Abstract
Elevated levels of myeloid-derived suppressor cells (MDSCs) induced by tumor-derived factors are associated with inhibition of immune responses in patients with gastrointestinal malignancies. We hypothesized that pro-MDSC cytokines and levels of MDSC in the peripheral blood would be elevated in pancreatic adenocarcinoma patients with progressive disease. Peripheral blood mononuclear cells (PBMCs) were isolated from 16 pancreatic cancer patients undergoing chemotherapy and phenotyped for MDSC using a five antigen panel (CD33, HLA-DR, CD11b, CD14, CD15). Patients with stable disease had significantly lower MDSC levels in the peripheral blood than those with progressive disease (1.41 ± 1.12 vs. 5.14 ± 4.58 %, p = 0.013, Wilcoxon test). A cutoff of 2.5 % MDSC identified patients with progressive disease. Patients with ECOG performance status ≥2 had a weaker association with increased levels of MDSC. Plasma was obtained from 15 chemonaive patients, 13 patients undergoing chemotherapy and 9 normal donors. Increases in the levels of pro-MDSC cytokines were observed for pancreatic cancer patients versus controls, and the pro-MDSC cytokine IL-6 was increased in those patients undergoing chemotherapy. This study suggests that MDSC in peripheral blood may be a predictive biomarker of chemotherapy failure in pancreatic cancer patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1618-8) contains supplementary material, which is available to authorized users.
Keywords: MDSC, Pancreas cancer, Cytokines, Progression
Introduction
Pancreatic cancer is a lethal malignancy, with less than 5 % of patients living beyond 5 years of diagnosis [1]. The average life expectancy of patients with stage IV adenocarcinoma is 6 months, and even with the most aggressive chemotherapy of 5-FU, oxaliplatin and irinotecan (FOLFIRINOX) survival is only improved by a few months [2–4]. More recently, new therapies in combination with gemcitabine have been studied. The MPACT study evaluated the use of nab-paclitaxel in combination with gemcitabine and demonstrated an increase in 1-year survival from 22 to 35 % [5, 6]. Recent studies suggest that the anti-tumor effects of cytotoxic chemotherapy may be mediated, in part, via an ability to alter the profile of the intra-tumoral immune infiltrate [7, 8]. Concurrently, there have been significant advances in our ability to generate a productive anti-tumor immune response simply via the blockade of T cell inhibitory pathways with neutralizing monoclonal antibodies [9, 10]. In the setting of pancreatic cancer, immune-based therapies represent a largely untapped therapeutic approach. Pancreatic cancers can be recognized by the host immune system, but the anti-tumor activity of T cells and natural killer cells is abrogated by several tumor-mediated mechanisms [11, 12]. Work in murine models of pancreatic cancer suggests that immune suppression is an early hallmark of the disease process. Thus, there is accumulating evidence of an important role for the immune system in the development, progression and effective treatment of pancreatic cancer [11].
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous group of immature myeloid cells present in the circulation and tumors of patients with a variety of cancers. They tend to be immunosuppressive and function to inhibit the immune response to cancer [13]. The mechanisms employed by these cells include the depletion of nutrients from the tumor microenvironment, production of reactive oxygen and nitrogen species with the ability to inhibit key immunologic pathways, secretion of immune-suppressive cytokines and the induction of inhibitory immune cells [11, 12].
Our group has shown that patients with gastrointestinal cancers have high levels of functionally suppressive MDSC which are characterized by the lack of HLA-DR and the presence of myeloid markers CD33 and CD11b [14]. Patients with pancreatic adenocarcinoma tend to have increased levels of granulocytic MDSCs in the peripheral blood as determined by the phenotypic marker CD15 [14, 15].
Our group has studied MDSC in gastrointestinal malignancies and has found that HLA-DRneg, CD33+ and CD11b+ MDSC are able to inhibit the T cell response to interferons [14]. Studies suggest that increased levels of MDSCs and immune suppression begin with premalignant lesions and increase with tumor bulk [16]. Numbers of MDSC correlate with stage in pancreatic cancer patients [17]. However, these previous studies did not distinguish between those patients receiving therapy and those that were treatment naïve. Increasing levels of MDSC could represent an early indicator of disease progression. Based on these studies, it can be postulated that patients who are refractory to treatment and exhibit increasing tumor bulk may also have increasing numbers of MDSC in the peripheral blood. This hypothesis forms the basis for the present investigation. Indeed, we found elevated levels of pro-MDSC cytokines in the peripheral blood of pancreatic cancer patients and levels of MDSC in the peripheral blood may be used a predictive biomarker for progressive disease in pancreatic adenocarcinoma.
Methods
Cytokine analysis
Plasma samples were analyzed utilizing the BioPlex® assay as per the manufacturer’s instructions (Bio-Rad, Hercules, CA). The enzyme-linked immunosorbent assay (ELISA)for S100A9 was purchased from Life Sciences Advanced Technologies, Inc. (Saint Petersburg, FL), and S100A9 levels were measured according to the manufacturer’s instructions. Pathway analysis of patient cytokine profiles obtained from the BioPlex® analysis was performed using Ingenuity Pathway Analysis software (Ingenuity Systems, Inc., Redwood City, CA).
Collection of patient peripheral blood samples
Patients were consented under an institutional review board (IRB) approved tumor banking protocol (OSU-06002). Historical cases were also collected under the same mechanism [14]. Samples were first processed by centrifugation to remove the plasma layer. Afterward, ficoll centrifugation was used to isolate PBMCs as previously described [14]. PBMCs were cryopreserved for further analysis (Table 1). To determine whether MDSC percentage was associated with progressive disease, 17 patients were evaluated. Plasma from normal donors (n = 9, ages 35–62) was purchased from Innovative Research, Inc. (Novi, MI).
Table 1.
