Abstract
We sought to examine the influence of social and clinical factors on risk of progression of chronic kidney disease (CKD) to end-stage renal disease (ESRD) in the urban poor. We studied 15,353 individuals with moderate-to-advanced CKD who received ambulatory care within a large public health system during 1996–2005. The primary outcome was progression to ESRD. Overall, 559 cases of ESRD occurred over a median follow-up of 2.8 years. Among traditional predictors of ESRD, younger age, male sex, non-White race/ ethnicity, public health insurance coverage, diabetes, lower kidney function, higher proteinuria, lower hemoglobin level, and lower serum albumin concentration were significantly associated with a higher adjusted ESRD risk (p < .001 for all). There was no significant association between HIV/AIDS (p=.07), viral hepatitis (p=.11), or non-English language (p=.27) and ESRD risk. Our results highlight the importance of addressing traditional risk factors for progressive CKD to reduce the disproportionate burden of ESRD among disadvantaged populations.
Keywords: End-stage renal disease, chronic kidney disease, urban poor, race or ethnicity, public health care, disparities
In the U.S., end- stage renal disease (ESRD) disproportionately affects the poor and members of racial/ethnic minority groups.1–4 Recent data from the U.S. Renal Data System indicate that nearly one-third of people initiating treatment for ESRD are now uninsured or covered by Medicaid, the U.S. health insurance program for people of severely limited financial means.5
Due to their relatively limited options for ongoing ambulatory care, many of America’s poor and underinsured seek medical care from urban public hospitals and safety-net health clinics. Collectively, these facilities provide health care to millions of uninsured and underserved individuals across the nation.6,7 Because chronic kidney disease (CKD) has historically been poorly coded in administrative data and because there is no system for tracking the care of patients who are uninsured or covered by Medicaid within the U.S.,8,9 few studies have examined predictors of progression of CKD to ESRD in these underserved populations.10 Prior studies in insured or universally screened populations have identified non-White race, hypertension, diabetes, proteinuria, and index kidney function as the most important predictors of incident ESRD.1,2,4,11,12 However, the influence of social or societally- driven clinical factors (i.e., factors that influence health by affecting exposure and vulnerability to disease, and access to health coverage or health care) such as poverty, substance abuse, and limited English proficiency on incident ESRD is unknown. Although kidney disease is a well- recognized consequence of infection with human immunodeficiency virus (HIV),13,14 little is known about the role of chronic viral diseases on subsequent progression from established CKD to ESRD.
To understand better whether these social and clinical factors predict progression of established CKD to ESRD in the urban poor, we examined longitudinal data from a diverse cohort of adults with non-dialysis dependent CKD who received ambulatory care in the Community Health Network, a large safety- net health care provider operated by the City and County of San Francisco, California. We hypothesized that in this population, chronic viral diseases, substance abuse, and non-English language would be positively associated with an increased risk of ESRD independent of age, sex, race/ ethnicity, kidney function, proteinuria, and other traditional ESRD risk factors.
Methods
Study design and setting
We conducted a retrospective cohort study of subjects with non-dialysis dependent CKD stages 3– 5 who received ambulatory care in the Community Health Network (CHN) from January 1, 1996 to December 31, 2005. The CHN is the health care delivery system of the Department of Public Health of the City and County of San Francisco. Along with a consortium of not- for- profit primary care clinics (Consortium), the CHN forms the backbone of San Francisco’s health care safety- net system and offers an array of health care services including primary care, specialty care and acute care. The CHN includes an acute care hospital (San Francisco General Hospital) with on- site primary and specialty care clinics, as well as 11 community based primary care clinics. Providers in these clinics, as well as those practicing in the Consortium clinics rely on San Francisco General Hospital for a significant portion of their laboratory testing, specialty referrals, and inpatient care. All of the CHN and Consortium clinics have access to the San Francisco Department of Public Health’s electronic health information system to assist in shared patient care. The CHN provides ambulatory and acute care to the majority of the estimated 130,000 uninsured residents of San Francisco. Services are available for free, or on a sliding scale based on income.15
Study subjects
The study cohort comprised 15,353 adults aged 20 years or older with non-dialysis dependent CKD stages 3– 5 who received routine ambulatory care in the CHN from January 1, 1996 to December 31, 2005. We defined CKD based on at least two outpatient estimated glomerular filtration rate (eGFR) measurements < 60 mL/ min/ 1.73 m2 as calculated by the re-expressed Modification of Diet in Renal Disease (MDRD) study equation based on calibrated serum creatinine, age, race and sex that were separated by at least one year.16,17 We considered participants to be receiving regular ambulatory care if they had at least one additional CHN outpatient encounter subsequent to the index serum creatinine date. We imposed these restrictions to ensure that the study cohort included people who meet the National Kidney Foundation definition for CKD stages 3–5 and who had access to ambulatory care, rather than individuals with misclassified acute kidney injury or those who transiently visited the CHN.
Outcome measures
The primary outcome measure was progression to ESRD, defined as having a first service date for maintenance dialysis or kidney transplantation. To ascertain ESRD, we performed linkage blinded to exposure status with the United States Renal Data System (USRDS) files based on patient last name, first name, date of birth, and Social Security number. We considered patients matched on at least three of the four identifiers as having ESRD. To address the possibility of informative censoring due to death, we performed identifier matching with the California Department of Health Services Death Registry, blinded to exposure status, using Social Security number, first and last name, sex, and date of birth. We assessed both ESRD and death through 31 December 2005, the last date that data were available for both outcomes at the time of identifier linkage. We defined survival time as the time from the second outpatient eGFR date until ESRD, death or the end of follow-up through December 31, 2005, whichever occurred first.
