. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Recommendation of the Immunization Practices Advisory Committee (ACIP). Inactivated hepatitis B virus vaccine. MMWR Morb Mortal Wkly Rep. 1982;31(24):317–322, 327–318. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep. 1991;40(RR-13):1–25. [PubMed] [Google Scholar]
- 5.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 6.Clemens R, Safary A, Hepburn A, Roche C, Stanbury WJ, Andre FE. Clinical experience with an inactivated hepatitis A vaccine. J Infect Dis. 1995;171(suppl 1):S44–S49. [DOI] [PubMed] [Google Scholar]
- 7.McMahon BJ, Williams J, Bulkow L, et al. Immunogenicity of an inactivated hepatitis A vaccine in Alaska Native children and Native and non-Native adults. J Infect Dis. 1995;171(3):676–679. [DOI] [PubMed] [Google Scholar]
- 8.Ashur Y, Adler R, Rowe M, Shouval D. Comparison of immunogenicity of two hepatitis A vaccines—VAQTA and HAVRIX—in young adults. Vaccine. 1999;17(18):2290–2296. [DOI] [PubMed] [Google Scholar]
- 9.Balcarek KB, Bagley MR, Pass RF, Schiff ER, Krause DS. Safety and immunogenicity of an inactivated hepatitis A vaccine in preschool children. J Infect Dis. 1995;171(suppl 1):S70–S72. [DOI] [PubMed] [Google Scholar]
- 10.Horng YC, Chang MH, Lee CY, Safary A, Andre FE, Chen DS. Safety and immunogenicity of hepatitis A vaccine in healthy children. Pediatr Infect Dis J. 1993;12(5):359–362. [DOI] [PubMed] [Google Scholar]
- 11.Horng YC, Chang MH, Lee CY, Safary A, Andre FE, Chen DS. Safety and immunogenicity of hepatitis A vaccine in healthy adult volunteers. J Gastroenterol Hepatol. 1993;8(4):338–341. [DOI] [PubMed] [Google Scholar]
- 12.Coates T, Wilson R, Patrick G, Andre F, Watson V. Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther. 2001;23(3):392–403. [DOI] [PubMed] [Google Scholar]
- 13.Leroux-Roels G, Abraham B, Fourneau M, De Clercq N, Safary A. A comparison of two commercial recombinant vaccines for hepatitis B in adolescents. Vaccine. 2000;19(7–8):937–942. [DOI] [PubMed] [Google Scholar]
- 14.Andre FE. Summary of safety and efficacy data on a yeast-derived hepatitis B vaccine. Am J Med. 1989;87(3A):14S–20S. [DOI] [PubMed] [Google Scholar]
- 15.Zajac BA, West DJ, McAleer WJ, Scolnick EM. Overview of clinical studies with hepatitis B vaccine made by recombinant DNA. J Infect. 1986;13(suppl A):39–45. [DOI] [PubMed] [Google Scholar]
- 16.Kuter BJ, Ngai A, Patterson A, et al. Safety, tolerability, immunogenicity of two regimens of Oka/Merck varicella vaccine (Varivax) in healthy adolescents and adults. Oka/Merck Varicella Vaccine Study Group. Vaccine. 1995;13(11):967–972. [DOI] [PubMed] [Google Scholar]
- 17.Gershon AA, Steinberg SP, LaRussa P, Ferrara A, Hammerschlag M, Gelb L. Immunization of healthy adults with live attenuated varicella vaccine. J Infect Dis. 1988;158(1):132–137. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. National Immunization Survey home page. Available at: http://www.cdc.gov/nis. Accessed May 1, 2008.
- 19.Centers for Disease Control and Prevention. Vaccination coverage among adolescents aged 13–17 years—United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(40):1100–1103. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. National Health Interview Survey home page. Available at: http://www.cdc.gov/nchs/nhis.htm. Accessed May 1, 2008.
- 21.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System home page. Available at: http://www.cdc.gov/brfss. Accessed May 1, 2008.
