Abstract
We present a method to identify single-stranded PCR products of varying lengths by hybridization of n-alkylated peptide nucleic acids (PNA amphiphiles) to the products, followed by separation in micellar electrokinetic chromatography (MEKC). These end-attached PNA amphiphiles partition to nonionic micelles in the running buffer (Triton X-100), linking the tagged DNA to the micellar drag-tag. This linkage shifts the electrophoretic mobility of a tagged component away from both untagged DNA and tagged DNA of different lengths. The mobility of the tagged DNA is established by its extent of partitioning to the micelle phase as well as its size relative to the attached micelle. A model is presented that can be used to determine the length of an unknown oligomer given an experimentally obtained mobility. We find that the collective action of micelles that transiently attach to the tagged DNA impart about the same hydrodynamic drag as covalently bound “drag-tags” of a similar size. Using the PNAA-MEKC method, PCR products 88, 134, 216, and 447 bases are clearly resolved in less than three minutes. To our knowledge, this work represents the first use of surfactant micelles as drag-tags to separate DNA in capillary electrophoresis. Furthermore, the PNAA tag only attaches to DNA containing a target sequence, helping ensure that only desired PCR products are analyzed.
Introduction
Length-based DNA separations are required for fundamental molecular biology applications such as DNA sequencing and analysis of restriction digests and PCR products. Capillary gel electrophoresis (CGE) methods have replaced traditional slab-gel electrophoresis formats for sequencing and high-throughput genotyping applications. Since the electrophoretic mobility of DNA in free solution does not change appreciably with molecular weight, 1-5 CGE methods use polymers to act as sieving matrices to provide length-dependent mobility shifts by a mechanism akin to reptation.6-12 End-labeled free-solution electrophoresis (ELFSE), in which monodisperse, uncharged polymers or proteins (“drag-tags”) are covalently end-attached to DNA strands prior to electrophoresis13 has emerged as a promising alternative to CGE. This method is attractive, as the mobility of DNA is much higher and complications arising from introduction of sieving matrices into tiny capillaries or microchannels are avoided. The drag-tag and its attached DNA form a polyampholyte, whose mobility is more negative as the length of the DNA section increases.
Here, we demonstrate that the transient attachment of nonionic surfactant micelles to the DNA strands yields a length-based, ELFSE-like separation of DNA in free-solution capillary electrophoresis (CE). Since unmodified DNA does not interact with nonionic surfactant micelles, the interaction is promoted selectively by hybridization of a PNA amphiphile (PNAA, Figure 1) to a binding sequence near the end of the DNA oligomer. The PNAA used is a 10-base peptide nucleic acid with a terminal 18-carbon n-alkane 14-17. PNA peptides are chosen as they bind their ssDNA complements tightly and with high sequence specificity18-21. The PNAA probes used here form tight complexes with their complements (Tm = 50.4°C 14) and binding can be regarded as irreversible at the operating temperature of the CE instrument (22°C).
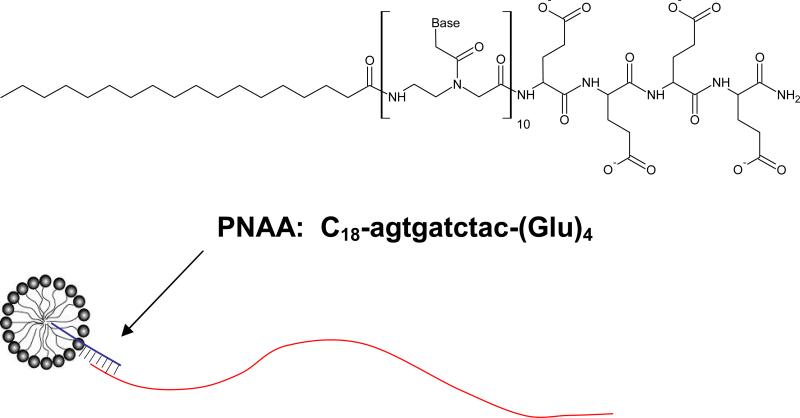
Figure 1.
Structure of the PNA amphiphile C18-agtgatctac-(Glu)4 used in this study, allowing tagged DNA oligomers to partition to uncharged carrier micelles of Triton X-100.
Following this off-line tagging step, the PNAA/DNA mixtures are injected into an uncoated CE capillary pre-filled with a running buffer containing the nonionic surfactant Triton X-100, at concentrations above its critical micelle concentration (CMC = 0.33 mM). On application of electric field, a combination of electro-osmotic flow (EOF) and electrophoresis (EP) causes the micelles to move faster than the DNA, overtaking the oligomers in the injection plug. Tagged DNA oligomers will then partition to the fast-moving micelle pseudophase, increasing their velocity relative to the untagged oligomers. The migration time of the tagged DNA is set not only by the dimensions of the DNA and its attached micelle, but by the fraction of time the tagged DNA spends attached to micelle pseudophase, which is a function of the amount of surfactant added to the running buffer.
Transient attachment of ELFSE drag-tags may have several advantages over covalent attachment, particularly when working with samples containing clusters of DNA oligomers of similar length. Micelle sizes and concentrations can be tuned for optimal resolution of each cluster without additional synthetic steps. Furthermore, we expect that a wide range of surfactant microstructures, including liposomes 22, 23 and microemulsions24, 25, may be used as pseudophases for this DNA separation. The use of transiently attached micelles may also overcome the requirement of extreme monodispersity of the ELFSE drag-tag. Each tagged DNA is expected to be bound to an individual micelle for only a fraction of a second, and the overall migration time will therefore represent the average interaction with many thousands of micelle tags.
The method presented here may be considered an instance of micellar electrokinetic chromatography (MEKC). MEKC, originally introduced in the mid 80's 26, 27, uses a micellar pseudophase to solubilize nonpolar analytes in CE. Resolution is provided by differences in the partitioning of analytes into the hydrophobic core of micelles, which usually are negatively charged. In our case, only the PNAA tag is expected to be solubilized in the micelle core, not the oligomer of interest. MEKC has been implemented to assess the extent of DNA methylation in tumor cells 28 and to quantify nucleosides that serve as markers of DNA oxidation. 29-31 However, to our knowledge, the work we present here represents the first use of MEKC for an ELFSE-like separation of intact DNA oligomers.