Independent variables
We extracted data on important sociodemographic and clinical factors that we hypothesized might predict progression of established CKD to ESRD in the urban health care safety net based on prior studies.1,2,11–14 Covariates were defined within the two- year period preceding and closest to the index qualifying eGFR measurement. Individual-level sociodemographic covariates included patient age, sex, race/ ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Asian/Pacific Islander, or other), health insurance coverage (uninsured, Medicaid, Medicare, or commercial/ other), primary spoken language (English, Spanish, Cantonese, or other), housing status (domiciled or homeless), and annual household income based on administrative data. We ascertained comorbid conditions based on established algorithms using discharge diagnostic codes, ambulatory diagnostic codes and procedural codes (see the Appendix) for diabetes, hypertension, cardiovascular disease (defined as coronary artery, cerebrovascular, or peripheral vascular disease), hepatitis B virus (HBV), hepatitis C virus (HCV), HIV or AIDS, alcoholism, and drug abuse. Laboratory predictors included eGFR, hemoglobin and serum albumin concentrations, and the presence and severity of proteinuria. We classified proteinuria as normal (urine albumin- to-creatinine ratio [ACR] < 30 mg/ g or urine dipstick negative), mild (ACR 30– 300 mg/ g or urine dipstick trace-1+), or heavy (ACR >300 mg/g or urine dipstick ≥2+).12,18
Statistical analysis
We examined the distribution of covariates and outcomes using side-by-side boxplots and histograms where appropriate. To examine the independent associations of these predictors on risk of incident ESRD, we used Cox proportional hazards regression models.19 In the adjusted model, we incorporated fixed demographic factors (age, sex, and race/ ethnicity), social factors (income, health insurance coverage, and primary spoken language), comorbid conditions that were known to associate with the aforementioned social factors (HBV, HCV, HIV or AIDS, and substance abuse), established risk factors for death and progressive CKD (diabetes, hypertension, and cardiovascular disease) and laboratory measures associated with CKD severity (eGFR, proteinuria, hemoglobin, and serum albumin). To test whether the associations of proteinuria, index eGFR and risk of ESRD might differ according to age, race/ ethnicity, sex or diabetes status, we incorporated interaction terms between proteinuria or eGFR and each covariate of interest and tested whether the estimated coefficients for the interaction terms differed from zero using the Wald test. We examined the adequacy of the functional form of each covariate by examining plots of Martingale residuals against each covariate. For all models, we tested for violations of the proportional hazards assumption by examining plots of -log(-log [survival rate]) against log (survival time). To reduce potential bias caused by excluding patients with missing data, we performed multiple imputation using the Markov chain Monte Carlo method with 10 imputations for these variables.20 We considered a two-tailed p-value <.05 as statistically significant without adjustment for multiple comparisons. We used Stata statistical soft ware for all analyses (Stata MP version 11.0, Stata Corp, College Station, TX). The United States Renal Data System and the respective Institutional Review Boards at the University of Washington and University of California San Francisco approved the study protocol.
Results
Patient characteristics
During the study period 15,353 adults with non-dialysis dependent CKD stages 3–5 received ambulatory care within CHN. In contrast with CKD cohorts from other North American studies, approximately one-half of subjects were younger than 60 and over one-fourth were younger than 50. The distribution of race/ ethnicity (72% non-White) and health insurance coverage (41% uninsured or enrolled in Medicaid) were consistent with estimates of populations generally served by members of the National Association of Public Hospitals.21 The prevalence of alcoholism (8%), drug abuse (16%), and chronic viral diseases including viral hepatitis (5%) and HIV (3%) were also remarkable as compared with the near absence of these comorbidities in prior CKD studies conducted in universally insured populations.22,23
As shown in Table 1, patients who eventually developed ESRD were younger and more likely to be male, of Black race, covered by Medicaid and speak English as their primary language as compared with those who did not develop ESRD. Patients who developed ESRD also had a higher prevalence of diabetes and lower prevalence of hypertension, cardiovascular disease and substance abuse than those who did not progress to ESRD (Table 1). In contrast with subjects who did not progress to ESRD, many of whom had moderate CKD (eGFR of 30 to 59 ml/ min/ 1.73 m2) and little to no proteinuria, most subjects who progressed to ESRD had advanced CKD (eGFR <30 ml/min/1.73 m2) and mild to heavy proteinuria at baseline. They also had lower mean levels of hemoglobin and serum albumin as compared with patients who did not progress to ESRD (Table 1).
Table 1.
