Abstract
Introduction
Isolated limb infusion (ILI) is a therapeutic option for patients with recurrent, unresectable extremity malignancies.
Methods
A prospectively collected single institution database of patients undergoing ILI was analyzed for preoperative, intraoperative, and postoperative parameters and outcomes.
Results
From 5/2007-1/2012, 76 patients successfully underwent initial ILI, and 28 following either previous hyperthermic isolated limb perfusion (HILP) or ILI. Seventy-nine (74%) patients had melanoma, 24 (22%) sarcoma, 3 (3%) Merkel cell and 1 (1%) squamous cell carcinoma. There were 55 (72%) initial and 22 (79%) repeat lower extremity (LE) ILI’s, and 21 (78%) initial and 6 (22%) repeat upper extremity (UE) ILI’s. Serologic toxicity, measured by serum creatine kinase (CK), peaked higher and later in LE ILI’s, median 620 vs. 124 IU/L and postoperative day 4 vs. 2, respectively, P<0.05. LE ILI’s had a longer hospital length of stay (LOS), median 6 vs. 5 days (P<0.0001). A median of grade II Wieberdink regional toxicity was observed. Three-month follow-up was available in 94 (90%). A response (ORR) was seen in 72% of ILI’s performed for melanoma and 58% for sarcoma. No difference in response was observed between UE vs. LE or between initial vs. repeat ILI’s. Repeat UE ILI’s, however, appeared to have an improved ORR than repeat LE ILI’s, 83% vs. 64%.
Conclusion
ILI may be successfully performed for cutaneous and soft tissue malignancies. LE ILI’s have higher CK levels and slightly longer LOS. Repeat ILI’s are not associated with increased toxicity and similar ORR. UE ILI’s may have better overall response rates.
Keywords: isolated limb infusion, limb preservation, melanoma and sarcoma
Introduction
Isolated limb infusion (ILI) is a less invasive method of delivering regional therapy for limb preservation, when compared with the open technique hyperthermic isolated limb perfusion (HILP). ILI was first adapted from HILP by Thompson, et al. in the early 1990’s to treat in-transit, unresectable melanoma of the extremities <1>. More recently, ILI has been utilized in treating unresectable extremity soft tissue sarcoma and other cutaneous malignancies, although published series demonstrate variable results <2, 3>.
A single-institution series describing the experience from the Melanoma Institute Australia demonstrated overall response (complete response and partial response) rates of 84% following ILI for melanoma and 90% for sarcoma <2, 4>. Of note, the Australian experience for melanoma includes treatment of stage I-II melanoma with ILI <4>. Multivariate analysis demonstrated only a complete response to be predictive of long-term survival, and stage was not predictive of disease response <5>. While single-institution series in the U.S. are not as robust, a multi-institutional review of patients treated for stage III/IV extremity melanoma demonstrated an overall response rate (ORR) of 64% (31% complete response, 33% partial response)<6>.
Because significant variability exists in patient selection and ILI technique, response rates may be affected by the heterogeneity amongst the reported series. The current study aims to evaluate a single-institution cohort of patients treated with ILI for in-transit melanoma metastases of the extremity, unresectable or maximally-treated softtissue sarcoma, or other cutaneous malignancies, performed under nearly uniform conditions.
Methods
A prospectively maintained database of patients undergoing ILI at our institution was reviewed for indication for limb infusion, patient demographics, intraoperative and perioperative parameters, and postoperative outcome.
Statistical Analyses
All data analyses were performed using SAS (version 9.2, SAS Institute Inc., Cary, NC), with significance considered when the two-sided p-value ≤ 0.05. Data was natural log transformed prior to applying parametric tests, and generalized linear mixed models or multivariate models applied where applicable.
Operative Technique
Each limb infusion involved placement of 5 French arterial and 6 French venous catheters to the affected limb under fluoroscopic guidance via a percutaneous approach. The patient was systemically heparinized with 300 units per kg body weight to achieve an activated clotting time (ACT) of greater than 400 seconds. Under general anesthesia in the operating room, 60 mg of Papaverine was administered into the ILI circuit after the tourniquet was inflated to 250 mmHg for upper extremities and 300 mmHg for lower extremities. Actinomycin-D (100μg/L) and Melphalan (7.5mg/L for LW and 10 mg/L for UE) were dose based on limb volume. Melphalan dose was further corrected based on the patient’s ideal body weight (IBW) as follows: Dose (mg) × Limb volume (L) × IBW / Actual body weight. The majority (90%) had chemotherapy dose-correction for IBW. Chemotherapy was circulated for 30 minutes while the extremity was externally warmed to greater than 37°C. The limb was washed out with saline prior to tourniquet release.
