Abstract
Introduction
Isolated limb infusion (ILI) is a limb-preserving treatment for in-transit extremity melanoma. The benefit of resecting residual disease following ILI is unclear.
Methods
A multi-institutional experience was analyzed comparing patients who underwent ILI plus resection of residual disease (ILI+RES) versus ILI-alone.
Results
176 patients were included: 154 ILI-alone and 22 ILI+RES. There were no differences between the groups with respect to gender, age, extremity affected, or time from diagnosis to ILI. All surgical resections were performed as an outpatient procedure, separate from the ILI. Within the ILI+RES group, fifteen (68%) had a partial response (PR), two (9%) stable disease (SD), and 5 (23%) progressive disease (PD). The ILI-alone group had 52 (34%) CR, 30 (19%) PR, 15 (10%) SD, and 46 (30%) PD. Eleven (7%) ILI-alone patients did not have 3-month response available for review. Evaluating overall survival (OS) from date of ILI, the ILI-alone group had a median OS of 30.9 months, whereas the ILI+RES group had not reached median OS, p=0.304. Although the ILI+RES group had a slightly longer disease free survival (DFS) compared to those with a CR after ILI-alone (12.4 vs. 9.6), this was not statistically significant, p=0.978. Within the ILI+RES group, those with an initial PR following ILI had improved DFS vs. those with SD or PD following ILI, p<0.0001.
Conclusion
Resection of residual disease following ILI offers a DFS and OS similar to those who have a CR after ILI-alone, and may offer a treatment strategy that benefits more patients undergoing ILI.
Keywords: Isolated limb infusion, Melanoma, Resection
Introduction
Melanoma represents the 5th and 6th most common cancer in the United States in men and women, respectively, with an incidence that has risen over the last decade(1). Although melanoma accounts for <5% of skin cancer, it accounts for the majority of skin cancer-related deaths (1). In-transit disease from extremity melanoma is currently classified as stage IIIB or IIIC disease, with five year survival rates of 59% and 40% (2, 3). Although the risk of disease relapse is high in these advanced-stage patients, 71% for stage IIIB and 85% for IIIC disease, often the recurrence is local or occurs in an in-transit fashion (30% and 22% for stage IIIB and IIIC, respectively) (4).
Isolated limb infusion (ILI) is a minimally invasive technique utilized to treat in-transit melanoma of the extremity. First modified from the isolated limb perfusion (ILP) by Thompson, et al. in the 1990's(5), experience with utilizing ILI as a limb-salvage technique has been quickly growing. Although studies have typically been small in size with short-term follow-up, the results have been encouraging, with limb salvage achieved in up to 86% (6). A three-month complete response (CR) in the limb is seen in up to 46% of patients, with duration of response lasting three years or longer (7, 8). While ILP has been considered superior to ILI in terms of overall response rate (ORR) and durability of response, ILP is associated with a greater risk of limb loss and is associated with higher toxicity (9-13). Overall survival (OS) of patients who achieve a complete response (CR) with ILI is similar to OS seen in those with a CR following ILP (7). However, ILI is associated with less morbidity than ILP and is considered as a first-line regional therapy at several institutions for in-transit melanoma, including the authors (7, 11, 12).
Use of ILI in attempt to downstage or control the burden of disease in a limb prior to surgical resection has not yet been qualified. In this study, the authors evaluate the impact of surgical resection of remaining disease following ILI, comparing overall and disease-free outcomes with ILI-alone.
Patients and Methods
After Institutional Review Board (IRB) approval, a retrospective review was conducted from the databases maintained at Moffitt Cancer Center and Duke University Medical Center. From 2004 to 2011, patients with in-transit, stage IIIB or IIIC melanoma, who were treated with isolated limb infusion and subsequent resection of residual or persistent disease (ILI+RES) were included in this analysis. This cohort was compared with contemporaneous patients who underwent ILI alone. Demographic features and operative parameters were recorded and compared between the ILI-alone and ILI+RES group. Tumor burden was classified as low or high. High burden of disease was defined as ten or more lesions or any single lesion 3 cm in diameter or larger.
