Abstract
Patient: Male, 86
Final Diagnosis: Gastric bezoar
Symptoms: —
Medication: Cola
Clinical Procedure: Endoscopic fragmentation after gastric lavage with Pepsi-Cola
Specialty: Gastroenterology and Hepatology
Objective:
Unusual setting of medical care
Background:
Although bezoar dissolution by Coca-Cola® has been described in case reports and case series, to the best of our knowledge, the usefulness of other cola products such as Pepsi-Cola® has never been reported in the English literature.
Case Report:
An 86-year-old Taiwanese man was diagnosed with a gastric bezoar. Endoscopic fragmentation with a polypectomy snare was attempted twice but failed to remove the bezoar. Subsequently, 500 mL of Pepsi NEX Zero® was administered daily for 4 days via nasogastric tube. The bezoar was softened and successfully fragmented by the polypectomy snare and needle-knife devices on the third attempt.
Conclusions:
This report presents the first case of a gastric bezoar successfully treated by endoscopic fragmentation in combination with Pepsi-Cola® administration, suggesting the possible utility of cola beverages in bezoar treatment, regardless of product brands.
MeSH Keywords: Bezoars; Cola; Endoscopy, Gastrointestinal; Foreign Bodies
Background
The formation of a bezoar, which is an indigestible conglomeration trapped in the gastrointestinal tract, is a relatively infrequent disorder. Since the first successful treatment achieved with Coca-Cola® lavage reported in 2002 by Ladas et al. [1], the administration of Coca-Cola, in combination with or without endoscopic fragmentation techniques, has been well-known among gastroenterologists as a conservative treatment option for gastric bezoars [2,3]. Bezoar dissolution by Diet Coke®, Coca-Cola Light®, and Coca-Cola Zero® has also been documented in previous reports, suggesting that these sugar-free beverages produce a similar action for bezoar lysis as the regular version of Coca-Cola [4,5]. Despite the clinical success of bezoar dissolution by cola products manufactured by the Coca-Cola Company, to the best of our knowledge, the usefulness of other carbonated beverages such as Pepsi-Cola® has never been reported in the English literature.
We recently treated a patient with a gastric bezoar who presented with coffee-ground emesis. Endoscopic fragmentation was attempted, but it failed because of the solidity of the bezoar. However, lavage with Pepsi NEX Zero® via a nasogastric tube resulted in softening of the bezoar, which was then successfully fragmented by endoscopic polypectomy snare and needle-knife devices. To the best of our knowledge, this report presents the first case of a gastric bezoar treated with endoscopic fragmentation after gastric lavage with Pepsi-Cola.
Case Report
An 86-year-old Taiwanese man underwent esophagogastroduodenoscopy to investigate the coffee-ground emesis that had begun 1 week earlier. The patient had alcoholic liver damage and dementia, but had been taking no medications. He had no previous history of gastrointestinal disease or diabetes mellitus. A physical examination revealed no abnormalities, and there was no evidence of anemia. Laboratory findings revealed a slight elevation of liver enzymes (aspartate amino-transferase [AST], 37 IU/L; gamma-glutamyl transferase [GGT], 120 IU/L), white blood cells (10 190/mcL), and plasma glucose (165 mg/dL). Red blood cells and C-reactive protein were within normal ranges. Esophagogastroduodenoscopy revealed a gastric bezoar, in addition to ulcers in the gastric angle and duodenal bulb (Figure 1). The pyloric ring was stenotic due to the presence of the duodenal ulcer. Endoscopic fragmentation with a polypectomy snare device and removal of the bezoar was attempted twice, but failed because of the solidity of the bezoar.
Figure 1.