Demographics for all patients
| Number of pancreatic adenocarcinoma patients | 37 |
| Sex (n) | |
| Male | 20 |
| Female | 17 |
| Age (years) | |
| Mean | 65.1 |
| Median | 65 |
| Range | 48–84 |
| Tumor extent | |
| Local | 11 |
| Stage I | 1 |
| Stage II | 9 |
| Advanced | 27 |
| Stage III | 8 |
| Stage IV | 19 |
| ECOG Performance Status (n) | |
| 0 | 18 |
| 1 | 14 |
| ≥2 | 5 |
MDSC immunophenotyping
PBMCs were analyzed for MDSC via flow cytometry for a population of cells that expressed low levels of HLA-DR and high levels of CD11b and CD33. The CD15 (granulocytic) and CD14 (monocytic) subsets were also characterized as previously described [14]. Antibodies for HLA-DR, CD11b, CD33 and CD15 were obtained from Beckman Coulter (Indianapolis IN). A CD14 antibody and the isotype control were obtained from BD Biosciences (San Jose, CA). Compensation beads (Life Technologies Grand Island, NY) were utilized to aid in compensation in cases where single color positives were low in abundance. Flow data were analyzed in FlowJo (Tree Star, Ashland, OR) and FCS express (De Novo Software, Los Angeles, CA). The data were analyzed by members of the research team who were blinded as to the clinical status of the patients.
Statistical analysis
The nonparametric Wilcoxon test was used to compare the plasma cytokine levels between normal donors versus untreated/treated pancreatic adenocarcinoma cancer patients. The Bonferroni method was used to adjust for the simultaneous comparisons of 27 cytokines in each experiment. The Wilcoxon test was also used to evaluate the difference in the mean levels of MDSC in peripheral blood in pancreatic adenocarcinoma patients with progressive versus stable disease. In order to determine whether patients exhibited progressive disease, computerized tomography (CT) imaging was performed as per standard of care. A S100A9 ELISA was performed on chemonaive patients, and the Wilcoxon test was used to compare levels between patients with different stages of cancer. The chi-squared (χ 2) test was used to test whether greater than 2.5 % HLA-DR CD33+ cells in peripheral blood was associated with progression of pancreas cancer based on a natural break in the data determined by visual inspection between the levels of MDSC in progressing versus non-progressing patients. The Wilcoxon signed-rank test was utilized to test whether granulocytic MDSC is in higher abundance than monocytic MDSC in patients who were receiving chemotherapy. Sensitivity analyses were conducted to examine the robustness of the conclusions from the nonparametric testing procedures using corresponding parametric statistical testing procedure with proper transformation. A significance level of α = 0.05 was used for each experiment.
This study tests the hypothesis that measurement of MDSC levels in the peripheral blood at the time of a clinic visit can predict disease progression. This hypothesis is tested in the setting of patients with pancreatic adenocarcinoma undergoing chemotherapy regardless of the stage of the disease. Notably, most patients receiving chemotherapy for pancreatic cancer have stage III or IV disease.
Results
MDSC-inducing cytokines are elevated in plasma obtained from pancreas cancer patients
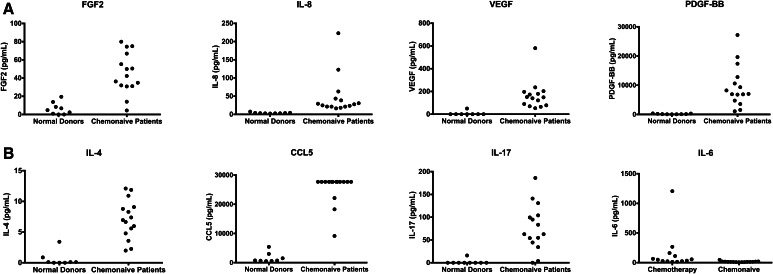
An analysis of plasma obtained from the peripheral blood of pancreas cancer patients was undertaken to see whether factors were present in the plasma that could explain the tendency for increased numbers of MDSC in these patients. Plasma was obtained from 28 pancreatic adenocarcinoma patients. These patients could be divided into two distinct groups. The first group consisted of 15 chemonaive patients (10 stage III or IV, 5 stage II). The second group of patients consisted of 13 patients who had received treatment for their pancreatic cancer (3 stage II, 10 stage III or IV). In the treated patients, 9/13 (69 %) had received gemcitabine-based treatments and the remainder had received 5-fluorouracil-based chemotherapy (3/13) or were enrolled in a clinical trial of carboplatin/paclitaxel with Reolysin™ (1/13) (clinicaltrials.gov NCT01280058). The third group consisted of nine normal donors whose plasma was purchased from a commercial source. It was hypothesized that pancreatic cancer patients would have increased levels of circulating cytokines responsible for MDSC recruitment, function and/or expansion. Pancreatic cancer patients as a whole (n = 28) had statistically significant increases in the following cytokines as compared to normal controls: platelet-derived growth factor beta (PDGF-bb), IL-1β, IL-4, IL-8, IL-12, IL-17, basic fibroblast growth factor (FGF-2), granulocyte colony-stimulating factor (G-CSF), monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein-1alpha (MIP-1α), chemokine (C-C motif) ligand 5 (CCL5) and vascular endothelial growth factor (VEGF). Untreated patients (n = 15) had statistically significant increases in the following cytokines as compared to normal controls: PDGF-bb, IL-4, IL-8, IL-17, FGF-2, CCL5 and VEGF (See Table 2; Fig. 1). Patients receiving chemotherapy had significantly increased levels of IL-6 in the plasma compared to chemonaive patients.
Table 2.