Baseline Characteristics of 15,353 Community Health Network Patients with Moderate to Advanced CKD who did and did not Develop End-Stage Renal Disease
| Characteristic | No ESRD (n=14,794) | ESRD (n=559) |
|---|---|---|
| Age, mean (SD), years | 59.5 (13.8) | 52.7 (12.9) |
| Age category, N (%), years | ||
| < 40 | 1213 (8) | 103 (18) |
| 40–49 | 2512 (17) | 137 (25) |
| 50–59 | 3563 (24) | 159 (28) |
| 60–69 | 4402 (30) | 106 (19) |
| ≥ 70 | 3104 (21) | 54 (10) |
| Female, N (%) | 7935 (53) | 216 (39) |
| Race/ethnicity, N (%) | ||
| Non-Hispanic White | 4243 (29) | 58 (10) |
| Non-Hispanic Black | 2928 (20) | 263 (47) |
| Hispanic | 2759 (19) | 84 (15) |
| Asian | 4475 (30) | 145 (26) |
| Other race/ethnicity | 389 (3) | 9 (2) |
| Primary spoken language, N (%) | ||
| English | 9863 (67) | 435 (78) |
| Spanish | 1721 (12) | 50 (9) |
| Cantonese | 1723 (12) | 36 (6) |
| Other | 1487 (10) | 38 (7) |
| Annual income ≤$10 000 USD, N (%) | 7968 (54) | 254 (45) |
| Primary health insurance, N (%) | ||
| Uninsured/none | 2683 (18) | 28 (5) |
| Medicaid | 3405 (23) | 154 (28) |
| Medicare | 5890 (40) | 214 (38) |
| Commercial or Other | 321 (2) | 8 (1) |
| Missing | 2495 (17) | 155 (28) |
| Homeless, N (%) | 827 (6) | 31 (6) |
| Comorbid conditions, % | ||
| Diabetes | 3154 (21) | 210 (38) |
| Hypertension | 6873 (46) | 208 (37) |
| Cardiovascular disease | 2604 (18) | 62 (11) |
| AIDS/HIV | 653 (4) | 14 (3) |
| Hepatitis C virus infection | 629 (4) | 11 (2) |
| Hepatitis B virus infection | 166 (1) | 1 (0) |
| Alcoholism | 1155 (8) | 15 (3) |
| History of drug use | 2365 (16) | 56 (10) |
| Laboratory measures, % | ||
| Estimated GFR category | ||
| 45–59 ml/min/1.73 m2 | 12,062 (81) | 200 (36) |
| 30–44 ml/min/1.73 m2 | 1926 (13) | 123 (22) |
| 15–29 ml/min/1.73 m2 | 631 (4) | 145 (26) |
| <15 ml/min/1.73 m2 | 175 (1) | 91 (16) |
| Proteinuria categorya | ||
| Normal (None or <30 mg/g) | 6545 (44) | 42 (5) |
| Mild (trace to 1+ or 30–300 mg/g) | 3891 (26) | 391 (70) |
| Heavy (≥ 2+ or >300 mg/g) | 202 (1) | 88 (16) |
| Missing | 4156 (28) | 54 (10) |
| Hemoglobin, mean (SD), g/dL | 12.9 (2.0) | 11.4 (2.2) |
| Serum albumin, mean (SD), g/dL | 4.0 (0.7) | 3.3 (0.7) |
Proteinuria determined either by dipstick urinalysis or urinary albumin-to-creatinine ratio
ESRD = End Stage Renal Disease
GFR = Glomerular Filtration Rate
Progression to end-stage renal disease
Overall, 559 patients reached ESRD and 984 died during the follow up period. In analyses adjusted for age group, sex, race/ ethnicity, income, health insurance coverage, primary language, comorbid conditions, eGFR, proteinuria, and hemoglobin and serum albumin levels, several factors independently predicted progression to ESRD (Table 2). Lower eGFR category and higher degrees of proteinuria were the most potent predictors of progression to ESRD. Other covariates associated with a significantly higher adjusted risk of progression to ESRD included younger age, male sex, non-White race/ ethnicity, diabetes and Medicaid and Medicare health insurance coverage (p<.001 for all comparisons). Substance abuse was the only ascertained comorbidity that was associated with a significantly lower risk of ESRD (p<.001). There was no significant association between HIV/ AIDS (p=.07), viral hepatitis (p=.11), or non-English language (p=.27) and risk of incident ESRD after adjustment (Table 2).
Table 2.
Unadjusted and Adjusted Hazard Ratios (95% Confidence Intervals) among Predictors of Time to End-Stage Renal Disease (ESRD)
| Predictor | Crude hazard ratio (95% confidence interval) |
Adjusted hazard ratioa (95% confidence interval) |
|---|---|---|
| Sociodemographics | ||
| Sex | ||
| Men (vs. women) | 1.69 (1.45, 1.97) | 1.51 (1.26, 1.82) |
| Health insurance coverage | ||
| Uninsured/none | Referent | Referent |
| Medicaid | 3.59 (2.40, 5.36) | 2.34 (1.54, 3.54) |
| Medicare | 2.48 (1.67, 3.67) | 2.90 (1.92, 4.39) |
| Commercial | 1.65 (0.58, 4.72) | 1.57 (0.54, 4.59) |
| Other | 2.87 (1.91, 4.29) | 1.49 (0.51, 4.34) |
| Age group × estimated GFRb | — | — |
| Race/ethnicity × estimated GFRb | ||
| Clinical predictors of ESRD | ||
| Diabetes | 2.59 (2.18, 3.08) | 2.00 (1.64, 2.46) |
| Cardiovascular disease | 0.69 (0.53, 0.90) | 0.93 (0.70, 1.23) |
| Hypertension | 0.86 (0.72, 1.02) | 0.95 (0.77, 1.17) |
| Log proteinuria per mg/g | 2.02 (1.91, 2.13) | 1.74 (1.64, 1.85) |
| Hemoglobin per g/L | 0.76 (0.73, 0.48) | 0.87 (0.83, 0.91) |
| Serum albumin per g/dL | 0.44 (0.40, 0.48) | 0.74 (0.65, 0.84) |
| Non-traditional predictors of ESRD | ||
| HIV/AIDS | 0.99 (0.80, 1.22) | 0.58 (0.36, 1.04) |
| Hepatitis B or C virus | 0.72 (0.40, 1.27) | 0.61 (0.33, 1.12) |
| History of substance abuse | 0.67 (0.51, 0.87) | 0.45 (0.33, 0.60) |
| Non-English speaker | 0.63 (0.51, 0.76) | 0.87 (0.67, 1.12) |
Model estimates are based on 10 imputations and are adjusted for baseline age group (referent group is 70+ years), sex, race/ethnicity (referent group is White), health insurance coverage (referent group is uninsured), diabetes, cardiovascular disease, hypertension, estimated glomerular filtration rate (EGFR referent group is 45–59 ml/min/1.73 m2), log proteinuria, HIV/AIDS, viral hepatitis, substance abuse, housing status, non-English language, hemoglobin level and serum albumin concentration.