For this preliminary study, we demonstrate that micelle tagging can be used to separate a population of several ssDNA oligomers, ranging in size from 88 to 447 bases, in less than three minutes. We also develop a model that predicts the mobility of tagged DNA, in the presence of a Triton X-100 pseudophase, as a function of its length and the concentration of micelles in the pseudophase.
Materials and Methods
The 10-base PNAA C18-agtgtacatc-(Glu)4 (listed N → C, Figure 1) was synthesized following Fmoc-protected solid phase synthesis techniques described elsewhere 14, 15. DNA oligomers (Table 2) were obtained from Integrated DNA Technologies (Coralville, Iowa) and used as received. DNA stock solutions (about 2.5 mM) were prepared in 50 mM Tris MES buffer, pH 8.0. 48 mM Stock solutions of Triton X-100 (Fluka) were prepared daily by vortexing a suitable amount of Triton X-100 in a Tris MES buffer. Aliquots were prepared at concentrations ranging from 1.2 to 48 mM, vortexed, and centrifuged to remove any air bubbles. The Tris MES buffering system was chosen to minimize fronting of the DNA peak caused by electrodispersion 32.
Table 2.
Partitioning model parameters for the 7 synthetic DNA targets.
| DNA Sequence (5’-3’) | DNA length (bases) | μdupo (10−4 cm2/Vs) | μmico (10−4 cm2/Vs) | K (mM−1) |
|---|---|---|---|---|
| GTAGATCACT | 10 | −2.84 ± 0.05 | −0.73 ± 0.05 | 400 ± 63 |
| CGTAGATCACTC | 12 | −2.85 ± 0.01 | −0.74 ± 0.06 | 260 ± 78 |
| CCGTAGATCACTCC | 14 | −2.87 ± 0.01 | −0.84 ± 0.05 | 270 ± 51 |
| CCCGTAGATCACTCCC | 16 | −2.89 ± 0.01 | −0.91 ± 0.04 | 180 ± 26 |
| CCCCGTAGATCACTCCCC | 18 | −2.89 ± 0.02 | −0.91 ± 0.03 | 110 ± 4 |
| CCCCCGTAGATCACTCCCCC | 20 | −2.91 ± 0.01 | −0.98 ± 0.02 | 90 ± 2 |
ssDNA Oligomer Synthesis by Asymmetric PCR
Homogeneous populations of ssDNA oligomers 88, 134, 216, and 447 bases in length were synthesized by asymmetric PCR (aPCR). Primer design criteria were taken largely from those required for linear-after-the-exponential PCR 33, 34. Briefly, primers were chosen (Table 1) such that the concentration-corrected melting temperature of the reverse (limiting) primer was greater than that of the forward (excess) primer. The concentration-dependent melting temperature of each primer was found using MELTING, a nearest-neighbor nucleic acid melting temperature calculator available at http://bioweb.pasteur.fr/seqanal/interfaces/melting.html 35. The Allawi et al. nearest-neighbor set and the Santalucia salt correction factor were used 36, 37. The PNA recognition sequence, denoted by the underlined portion of the forward primer pSP64 EH13, was included it in the forward primer.
Table 1.
Forward (excess) and reverse (limiting) primers used to generate aPCR products. The PNAA binding sequence (underlined) is not complementary to pSP64. Melting temperatures (Tm) for both the original forward primer and the extended forward primer containing the PNAA binding sequence (italics) are listed.
| Forward primer | Sequence | Tm (°C) |
|---|---|---|
| pSP64 EH13 | CGCGGTAGATCACTCCGAATTCGTAATCATGTCATAGC | 55.8 (66.5) |
| Reverse primers | Sequence | Tm (°C) |
|---|---|---|
| pSP64 rp1 | AGCTCACTCATTAGGCACCCCAGG | 60.0 |
| pSP64 rp2 | TCGTATGTTGTGTGGAATTGTGAGCGGA | 60.6 |
| pSP64 rp3 | TGGCCGATTCATTAATGCAGCTGGCA | 61.5 |
| pSP64 rp4 | CGGAGCCTATGGAAAAACGCCAGCA | 61.7 |
All aPCR reactions were conducted in a Smart Cycler (Cepheid) at a total volume of 100 μl. aPCR reaction mixtures contained 1000 nM excess primer, 75 nM limiting primer, 0.2 mM dNTP mixture, 1.5 mM MgCl2, 1× PCR reaction buffer [50 mM KCl, 10 mM Tris-HCl (pH 9), 0.1% TX-100 (Promega)], 0.04 ng/μl plasmid pSP64 template (Promega), and 0.04 Units/μl Taq DNA Polymerase (Promega). Plasmid pSP64 was cloned in Subcloning Efficiency DH5α Competent Escherichia coli cells (Invitrogen). For the generation of 88, 134, and 216 base DNA strands, an initial denaturation step of 120 seconds at 95°C was followed by 50 cycles of 95°C for 10 seconds, 60°C for 15 seconds, and 72°C for 15 seconds. 447-base DNA strand generation required lengthening the denaturation, annealing, and extension steps to 15, 30, and 30 seconds, respectively. The purity of each PCR reaction was assessed on 8% non-denaturing polyacrylamide gel electrophoresis (BioRad). The products of three 100 μl aPCR reactions were diluted with 50 mM Tris MES (pH 8) to a total volume of 500 μl. Samples then underwent 3 buffer exchanges with 50 mM Tris MES (pH 8) using a 30,000 Dalton cutoff Microcon spin filter (Millipore). Each was centrifuged at 13,400g until a sample volume of about 25 μl was reached (~10 mins.). Final strand concentration was between 1 and 10 μM. 25 μM of the PNAA C18-agtgatctac-(Glu)4 (Figure 1) was then added to each DNA sample and the mixture was incubated at room temperature for 5-10 minutes to ensure maximum hybridization. Each sample was subsequently loaded and stored at 10°C prior to CE analysis.