Post-Operative Followup
Patients were monitored with daily physical examination of the extremity and twice daily assessment of creatine kinase (CK) levels, a measure of systemic toxicity. If CK levels peaked above 1000 IU/L, intravenous dexamethasone and normal saline infusion was initiated in an effort to decrease the acute inflammatory reaction in the affected extremity and prevent rhabdomyolysis-induced acute tubular necrosis. Once the CK level peaked, patients were discharged. If patients did not exhibit a CK elevation or limb toxicity, they were generally discharged on post-operative day 4. All patients received routine deep vein thrombosis (DVT) prophylaxis with Fragmin 5,000 units subcutaneous injection daily while hospitalized and were discharged home on aspirin 325 mg daily for three months.
Patients were seen in follow-up at 2, 6 and 12 weeks following ILI. Response to treatment was judged by clinical assessment of disease burden and PET/CT. Clinical assessment was used for dermal and cutaneous lesions; PET/CT imaging was routinely obtained 3 months following ILI to assess for both regional response and distant disease, in keeping with an algorithm previously published by Beasley, et al. <8>. Complete response (CR) was defined as resolution of all nodules; partial response (PR) as a ≥ 30% decrease in diameter or disappearance of at least 30% of the nodules in field; stable disease (SD) was failure to meet criteria for PR but without progression of disease; and progressive disease (PD) was at least a 20% increase in diameter of disease nodules or progression of disease as evidenced by new nodules after ILI. Overall response rate (OR) was defined as the number of patients with CR or PR in the cohort.
As described by Chai et al., an algorithm was proposed for repeat ILI’s<9>. Repeat ILI was generally offered for patients who had a CR or PR after initial HILP or ILI with duration of response >3 months before disease recurrence or progression <9>.
Outcome
Clinical response to ILI was determined at three months following ILI. Because melanoma and sarcoma represented the majority of patients, clinical response is reported here for those two groups. Ninety-four procedures were evaluated.
Results
Patient Cohort
From 5/2007 to 1/2012, 107 patients underwent attempted ILI at our institution. Three procedures (3%) were terminated before ILI was performed. In one patient, the tourniquet failed to achieve arterial occlusion in the affected limb. In one patient, the vessels were too small or tortuous and would not accommodate a 5 or 6 French sheath, and one patient sustained an arterial intimal flap upon placement of the catheters, and the ILI was not performed. Of the 104 ILI’s performed, the majority of patients were female (N=63, 59%) with a median age of 73 (range 19-92) years. Seventy-nine (74%) patients had in-transit melanoma metastases, not amenable to surgical resection. Twenty-four (22%) had unresectable extremity sarcoma; multiple histologic subtypes were treated, most commonly high-grade undifferentiated pleomorphic sarcoma (N=11, 46%) and Kaposis sarcoma (N=3, 13%). Less common indications included Merkel cell carcinoma (3, 3%) and squamous cell carcinoma (1, 1%), Table 1.
Table 1.
Patient Cohort demographics.
| Patient Characteristics | |
|---|---|
| Age | 73 (Median), range 19-92 years |
| Gender | |
| Female | N=63 (59%) |
| Male | N=44 (41%) |
| Indication for Isolated Limb Infusion | |
| Melanoma | N=79 (74%) |
| Sarcoma | N=24 (22%) |
| Undifferentiated Pleomorphic Sarcoma | N=11 (46%) |
| Synovial Cell Sarcoma | N=2 (8%) |
| Kaposis Sarcoma | N=3 (13%) |
| Angiosarcoma | N=1 (4%) |
| Pleomorphic Sarcoma | N=1 (4%) |
| Rhabdomyosarcoma | N=1 (4%) |
| Dedifferentiated Liposarcoma | N=1 (4%) |
| Epithelioid Sarcoma | N=1 (4%) |
| Rhabdomyosarcoma | N=1 (4%) |
| Myxoinflammatory Fibroblastic Sarcoma | N=1 (4%) |
| Leiomyosarcoma | N=1 (4%) |
| Merkel Cell Carcinoma | N=3(3%) |
| Squamous cell carcinoma | N=1(1%) |
Seventy-six patients underwent ILI as an initial regional therapeutic procedure; of these, 55 (72%) were in the lower extremity (LE) and 21 (28%) in the upper extremity (UE). Twenty-eight patients underwent a repeat regional therapeutic procedure as an ILI after either an initial HILP or ILI, 22 (79%) of whom had LE disease and 6 (21%) with UE disease (Figure 1).