Cytokine analysis comparing plasma cytokine levels from peripheral blood between 15 chemotherapy naïve pancreatic adenocarcinoma patients and 9 normal controls
| Cytokine | Plasma levels in untreated pancreatic cancer patients (pg/mL) | Plasma levels in normal donors (pg/mL) | p value |
|---|---|---|---|
| PDGF-bb | 9,575 (1,074, 27,198) | 137 (31.4, 335) | 0.016 |
| IL-4 | 7.08 (1.98, 12.1) | 0.57 (0,3.42) | 0.032 |
| IL-8 | 48.3 (16.4,223) | 3.71 (2.26,7.51) | 0.016 |
| IL-17 | 77.2 (0, 186) | 1.77 (0, 16.0) | 0.030 |
| FGF2 | 45.2 (4.42, 78.0) | 6.25 (0, 19.4) | 0.038 |
| RANTES (CCL5) | 25,445 (91,565, 276,765) | 1,652 (537, 5,415) | 0.011 |
| VEGF | 166 (52.7, 581) | 6.27 (0, 50.2) | 0.022 |
Five patients had stage II disease, five patients had stage III disease, and five patients had stage IV disease
Fig. 1.
a Levels of cytokines are increased in the plasma of chemonaive patients versus normal donors. The MDSC levels in 15 patients with pancreatic cancer naive to chemotherapy were compared to nine normal donors. Five patients had stage IIB disease, five patients had stage III disease, and five patients had stage IV disease. b Levels of IL-6 are increased in the plasma of pancreatic adenocarcinoma patients undergoing chemotherapy as compared to chemonaive patients; 15 chemonaive patients (population described in a) were compared to 13 patients undergoing chemotherapy. In the patients undergoing chemotherapy, three patients had stage II disease, two patients had stage III disease, and eight patients had stage IV disease
Pathway analysis of cytokine data demonstrates that chemotactic cytokines for MDSC are elevated
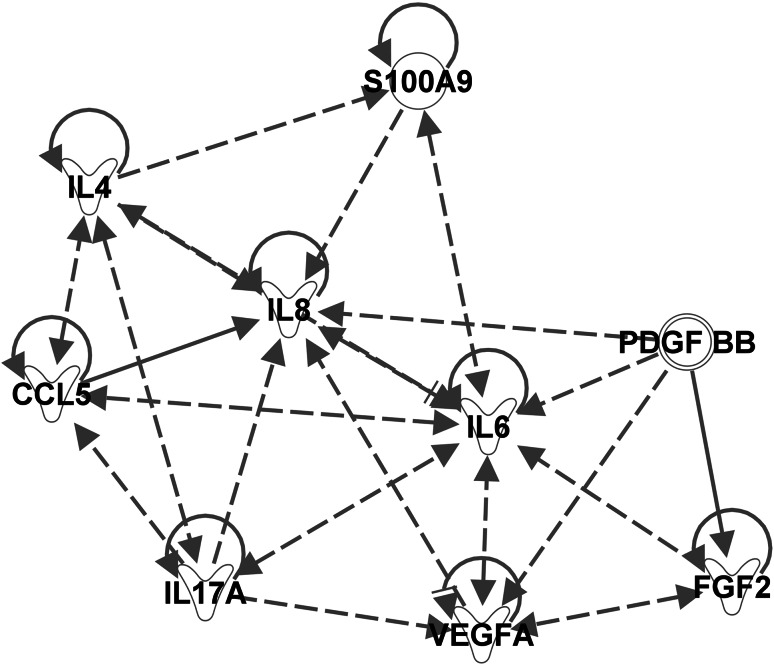
Ingenuity pathway analysis (IPA) is a software application that uses data from a variety of experimental platforms to identify potential relationships in and between biological pathways. IPA was used to evaluate the pathway relationships between the cytokines that were found to be elevated in chemonaive pancreatic cancer patients as compared to normal controls. IPA categorizes relationships between molecules as either direct or indirect, based on the absence or presence, respectively, of an intermediary molecule. We searched IPA for biological functions involving the circulating factors found in pancreatic cancer patients. The top four biological functions that were identified by IPA analysis as being induced by the circulating factors included the following: cell-to-cell signaling and interaction, cellular movement, hematological system development and function, and immune cell function. Within these functions, the myeloid cell chemotaxis pathway was found to be up-regulated. The myeloid cell chemotaxis pathway provided by IPA was visualized using a pathway map (myeloid cell chemotaxis pathway map figure embedded in IPA). Molecules in this pathway map were kept in the model if they were found by an IPA query to be involved in human cancers. The five molecules remaining after removing all “non-cancer” elements were as follows: secretory leukocyte peptidase inhibitor, thrombospondin 4, alpha 1 defensin, S100A8 and S100A9. S100A9 had the highest number of citations in the PubMed database pertaining to MDSC biology. To create a better visualization of potential relationships between all the cytokines elevated in chemonaive patients, a second pathway map was constructed (Fig. 2). This map illustrates the direct and indirect relationships between the important cytokines identified via the BioPlex® analysis. S100A9 was added to the chemonaive elevated cytokine pathway map due to its predicted role in the myeloid cell chemotaxis pathway. This relationship was confirmed using a dedicated ELISA (Supplementary Figure 1) which revealed elevated levels of this protein in the plasma of patients with pancreatic cancer. IL-6 was the only cytokine elevated when comparing the BioPlex® analyses of chemonaive and chemotherapy-treated pancreatic cancer patients. This finding indicates that IL-6 has a unique regulatory role in both chemonaive and chemotherapeutic pancreatic cancer patients, thus justifying its addition to the chemonaive elevated cytokine pathway (Fig. 2). The main conclusion of this exercise is that all of the cytokines that were found to be up-regulated in the peripheral blood of patients were, in fact, also found via IPA to be interconnected by biochemical pathways important for MDSC regulation. Furthermore, IPA predicts that the levels of S100A9 should be up-regulated in patients with increasing stage of pancreas cancer. Therefore, levels of S100A9 were measured on newly diagnosed chemonaive patients, and increased levels of S100A9 were associated with more advanced disease (Supplementary Figure 1). These data raise the possibility that levels of MDSC increase with progression of pancreas adenocarcinoma.
Fig. 2.