All ILIs were performed as previously described from both Duke University Medical Center and Moffitt Cancer Center (12, 14). Briefly, percutaneous catheters were placed by Interventional Radiology under fluoroscopic guidance to the affected limb. The limb was externally warmed to >37°C. Under tourniquet occlusion, Melphalan and Actinomycin-D were then circulated for 30 minutes. The limb was washed out with saline prior to tourniquet release. The majority (N=155, 86%) had dose-correction of Melphalan for ideal body weight.
Three-month response was determined by clinical assessment with physical exam and PET imaging. Patients were considered to have a complete response (CR) if no evidence of disease was present, partial response (PR) as a ≥30% reduction in disease burden, stable disease (SD) if no change was evident, and progressive disease (PD) if tumor burden had increased. Patients with persistent disease, deemed surgically resectable, were offered resection to render them clinically disease-free (NED). Patients who achieved a CR at three months following ILI who then recurred and underwent surgical resection were excluded from this analysis. All surgical resections were performed as outpatient procedures. Surgical resectability was determined by the surgeon and was generally considered as a lesion(s) that could be resected primarily without causing significant disfigurement or dysfunction to the extremity. Only patients with residual disease that could be resected so that no remaining disease was evident in the extremity were included in this analysis.
Statistical Analyses
All statistical analyses were performed using SAS (version 9.2, SAS Institute Inc., Cary, NC), with significance considered when the two-sided p-value ≤ 0.05. Categorical variables were compared using Fisher's exact tests; continuous variables were compared using Wilcoxon rank-sum tests, and Kaplan Meier and log rank tests used for survival analyses. Overall survival was defined as time from date of ILI to date of last follow-up or death from any cause. To compare disease free survival (DFS) between cohorts, only those patients who achieved a CR at 3 months were compared to the ILI+RES group. Disease free survival was determined as time from date of ILI (ILI-alone patients) or date of surgical resection (ILI+RES) to date of disease recurrence.
Results
Demographics and Perioperative Parameters
176 patients were included in this analysis, 154 ILI-alone and 22 ILI+RES. There was no difference with respect to gender or median age between the ILI-alone and ILI+RES groups (Table 1). There was a similar distribution of disease in upper vs. lower extremities between groups. Burden of disease was recorded in 164 (93%); there was no difference in disease burden between groups, p=0.821 (Table 1).
Table 1.
Demographics of the ILI-alone and ILI+RES cohorts.
| Demographic | ILI -Alone, N=154 (N, %) | ILI + RES, N=22 (N, %) | P value |
|---|---|---|---|
| Gender | 1.0 | ||
| Female | 89 (58%) | 13 (59%) | |
| Male | 65 (42%) | 9 (41%) | |
| Age, Median (range) | 70 (29-94) | 65 (34-89) | 0.637 |
| Extremity Affected | 1.0 | ||
| Lower | 122 (79%) | 18 (82%) | |
| Upper | 32 (21%) | 4 (18%) | |
| Burden of Disease | 0.821 | ||
| Low | 76 (49%) | 11 (50%) | |
| High | 66 (43%) | 11 (50%) | |
| Missing | 12 (8%) | 0 (0%) |
With respect to perioperative parameters, median ischemia time was similar between groups, 63 minutes in the ILI-alone group and 68 minutes in the ILI+RES group, p=0.77. Serum creatine phosphokinase (CPK) levels were used to monitor systemic toxicity. The CPK peak and the post-operative day when the CPK peak occurred were also similar between groups, peaking at a median of 584 U/L in the ILI alone group and 1054 U/L in the ILI+RES group, p=0.121, on post-operative day 4 for both groups (Table 2). The Wieberdink toxicity score was used to classify limb toxicity. Although the ILI-alone group had 11 (7%) patients with a grade 4 Wieberdink toxicity vs. 0 (0%) in the ILI+RES group, there was no significant difference in overall toxicity between the two groups, p=0.62. The ILI+RES group did exhibit a longer median hospital stay, 7 vs. 6 days, p=0.047 (Table 2). Since the hospital length of stay was related only to the ILI procedure, it is unclear why the ILI+RES patients had a one day longer length of stay from their ILI since the ILI and surgical resection were performed in two separate settings.
Table 2.