Figure 1.

Patient cohort stratified by initial or repeat infusion as well as upper or lower extremity. 108 total patients underwent Isolated Limb Infusion (ILI) at our institution, the majority for melanoma and sarcoma. The majority of these patients underwent initial ILI, of whom most were performed in the lower extremity. 26% of patients had repeat ILI, again the majority performed in the lower extremity.
Intraoperative and Perioperative Parameters
While the standard circulation time for chemotherapy was 30 minutes for all patients, total ischemia time varied due to time to prime the ILI circuit, ensuring adequate tourniquet occlusion, achieving limb temperature >37° prior to chemotherapy instillation, and washout of the limb after a 30 minute infusion. Median ischemic time for the overall cohort was 50 minutes (range 42-82). There was no difference in ischemia time between LE and UE ILI (P=0.70), or in initial vs. repeat ILI (P=0.39), Table 2.
Table 2.
Intra-operative and post-operative parameters during and following ILI.
| Overall Cohort | Initial LE | Initial UE | Repeat LE | Repeat UE | |
|---|---|---|---|---|---|
| Intra-operative Parameters | |||||
| Median Ischemic Time (minutes) | 50 | 50 | 47 | 51 | 47 |
| Median Perfusate Base Excess at 30 minutes (mEq/L) | -9 | -8 | -14 | -8 | -13 |
| Median Perfusate pH at 30 minutes | 7.2 | 7.2 | 7.1 | 7.2 | 7.1 |
| Post-operative Parameters | |||||
| Median peak CK level (IU/l) | 392 | 535 | 124 | 963 | 122 |
| Day of peak CK level | 4 | 5 | 2 | 4 | 2 |
| Length of Hospital Stay (days) | 6 | 6 | 5 | 6 | 5 |
| Wieberdink Toxicity Score | 2 | 2 | 2 | 2 | 2 |
| Complications (30 day) | |||||
| Deep vein thrombosis | 3 (3%) | 3 (5%) | |||
| Stroke | 1 (1%) | 1 (5%) |
The median base excess, seen on perfusate blood gas analysis, as well as the median perfusate pH at 30 minutes, were evaluated. The overall cohort had a median perfusate base excess of -9 mEq/L (range – 3 to -22.8) with a median pH at 30 minutes of 7.2 (range 6.92-7.72). UE ILI’s had greater base excess when compared to LE procedures, -14 vs. -8 mEq/L, and were associated with a lower pH, 7.1 vs. 7.21, P<0.001. Repeat UE and LE ILI’s did not exhibit greater base excess than initial infusions, median -13 vs. -14 mEq/L and -8 vs. -8 mEq/L, respectively. Repeat ILI’s were not associated with any difference in pH than initial ILI’s, P=0.99 (Table 2).
Postoperatively, serologic toxicity was assessed by measurement of CK levels. While the median peak CK level for the cohort was 421 IU/L (range 21-9626), LE procedures achieved a higher peak CK level than UE procedures, 620 vs. 124 IU/L, P=0.001. Repeat LE procedures tended to achieve an even higher peak CK level, median 963 IU/L. Repeat UE procedures exhibited similar peak CK levels as initial ILI’s, 122 vs. 124 IU/L, respectively. LE ILI’s achieved a peak CK level on a later postoperative day than UE ILI’s, day 4 vs. 2, P<0.05. There was no difference in day of peak CK level when comparing initial ILI vs. repeat ILI, P=0.46 (Table 2).