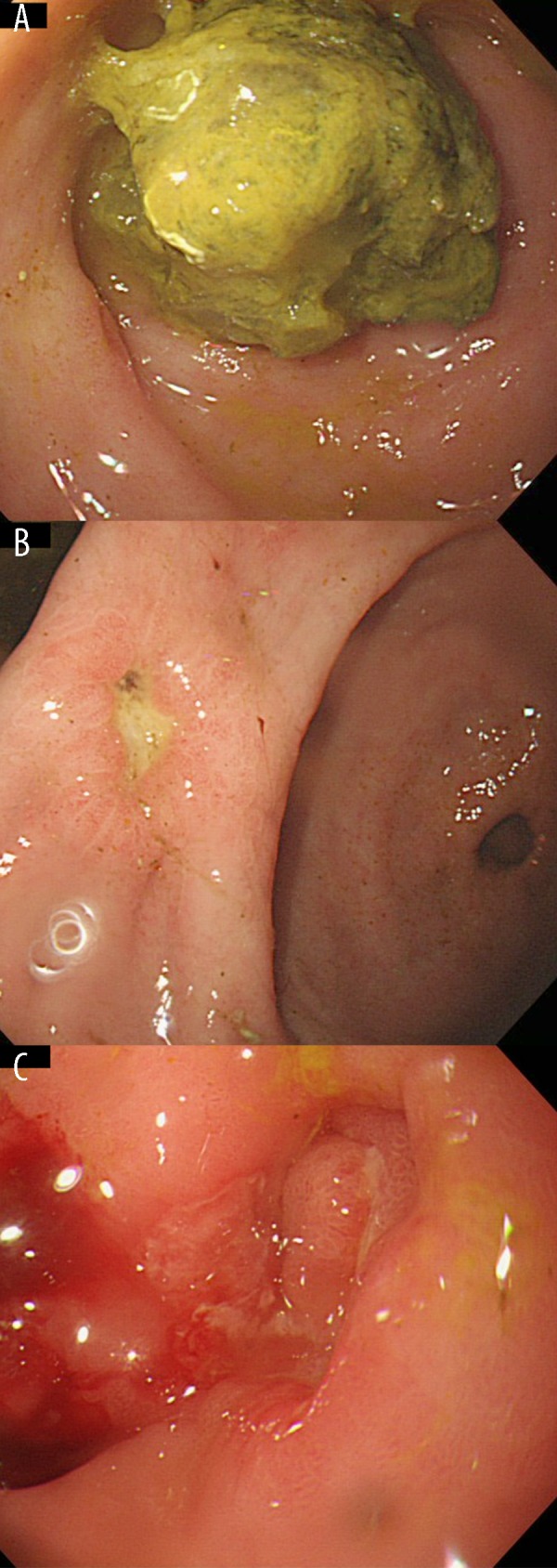
Esophagogastroduodenoscopy images at the first presentation of the patient. A yellow concretion was observed in the stomach (A). Ulcers were also seen in the gastric angle (B) and duodenal bulb (C).
To dissolve the gastric bezoar, a nasogastric tube was inserted and 500 mL of Pepsi NEX Zero® was administered daily for 4 days via the tube. After the administration of the cola, the bezoar was softened and successfully fragmented by a polypectomy snare and needle-knife devices on the third attempt (Figure 2). The fragments were retrieved by a net device. No component analysis of the bezoar fragments was performed. The patient was assumed to have Helicobacter pylori infection based on the presence of atrophic gastritis, although no test to detect the infection was performed. Esophagogastroduodenoscopy performed 16 days after the bezoar removal revealed no remaining fragments or recurrence of the bezoars.
Figure 2.
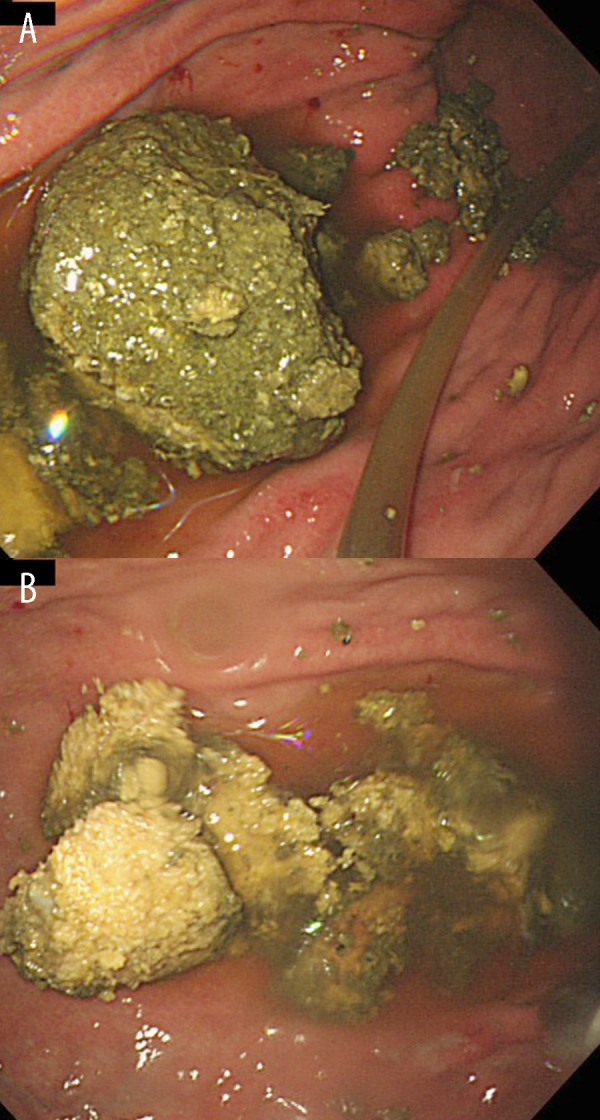
Esophagogastroduodenoscopy images after lavage with Pepsi NEX Zero. The gastric bezoar was partly fragmented (A). The bezoar was softened and completely fragmented by a polypectomy snare and needle-knife devices (B).