Pathway analysis of cytokines determined to be up-regulated in plasma collected from pancreatic adenocarcinoma patients. The pathway map was generated by adding the cytokines increased in the plasma of untreated pancreatic adenocarcinoma and those cytokines that were found to be increased in chemotherapy-treated pancreatic adenocarcinoma versus chemonaive into a new pathway map in IPA and have IPA determine the relationships. S100A9 was then added to the map given the importance of S100A9 in the myeloid chemotaxis pathway, and IPA was utilized to predict the relationships between the cytokines and S100A9. IL-6 is a central regulator of MDSC biology and pathway analysis using Ingenuity Pathway Analysis software confirmed this finding. In addition, the protein S100A9, although not part of the BioPlex® analysis, appears to be important in signaling in pancreatic adenocarcinoma patients. Dashed line arrows signify indirect relationships. Solid lines with arrows represent direct relationships. A solid line parallel to the arrow signifies inhibition. Proteins that regulate themselves have a solid line pointed to themselves (e.g., S100A9, IL-4, CCL5 (RANTES), IL-6). The proteins have different shapes based on their categorization in IPA. Cytokines have a Y shape. S100A9 and PDGF-BB are not placed in any one particular group which is why they have circular shapes
Retrospective review of phenotyping data demonstrates that levels of MDSC increased in pancreatic adenocarcinoma patients with progressive disease
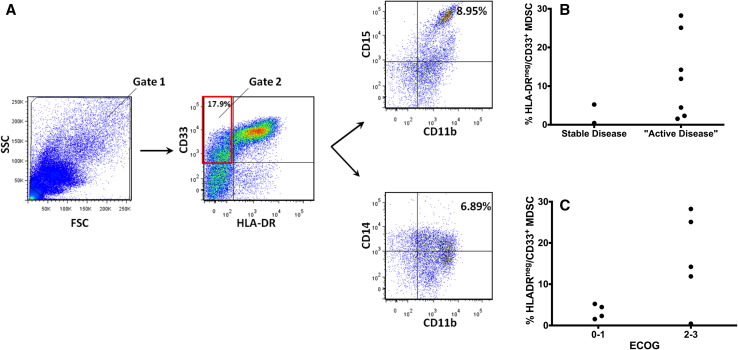
It was previously reported by our group that levels of MDSCs in the peripheral blood are increased in patients with pancreatic cancer [14]. The records of these nine pancreatic cancer patients were reviewed for both Eastern Cooperative Oncology Group (ECOG) performance status and clinical course (progression of disease) at the time of analysis. Of these patients, one patient had localized stage II disease, one patient had locally advanced stage III disease, and seven patients were diagnosed with metastatic stage IV disease. The MDSC populations were analyzed in two ways. The five antibody panel (HLA-DR, CD33, CD11b, CD14 and CD15) was found to be useful for determining the abundance of granulocytic and monocytic MDSC. Two markers HLA-DR and CD33 seemed sufficient to estimate the relative numbers of MDSC in the clinical setting (Fig. 3). From this retrospective analysis, it was determined that HLA-DR and CD33 status may provide a useful measure of the number of MDSC in the patient’s peripheral blood. Levels of MDSCs in this retrospective analysis tended to be greater in patients who had active disease (progressive disease and new diagnosis; Fig. 3b). When analyzing this subset, it was noted that there was approximately a threefold increase in the numbers of MDSC on average when patients were designated as having exhibited progressive disease while receiving chemotherapy at the time of immune cell analysis. An association between ECOG performance status and levels of HLA-DRnegCD33+ MDSC was also observed. Patients with poor performance status (PS > 2) typically had greater than 5 % MDSC in the peripheral blood (Fig. 3c), whereas those with performance 0 or 1 had less than 5 % MDSC. Given the increased numbers of MDSC in the peripheral blood in this retrospective analysis, additional MDSC analyses were conducted in a prospective fashion.
Fig. 3.
a Representative scatter plots for a pancreatic adenocarcinoma patient. In the left most panel, the scatter plot (side scatter vs. forward scatter) is presented. The center plot shows the staining for CD33 and HLA-DR. The scatter plot was then created for CD15 versus CD11b to determine the percentage of granulocytic MDSC. CD14 versus CD11b was plotted for monocytic MDSC. b HLA-DRneg/CD33+ percentages in PBMCs measured as a function of whether they had stable (n = 2), or “active disease” (n = 7) in a retrospective study of nine patients with pancreatic adenocarcinoma. Active disease was defined by progressive disease on chemotherapy or a new diagnosis of pancreatic adenocarcinoma. One patient had stage II disease, one patient had stage III disease, and seven patients had stage IV disease. This analysis led to the hypothesis that the MDSC percentage increases with progressive disease that was tested with a prospectively collected cohort of patients. c HLA-DRneg/CD33+ percentages in PBMCs obtained from peripheral blood measured as a function of ECOG performance status. An ECOG score of 0–1 indicates good performance status (n = 4) and a score of 2–3 indicates poor performance status (n = 5)
Levels of HLA-DRnegCD33+ MDSC are associated with progressive disease
PBMCs were obtained from the peripheral blood of 16 additional pancreatic cancer patients. PBMCs were obtained from eight chemotherapy-treated patients that previously had undergone cytokine analysis. Nine additional patients undergoing chemotherapy were phenotyped to increase the size of the cohort (Table 3). Of these 16 patients, one patient had stage 1 disease, two patients had stage II disease, four patients had stage III disease and nine patients had stage IV disease. Of these patients, four were receiving the recently approved FOLFOXIRI regimen [2] and three patients were on a clinical trial. One patient received carboplatin, paclitaxel and reolysin (clinicaltrials.gov NCT01280058), and one patient received gemcitabine with Algenpantucel-L (NCT01072981) in the context of a clinical trial. In all, nine of the sixteen patients received gemcitabine-based therapy including the two patients that were on clinical trial. One patient received treatment with the FOLFIRI regimen. The patients were grouped by whether they were determined to have stable disease or progressive disease. Patients being followed at The Ohio State University Comprehensive Cancer Center are staged every 2 months with CT scans to determine whether they are stable on their current course of chemotherapy. For this cohort, there was no requirement for the patients to be on a particular regimen of chemotherapy. The blood sample was obtained before the administration of chemotherapy. Typically, this was 2–3 weeks after the last chemotherapy administration. The determination of stable/progressive disease was made at the clinic visit or by the weekly tumor board prior to the analysis of the phenotyping data using RECIST criteria. It was found that patients with stable disease had significantly lower MDSC levels than those patients with progressive disease (1.41 ± 1.12 vs. 5.14 ± 4.58 %), p = 0.013 based on the Wilcoxon test, Fig. 4a). Overall, progressive disease patients had a 3.6-fold increase in MDSC as compared to stable disease patients. In this 16 patient cohort, 10 subjects had MDSC ≥ 2.5 % and 6 had MDSC < 2.5 %. Among the 10 patients with a HLA-DRnegCD33+ population greater than 2.5 % (based on a natural break determined upon visual inspection of the numbers of MDSCs in patients with progressive disease vs. stable disease), nine (90 %) were declared to have progressive disease at the time of clinical evaluation, while only 2 of 6 (33 %) patients with a HLA-DRneg/CD33+ population <2.5 % had progressive disease. (Fig. 4a, p = 0.017).