Overall median length of stay was 6 days for the cohort and was shorter for UE ILI’s vs. LE, median 5 vs. 6 days, P<0.0001. Repeat ILI’s were not associated with any longer length of hospital stay, P=0.44. A median Wieberdink grade 2 toxicity was seen in the overall cohort (range of 1-3). Twenty-four (23%) patients had Wieberdink grade 3 toxicity, 23 (96%) occurring in LE ILI’s and 1 (4%) in an UE ILI. LE ILI’s exhibited greater toxicity than UE ILI’s, P=0.0004 (Table 2). Repeat perfusions were not found to have a significant increase in toxicity, and no patients required fasciotomy or had treatment-related limb loss (Wieberdink grade 4 or 5 toxicity).
Complications were seen in 4 (4%) patients within a 30-day period. This included 3 (3%) patients who developed a deep vein thrombosis (DVT) in the affected limb. All patients were treated with anticoagulation and did not suffer any adverse consequences. One (1%) patient developed an ischemic stroke immediately following the procedure. With the exception of this patient who had residual hemiparesis of the upper extremity nearly six months following ILI, no patients had long-term toxicity or sequelae following ILI.
Response to ILI in Melanoma Patients
Overall, a CR was achieved in 32% of ILI’s for melanoma, and a PR was seen in 40%. Ten percent of melanoma ILI’s demonstrated SD. Eighteen percent of melanoma had PD after ILI (Figure 2).
Figure 2.
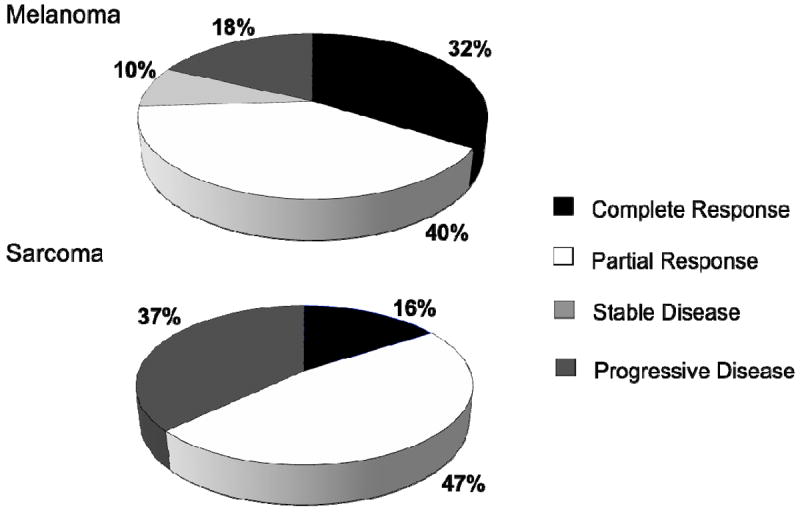
Disease response at 3 months, melanoma and sarcoma.
When examining response by LE vs. UE as well as by initial vs. repeat ILI, CR was seen in 35% of initial LE ILI’s for melanoma; repeat LE ILI had a 21% CR. Upper extremity ILI’s for melanoma achieved a 42% initial CR and a 25% repeat CR (Table 3).
Table 3.
Disease response at 3 months, stratified by melanoma and sarcoma, upper versus lower extremity, and initial and repeat limb infusions.
| Complete Response | Partial Response | Stable Disease | Progressive Disease | |
|---|---|---|---|---|
| Melanoma | ||||
| Initial Lower Extremity (N=37) | N= 13 (35%) | N= 14 (38%) | N= 3 (8%) | N= 7 (19%) |
| Repeat Lower Extremity (N=19) | N= 4 (21%) | N=8 (42%) | N= 3 (16%) | N=4 (21%) |
| Initial Upper Extremity (N= 12) | N= 5 (42%) | N=4 (33%) | N=1 (8%) | N= 2 (17%) |
| Repeat Upper Extremity (N=4) | N=1 (25%) | N=3 (75%) | 0% | 0% |
| Sarcoma | ||||
| Initial Lower Extremity (N=13) | N=3 (23%) | N= 4 (31%) | 0% | N=6 (46%) |
| Repeat Lower Extremity (N=3) | 0% | N=2 (67%) | 0% | N=1 (33%) |
| Initial Upper Extremity (N=4) | 0% | N=2 (50%) | 0% | N=2 (50%) |
| Repeat Upper Extremity (N=1) | 0% | N=1 (100%) | 0% | 0% |
Partial response was seen in approximately one third of patients who underwent initial ILI in either LE (38%) or UE (33%) for melanoma. PR was seen more often in repeat infusions (ILI after initial ILI or HILP) for melanoma, with 42% and 75% of LE and UE repeat ILI’s achieving PR, respectively.