Discussion
In 2002, Ladas et al. first reported a case series regarding the efficacy of Coca-Cola in dissolving gastric bezoars [1]. They treated 5 gastric phytobezoar patients with 3 L of Coca-Cola lavage over 12 h via nasogastric tube, and the procedure resulted in complete bezoar dissolution in a single session in all cases. Subsequent successful outcomes by Coca-Cola lavage via a nasogastric tube or by drinking Coca-Cola have been reported by various authors [2–6]. In a recent review article by Ladas et al. [2], 24 publications describing 46 patients were summarized, and the authors noted that bezoar resolution was achieved by Coca-Cola administration in 91.3% of the cases, either as the sole treatment or in combination with additional endoscopic procedures. The cola beverages used to date in the treatment of bezoars include Diet Coke, Coca-Cola Light, Coca-Cola Zero, and regular Coca-Cola [3–6]. However, to the best of our knowledge, the usefulness of other cola products, such as Pepsi-Cola, has never been reported in the English literature. For example, 20 articles and 10 articles are retrieved by searches in PubMed with the terms “bezoar and Coca-Cola” and “phytobezoar and Coca-Cola,” respectively, while no articles were retrieved by searching for “bezoar and Pepsi-Cola” or “phytobezoar and Pepsi-Cola.” Therefore, we believe this to be the first case report showing the clinical utility of beverages produced by PepsiCo.
In the present case, we used Pepsi NEX Zero to dissolve the bezoar. This beverage is a calorie-free product distributed in Japan and South Korea and sold under license from PepsiCo. After lavage with Pepsi NEX Zero for 4 days, the bezoar had softened and was able to be broken by endoscopy devices. In our previous study, similar effects of bezoar softening were observed in vitro by Coca-Cola and Coca-Cola Zero [7]. After a 12-h incubation of persimmon phytobezoar fragments extracted from a patient and hydrochloric acid-potassium chlo-ride buffer (pH 2.0) with Coca-Cola or Coca-Cola Zero, the bezoar fragments were more softened and broken than those incubated with distilled water (control). Our in vitro study indicates that Coca-Cola Zero has a lytic action similar to that of regular Coca-Cola. The mechanism by which Coca-Cola beverages dissolve bezoars has not yet been fully revealed, but the suspected mechanisms include: i) enhancement of bezoar digestion based on mucolysis by sodium bicarbonate, ii) acidification by carbonic acid and phosphoric acid, and iii) destruction of bezoar structure by carbon dioxide bubbles [1,2,4,8,9]. We believe that Pepsi NEX Zero is as effective as Coca-Cola for bezoar treatment because both beverages contain sodium bicarbonate, carbonic acid, phosphoric acid, and carbon dioxide bubbles. Furthermore, there have been some articles written in Japanese describing successful outcomes in bezoar treatment with Pepsi-Cola and even carbonated water [10,11]. Based on the positive results in the present patient and previous studies, we consider beverages with sodium bicarbonate, regardless of product brand, as useful in dissolving bezoars and preventing further formation and recurrence.