GFR = Glomerular Filtration Rate
Modification of progression
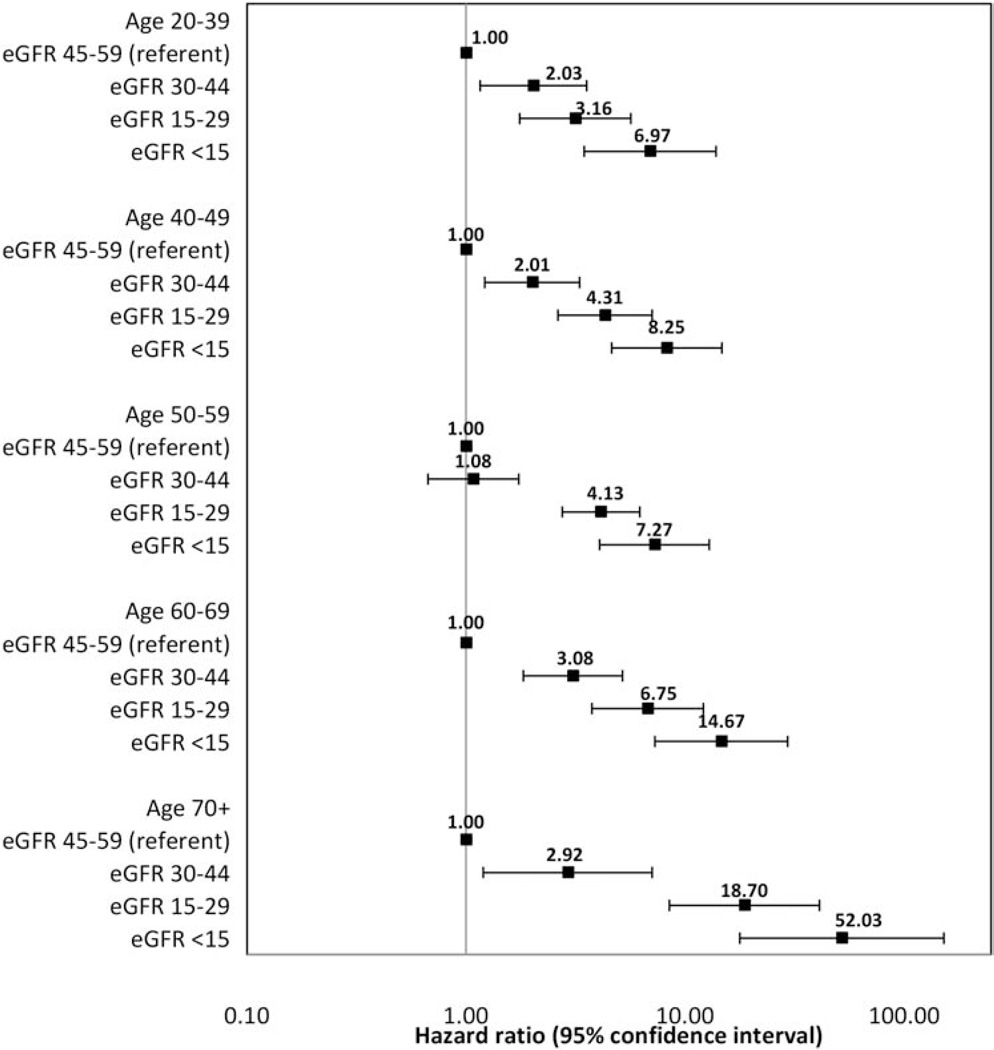
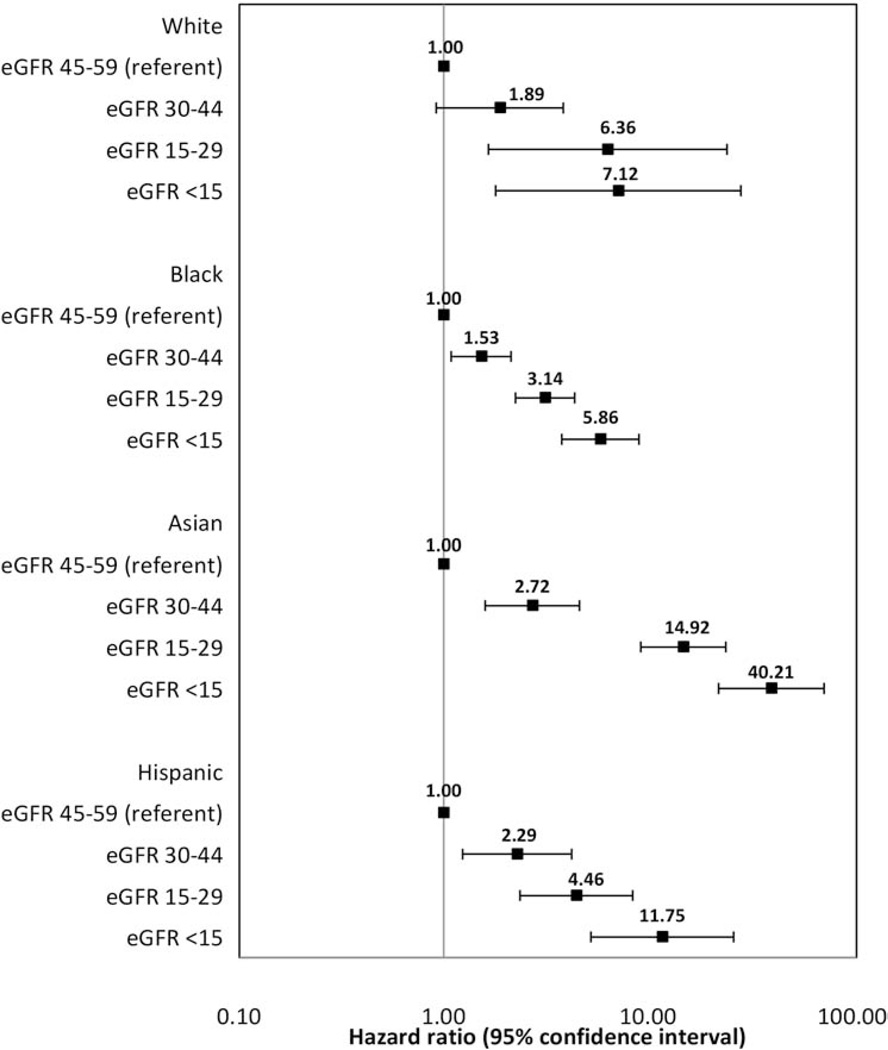
There was, however, strong evidence that the relations of eGFR category and time to ESRD differed according to age group (Wald test p- value=.008) and race/ ethnicity (p<.001). The association of lower eGFR category and increased risk of ESRD was more pronounced among older than among younger individuals (Figure 1), and among Asian and Hispanic than among Black and White patients (Figure 2). In contrast, there was no strong evidence that the relationship between eGFR category and time to ESRD differed significantly according to sex (p=.16), proteinuria (p=.96) or diabetes status (p=.47). Similarly, there was no strong evidence that the relationship between proteinuria and time to ESRD differed significantly according to age (p=.86), sex (p=.99), or race/ ethnicity (p=.51), but there was strong evidence that this association differed by diabetes status (p=.007). The association of higher proteinuria and increased risk of ESRD was more pronounced among patients without diabetes (HR [95%CI or Confidence Interval]: 1.86 [1.72, 2.02]) as compared with those with diabetes (1.63 [1.49, 1.79]).
Figure 1.
Adjusted hazard ratios (95% confidence intervals) for the association of initial eGFR Glomerular Filtration Rate category and time to end-stage renal disease stratified by age group
Figure 2.
Adjusted hazard ratios (95% confidence intervals) for the association of initial eGFR Glomerular Filtration Rate category and time to end- stage renal disease stratified by race/ethnicity
Discussion
In the U.S., the rates of ESRD differ markedly by race/ ethnicity and socioeconomic status.5,24 Despite widespread recognition of these disparities, few studies have examined predictors of ESRD among disadvantaged populations.5 In this public health care setting, we confirmed that younger age, male sex, non-White race/ ethnicity, health insurance coverage, diabetes, lower eGFR, higher proteinuria, lower hemoglobin level, and lower serum albumin concentration were significantly associated with a higher adjusted risk of progression to ESRD. In contrast, we found no significant association between social or societally- determined clinical factors including substance abuse, HIV/AIDS, viral hepatitis (HBV or HCV), and non-English language with higher risk of ESRD after concurrent adjustment for the aforementioned variables.