Stable disease (SD) was seen in 8% of melanoma patients who underwent initial LE or UE ILI’s. Sixteen percent and 0% of the repeat LE and UE ILI’s, respectively, had SD. Similar proportions of patients with melanoma demonstrated PD after undergoing initial ILI, 19% and 17% for LE and UE, respectively. Repeat infusions demonstrated similar PD response in LE ILI’s, 21%, but none of the repeat UE ILI’s had PD (Table 3).
Response to ILI in Sarcoma Patients
Overall, a CR was seen in 16% of ILI’s performed for sarcoma. Partial response was seen in 47%. None of the sarcoma ILI’s had SD, and 37% had PD after ILI.
When examining disease response by LE vs. UE and initial vs. repeat, disease response in ILI’s performed for sarcoma appeared to demonstrate less favorable outcomes, with a 23% and 0% CR for initial and repeat LE ILI’s, respectively, although was not statistically significant. Complete response was not achieved in any UE ILI performed for sarcoma (Table 3).
Sarcoma patients had greater proportions of patients achieving PR than melanoma patients, seen in 31% and 50% of initial LE and UE ILI’s, respectively. Although there were only four sarcoma patients who underwent repeat infusions, they achieved PR rates of 67% and 100% in repeat LE and UE ILI’s, respectively (Table 3).
None of the patients with sarcoma who underwent ILI had stable disease, either demonstrating disease response or disease progression. Progressive disease was seen in nearly half (46% and 50% for LE and UE, respectively) of patients who had an initial ILI for sarcoma. Repeat infusions, however, demonstrated disease progression in one of the three LE sarcoma patients (33%). None of the UE patients, however, had PD.
Overall response rates between LE vs. UE ILI’s, P=0.44 (C.I. 0.17-2.12), or initial vs. repeat ILI’s, P=0.9 (C.I. 0.47-2.34), was not significantly different in either the melanoma or sarcoma groups.
Discussion
Development of in-transit melanoma metastases is generally perceived to be an indicator of aggressive biologic behavior in cutaneous melanoma and presents a therapeutic conundrum. Classified as stage III disease by the American Joint Committee on Cancer Staging (AJCC) <10>, in-transit metastases are seen to develop in 2-3.5% of sentinel lymph node-negative patients and up to 12% of sentinel-lymph node positive patients <11, 12>. Development of in-transit metastases is associated with a median overall 5-year survival of 54% <12>. Management is controversial, ranging from hyperthermic limb perfusion or ILI to local intratumoral injections to systemic therapy, with no therapy definitively improving overall survival <13, 14>. Locally advanced or unresectable soft tissue sarcoma, likewise, poses a similar challenge, with amputation historically being the treatment option of choice. Limb salvage options, including regional therapy with hyperthermic limb perfusion and ILI have been proposed with limb salvage rates of up to 80% but also without definitive impact on overall survival <15, 16>.
Isolated limb perfusion (ILP) was first introduced as a regional therapy in the 1950’s, designed to achieve higher concentrations of chemotherapy in the extremity than what could be achieved via systemic administration, while avoiding toxicity <13, 17, 18>. Hyperthermia to the extremity was introduced in the 1960’s, with improved response rates compared to normothermic perfusion <19, 20>. Isolated limb infusion was presented as a simpler, less morbid alternative to HILP <1>.
While the Melanoma Institute Australia experience reports comparable ORR and duration of response with ILI for melanoma compared with conventional HILP (84% ORR with ILI compared with 85% with ILP), the CR rate was higher for HILP than ILI (69% vs. 38%, respectively) <4, 21>. One single-institution U.S. series reported a lower ORR with ILI vs. HILP, 44% ORR with ILI vs. 88% with HILP <22>; however more recent series have shown improved results with ILI for melanoma, with ORR up to 88% <6>. It may not be valid to compare the U.S. experience with the Australians, as the U.S. excludes early stage melanoma. Our institutional experience with ILI for melanoma demonstrated an ORR of 74%, with a CR rate of 34%.