Capillary Electrophoresis
Capillary Electrophoresis was performed on a P/ACE MDQ (Beckman Coulter, Fullerton CA) equipped with a UV absorbance detector. A 50 um I.D. fused silica capillary (Beckman Coulter), with 31 cm total length, 21 cm length to detector, was used. After filling the capillary with the running buffer of interest, samples were injected into the capillary hydrodynamically, at 0.5 psi for 5 sec. Electrophoretic separation was conducted under normal polarity (from anode to cathode) with electric field strength of 700 V/cm. UV detection was performed at 254 nm and the capillary thermostated at 22°C. 10% methanol was added to the sample buffer to determine the electroosmotic velocity of the running buffer in instances where a decrease in the Triton X-100 baseline was not sufficient. Care was taken to prevent contact between the surfactant-laden buffers and the rubber caps of vials used in the P/ACE MDQ system, so the caps would not pop off during the runs.
Data collection and analysis was performed using 32 Karat software (Beckman Coulter). To account for small drifts in the EOF (likely due to small temperature changes), the EOF was measured by recording the time required for the Triton-free sample plug to cross the detector window (observed as a slight decrease in absorbance). To remove effects of small changes in EOF from run to run, we convert the migration time axis in the electropherograms to an “electrophoretic mobility” axis. This is accomplished by recasting the migration time as a migration velocity, subtracting the EOF velocity, and scaling the residual velocity (deemed to be EP only) by the electric field (E). Velocities (v) of the EOF and those of the migrating species are calculated by:
| (1) |
where l is the length to the detector, L is the total capillary length and V is the applied voltage. The effective electrophoretic mobility (μeff) of the various components is then obtained by subtracting the EOF velocity from the velocity of each detected component (vapp):
| (2) |
where E is the applied electric field. Hence, we deem all non-EOF migration to result from electrophoresis alone, and refer to that residual velocity (scaled by the electric field) to be the “effective” electrophoretic mobility.
Results and Discussion
The effective electrophoretic mobility (μeff) observed for the PNAA-tagged DNA is established mainly by two main parameters; the fraction of time it spends attached to micelles (fmic), and its mobility (in surfactant-free buffer) when attached to a micelle (μmDNA0):