In a retrospective cohort study of 2,015,891 U.S. Veterans with and without CKD, Choi et al. reported that HIV seropositivity was associated with a higher risk of developing ESRD among Black but not among White patients after adjustment for age, sex, eGFR level, and comorbid conditions.25 Like Choi et al., we found no evidence of an overall association between HIV seropositivity and risk of ESRD. However, in contrast with Choi et al., we found no evidence that the association of HIV and risk of ESRD differed according to race or ethnicity. While the vast majority of patients with HIV who developed ESRD in our study (13 of 14) were Black, our analyses were limited by the paucity of overall ESRD events in this subgroup. Differences in subject selection (i.e., only patients with moderate to advanced CKD were included in our study as compared with the largely non-CKD Veteran cohort of Choi et al.) may have also contributed to the disparate findings.
As with HIV, we found no significant association between the presence of HCV infection and higher risk of ESRD. In a retrospective cohort study of 474,369 U.S. Veterans, Tsui et al. reported that HCV seropositivity was associated with an approximate three- fold higher adjusted risk of developing ESRD. In contrast with this national cohort of Veterans in which CKD was relatively uncommon (baseline prevalence <14%), all subjects in our study had moderate to advanced CKD. The more homogeneous nature of our study cohort with respect to kidney disease and socioeconomic status combined with shorter follow-up (median 3.6 vs. 2.8 years in our cohort) and relative paucity of ESRD events among HCV- infected subjects (n=11) likely contributed to differences in study findings due to insufficient power to test this association. Likewise, we observed only a single case of ESRD among HBV- infected subjects in our study which precluded substantive inference.