Few series examine the utility of ILI for soft-tissue-sarcoma, although small series have demonstrated ORR up to 90% <2, 3>. Combination with external beam irradiation has been described, achieving limb salvage in 83% <23>. While these results are encouraging, soft tissue sarcoma encompasses an enormous range of histologic subtypes, with varying biologic and clinical behavior. Our experience with soft-tissue-sarcoma and ILI showed ORR of 63%.
Previous reports of patients who underwent repeat ILI procedures for melanoma showed ORR ranging from 33% to 83% <9, 24>. The Melanoma Institute Australia demonstrated an ORR of 83% and a CR of 23% after repeat ILI. Their cohort, however, included early stage melanoma and planned double ILI procedures <24>. Chai et al reported a multi-institutional U.S experience with repeat ILI following initial ILI and found an ORR of 40% and a CR rate of 24% <9>. Data regarding repeat ILI for sarcoma is quite limited. Our experience with repeat ILI for melanoma demonstrated improved ORR for repeat UE ILI (ORR of 100%) whereas repeat LE ILI had a lower ORR when compared with initial ILI (63% vs. 73%, respectively). Complete response rates were similar between repeat UE and LE ILI’s. Repeat ILI for sarcoma, likewise, appeared to have improved ORR compared with initial ILI, with repeat UE ILI achieving a 100% ORR, although cohort size was small. Despite an improved ORR, there was no CR in repeat ILI’s performed for sarcoma.
While ORR vary between institutions, toxicity and complications were shown to be lower with ILI than with ILP, an advantage for ILI <24>. In one U.S. single-institution study comparing ILI to ILP, ILI had 0% treatment-related limb loss vs. a 3% risk with ILP. This same study also reported a 4% development of deep venous thrombosis (DVT) with ILI vs. 11% with ILP. Twenty percent of the ILI cohort also developed ≥ Grade 3 Wieberdink toxicity, whereas ILP was associated with a 27% risk of ≥ Grade 3 toxicity <25>. The Sydney Melanoma Unit recently reported a 0% limb loss rate with ILI for melanoma, but observed a 35% rate of ≥ Grade 3 toxicity <26>. Santillan et al found toxicity after ILI to be associated with female sex, papaverine, and high peak CK levels. Adjustment of the Melphalan dose for IBW, however, was significantly associated with lower toxicity<7>.
In this study, we also report a 0% limb loss rate. The median CK level increased with repeat LE ILI, and we found higher toxicity in LE ILI’s than. UE ILI’s. UE ILI’s demonstrated an earlier peak CK level and significantly shorter hospital length of stay. There was no difference in hospital length of stay for repeat infusions, either UE or LE. No patient suffered any major adverse toxicity from ILI.
Even in the modern era of melanoma treatment, HILP appears to improve overall survival (OS) in only a small subset of patients, with a 34% 10 year actuarial survival <27>. As there have been no prospective trials comparing ILP to ILI, it is difficult to determine whether the two modalities are equivalent. The Duke University experience, when retrospectively analyzed, found no difference in OS in patients treated with either HILP or ILI over 15 years <25>. One must note that a fair comparison between ILI and HILP was not made in that review since disease burden and techniques differed, and the review was not performed in the context of a randomized clinical trial. However, a survival benefit was seen in patients who attained a CR with treatment, either HILP or <25>. In this study, we demonstrate that ILI may achieve ORR of 50-75%, depending on the indication and extremity, with CR achieved in up to 42%. ILI performed for sarcoma achieves similar ORR as melanoma, and repeat infusions offer similar response rates as initial infusions. It is important, however, to note that although advanced extremity sarcoma patients have few treatment options available, Melphalan-based ILI offers a greater potential benefit to melanoma patients. While repeat UE ILI’s appeared to offer clinical benefit, with no patients demonstrating disease progression, the sample size was limited.
Conclusion
ILI is an effective alternative to ILP and may be successfully performed for the majority of extremity in-transit melanoma metastases, unresectable soft-tissue sarcoma and other non-melanoma cutaneous neoplasms. Repeat ILI is feasible, with a similar toxicity profile as initial ILI, and offers similar disease response rates. UE ILI’s may have an improved disease response and warrants further investigation. Due to the low complication rate and 0% amputation rate, ILI presents an attractive treatment modality and should be incorporated into the therapeutic armamentarium. Further investigation is warranted to define its true impact on overall survival.