| (3) |
| (4) |
Here, μhDNA0 is the mobility of the tagged DNA in surfactant-free buffer, K is the partition coefficient of tagged DNA to the micelle phase (units mM−1), Cvisc is a viscosity constant (0.53 ± 0.02 mM−1 for TX-100), and [M] is the concentration of micelles in the running buffer (full derivation in Supporting Information). Therefore, for a true understanding of the process we need to obtain values for K and μmDNA0 for different lengths of DNA.
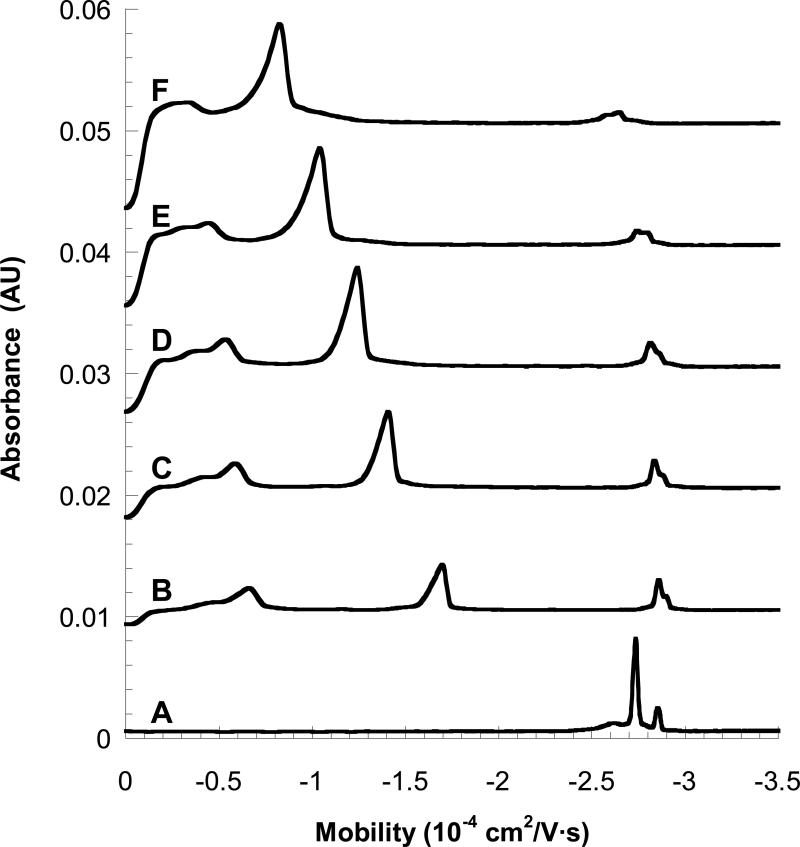
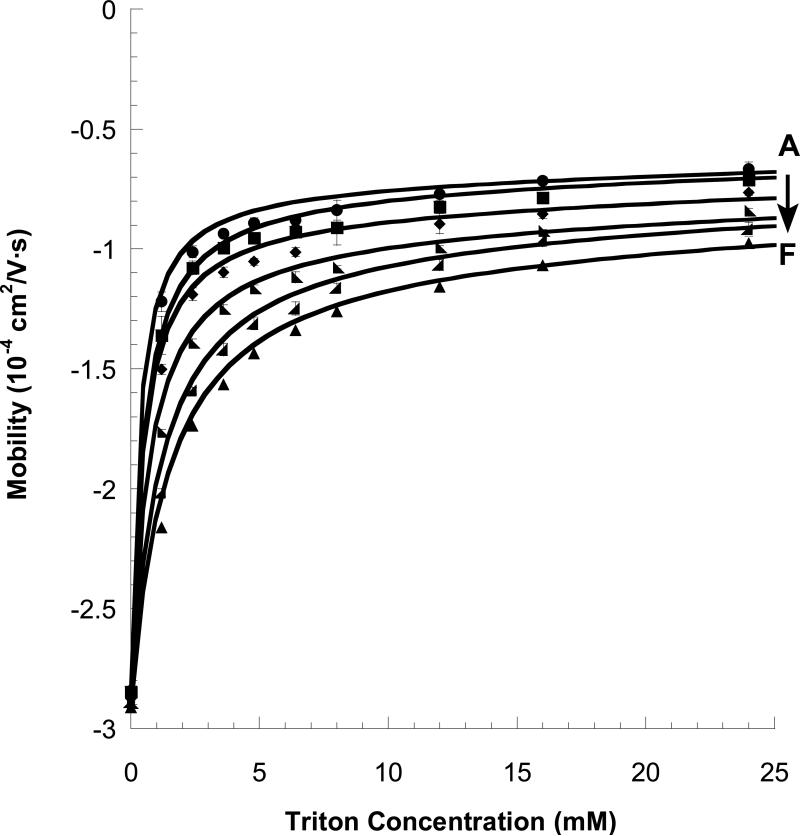
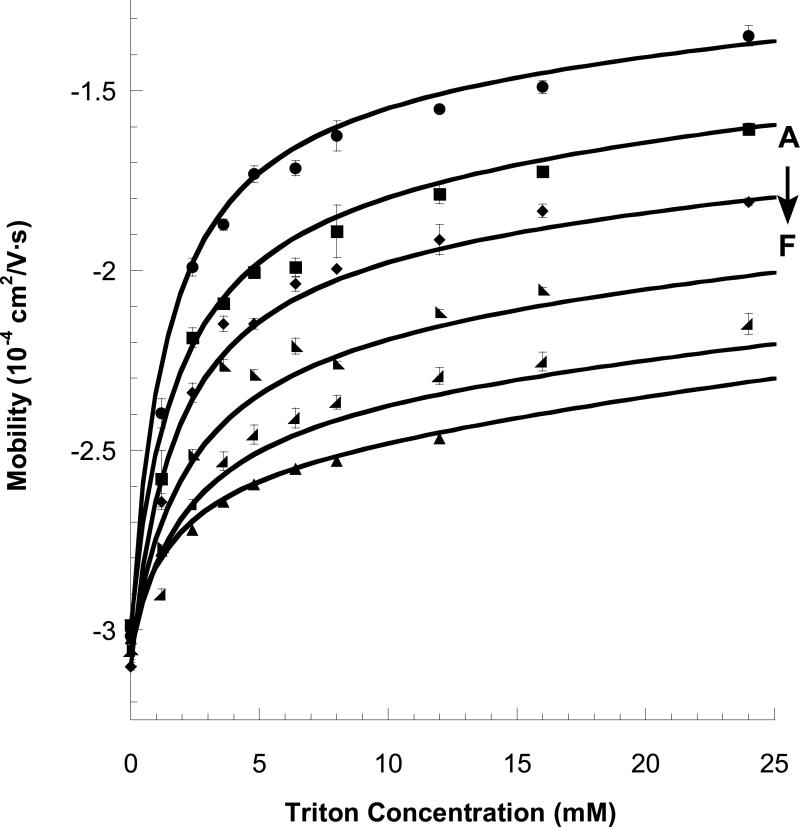
Figure 2 shows electropherograms for elution of PNAA tagged, 20-base synthetic DNA oligomers in MEKC. The left-most peaks are unbound PNAA, the large middle peaks are the PNAA-tagged DNA, and the small right-most peaks are a small residue of unbound DNA, in each case. The mobility of the tagged DNA (μeff) becomes less negative with increasing [M] due to both the stronger partitioning to the micelle phase and the increasing viscosity of the running buffer (details in Supporting Information). The peak for the untagged DNA also shifts slightly with increasing [M], due solely to the increasing viscosity effect. The mobilities of the PNAA-tagged DNA of varying lengths (from 10-20 bases) and with varying TX-100 concentration (0 to 36 mM) are plotted in Figure 3 and fit to a linearized form of eq 3 (see Supporting Information, Figure S-2). The TX-100 micelle concentration was calculated using cmc = 0.33 mM and aggregation number = 128. 17 The fitted parameters K and μmDNA0 are given in Table 2. μmDNA0 becomes more negative with longer DNA, as expected. The partition coefficient (K) also is largest for the shortest DNA, suggesting some electrosteric repulsion is observed between overhanging DNA and the micelle.
Figure 2.
Electropherograms of 50 μM C18-agtgatctac-E4 PNAA + 50 μM complementary 20 base DNA in a 50 mM Tris-MES running buffer (pH 8.0) with varying concentrations of Triton X-100. (A) 0 mM Triton X-100; (B) 2.4 mM Triton X-100; (C) 4.8 mM Triton X-100; (D) 8 mM Triton X-100; (E) 16 mM Triton X-100; (F) 36 mM Triton X-100.
Figure 3.
Effective electrophoretic mobility (μeff) of the tagged DNA (PNAA/DNA duplex) as a function of TX-100 micelle concentration for various lengths of target DNA oligomers. (A) 10 base DNA ● ;(B) 12 base DNA ■ ;(C) 14 base DNA ◆ ;(D) 16 base DNA ◣ ;(E) 18 base DNA ◢ ;(F) 20 base DNA ▲. Lines are fits to eq 3.