Table 3.
The MDSC levels in 16 patients with pancreatic cancer undergoing chemotherapy were measured
| Patient | HLADRneg/CD33+ | HLADRneg/CD33+/CD15+/CD11b+ | HLADRneg/CD33+/CD14+/CD11b+ | HLADRneg/CD33+/CD14neg/CD15neg | Stage | ECOG | CA-19-9 |
|---|---|---|---|---|---|---|---|
| 1 | 1.51 | 1.37 | 0.60 | 0.17 | 3 | 1 | 909.53 |
| 2 | 0.97 | 0.65 | 0.45 | 0.29 | 1 | 0 | <15 |
| 3 | 0.60 | 0.28 | 0.23 | 0.27 | 2 | 0 | 19.7 |
| 4 | 0.59 | 0.47 | 0.43 | 0.15 | 4 | 1 | 11,658 |
| 5 | 3.36 | 2.35 | 0.40 | 1.11 | 4 | 0 | 3,072.82 |
| 6 | 4.31 | 3.57 | 1.65 | 0.80 | 4 | 2 | <15 |
| 7 | 3.00 | 0.90 | 1.26 | 1.56 | 4 | 1 | 220.74 |
| 8 | 17.9 | 8.95 | 6.87 | 8.32 | 4 | 3 | 6,300 |
| 9 | 3.30 | 2.18 | 1.15 | 1.19 | 3 | 1 | 653.57 |
| 10 | 1.18 | 0.57 | 0.36 | 0.71 | 4 | 1 | 47,901 |
| 11 | 4.35 | 3.14 | 0.02 | 1.82 | 4 | 1 | 59,595 |
| 12 | 3.67 | 2.42 | 1.53 | 1.16 | 4 | 2 | 314.38 |
| 13 | 8.05 | 7.27 | 5.92 | 0.64 | 3 | 1 | 3,064 |
| 14 | 3.49 | 2.97 | 0.25 | 0.49 | 3 | 0 | 64.82 |
| 15 | 2.22 | 0.81 | 0.50 | 1.40 | 2 | 2 | 11,976.55 |
| 16 | 5.07 | 4.44 | 0.16 | 0.75 | 4 | 2 | 9,072 |
Of these 16 patients, one patient had stage I disease, two patients had stage II disease, four patients had stage III disease, and nine patients had stage IV disease
Fig. 4.
a PBMCs were measured using two markers for cell populations negative for HLA-DR and positive for CD33. Patients were categorized according to whether they had stable (n = 5) or progressive disease (n = 11). A HLA-DRnegCD33+ percentage greater than 2.5 % is suggestive of progressive disease. Of these 16 patients, 1 patient had stage I disease, 2 patients had stage II disease, four patients had stage III disease, and 9 patients had stage IV disease. b Measurement of HLA-DRnegCD33+CD14negCD15neg in the same patient populations suggests a percentage greater than 0.5 % lineage-negative MDSC is suggestive of progressive disease. c Percentages of HLA-DRnegCD33+ cells were measured as a function of ECOG performance status. Worse performance status is associated with increasing numbers of MDSC. Patients with ECOG ≥ 2 (n = 5) tended to have higher levels of MDSC
Interestingly, there was one patient with a HLA-DRneg CD33+ population of 3.49 % in the stable disease group who was determined to be clinically stable at the time of the office visit by current clinical guidelines but in retrospect had evidence of peritoneal seeding on CT scan. Therefore, the test of increasing levels of MDSC would have placed this patient in the progressive group and treatment could have been changed. The patient subsequently went on to have obvious progressive disease at the next CT scan a few weeks later. One of these two progressive disease patients with HLA-DRneg/CD33+ < 2.5 % had recently undergone biliary drain placement for hyperbilirubinemia secondary to a bile duct stricture which could have led to a decrease in the numbers of MDSC due to decreased local inflammation. The second patient had a small local recurrence after a pancreas resection.
Next, it was considered whether an evaluation of the granulocytic versus monocytic populations of MDSC would help in the clinical decision-making process. The numbers of granulocytic CD15+ MDSCs were found to be statistically significantly greater than monocytic CD14+ MDSCs in these patients (p = 0.031 based on the Wilcoxon signed-rank Test; Table 3). Patients with stable disease had lower numbers of HLA-DRnegCD33+CD15+ MDSC (1.02 ± 0.85 %) than those patients with progressive disease (3.38 ± 2.66 %; p = 0.012). Patients with stable disease had lower numbers of HLA-DRnegCD33+CD15negCD14neg lineage-negative MDSC (n = 5, 0.40 ± 0.40 %) than those patients with progressive disease (n = 11, 1.71 ± 2.23 %; p = 0.013; Fig. 4b). The natural break of the data of 0.5 % HLA-DRnegCD33+CD15negCD14neg for determination of low versus high levels of MDSC was determined upon visual inspection and was found to be associated with the progression status (p = 0.004). This assay was robust as was the measurement of the HLA-DRnegCD33+ population that does not include the markers for CD15 and CD14 in the assay. Further studies will need to be done to determine whether it is necessary and cost effective to include the CD14 and CD15 antigens in a larger patient cohort or whether the two antigens HLA-DR and CD33 are enough to predict which patients are failing chemotherapy.