Footnotes
Presented in part at the 7th International Symposium on Regional Cancer Therapies, Captiva, FL February 2012
References
- 1.Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Seminars in Surgical Oncology. 1998;14:238–47. doi: 10.1002/(sici)1098-2388(199804/05)14:3<238::aid-ssu8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Moncrieff MD, Kroon HM, Kam PC, Stalley PD, Scolyer RA, Thompson JF. Isolated Limb Infusion for Advanced Soft Tissue Sarcoma of the Extremity. Annals of Surgical Oncology. 2008;15(10):2749–2756. doi: 10.1245/s10434-008-0045-5. [DOI] [PubMed] [Google Scholar]
- 3.Turaga KK, Beasley GM, Kane JM, et al. Limb Preservation With Isolated Limb Infusion for Locally Advanced Nonmelanoma Cutaneous and Soft-Tissue Malignant Neoplasms. Archives of Surgery. 2011;146(7):870–875. doi: 10.1001/archsurg.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes Following Isolated Limb Infusion for Melanoma. A 14-Year Experience. Annals of Surgical Oncology. 2008;15(11):3003–3013. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]
- 5.Sanki A, Kam PC, Thompson JF. Long-term Results of Hyperthermic, Isolated Limb Perfusion for Melanoma A Reflection of Tumor Biology. Annals of Surgery. 2007;245(4):591–596. doi: 10.1097/01.sla.0000251746.02764.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beasley GM, Caudle A, Petersen RP, et al. A Multi-Institutional Experience of Isolated Limb Infusion: Defining Response and Toxicity in the US. Journal of the American College of Surgeons. 2009;208:706–717. doi: 10.1016/j.jamcollsurg.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Santillan AA, Delman KA, Beasley GM, et al. Predictive Factors of Regional Toxicity and Serum Creatine Phosphokinase Levels After Isolated Limb Infusion for Melanoma: A Multi-Institutional Analysis. Annals of Surgical Oncology. 2009;16(9):2570–8. doi: 10.1245/s10434-009-0563-9. [DOI] [PubMed] [Google Scholar]
- 8.Beasley GM, Parsons C, Broadwater G, et al. A Multicenter Prospective Evaluation of the Clinical Utility of F-18 FDG-PET/CT in Patients With AJCC Stage IIIB or IIIC Extremity Melanoma. Annals of Surgery. 2012 Jun 11; doi: 10.1097/SLA.0b013e318256d1f5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai CY, Deneve JL, Beasley GM, et al. A Multi-institutional Experience of Repeat Regional Chemotherapy for Recurrent Melanoma of Extremities. Annals of Surgical Oncology. 2011 Dec 6; doi: 10.1245/s10434-011-2151-z. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. pp. 325–44. [Google Scholar]
- 11.Zogakis TG, Essner R, Wang HJ, Foshag LJ, Morton DL. Natural History of Melanoma in 773 Patients with Tumor-Negative Sentinel Lymph Nodes. Annals of Surgical Oncology. 2007;14(5):1604–1611. doi: 10.1245/s10434-006-9267-6. [DOI] [PubMed] [Google Scholar]
- 12.Pawlik TM, Ross MI, Johnson MM, et al. Predictors and Natural History of In-Transit Melanoma After Sentinel Lymphadenectomy. Annals of Surgical Oncology. 2005;12(8):587–596. doi: 10.1245/ASO.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Testori A, Verhoef C, Kroon HM, Pennacchioli E, Faries MB, Eggermont AM, Thompson JF. Treatment of melanoma metastases in a limb by isolated limb perfusion and isolated limb infusion. Journal of Surgical Oncology. 2011;104(4):397–404. doi: 10.1002/jso.22028. [DOI] [PubMed] [Google Scholar]
- 14.Testori A, Faries MB, Thompson JF, et al. Local and intralesional therapy of intransit melanoma metastases. Journal of Surgical Oncology. 2011;104(4):391–6. doi: 10.1002/jso.22029. [DOI] [PubMed] [Google Scholar]