Peri-operative ILI parameters for the ILI-alone and ILI+RES cohorts.
| ILI Alone, N=154 (N, %) | ILI + RES, N=22 (N, %) | P value | |
|---|---|---|---|
| ILI parameter (Median) | |||
| Ischemia Time (minutes) | 63 | 68 | 0.77 |
| CPK peak (U/L) | 584 | 1054 | 0.121 |
| CPK peak post operative day | 4 | 4 | 0.269 |
| Length hospital stay (days) | 6 | 7 | 0.047 |
| Wieberdink Toxicity Score | 0.62 | ||
| Grade I/II | 96 (62%) | 11 (50%) | |
| Grade III/IV | 54 (35%) | 8 (36%) | |
| Missing | 4 (3%) | 3 (14%) |
3-Month Response
At 3-month follow-up after ILI, the ILI+RES group had 15 (68%) PR, two (9%) SD, and five (23%) PD. The median time to resection from ILI was 3.3 months for the entire group (range 0.2-11.2 months). Although two (9%) underwent resection of residual disease after only six weeks, the majority (N=14, 64%) underwent resection after 3-month follow-up. The ILI-alone group demonstrated 3-month response rates of: 52 (34%) CR, 30 (19%) PR, 15 (10%) SD, and 46 (30%) PD. Eleven patients (7%) in this group had unrecorded 3-month clinical response. The median time from melanoma diagnosis to ILI was similar between groups, 25 months for ILI-alone vs. 37 months for ILI+RES, p=0.222 (Table 3).
Table 3.
Response rates, time from diagnosis, and disease recurrence following ILI for the ILI-alone and ILI+RES cohorts. ORR (overall response rate).
| ILI Alone, N=154 (N, %) | ILI + RES, N=22 (N, %) | P value | |
|---|---|---|---|
| Three month clinical response | |||
| Complete Response | 52 (36%) | N/A | 0.364 |
| Partial Response | 30 (21%) | 15 (68%) | |
| Stable Disease | 15 (10%) | 2 (9%) | |
| Progressive Disease | 46 (32%) | 5 (23%) | |
| Time from diagnosis to ILI (median, months) | 25 | 37 | 0.222 |
| Disease Recurrence (CR cohort) | 31 (20%) | 13 (59%) | 0.795 |
Overall and Disease Free Survival
Median follow-up from ILI for the cohort was 16.6 months (range 0.2-71.2 months). Median OS from time of ILI was 30.9 months in the ILI-alone group; median OS was not reached in the ILI+RES group. There was no significant difference in OS between the ILI-alone and ILI+RES groups, p=0.304 (Figure 1). Of those in the ILI-alone group who achieved a 3-month CR, 31 (63%) developed disease recurrence. In the ILI+RES group, 13 (59%) developed disease recurrence, p=0.795. Although median DFS was longer in the ILI+RES group (12.4 months vs. 9.6 months for ILI-alone), this was not significant, p=0.978 (Figure 1).
Figure 1.
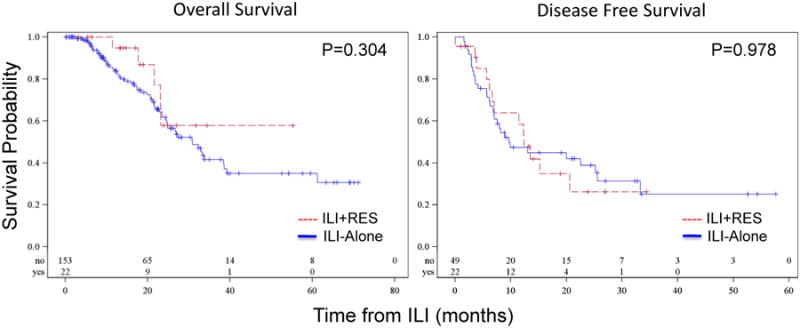
Overall and disease free survival of patients who underwent ILI + RES to achieve NED (red) versus ILI alone (blue). Median OS was 30.9 months in the ILI alone group and not reached in the ILI+ RES group. DFS was similar for both ILI + RES and ILI-alone.
The ILI-alone group was then compared with ILI+RES according to the 3-month clinical response of ILI-alone. Patients with a 3-month CR following ILI-alone had a median OS of 33.7 months; those with a 3-month PR had a median OS of 38.4 months. This was similar to patients who underwent ILI+RES, who did not achieve median OS at the time of this analysis, p=0.506 (Figure 2). Evaluating DFS of those who achieved a CR following ILI-alone, median DFS of the ILI+RES group who had a 3-month PR was longer over CR with ILI-alone, 15.2 vs. 9.6 months, respectively (Figure 2). Those who underwent ILI+RES who had SD or PD, however, had a shorter median DFS of 3.8 months, p=0.018 (Figure 2).