Trends in ECOG performance status with increasing levels of MDSC in 16 patients with pancreatic cancer
Poor ECOG performance status was found to be associated with increased levels of MDSC in the peripheral blood. The patients with ECOG ≥ 2 (n = 5, 6.63 ± 6.38 %) had marginally higher levels of HLA-DRnegCD33+ MDSC than those with ECOG ≤ 1 (n = 11, 2.76 ± 2.2 % p = 0.07, Fig. 4c). Notably, when ECOG status was measured as a function of HLA-DRnegCD33+CD11b+CD15+ MDSC, the analysis was less robust and did not reach statistical significance (p = 0.09).
Trends in MDSC levels with various clinical markers
MDSC levels were associated with disease progression as determined by CT scans of the abdomen and/or clinical findings (e.g., ascites as an indicator of carcinomatosis). In these patients, there was a trend of increasing levels of CA-19-9 with increasing levels of MDSC (Spearman nonparametric correlation coefficient, r = 0.278, p = 0.30); however, this trend did not reach significance. Notably, not all pancreatic cancers secrete CA-19-9, and levels of CA-19-9 can vary widely in pancreatic cancer patients who have the same stage of disease [18]. Lineage-negative MDSC in patients without liver metastasis (n = 7) was 0.49 % versus 1.11 % (n = 9) in patients with liver metastasis (p = 0.14). However, patients with liver metastases who were stable on their chemotherapy have less than 0.5 % lineage-negative MDSC.
Discussion
In a study published by our group, the MDSC phenotype in gastrointestinal malignancies was correlated with a functional analysis (decreased interferon stimulation of T cells) and it was found that measurement of CD11b, CD15 and CD14 in HLA-DRnegCD33+ cells provided an accurate measure of MDSC populations [14]. Given this observation, levels of cytokines responsible for MDSC induction were measured and were found to be elevated in pancreatic adenocarcinoma patients as compared to normal controls. Bioinformatic analysis of the cytokine data revealed that levels of S100A9 should be elevated with increasing disease bulk in chemonaive patients and this was confirmed via ELISA. In this study, a retrospective analysis of the nine patients previously evaluated only for MDSC phenotype demonstrated that ECOG performance status tended to correlate with MDSC levels [14]. In addition, those patients with progressive disease tended to have higher levels of MDSC. From these experiments, it was hypothesized that patients with increased numbers of HLA-DRneg CD33+ cells would have progressive disease. These data are consistent with the study by Solito et al. [19] demonstrating that the promyelocytic population of MDSC is important for suppressive activity in breast and colorectal cancer. A cohort of patients was collected prospectively, and it was found that MDSC levels are a potential biomarker to predict disease progression in patients with pancreatic adenocarcinoma. Measurement of the granulocytic subset of MDSC does not help in predicting who will progress while on chemotherapy.
Factors indicating a poor prognosis in pancreatic cancer include metastatic disease at time of diagnosis, advanced age and evidence of immune suppression [15]. In one review of prognostic markers in surgical patients, the following were associated with a worse overall survival: abdominal and back discomfort, abdominal mass palpable at clinical visit, hemoglobin <12 g/dL and serum albumin <2.8 g/dL, perineural and lymph node infiltration, advanced stage, and R1 or R2 resection (the presence of microscopic (R1) or macroscopic (visible R2) residual tumor) [20]. In general, multiple prognostic factors can be utilized to estimate prognosis. In the current study, levels of MDSCs were shown to have some predictive value in determining whether a patient was going to progress while on chemotherapy.
Tumor-derived cytokines are important in recruiting and maintaining MDSC populations. In this study, patients with adenocarcinoma of the pancreas treated with chemotherapy had elevated levels of the immunosuppressive cytokine IL-6. Levels of S100A9 were also found to be increased with increasing stage. S100A9 is secreted by myeloid cells and also acts as a chemotaxis agent for MDSCs. S100A9 has direct chemotactic properties for MDSC and S100A9 also binds directly to the Receptor for Advanced Glycation Endproducts (RAGE) on MDSC or endothelium [21–25]. RAGE signaling is important for IL-6 secretion in myeloid cells (e.g., MDSC) and functions by activating the signal transducer and activator of transcription 3/suppressor of cytokine signaling 3 (STAT3/SOC3) in pancreatic cancer precursor cells. The activation of the STAT3/SOC3 pathway leads to the progression of pancreatic intraepithelial neoplasia (PanIN) [26]. IL-6 production also directly stimulates phosphorylation of STAT3 in pancreatic stellate cells thus contributing to an immunosuppressive tumor microenvironment [27]. Pancreatic stellate cells themselves produce IL-6, thus further promoting MDSC expansion [27]. It has also been shown that knockout of RAGE signaling leads to reduced pancreatic cancer tumor growth in mice even in the presence of an oncogenic KRAS mutation [28, 29]. Therefore, it is very interesting that of all the cytokines measured in this assay, IL-6 is the only one that is elevated with statistical significance between those patients who were treated with chemotherapy versus those who were not treated with chemotherapy. It suggests that elevation of IL-6 is associated with greater MDSC-mediated immune suppression and subsequent worse outcomes in those patients failing chemotherapy.