- 15.Bonvalot S, Laplanche A, Lejeune F, et al. Limb salvage with isolated perfusion for soft tissue sarcoma: could less TNF-alpha be better? Annals of Oncology. 2005;16(7):1061–8. doi: 10.1093/annonc/mdi229. [DOI] [PubMed] [Google Scholar]
- 16.Deroose JP, Eggermont AM, Van Geel AN, Burger JW, den Bakker MA, de Wilt JH, Verhoef C. Long-Term Results of Tumor Necrosis Factor ∝-and Melphalan-Based Isolated Limb Perfusion in Locally Advanced Extremity Soft Tissue Sarcomas. Journal of Clinical Oncology. 2011;29(30):4036–44. doi: 10.1200/JCO.2011.35.6618. [DOI] [PubMed] [Google Scholar]
- 17.Klopp CT, Alford TC, Bateman J, Berry GN, Winship T. Fractionated intra arterial cancer chemotherapy. Annals of Surgery. 1950;132:811–832. doi: 10.1097/00000658-195010000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creech OJ, Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer; Regional perfusion using an extracorporeal circuit. Annals of Surgery. 1958;148:616–632. doi: 10.1097/00000658-195810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stehlin JS. Hyperthermic perfusion with chemotherapy for cancers of the extremity. Surgery Gynecology and Obstetrics. 1969;129:305–308. [PubMed] [Google Scholar]
- 20.Cumberlin R, De Moss E, Lassus M, Friedman M. Isolated Perfusion for Malignant Melanoma of the Extremity: A Review. Journal of Clinical Oncology. 1985;3(7):1022–1031. doi: 10.1200/JCO.1985.3.7.1022. [DOI] [PubMed] [Google Scholar]
- 21.Sanki A, Kam PCA, Thompson JF. Long-term Results of Hyperthermic, Isolated Limb Perfusion for Melanoma. A Reflection of Tumor Biology. Annals of Surgery. 2007;245(4):591–596. doi: 10.1097/01.sla.0000251746.02764.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beasley GM, Petersen RP, Yoo J, et al. Isolated Limb Infusion for In-Transit Malignant Melanoma of the Extremity: A Well-Tolerated but Less Effective Alternative to Hyperthermic Isolated Limb Perfusion. Annals of Surgical Oncology. 2008;15(8):2195–2205. doi: 10.1245/s10434-008-9988-9. [DOI] [PubMed] [Google Scholar]
- 23.Hegazy MA, Kotb SZ, Sakr H, El Dosoky E, Amer T, Hegazi RA, Farouk O. Preoperative isolated limb infusion of Doxorubicin and external irradiation for limb-threatening soft tissue sarcomas. Annals of Surgical Oncology. 2007;14(2):568–76. doi: 10.1245/s10434-006-9138-1. [DOI] [PubMed] [Google Scholar]
- 24.Kroon HM, Lin DY, Kam PC, Thompson JF. Efficacy of Repeat Isolated Limb Infusion With Melphalan and Actinomycin D for Recurrent Melanoma. Cancer. 2009;115:1932–40. doi: 10.1002/cncr.24220. [DOI] [PubMed] [Google Scholar]
- 25.Raymond AK, Beasley GM, Broadwater G, et al. Current Trends in Regional Therapy for Melanoma: Lessons Learned from 225 Regional Chemotherapy Treatments between 1995 and 2010 at a Single Institution. Journal of the American College of Surgeons. 2011;213:306–318. doi: 10.1016/j.jamcollsurg.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huismans AM, Kroon HM, Kam PC, Thompson JF. Does Increased Experience with Isolated Limb Infusion for Advanced Limb Melanoma Influence Outcome? A Comparison of Two Treatment Periods at a Single Institution. Annals of Surgical Oncology. 2011;18(7):1877–83. doi: 10.1245/s10434-011-1646-y. [DOI] [PubMed] [Google Scholar]
- 27.Alexander HR, Fraker DL, Bartlet DL, Libutti SK, Steinberg SM, Soriano P, Beresnev T. Analysis of Factors Influencing Outcome in Patients With In-Transit Malignant Melanoma Undergoing Isolated Limb Perfusion Using Modern Treatment Parameters. Journal of Clinical Oncology. 2010;28(1):114–8. doi: 10.1200/JCO.2009.23.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]