Figure 2.
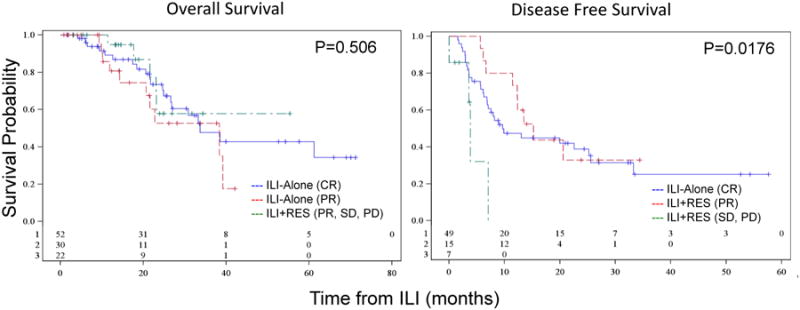
Overall survival of patients who underwent ILI-alone and achieved a CR (blue) or PR (red) versus ILI + RES (green) and disease-free survival of patients who had a CR following ILI-alone (blue) versus ILI+RES who had a 3-month PR (red) or SD/PD (green).
Within the ILI+RES cohort, survival was also compared by the 3-month clinical response following ILI. There was no difference in OS between patients who achieved PR vs. SD vs. PD, p=0.135. However, median DFS was significantly improved in patients with a PR at 3-months following ILI compared with patients with SD or PD (15.2 vs. 1.9 vs. 7.0 months, respectively), p<0.0001.
Discussion
Experience with ILI as a regional therapy for in-transit melanoma is growing. One of the larger studies explores the Melanoma Institute Australia (MIA) 14-year experience following 185 patients undergoing ILI, demonstrating an overall response rate (ORR) of 84%, with a median duration of response of 13 months. Those patients with a CR following ILI had a significantly improved median survival of 22 months vs. 9 months with PR, p=0.012 (15). Median overall survival for their cohort was 38 months. Those with a CR had significantly improved OS compared to non-CR (53 vs. 25 months, p=0.005). They concluded that ILI produced outcomes and duration of response comparable to HILP(15). A recently updated publication by the MIA showed a slightly decreased ORR of 77% for their more recent ILIs; however, no significant survival differences were observed (16).
The experience in the United States is growing at a rapid pace. Differences in methodology from those in Australia may contribute to the differences in outcome seen so far. In a recent multi-institutional United States experience, ILI was offered as a regional therapy for patients with in-transit melanoma, stage IIIB or IIIC; this is contrary to the MIA which reports on ILI inclusive of stage I and II disease as well as planned double or repeat ILI(14). Additionally, response at our institute and Duke University Medical Center is typically recorded at 3-month follow-up, based on both clinical exam and PET imaging, while MIA reports response by two clinical observations <4 weeks apart (15).
An early multi-center U.S. experience of 128 patients reported ORR of 64%, with 31% achieving CR. With low toxicity being seen, ILI was considered an acceptable alternative to HILP (17, 18). A recent study from Duke University Medical Center followed 126 patients who underwent ILI for the first time for melanoma. Although the ORR was low (43%), CR was achieved in 30% (11). Median OS for CR was improved at 35 months vs. 15 months for non-CR (11). Survival following CR from ILI was comparable to CR following HILP (11). Clearly, obtaining a CR following ILI is an important prognostic indicator for prolonged overall and disease free survival and offers comparable disease control as a CR with HILP. In a recent study by Sharma, et al., exploring patterns of disease recurrence following a CR from ILI, 56% of patients had developed an in-field recurrence at 3-year follow-up (19).
Of various methods utilized to address recurrences in the limb following ILI, wide local resection was the most commonly used procedure, producing an 8-month duration of response (19). Although considered somewhat extreme, limb amputation may even be palliative following failed ILI. In a study of 14 patients treated at MIA who had persistent disease or disease recurrence following ILI and underwent limb amputation, an ORR of 71% was seen with a median duration of response of 7 months. The median time from ILI to amputation was 11-months(20). Median survival following amputation was 13 months, range 1-84 months; however, effective symptom relief was achieved in all patients (20). Clearly, a role for surgical intervention exists following failed ILI for control of limb disease from melanoma.