Notably, one- third of individuals with CKD stages 3– 5 in our study cohort spoke a primary language other than English. Prior studies in non-CKD populations have described suboptimal processes of care among adults with limited English proficiency such as poorer glycemic control among patients with diabetes.26,27 In our study, we found no evidence to suggest that non-English speakers were at higher risk for progressing to ESRD compared with English speakers. The CHN provides a wide range of interpreter services and health information in several foreign languages, and it is possible that these services reduced linguistic barriers to care in our diverse patient population. In contrast with prior studies which were unable to account for differences in socioeconomic status and access to care among participants, we controlled for several measures of these important variables including household income and health insurance coverage. Based on our results, we posit that unfavorable outcomes associated with linguistic barriers might be attenuated in the presence of readily available interpreter services and access to ambulatory care as were available in the CHN.
In our study, having a history of substance abuse (drug use or alcoholism) was associated with an estimated 55% lower adjusted risk of progressing to ESRD. Several case- series and cross- sectional studies have reported associations between intravenous drug use, higher levels of proteinuria and more severe kidney disease.28–31 However, most of these reports preceded widespread testing and recognition of HIV: these reports may have actually been describing the renal manifestations of untreated HIV (i.e., HIV-associated nephropathy) rather than a true link between substance abuse and kidney function. Moreover, in our study, the mean death rates were nearly twofold higher among patients with (29.2 [95% CI: 25.8, 33.0] per 1000 person-years) versus those without (15.0 [95% CI: 13.9, 16.1]) a history of substance abuse. Thus, it is likely that the association of substance abuse and reduced risk of ESRD was at least partly attributable to a higher risk of death in patients with (versus without) substance abuse. Considering the higher prevalence of proteinuria among patients with versus those without a history of substance in our study, it is also unlikely that differences in underlying cause of CKD (i.e., progressive vs. non-progressive CKD) accounted for this observation.
Consistent with prior reports, we found that more severe proteinuria and lower levels of kidney function were significantly associated with higher risk of ESRD.11,32 Iseki et al. and Hsu et al. described independent, graded increases in ESRD risk associated with higher levels of dipstick proteinuria based on universally screened and insured cohorts from Japan and the U.S., respectively.11,32 Our risk estimates for ESRD associated with more severe proteinuria or lower levels of kidney function were substantially higher than those reported in the aforementioned studies.11,32 This observation was most likely due to differences in subject selection where younger patients with more advanced kidney disease were present in higher proportions in our cohort.
Neither cardiovascular disease nor hypertension was associated with the development of ESRD after adjustment. The higher prevalence of cardiovascular disease among patients who did not develop ESRD may partly reflect a higher risk of death even with moderate to advanced CKD. Among patients from an integrated health system, the risk of all- cause death and cardiovascular events increased non-linearly at lower levels of kidney function.33 In contrast with prior studies,2,32 we found no significant association between the presence of hypertension and increased ESRD risk. Although blood pressure is clearly important for diagnosis and management of CKD, the course of hypertensive CKD is typically one of slow decline in kidney function over many years.2,22,32 Thus, it is plausible that the modest duration of follow up may have precluded observation of the long- term effects of hypertension on ESRD risk. Alternatively, our reliance on diagnostic codes rather than clinical measurement to ascertain hypertension may have rendered our assessment of this particular exposure relatively insensitive.22
Even in this resource- poor environment, we also found that non-White race/ ethnicity, and, in particular, Black race was associated with higher risk of developing ESRD compared with White race. The risk estimates for ESRD among members of racial/ ethnic minority groups in our study were consistent with those from CKD cohorts in other U.S. health care settings.23,34,35 However, the persistence of racial/ethnic differences in ESRD risk in this low-income population suggests that factors other than socioeconomic status may play a central role in the progression of established CKD to ESRD than previously suggested. Recent studies have linked APOL1 gene mutations with certain types of progressive kidney disease.36,37 Due partly to the protective effects conferred by APOL1 mutations against trypanosomal disease, these mutations appear to be relatively common among individuals of African descent but virtually absent among those from Europe.36 *Similar associations between APOL1 mutations and American Indian race or Hispanic ethnicity have not been observed, and reasons for the substantially higher risk of ESRD in these groups relative to Whites remain unclear. Other studies suggest that racial differences in chronic stress, medical mistrust, and nephrotoxin use might also contribute to the elevated risk of ESRD among non-White versus White patients.4,38–40 Such factors warrant additional inquiry.
Consistent with prior studies, male sex and younger age were significant, independent predictors of progression to ESRD.5,24,41 While the mechanisms underlying sex differences in ESRD risk among humans are inadequately understood, some investigators report that estrogen may have beneficial effects on collagen metabolism and mesangial cell biology.41 With respect to age, we confirmed a strong stepwise association between younger age and higher risk of progression to ESRD in this largely poor clinical cohort.24 Based on more pronounced attenuation of ESRD risk estimates among younger subjects after adjustment for baseline comorbidities (Table 2), the age- ESRD relations appear to be at least partly related to a higher frequency of risk factors for CKD progression among younger subjects. In the context of these findings, the high fraction of relatively young adults with moderate to advanced CKD in our study should prompt further inquiry into the potential impact of targeted CKD risk factor management interventions within public health systems. Lastly, the more pronounced association of lower eGFR and increased risk of ESRD among older patients and among Asians and Hispanics (relative to younger patients and Blacks and Whites, respectively) may reflect age or racial differences in CKD etiology, comorbid burden or perhaps tolerance of uremia among members of these groups at similar initial levels of eGFR.24 It is also possible that these differences reflect some degree of eGFR misclassification as the MDRD equation has not been validated across the full range of age, racial groups and eGFR levels examined here.17
Strengths and limitations
Our study is strengthened by the inclusion of adults with moderate to advanced CKD from the urban health care safety net—a population rarely captured in U.S.-based studies of kidney disease. In addition to providing detailed demographic and clinical data, we were able to link our cohort to statewide and national registries to obtain complete or nearly complete capture of treated ESRD and vital status. Our study also had several limitations. First, while diverse populations were well represented in our study, our cohort may not be fully reflective of people receiving care from other U.S. public hospitals or safety- net health systems.7 Second, we were unable to account for changes in exposures such as health insurance coverage or laboratory measures over the period of follow-up. Third, our assessment of comorbid conditions was based on diagnostic codes and thus may underestimate the prevalence of comorbidities such as cardiovascular disease, diabetes, and hypertension in this population; moreover, while we incorporated laboratory measures that often reflect disease severity, we could not directly determine the severity or duration of most of the comorbid conditions. Fourth, while it is possible that we have misclassified some people with acute kidney injury or with near normal kidney function as having CKD, we attempted to reduce this potential misclassification by requiring at least two outpatient eGFR determinations for study inclusion. Misclassification of CKD and its severity using population- based GFR estimating equations may also be operative since the MDRD study equation was derived in a population of largely White and Black patients with moderate to advanced CKD, very few of whom had diabetes.17 Finally, we were limited in our ability to examine the association of ESRD and certain exposures including chronic viral hepatitis and HIV due to the limited sample size of these groups.