Inhibiting MDSCs in patients with pancreatic adenocarcinoma may reverse immune suppression [30]. It has recently been shown that soluble factors contribute to the up-regulation of murine MDSCs in the murine mode and immune dysfunction of T cells. Similar to our study, G-CSF, IL-6 and VEGF were elevated in a murine model of pancreatic cancer [31]. New immunotherapeutic approaches are being explored to integrate the suppression of MDSCs with immune-modulatory agents in combination with traditional chemotherapeutics [32]. Various mechanisms to deplete MDSC are available to clinicians. For example, gemcitabine and capecitabine administration resulted in 42 % of the patients having decreased levels of MDSC, whereas 86 % of the patients treated with gemcitabine and capecitabine in combination with the GV1001 (Telomerase Peptide Vaccine) had a decrease in the levels of circulating MDSC in the peripheral blood [33]. In another example, murine experiments demonstrated that the combination of gemcitabine and rosiglitazone successfully reduced the numbers of MDSC and abrogated MDSC inhibitory effects on T cells [34]. Therefore, traditional chemotherapeutic regimens may potentially be used to target MDSCs and increase the effective of immunologic therapies in patients with pancreas cancer.
In this study, cytokines with proangiogenic activity such as IL-8 and VEGF were elevated in the plasma of a pancreatic adenocarcinoma patient population compared to normal controls. Angiogenic cytokines have been associated with increased expansion of MDSC populations [13]. The mechanism likely includes both direct expansion from progenitors in response to stimulation of the VEGF receptor and the chemotactic properties of these cytokines at the level of the tumor microenvironment [13, 35]. VEGF is elevated in pancreatic cancer, and multikinase inhibitors are currently being explored as potential therapeutics [36, 37]. Although angiogenesis is a known target in pancreatic adenocarcinoma, it has not demonstrated significant clinical efficacy as of the publication by Assifi et al. [38]. It is possible that this may be secondary to the dense stroma in the pancreatic cancers. If the tumor stroma is digested in murine models, the survival time is doubled [39].
Pro-MDSC cytokines are elevated in the plasma collected from pancreas cancer patients. Therefore, MDSC populations were phenotyped prospectively in a series of 17 patients undergoing chemotherapy, and it was found that MDSC levels were associated with progressive disease (Fig. 4). The literature in animal models supports the conclusion that decreasing levels of MDSC are associated with less tumor bulk. Generally, in murine models, administration of chemotherapy results in decreasing tumor size, decreased release of pro-MDSC cytokines and ultimately decreased numbers of MDSC [13]. Gemcitabine administration leads to decreases in the numbers of MDSC in the blood obtained from pancreatic cancer patients in the setting of decreasing tumor volume, but MDSC numbers do not decrease in those patients who do not experience a clinical response as measured by RECIST criteria [33]. This lack of a decrease could either be due to a direct effect of the gemcitabine over time (e.g., via stimulation of the inflammasome [40]). Of note, in one patient with ~18 % MDSC, the total peripheral monocyte population was increased to 11 %. The promyelocytic population of MDSC (HLA-DRnegCD14neg) is possibly a precursor to the population of inflammatory monocytes CD14+CCR2+CD16negCX3CR1low) described to be up-regulated in pancreatic adenocarcinoma that are associated with a worse overall survival [41]. In this study, increasing numbers of MDSCs are associated with progressive disease. Measurement of levels of MDSCs in the peripheral blood could be a very sensitive and inexpensive way to evaluate the clinical course. In the cases where it is sometimes difficult to assess disease burden (liver, peritoneal carcinomatosis), measurement of MDSC in peripheral blood may be useful as a predictive test to determine whether a patient has progressing disease on chemotherapy.
In conclusion, this study suggests that MDSC may be a predictive biomarker to assess whether a patient with pancreas cancer is responding to chemotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to acknowledge National Institute of Health grant T32CA090223 (William Edgar Carson, III) and the Pelotonia Postdoctoral Fellowship (to J. Markowitz). Taylor Brooks was supported by the Pelotonia undergraduate fellowship program. Any opinions, findings and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the Pelotonia Fellowship Program. We would also like to acknowledge the National Cancer Institute grant P01CA095426.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- CCL5
Chemokine (C-C motif) ligand 5 [synonym: RANTES]
- CD
Cluster of differentiation
- CT
Computerized tomography
- FGF2
Basic fibroblast growth factor
- FOLFIRINOX
Chemotherapy consisting of 5-FU, leucovorin, irinotecan and oxaliplatin
- G-CSF
Granulocyte colony-stimulating factor
- HLA
Human leukocyte antigen
- IPA
Ingenuity pathway analysis
- MCP-1
Monocyte chemotactic protein 1
- MDSC
Myeloid-derived suppressor cell
- MIP-1α
Macrophage inflammatory protein-1alpha
- MPACT
Metastatic pancreatic adenocarcinoma clinical trial
- PBMC
Peripheral blood mononuclear cell
- PDGF-bb
Platelet-derived growth factor–beta
- RAGE
Receptor for advanced glycation endproducts
- RANTES
Regulated on activation, normal T cell expressed and secreted [synonym: CCL5]
- STAT3/SOC3
Signal transducer and activator of transcription 3/suppressor of cytokine signaling 3
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Kim R. FOLFIRINOX: a new standard treatment for advanced pancreatic cancer? Lancet Oncol. 2011;12(1):8–9. doi: 10.1016/S1470-2045(10)70237-0. [DOI] [PubMed] [Google Scholar]
- 4.Ying JE, Zhu LM, Liu BX. Developments in metastatic pancreatic cancer: Is gemcitabine still the standard? World J Gastroenterol. 2012;18(8):736–745. doi: 10.3748/wjg.v18.i8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kothari N, Saif MW, Kim R. First-line treatment for advanced pancreatic cancer. JOP. 2013;14(2):129–132. doi: 10.6092/1590-8577/1477. [DOI] [PubMed] [Google Scholar]
- 6.Patel J. Drug combination extends late-stage pancreatic cancer survival in phase III clinical trial. Expert Rev Clin Pharmacol. 2013;6(2):100. [PubMed] [Google Scholar]
- 7.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarboe J, Gupta A, Saif W. Therapeutic human monoclonal antibodies against cancer. Methods Mol Biol. 2014;1060:61–77. doi: 10.1007/978-1-62703-586-6_4. [DOI] [PubMed] [Google Scholar]