The current study found an overall response rate (CR+PR) of 57% in the ILI-alone group evaluated at 3 months after ILI. The groups were comparable with respect to demographics, time from diagnosis, and tumor burden. There were no differences seen in the peri-operative parameters between groups. Disease free survival was similar between the ILI-alone with 3-month CR and the ILI+RES group, suggesting that surgery with resection to achieve NED may offer the potential of improved disease free survival. Further analysis demonstrated that the group that had a 3-month PR following ILI and went on to resection demonstrated the best DFS and had a similar median DFS compared to those with a CR following ILI-alone and improved DFS than ILI+RES with SD or PD. Although no statistically significant OS differences were seen between the ILI-alone group and ILI+RES, median survival was not reached in the ILI+RES group. Longer follow-up may demonstrate improved survival in those patients who undergo ILI+RES.
Surgical resection represents one of many treatment options available for in-field recurrence of extremity melanoma. Repeat ILI has demonstrated similar ORR as initial ILI and is offered to patients with unresectable disease recurrence who previously demonstrated a response with an initial ILI or ILP (21). Other therapies such as electrochemotherapy (ECT) and intralesional immunotherapy have also shown promise, although long-term efficacy remains to be determined (22, 23).
The current study is one of few U.S. series evaluating the utility of ILI for advanced extremity melanoma and certainly one of the first to specifically evaluate the effect of surgical resection following ILI and its impact on disease recurrence as well as overall survival. Particularly for patients with continuing development of in-transit disease or multifocal disease, ILI offers an opportunity to provide non-invasive chemotherapy to the limb, with low morbidity and near zero risk of limb loss (18). The current treatment approach adopted by our center and Duke University Medical Center for treatment of in-transit melanoma is to offer ILI to patients with multifocal or multiply recurrent lesions. With growing experience using ILI in the treatment of advanced extremity melanoma, our study suggests that resecting residual disease in patients who have low disease burden after ILI is an effective strategy to optimally control regionally advanced disease in the limb.
Conclusion
In this study, we found a survival benefit for surgical resection of residual disease following ILI. Resection of residual disease offered equivalent overall survival and potentially improved disease-free survival as those who attained a CR from ILI-alone. This effect was particularly pronounced for patients who achieved a PR with ILI who then underwent surgical resection of residual disease. For appropriate candidates, particularly those who have a clinical response to ILI, surgical resection should be offered, as it may improve outcome.
References
- 1.American Cancer Society. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7th. New York, NY: Springer; 2010. Melanoma of the skin; pp. 325–44. [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong SJ, et al. Final Version of 2009 AJCC Melanoma Staging and Classification. Journal of Clinical Oncology. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romano E, Scordo M, Dusza SW, et al. Site and Timing of First Relapse in Stage III Melanoma Patients: Implications for Follow-Up Guidelines. Journal of Clinical Oncology. 2010;28:3042–3047. doi: 10.1200/JCO.2009.26.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Seminars in Surgical Oncology. 1998;14:238–47. doi: 10.1002/(sici)1098-2388(199804/05)14:3<238::aid-ssu8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Kroon HM, Lin DY, Kam PC, Thompson JF. Isolated limb infusion as palliative treatment for advanced limb disease in patients with AJCC stage IV melanoma. Annals of Surgical Oncology. 2009;16(5):1193–201. doi: 10.1245/s10434-009-0326-7. [DOI] [PubMed] [Google Scholar]
- 7.McClaine RJ, Giglia JS, Ahmad SA, et al. Quality of life outcomes after isolated limb infusion. Annals of Surgical Oncology. 2012;19(5):1372–8. doi: 10.1245/s10434-012-2239-0. [DOI] [PubMed] [Google Scholar]