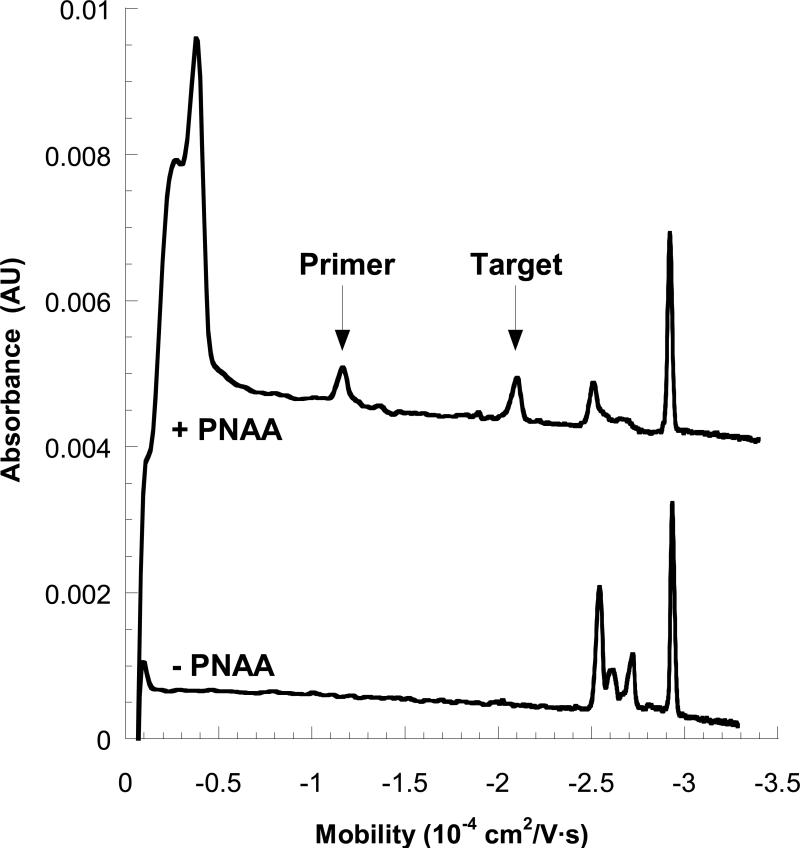
To test the method using longer DNA, we used an asymmetric PCR (aPCR) method to generate ssDNA oligomers up to 447 bases in length using a commercially available plasmid as a template (pSP64, Promega). 33, 34 The forward primer was selected to include the binding sequence for the PNAA tag and various reverse primers were selected to set the length of the resulting oligomers (Table 1). Products of the aPCR reaction, which include unextended primers, dsDNA oligomers, and the desired ssDNA oligomers were incubated with an excess of the PNAA tag and analyzed using the MEKC method. A typical MEKC electropherogram (48 mM Triton) is shown in Figure 4. The lower trace shows the aPCR products without the PNAA tag, revealing the existence of multiple components. A similar sample pre-incubated with the PNAA tag (upper trace) displays two new peaks; one with a large mobility shift (to about −1.1 × 10−4 cm2/V-s) and a second with a smaller mobility shift (to about −2.1 × 10−4 cm2/V-s). The former is presumed to be the unextended primer and the latter is the 447-base product. Two remaining, unshifted peak are the unextended reverse primer (−2.55 × 10−4 cm2/V-s) and the dsDNA product (−2.95 × 10−4 cm2/V-s). These populations were identified by control experiments containing only the primers, and also a symmetric PCR reaction mixture containing the dsDNA product (data not shown).
Figure 4.
Electropherograms for a sample containing aPCR products (including a 447 base ssDNA target) in a running buffer containing 50 mM Tris-MES (pH 8.0) and 48 mM Triton X-100. The lower curve represents the untagged aPCR products, and the upper one represents the same sample tagged with a complementary PNAA sequence tag. The binding site of PNAA probe was engineered into the forward primer (Table 2), resulting in the emergence of two shifted peaks, one corresponding to the tagged primer, and the other to the tagged PCR product target. Absorbance was measured at 254 nm.
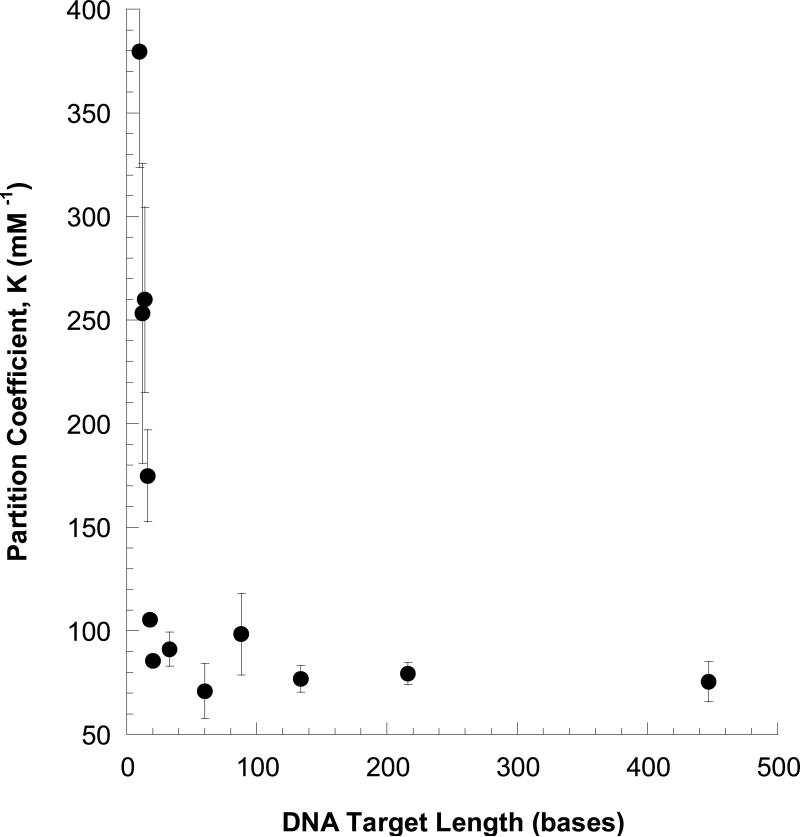
A summary of the mobilities observed in MEKC analysis of PNAA-tagged aPCR products (88, 134, 216, and 447 bases in length) is shown in Figure 5, again showing good agreement with eq 3 (fit given in Supporting Information, Figure S-3) Data for the tagged, unextended primer (30 bases in length) and a synthetic 60-base oligomer are also included. Figure 6 plots the fitted K values for all DNA samples as a function of DNA length (L), showing a strong dependence on L up to about 100 bases, then leveling off at K=75 for L>100 bases. We presume that DNA monomer located more than 100 bases from the PNAA target site are too far from the micelle to have an impact on K.
Figure 5.
Effective electrophoretic mobility (μeff) of the tagged DNA (PNAA/DNA duplex) as a function of Triton X-100 micelle concentration for various lengths of DNA oligomers, some generated by aPCR. (A) 33 base DNA ● (B) 60 base DNA ■ (C) 88 base DNA ◆ (D) 134 base DNA ◣ (E) 216 base DNA ◢ (F) 447 base DNA Lines are fits to eq 3.
Figure 6.
Partition coefficient (K) for tagged DNA to the micelle phase as a function of DNA length (in bases). Data were obtained from the fits of Figure 3 and Figure 5.