Conclusions
In the urban health care safety net, we found no evidence that social and socially- determined clinical factors including substance abuse, non-English language status, and chronic viral diseases significantly predicted higher risk of progressing to ESRD. While the importance of these factors in predicting ESRD may differ in populations that include a wider- range of patients, our results reinforce the importance of addressing traditional risk factors for progressive CKD to reduce the disproportionate burden of ESRD among underserved populations.
Supplementary Material
Acknowledgments
This project received support from NIH/ NCRR UCSF-CTSI grants UL1 RR024131 and KL2 RR024130, Satellite Health care’s Norman S. Coplon Extramural Grant Program, and NIH/ NIDDK grants K23 DK 087900 and K23 DK 080645. The funding organizations had no role in the design and conduct of the study; collection, analysis, or preparation of the data; or preparation, review, or approval of the manuscript. The findings and conclusions in this report are solely the responsibility of the authors and do not necessarily represent the official views of the U.S. Government, NIH, or the San Francisco Department of Health.
Footnotes
The mechanism(s) of how mutations in the APOL1 gene lead to progressive kidney disease have yet to be elucidated. Mutations in APOL1 are associated with odds ratios of 10.5 in idiopathic FSGS and 7.3 in hypertension-attributed ESRD. While the pathways linking APOL1 variants to ESRD remain unclear, researchers hypothesize that these kidney disease risk variants likely rose to high frequency in Africa because they confer resistance to trypanosomal infection and protect from African sleeping sickness.37,38
Notes
- 1.Karter AJ, Ferrara A, Liu JY, et al. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002 May 15;287(19):2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 2.Klag MJ, Whelton PK, Randall BL, et al. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997 Apr 23–30;277(16):1293–1298. [PubMed] [Google Scholar]
- 3.Hsu CY, Lin F, Vittinghoff E, et al. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003 Nov;14(11):2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 4.Hall YN, Hsu CY, Iribarren C, et al. The conundrum of increased burden of end-stage renal disease in Asians. Kidney Int. 2005 Nov;68(5):2310–2316. doi: 10.1111/j.1523-1755.2005.00691.x. [DOI] [PubMed] [Google Scholar]
- 5.Collins AJ, Foley RN, Herzog C, et al. U.S. Renal Data System 2010 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Am J Kidney Diseases. 2011 Jan;57(1 Suppl 1):e1–e526. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Gaskin DJ, Hadley J. Population characteristics of markets of safety-net and non-safety-net hospitals. J Urban Health. 1999 Sep;76(3):351–370. doi: 10.1007/BF02345673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regenstein M, Sickler D. Race, ethnicity, and language of patients: hospital practices regarding collection of information to adress disparties in health care. Washington, DC: National Public Health and Hospital Institute; 2006. Available at: http://www.naph.org/Main-Menu-Category/Our-Work/Health-Care-Disparities/raceethnicityandlanguageofpatients.aspx. [Google Scholar]
- 8.Kern EF, Maney M, Miller DR, et al. Failure of ICD-9-CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res. 2006 Apr;41(2):564–580. doi: 10.1111/j.1475-6773.2005.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powe NR, Plantinga L, Saran R. Public health surveillance of CKD: principles, steps, and challenges. Am J Kidney Dis. 2009 Mar;53(3 Suppl 3):S37–S45. doi: 10.1053/j.ajkd.2008.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall YN, Choi AI, Chertow GM, et al. Chronic kidney disease in the urban poor. Clin J Am Soc Nephrol. 2010 May;5(5):828–835. doi: 10.2215/CJN.09011209. Epub 2010 Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iseki K, Ikemiya Y, Iseki C, et al. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003 Apr;63(4):1468–1474. doi: 10.1046/j.1523-1755.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 12.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010 Feb 3;303(5):423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 13.Gupta SK, Eustace JA, Winston JA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2005 Jun 1;40(11):1559–1585. doi: 10.1086/430257. Epub 2005 Apr 22. [DOI] [PubMed] [Google Scholar]
- 14.Choi AI, Rodriguez RA, Bacchetti P, et al. The impact of HIV on chronic kidney disease outcomes. Kidney Int. 2007 Dec;72(11):1380–1387. doi: 10.1038/sj.ki.5002541. Epub 2007 Sep 5. [DOI] [PubMed] [Google Scholar]