- 8.Barbour AP, Thomas J, Suffolk J, et al. Isolated limb infusion for malignant melanoma: predictors of response and outcome. Annals of Surgical Oncology. 2009;16(12):3463–72. doi: 10.1245/s10434-009-0717-9. [DOI] [PubMed] [Google Scholar]
- 9.Alexander HR, Jr, Fraker DL, Bartlett DL, et al. Analysis of Factors Influencing Outcome in Patients With In-Transit Malignant Melanoma Undergoing Isolated Limb Perfusion Using Modern Treatment Paramenters. Journal of Clinical Oncology. 2010;28(1):114–118. doi: 10.1200/JCO.2009.23.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boesch CE, Meyer T, Washcke L, et al. Long-term outcome of hyperthermic isolated limb perfusion (HILP) in the treatment of locoregionally metastasized malignant melanoma of the extremities. International Journal of Hyperthermia. 2010;26(1):16–20. doi: 10.3109/02656730903236086. [DOI] [PubMed] [Google Scholar]
- 11.Raymond AK, Beasley GM, Broadwater G, et al. Current trends in regional therapy for melanoma: lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. Journal of the American College of Surgeons. 2011;213(2):306–16. doi: 10.1016/j.jamcollsurg.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Annals of Surgical Oncology. 2008;15(8):2195–205. doi: 10.1245/s10434-008-9988-9. [DOI] [PubMed] [Google Scholar]
- 13.Santillan AA, Zager JS. Isolated limb infusion for melanoma: a less morbid alternative to hyperthermic isolated limb perfusion in the US. Expert Opinion in Drug Metabolism and Toxicology. 2010;6(9):1033–7. doi: 10.1517/17425250903559881. [DOI] [PubMed] [Google Scholar]
- 14.Beasley GM, Sharma K, Wong J, et al. A multi-institution experience comparing the clinical and physiologic differences between upper extremity and lower extremity melphalan-based isolated limb infusion. Cancer. 2012;118(24):6136–43. doi: 10.1002/cncr.27676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroon HM, Moncrieff M, Kam PCA, Thompson JF. Outcomes Following Isolated Limb Infusion for Melanoma. A 14-Year Experience. Annals of Surgical Oncology. 2008;15(11):3003–3013. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]
- 16.Huismans AM, Kroon HM, Kam PCA, Thompson JF. Does Increased Experience with Isolated Limb Infusion for Advanced Limb Melanoma Influence Outcome? A Comparision of Two Treatment Periods at a Single Institution. Annals of Surgical Oncology. 2011;18:1877–1883. doi: 10.1245/s10434-011-1646-y. [DOI] [PubMed] [Google Scholar]
- 17.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. Journal of the American College of Surgeons. 2009;208(5):106–15. doi: 10.1016/j.jamcollsurg.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Santillan AA, Delman KA, Beasley GM, et al. Predictive factors of regional toxicity and serum creatine phosphokinase levels after isolated limb infusion for melanoma: a multi-institutional analysis. Annals of Surgical Oncology. 2009;16(9):2570–8. doi: 10.1245/s10434-009-0563-9. [DOI] [PubMed] [Google Scholar]
- 19.Sharma K, Beasley G, Turley R, et al. Patterns of Recurrence Following Complete Response to Regional Chemotherapy for In-Transit Melanoma. Annals of Surgical Oncology. 2012;19:2563–2571. doi: 10.1245/s10434-012-2315-5. [DOI] [PubMed] [Google Scholar]
- 20.Kroon HM, Lin DY, Kam PCA, Thompson JF. Major Amputation for Irresectable Extremity Melanoma After Failure of Isolated Limb Infusion. Annals of Surgical Oncology. 2009;16:1543–1547. doi: 10.1245/s10434-009-0394-8. [DOI] [PubMed] [Google Scholar]
- 21.Chai CY, Deneve JL, Beasley GM, et al. A Multi-institutional Experience of Repeat Regional Chemotherapy for Recurrent Melanoma of the Extremities. Annals of Surgical Oncology. 2012;19:1637–1643. doi: 10.1245/s10434-011-2151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Testori A, Intellisano A, Verrecchia F, et al. Alternatives for the treatment of local advanced disease: electrochemotherapy, limb perfusion, limb infusion, intralesional IL2. What is the role? Dermatologic Therapy. 2012;25(5):443–51. doi: 10.1111/j.1529-8019.2012.01486.x. [DOI] [PubMed] [Google Scholar]
- 23.Testori A, Faries MB, Thompson JF, et al. Local and Intralesional Therapy of In-Transit Melanoma Metastases. Journal of Surgical Oncology. 2011;104:391–396. doi: 10.1002/jso.22029. [DOI] [PubMed] [Google Scholar]