- 10.Drake CG. Combination immunotherapy approaches. Ann Oncol. 2012;23(Suppl 8):viii41–viii46. doi: 10.1093/annonc/mds262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67(19):9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 12.Evans A, Costello E. The role of inflammatory cells in fostering pancreatic cancer cell growth and invasion. Front Physiol. 2012;3:270. doi: 10.3389/fphys.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mundy-Bosse BL, Young GS, Bauer T, Binkley E, Bloomston M, Bill MA, Bekaii-Saab T, Carson WE, 3rd, Lesinski GB. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4(+) T cells from patients with GI malignancy. Cancer Immunol Immunother. 2011;60(9):1269–1279. doi: 10.1007/s00262-011-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60(10):1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao F, Obermann S, von Wasielewski R, Haile L, Manns MP, Korangy F, Greten TF. Increase in frequency of myeloid-derived suppressor cells in mice with spontaneous pancreatic carcinoma. Immunology. 2009;128(1):141–149. doi: 10.1111/j.1365-2567.2009.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, Gillanders WE, Linehan DC, Goedegebuure P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61(9):1373–1385. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R, Nicolini A, Topolcan O, Heinemann V. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21(3):441–447. doi: 10.1093/annonc/mdp332. [DOI] [PubMed] [Google Scholar]
- 19.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, Garrett-Mayer E, Montero AJ, Bronte V, Mandruzzato S. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118(8):2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Tovar J, Martin-Perez E, Fernandez-Contreras ME, Reguero-Callejas ME, Gamallo-Amat C. Identification of prognostic factors in pancreatic cancer. Cir Cir. 2011;79(4):313–322. [PubMed] [Google Scholar]
- 21.Markowitz J, Carson WE. 3rd (2013) Review of S100A9 biology and its role in cancer. Biochim Biophys Acta. 1835;1:100–109. doi: 10.1016/j.bbcan.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205(10):2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turovskaya O, Foell D, Sinha P, Vogl T, Newlin R, Nayak J, Nguyen M, Olsson A, Nawroth PP, Bierhaus A, Varki N, Kronenberg M, Freeze HH, Srikrishna G. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29(10):2035–2043. doi: 10.1093/carcin/bgn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, Hess J, Angel P. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205(2):275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjork P, Bjork A, Vogl T, Stenstrom M, Liberg D, Olsson A, Roth J, Ivars F, Leanderson T. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7(4):e97. doi: 10.1371/journal.pbio.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, Algul H. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, Fuchs JR, Eubank TD, Frankel WL, Bekaii-Saab T, Bloomston M, Lesinski GB. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73(10):3007–3018. doi: 10.1158/0008-5472.CAN-12-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vernon PJ, Loux TJ, Schapiro NE, Kang R, Muthuswamy R, Kalinski P, Tang D, Lotze MT, Zeh HJ., 3rd The receptor for advanced glycation end products promotes pancreatic carcinogenesis and accumulation of myeloid-derived suppressor cells. J Immunol. 2013;190(3):1372–1379. doi: 10.4049/jimmunol.1201151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vernon PJ, Zeh Iii HJ, Lotze MT. The myeloid response to pancreatic carcinogenesis is regulated by the receptor for advanced glycation end-products. Oncoimmunology. 2013;2(5):e24184. doi: 10.4161/onci.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goedegebuure P, Mitchem JB, Porembka MR, Tan MC, Belt BA, Wang-Gillam A, Gillanders WE, Hawkins WG, Linehan DC. Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr Cancer Drug Targets. 2011;11(6):734–751. doi: 10.2174/156800911796191024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21(6):822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghansah T, Vohra N, Kinney K, Weber A, Kodumudi K, Springett G, Sarnaik AA, Pilon-Thomas S. Dendritic cell immunotherapy combined with gemcitabine chemotherapy enhances survival in a murine model of pancreatic carcinoma. Cancer Immunol Immunother. 2013;62(6):1083–1091. doi: 10.1007/s00262-013-1407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annels NE, Shaw VE, Gabitass RF, Billingham L, Corrie P, Eatock M, Valle J, Smith D, Wadsley J, Cunningham D, Pandha H, Neoptolemos JP, Middleton G. The effects of gemcitabine and capecitabine combination chemotherapy and of low-dose adjuvant GM-CSF on the levels of myeloid-derived suppressor cells in patients with advanced pancreatic cancer. Cancer Immunol Immunother. 2014;63(2):175–183. doi: 10.1007/s00262-013-1502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bunt SK, Mohr AM, Bailey JM, Grandgenett PM, Hollingsworth MA. Rosiglitazone and Gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother. 2013;62(2):225–236. doi: 10.1007/s00262-012-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan RA, Restifo NP, Rosenberg SA. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res. 2012;18(6):1672–1683. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C, Huang R, Chang W, Jiang T, Huang K, Cao J, Sun X, Qiu Z. The expression and clinical significance of pSTAT3, VEGF and VEGF-C in pancreatic adenocarcinoma. Neoplasma. 2012;59(1):52–61. doi: 10.4149/neo_2012_007. [DOI] [PubMed] [Google Scholar]
- 37.Taeger J, Moser C, Hellerbrand C, Mycielska ME, Glockzin G, Schlitt HJ, Geissler EK, Stoeltzing O, Lang SA. Targeting FGFR/PDGFR/VEGFR impairs tumor growth, angiogenesis, and metastasis by effects on tumor cells, endothelial cells, and pericytes in pancreatic cancer. Mol Cancer Ther. 2011;10(11):2157–2167. doi: 10.1158/1535-7163.MCT-11-0312. [DOI] [PubMed] [Google Scholar]
- 38.Assifi MM, Hines OJ. Anti-angiogenic agents in pancreatic cancer: a review. Anticancer Agents Med Chem. 2011;11(5):464–469. doi: 10.2174/187152011795677463. [DOI] [PubMed] [Google Scholar]
- 39.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, Kanellopoulos J, Martin F, Rebe C, Apetoh L, Ghiringhelli F. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19(1):57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 41.Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, Wang-Gillam A, Eberlein TJ, Denardo DG, Goedegebuure SP, Linehan DC. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19(13):3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.