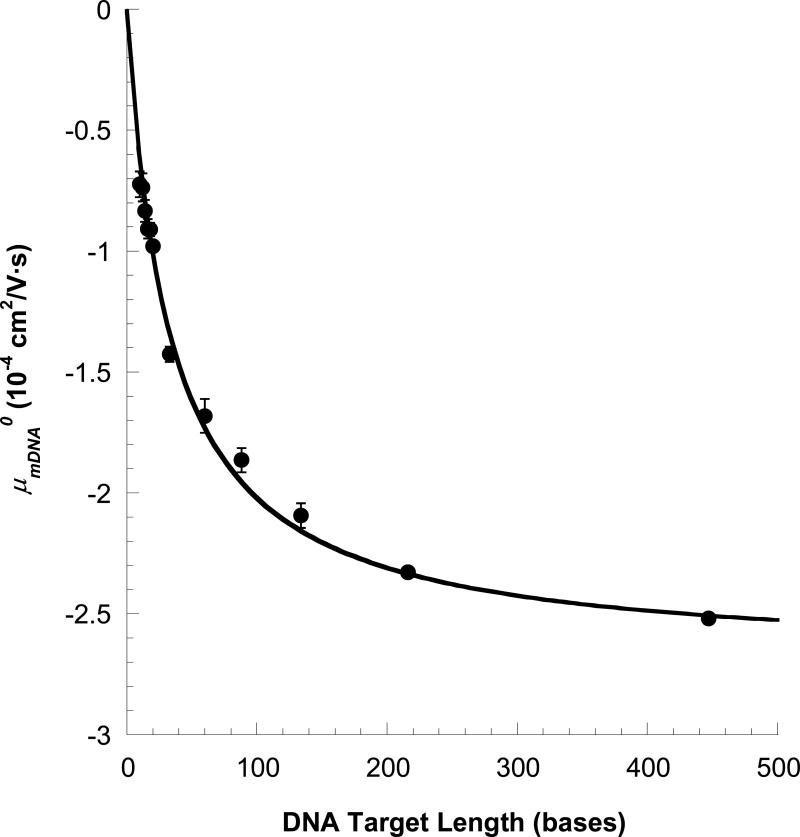
A plot of μmDNA0 vs. L (Figure 7) shows significant changes in the full range of 30 to 447 bases. Recall that the quantity μmDNA0 is the electrophoretic mobility of the tagged DNA when attached to a Triton micelle, in other words, it is the mobility of the fully bound micelle-tag-oligomer complex. For permanently attached drag-tags, the mobility of an end-labeled oligomer of length L is given by 13, 38, 39:
| (5) |
where μ0 represents the free-solution mobility of the unlabeled DNA oligomer and α is a drag parameter, representing roughly the equivalent drag of a hypothetical, uncharged DNA oligomer α bases long. Fitting of eq 5 to the data of Figure 7 (solid line) gives good agreement and a value of α = 33.5 bases, which compares favorably with previously reported values for covalently attached drag tags, including the protein streptavidin (α = 25-30 bases).38 This indicates that the collective action of numerous, transiently attached micelles shifts the mobility of DNA in a manner similar to that of a single, permanently attached drag-tag. Of course, in order to fully realize this benefit, the fraction of time the micelle is attached to the tagged DNA (fmic) must approach unity. This is easily achieved; using Triton concentrations greater than 18 mM, tagged oligomers longer than 100 bases (K = 75) will spend over 99% of their migration attached to a micelle (eq 4). At this point, the partitioning effect saturates, and further increases in [M] have almost no effect on resolution. The mobilities of all components will still shift, but only due to the viscosity effect, which to a first approximation does not favor one tagged DNA component over another.
Figure 7.
Electrophoretic mobility of tagged DNA attached to a micelle (μmDNA0) as a function of DNA length in bases. Data are from the fits of Figures 3 and 5.
This is not meant to imply that the tagged oligomers are attached to the same micelle for extended periods of time. Micelle lifetimes for Triton X-100 have been reported to be 3.6 seconds, 40 and the timescale for detachment of tagged DNA from micelles may be much shorter than this upper limit. Accordingly, tagged oligomers likely interact with hundreds (or possibly thousands) of different micelles during migration through the capillary. This may act to enhance peak sharpness, as effects of micelle polydispersity on migration time of tagged DNA populations are averaged over comparable distributions during the run. It should also be noted that the micelles themselves will dynamically exchange their monomer with those in solution, even while a tagged DNA is attached, providing additional sampling of the micelle size distribution.
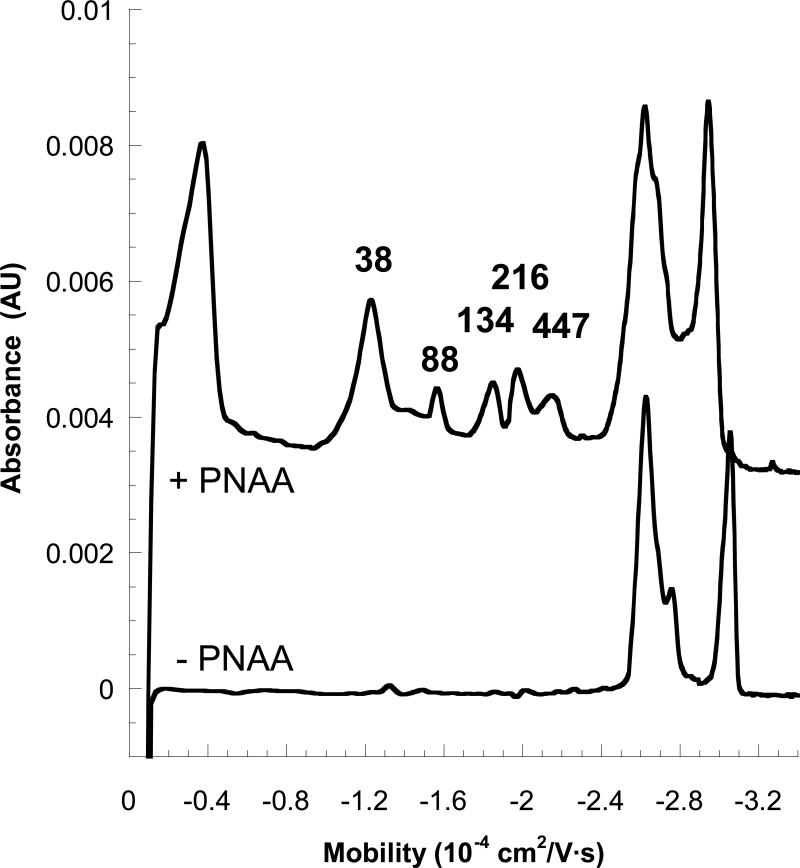
Simultaneous separation of multiple DNA targets using the PNAA-MEKC method give the same elution times as the components on their own. Figure 10 is an electropherogram collected during the MEKC-based separation of PCR-generated DNA 88, 124, 216, and 447 bases in length (48 mM Triton X-100) in the absence and presence of the PNAA tag. The four products are nearly baseline resolved and elute at the same mobilities predicted by the partitioning fits of Figures 6 and 7 (comparison given in Table 3). However, the peaks are somewhat broad, leading to a diminished resolution for the longer PCR products. In CGE analysis of DNA sequencing products, where single-base resolution is required, DNA bands are focused prior to separation by electrokinetic injection of DNA from organic solvents, a process that requires EOF suppression when implemented in bench-top CE instruments. EOF suppression is expected to give rise to longer run times as the components with lowest electrophoretic mobility (i.e., shortest, micellated DNA) will need to electrophorese across the entire capillary for detection, rather than being swept along by EOF, even after they are fully resolved.41 Therefore, we are currently investigating other methods of focusing the DNA sample so that EOF can be preserved. Sharper bands may also be obtained using laser-induced fluorescence (LIF) detection rather than UV detection.