- 22.International Classification of Diseases, Ninth Revision, Clinical Modification. Hyattsville, MD: National Center for Health Statistics; 1980. DHHS publication PHS 80-1260. [Google Scholar]
- 23.DeStefano F. The Vaccine Safety Datalink project. Pharmacoepidemiol Drug Saf. 2001;10(5):403–406. [DOI] [PubMed] [Google Scholar]
- 24.Lumley T, Kronmal RA, Ma S. Relative Risk Regression in Medical Research: Models, Contrasts, Estimators and Algorithms. Seattle: University of Washington; 2006. UW Biostatistics Working Paper Series, paper 293. Available at: http://www.bepress.com.offcampus.lib.washington.edu/uwbiostat/paper293. Accessed July 19, 2006. [Google Scholar]
- 25.Sansom S, Rudy E, Strine T, Douglas W. Hepatitis A and B vaccination in a sexually transmitted disease clinic for men who have sex with men. Sex Transm Dis. 2003;30(9):685–688. [DOI] [PubMed] [Google Scholar]
- 26.Campbell JV, Garfein RS, Thiede H, et al. Convenience is the key to hepatitis A and B vaccination uptake among young adult injection drug users. Drug Alcohol Depend. 2007;91(suppl 1):S64–S72. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald V, Dore GJ, Amin J, van Beek I. Predictors of completion of a hepatitis B vaccination schedule in attendees at a primary health care centre. Sex Health. 2007;4(1):27–30. [DOI] [PubMed] [Google Scholar]
- 28.Owen EC, Peddecord KM, Wang WW, et al. Hepatitis A vaccine uptake in San Diego County: Hispanic children are better immunized. Arch Pediatr Adolesc Med. 2005;159(10):971–976. [DOI] [PubMed] [Google Scholar]
- 29.Fiore A, Baxter LC, Bell BP, et al. Hepatitis A 2004 vaccination in children: methods and findings of a survey in two states. Am J Prev Med. 2007;33(4):346–352. [DOI] [PubMed] [Google Scholar]
- 30.Bardenheier B, Gonzalez IM, Washington ML, et al. Parental knowledge, attitudes, and practices associated with not receiving hepatitis A vaccine in a demonstration project in Butte County, California. Pediatrics. 2003;112(4):e269. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez IM, Averhoff FM, Massoudi MS, et al. Hepatitis B vaccination among adolescents in 3 large health maintenance organizations. Pediatrics. 2002;110(5):929–934. [DOI] [PubMed] [Google Scholar]
- 32.Middleman AB, Robertson LM, Young C, Durant RH, Emans SJ. Predictors of time to completion of the hepatitis B vaccination series among adolescents. J Adolesc Health. 1999;25(5):323–327. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Hepatitis B vaccination coverage among adults—United States, 2004. MMWR Morb Mortal Wkly Rep. 2006;55(18):509–511. [PubMed] [Google Scholar]
- 34.Rand CM, Shone LP, Albertin C, Auinger P, Klein JD, Szilagyi PG. National health care visit patterns of adolescents: implications for delivery of new adolescent vaccines. Arch Pediatr Adolesc Med. 2007;161(3):252–259. [DOI] [PubMed] [Google Scholar]
- 35.Humiston SG, Rosenthal SL. Challenges to vaccinating adolescents: vaccine implementation issues. Pediatr Infect Dis J. 2005;24(6 suppl):S134–S140. [DOI] [PubMed] [Google Scholar]
- 36.Bloom HG, Wheeler DA, Linn J. A managed care organization's attempt to increase influenza and pneumococcal immunizations for older adults in an acute care setting. J Am Geriatr Soc. 1999;47(1):106–110. [DOI] [PubMed] [Google Scholar]
- 37.Mullooly J, Drew L, DeStefano F, et al. Quality of HMO vaccination databases used to monitor childhood vaccine safety. Vaccine Safety DataLink Team. Am J Epidemiol. 1999;149(2):186–194. [DOI] [PubMed] [Google Scholar]