- 15.Schillinger D, Bibbins-Domingo K, Vranizan K, et al. Effects of primary care coordination on public hospital patients. J Gen Intern Med. 2000 May;15(5):329–336. doi: 10.1046/j.1525-1497.2000.07010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003 Jul 15;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem. 2009 May;46(Pt 3):205–217. doi: 10.1258/acb.2009.009007. [DOI] [PubMed] [Google Scholar]
- 19.Cox DR, Oakes D. Analysis of survival data. Boca Raton, FL: Chapman & Hall/ CRC Press; 1984. [Google Scholar]
- 20.Little RJA, Rubin DB. Statistical analysis with missing data. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 21.Zaman OS, Cummings LC, Spieler SS. America’s public hospitals and health systems, 2008: results of the annual National Association of Public Hospitals and Health Systems (NAPH) Hospital Characteristics Survey. Washington, DC: National Association of Public Hospitals and Health Systems; 2010. Available at: http://www.naph.org/Main-Menu-Category/Publications/Safety-Net-Financing/Characteristics-2008.aspx. [Google Scholar]
- 22.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011 Apr 20;305(15):1553–1559. doi: 10.1001/jama.2011.451. Epub 2011 Apr 11. [DOI] [PubMed] [Google Scholar]
- 23.Peralta CA, Shlipak MG, Fan D, et al. Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006 Oct;17(10):2892–2899. doi: 10.1681/ASN.2005101122. Epub 2006 Sep 7. [DOI] [PubMed] [Google Scholar]
- 24.O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007 Oct;18(10):2758–2765. doi: 10.1681/ASN.2007040422. Epub 2007 Sep 12. [DOI] [PubMed] [Google Scholar]
- 25.Choi AI, Rodriguez RA, Bacchetti P, et al. Racial differences in end-stage renal disease rates in HIV infection versus diabetes. J Am Soc Nephrol. 2007 Nov;18(11):2968–2974. doi: 10.1681/ASN.2007040402. Epub 2007 Oct 17. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez A, Schillinger D, Warton EM, et al. Language barriers, physician-patient language concordance, and glycemic control among insured Latinos with diabetes: the Diabetes Study of Northern California (DISTANCE) J Gen Intern Med. 2011 Feb;26(2):170–176. doi: 10.1007/s11606-010-1507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim G, Worley CB, Allen RS, et al. Vulnerability of older Latino and Asian immigrants with limited English proficiency. J Amer Geriatr Soc. 2011 Jul;59(7):1246–1252. doi: 10.1111/j.1532-5415.2011.03483.x. Epub 2011 Jun 30. [DOI] [PubMed] [Google Scholar]
- 28.Rhee MS, Schmid CH, Stevens LA, et al. Risk factors for proteinuria in HIV-infected and-uninfected Hispanic drug users. Am J Kidney Dis. 2008 Oct;52(4):683–690. doi: 10.1053/j.ajkd.2008.04.016. Epub 2008 Jun 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao TK, Nicastri AD, Friedman EA. Natural history of heroin-associated nephropathy. N Engl J Med. 1974 Jan 3;290(1):19–23. doi: 10.1056/NEJM197401032900105. [DOI] [PubMed] [Google Scholar]
- 30.Eknoyan G, Gyorkey F, Dichoso C, et al. Renal involvement in drug abuse. Arch Intern Med. 1973 Dec;132(6):801–806. [PubMed] [Google Scholar]
- 31.Bakir AA, Dunea G. Drugs of abuse and renal disease. Curr Opin Nephrol Hypertens. 1996 Mar;5(2):122–126. doi: 10.1097/00041552-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Hsu CY, Iribarren C, McCulloch CE, et al. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009 Feb 23;169(4):342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 34.Klag MJ, Whelton PK, Randall BL, et al. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997 Apr 23–30;277(16):1293–1298. [PubMed] [Google Scholar]
- 35.Hall YN, Hsu CY, Iribarren C, et al. The conundrum of increased burden of end-stage renal disease in Asians. Kidney Int. 2005 Nov;68(5):2310–2316. doi: 10.1111/j.1523-1755.2005.00691.x. [DOI] [PubMed] [Google Scholar]
- 36.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010 Aug 13;329(5993):841–845. doi: 10.1126/science.1193032. Epub 2010 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollak MR, Genovese G, Friedman DJ. APOL1 and kidney disease. Curr Opin Nephrol Hypertens. 2012 Mar;21(2):179–182. doi: 10.1097/MNH.0b013e32835012ab. [DOI] [PubMed] [Google Scholar]
- 38.Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health. 2010 May;100(5):933–999. doi: 10.2105/AJPH.2008.143446. Epub 2009 Oct 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saha S, Komaromy M, Koepsell TD, et al. Patient-physician racial concordance and the perceived quality and use of health care. Arch Intern Med. 1999 May 10;159(9):997–1004. doi: 10.1001/archinte.159.9.997. [DOI] [PubMed] [Google Scholar]
- 40.Ko RJ. Adulterants in Asian patent medicines. N Engl J Med. 1998 Sep 17;339(12):847. doi: 10.1056/nejm199809173391214. [DOI] [PubMed] [Google Scholar]
- 41.Tofovic SP, Dubey R, Salah EM, et al. 2-Hydroxyestradiol attenuates renal disease in chronic puromycin aminonucleoside nephropathy. J Am Soc Nephrol. 2002 Nov;13(11):2737–2747. doi: 10.1097/01.asn.0000031804.77546.f5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.