Table 3.
Effective mobilities of tagged PCR products of various lengths obtained from a single run (Figure 8), and compared to predictions from the partition fits of Figure 5.
| DNA length (bases) | μeff, Figure 8 (10−4 cm2/Vs) | μeff, predicted (10−4 cm2/Vs) |
|---|---|---|
| 88 | −1.56 | −1.65 |
| 134 | −1.85 | −1.85 |
| 216 | −1.98 | −2.04 |
| 447 | −2.15 | −2.10 |
Conclusions
We have shown that the oligomers 88, 124, 216, and 447 bases in length can be discriminated each other and from untagged oligomers of the same length using a transient micelle tagging method. The migration time of the tagged DNA can be predicted by a partitioning model that accounts for the partition coefficient, the electrophoretic mobility of the micelle with attached oligomer, and changes in buffer viscosity by addition of surfactant. The migration time for the tagged DNA can be manipulated by varying the micelle concentration, and an analysis of the migration times obtained gives the length of the tagged oligomer.
We envision many potential applications for this gel-free DNA analysis method. Rapid genotyping by length polymorphisms may be achieved using PNAA targeted to endonuclease digestion sites. As it stands, the system is ideally suited to analysis of shorter oligomers such as microRNAs, which are about 20-40 bases in length. 42 The method could be extended to separation of longer oligomers, such as those derived from mRNA, by using larger micelle tags to increase the drag coefficient α. The binding of PNAA to ssDNA targets is highly specific; DNA with only a single base mismatch shows no “tagged DNA” population (Supplemental Information, Figure S-4). MEKC methods that use larger surfactant assemblies, including liposomes 22, 23 and surfactant microemulsions 24, 25 have been reported and might be useful in that regard. These and other applications are currently under development in our lab.
Supplementary Material
Figure 8.
Electropherograms for a sample containing aPCR products 88, 134, 216, and 447 bases in length in a running buffer containing 50 mM Tris-MES (pH 8.0) and 48 mM Triton X-100. The lower curve represents the untagged aPCR products, and the upper one represents the same sample tagged with a complementary PNAA sequence tag. The unextended primer (38 bases in length) is also labeled. Absorbance was measured at 254 nm.
Acknowledgements
The authors would like to acknowledge the National Science Foundation (BES-0093538), the Arnold and Mabel Beckman Foundation, the Air Force Office of Scientific Research, and the DARPA SIMBIOSYS program for financial support of this work. J.M.S. was supported in part by an NIH training grant (5 T32 GM065100-03).
Footnotes
Supplementary Material Available: The linearized fits to the partitioning data of Figures 3 and 5 are provided (Figures S-2 and S-3), as well as the calculation of Cvisc (eq 3) using the Beckman-Coulter P/ACE MDQ system (Figure S-1), are available as Supporting Information. Also included is a full derivation of eq 3 and electropherograms using DNA containing a single-base mismatch as a control (Figure S-4). Current ordering information is available on any masthead page.