The predisposing risk factors of bezoar formation include prior gastric surgery, diabetes mellitus, peptic ulcer disease, carcinoma of the gastrointestinal tract, and hypothyroidism [12–14]. These factors result in reduced gastric acidity, gastric stasis, loss of pyloric function, and/or pyloric stenosis. Elderly individuals are also susceptible to bezoar formation because of their impaired gastric motility [15]. The present patient was of advanced age (86 years old) and had pyloric stenosis due to duodenal ulcers. Although this patient had no previous history of diabetes mellitus, blood examination revealed impaired glucose tolerance. All of these underlying factors would have contributed to delayed gastric emptying and the formation of the bezoar.
Conclusions
In summary, we treated a gastric bezoar patient with endoscopic fragmentation after lavage with Pepsi NEX Zero via a nasogastric tube. Our results indicate that cola beverages, regardless of product brand, are useful in bezoar treatment because they help to dissolve bezoars.
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References:
- 1.Ladas SD, Triantafyllou K, Tzathas C, et al. Gastric phytobezoars may be treated by nasogastric Coca-Cola lavage. Eur J Gastroenterol Hepatol. 2002;14:801–3. doi: 10.1097/00042737-200207000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Ladas SD, Kamberoglou D, Karamanolis G, et al. Systematic review: Coca-Cola can effectively dissolve gastric phytobezoars as a first-line treatment. Aliment Pharmacol Ther. 2013;37:169–73. doi: 10.1111/apt.12141. [DOI] [PubMed] [Google Scholar]
- 3.Iwamuro M, Okada H, Matsueda K, et al. Review of the diagnosis and management of gastrointestinal bezoars World J Gastrointest Endosc [in press] doi: 10.4253/wjge.v7.i4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ertuğrul G, Coşkun M, Sevinç M, et al. Treatment of gastric phytobezoars with Coca-Cola given via oral route: a case report. Int J Gen Med. 2012;5:157–61. doi: 10.2147/IJGM.S29453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer SJ, Pochapin MB. Gastric phytobezoar dissolution with ingestion of diet coke and cellulase. Gastroenterol Hepatol (NY) 2012;8(11):770–72. [PMC free article] [PubMed] [Google Scholar]
- 6.Iwamuro M, Tanaka S, Shiode J, et al. Clinical characteristics and treatment outcomes of nineteen Japanese patients with gastrointestinal bezoars. Intern Med. 2014;53:1099–105. doi: 10.2169/internalmedicine.53.2114. [DOI] [PubMed] [Google Scholar]
- 7.Iwamuro M, Kawai Y, Shiraha H, et al. In vitro analysis of gastric phytobezoar dissolubility by Coca-Cola, Coca-Cola Zero, cellulase, and papain. J Clin Gastroenterol. 2014;48:190–91. doi: 10.1097/MCG.0b013e3182a39116. [DOI] [PubMed] [Google Scholar]
- 8.Chung YW, Han DS, Park YK, et al. Huge gastric diospyrobezoars successfully treated by oral intake and endoscopic injection of Coca-Cola. Dig Liver Dis. 2006;38:515–17. doi: 10.1016/j.dld.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Sanderson I, Ibberson O, Fish EB. Gastric phytobezoar following gastrectomy. Can Med Assoc J. 1971;104:1115. [PMC free article] [PubMed] [Google Scholar]
- 10.Maekawa T, Onoyama M, Shimada T. [A case of phytobezoars treated by Pepsi Cola ingestion and endoscopic treatment] Shoukakika. 2007;45:345–48. [in Japanese with English abstract] [Google Scholar]
- 11.Mizushige T, Wato M, Inaba T, et al. [Two successful cases of dissolution therapy with carbonated water for gastric phytobezoars] Gastroenterological Endoscopy. 2014;56:3340–46. [in Japanese with English abstract] [Google Scholar]
- 12.Kumar GS, Amar V, Ramesh B, Abbey RK. Bizarre metal bezoar: a case report. Indian J Surg. 2013;75(Suppl.1):356–58. doi: 10.1007/s12262-012-0706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaFountain J. Could your patient’s bowel obstruction be a bezoar? Todays Surg Nurse. 1999;21:34–37. [PubMed] [Google Scholar]
- 14.Işik A, Deniz Firat Y, Peker K, et al. How could such a wide piece of tree root pass through the narrow pyloric orifice? An extremely rare case. Am J Case Rep. 2014;15:284–87. doi: 10.12659/AJCR.890713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders MK. Bezoars: from mystical charms to medical and nutritional management. Practical Gastroenterology. 2004;18:37–50. [Google Scholar]