References
- 1.Stellwagen NC, Gelfi C, Righetti PG. Biopolymers. 1997;42:687–703. doi: 10.1002/(SICI)1097-0282(199711)42:6<687::AID-BIP7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 2.Stellwagen E, Stellwagen NC. Electrophoresis. 2002;23:2794–2803. doi: 10.1002/1522-2683(200208)23:16<2794::AID-ELPS2794>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 3.Stellwagen E, Stellwagen NC. Electrophoresis. 2002;23:1935–1941. doi: 10.1002/1522-2683(200206)23:12<1935::AID-ELPS1935>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Stellwagen NC, Zhivkov AM. Abstracts of Papers, 225th ACS National Meeting, New Orleans, LA, United States, March 23-27, 2003. 2003:COLL–529. [Google Scholar]
- 5.Lu Y, Weers B, Stellwagen NC. Biopolymers. 2001;61:261–275. doi: 10.1002/bip.10151. [DOI] [PubMed] [Google Scholar]
- 6.Barron AE, Sunada WM, Blanch HW. Biotechnology and Bioengineering. 1996;52:259–270. doi: 10.1002/(SICI)1097-0290(19961020)52:2<259::AID-BIT7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Albarghouthi MN, Buchholz BA, Huiberts PJ, Stein TM, Barron AE. Electrophoresis. 2002;23:1429–1440. doi: 10.1002/1522-2683(200205)23:10<1429::AID-ELPS1429>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 8.Albarghouthi MN, Barron AE. Electrophoresis. 2000;21:4096–4111. doi: 10.1002/1522-2683(200012)21:18<4096::AID-ELPS4096>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 9.Kopecka K, Drouin G, Slater GW. Electrophoresis. 2004;25:2177–2185. doi: 10.1002/elps.200305951. [DOI] [PubMed] [Google Scholar]
- 10.Heller C. Electrophoresis. 2001;22:629–643. doi: 10.1002/1522-2683(200102)22:4<629::AID-ELPS629>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.Heller C. Electrophoresis. 1999;20:1978–1986. doi: 10.1002/(SICI)1522-2683(19990701)20:10<1978::AID-ELPS1978>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Heller C. Electrophoresis. 1999;20:1962–1977. doi: 10.1002/(SICI)1522-2683(19990701)20:10<1962::AID-ELPS1962>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Meagher RJ, Won J-I, McCormick LC, Nedelcu S, Bertrand MM, Bertram JL, Drouin G, Barron AE, Slater GW. Electrophoresis. 2005;26:331–350. doi: 10.1002/elps.200410219. [DOI] [PubMed] [Google Scholar]
- 14.Vernille JP, Kovell LC, Schneider JW. Bioconjugate Chemistry. 2004;15:1314–1321. doi: 10.1021/bc049831a. [DOI] [PubMed] [Google Scholar]
- 15.Vernille JP, Schneider JW. Biotechnology Progress. 2004;20:1776–1782. doi: 10.1021/bp049971z. [DOI] [PubMed] [Google Scholar]
- 16.Marques BF, Schneider JW. Langmuir. 2005;21:2488–2494. doi: 10.1021/la047962u. [DOI] [PubMed] [Google Scholar]
- 17.Lau C, Bitton R, Bianco-Peled H, Schultz DG, Cookson DJ, Grosser ST, Schneider JW. Journal of Physical Chemistry B. 2006;110:9027–9033. doi: 10.1021/jp057049h. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen PE, Egholm M, Berg RH, Buchardt O. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 19.Egholm M, Buchardt O, Christensen L, Behrens C, Freier SM, Driver DA, Berg RH, Kim SK, Norden B, Nielsen PE. Nature. 1993;365:566–568. doi: 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- 20.Ratilainen T, Holmen A, Tuite E, Haaima G, Christensen L, Nielsen PE, Norden B. Biochemistry. 1998;37:12331–12342. doi: 10.1021/bi9808722. [DOI] [PubMed] [Google Scholar]
- 21.Tomac S, Sarkar M, Ratilainen T, Wittung P, Nielsen PE, Norden B, Graeslund A. J. Am. Chem. Soc. 1996;118:5544–5552. [Google Scholar]
- 22.Bilek G, Kremser L, Blaas D, Kenndler E. J. Chromatography B. 2006;841:38–51. doi: 10.1016/j.jchromb.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Wiedmer SK, Holopainen JM, Mustakangas P, Kinnunen PK, Riekkola ML. Electrophoresis. 2000;21:3191–3198. doi: 10.1002/1522-2683(20000901)21:15<3191::AID-ELPS3191>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 24.Marsh A, Clark B, Broderick M, Power J, Donegan S, Altria K. Electrophoresis. 2004;25:3970–3980. doi: 10.1002/elps.200406112. [DOI] [PubMed] [Google Scholar]
- 25.Hansen SH. Electrophoresis. 2003;24:3900–3907. doi: 10.1002/elps.200305637. [DOI] [PubMed] [Google Scholar]
- 26.Terabe S, Otsuka K, Ichikawa K, Tsuchiya A, Ando T. Analytical Chemistry. 1984;56:111–113. [Google Scholar]
- 27.Terabe S, Otsuka K, Ando T. Analytical Chemistry. 1985;57:834–841. [Google Scholar]
- 28.Wirtz M, Stach D, Kliem HC, Wiessler M, Schmitz OJ. Electrophoresis. 2004;25:839–845. doi: 10.1002/elps.200305761. [DOI] [PubMed] [Google Scholar]
- 29.Guarnieri C, Muscari C, Stefanelli C, Giaccari A, Zini M, Di Biase S. J Chromatogr B Biomed Appl. 1994;656:209–213. doi: 10.1016/0378-4347(94)00044-1. [DOI] [PubMed] [Google Scholar]
- 30.Lyko F, Stach D, Brenner A, Stilgenbauer S, Dohner H, Wirtz M, Wiessler M, Schmitz OJ. Electrophoresis. 2004;25:1530–1535. doi: 10.1002/elps.200305830. [DOI] [PubMed] [Google Scholar]
- 31.Wirtz M, Schumann CA, Schellentrager M, Gab S, Vom Brocke J, Podeschwa MA, Altenbach HJ, Oscier D, Schmitz OJ. Electrophoresis. 2005;26:2599–2607. doi: 10.1002/elps.200410397. [DOI] [PubMed] [Google Scholar]
- 32.Beckers JL, Bocek P. Electrophoresis. 2003;24:518–535. doi: 10.1002/elps.200390060. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez JA, Pierce KE, Rice JE, Wangh LJ. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1933–1938. doi: 10.1073/pnas.0305476101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierce KE, Sanchez JA, Rice JE, Wangh LJ. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8609–8614. doi: 10.1073/pnas.0501946102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Novere N. Bioinformatics. 2001;17:1226–1227. doi: 10.1093/bioinformatics/17.12.1226. [DOI] [PubMed] [Google Scholar]
- 36.Allawi HT, SantaLucia J., Jr. Biochemistry. 1997;36:10581–10594. doi: 10.1021/bi962590c. [DOI] [PubMed] [Google Scholar]
- 37.Santalucia J., Jr. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1460–1465. doi: 10.1073/pnas.95.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heller C, Slater GW, Mayer P, Dovichi N, Pinto D, Viovy J-L, Drouin G. Journal of Chromatography, A. 1998;806:113–121. [Google Scholar]
- 39.Ren H, Karger AE, Oaks F, Menchen S, Slater GW, Drouin G. Electrophoresis. 1999;20:2501–2509. doi: 10.1002/(SICI)1522-2683(19990801)20:12<2501::AID-ELPS2501>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 40.Patist A, Kanicky JR, Shukla PK, Shah DO. J. Coll. Int. Sci. 2002;245:1–15. doi: 10.1006/jcis.2001.7955. [DOI] [PubMed] [Google Scholar]
- 41.McCormick LC, Slater GW. Electrophoresis. 2006;27:1693–1701. doi: 10.1002/elps.200500573. [DOI] [PubMed] [Google Scholar]
- 42.He L, Hannon GJ. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.