Abstract
Insulin analog patent expiry is likely to mean that, increasingly, copies of original biopharmaceutical products will be submitted for authorization. Experience with biosimilars in other therapeutic areas suggests that careful regulation and caution are needed. Published guidelines of regulatory authorities around the world on approval of biosimilars and, where available, insulin biosimilars were reviewed. Information was sourced through Internet searching and cross-referencing guidelines. As of August 2014, general biosimilar and insulin-specific guidelines are available in 34 countries and two countries/regulatory domains, respectively. Many guidelines are clearly related to, or partly derived from, the general and insulin-specific European Medicines Agency (EMA) guidelines. Areas covered by these guidelines are fairly consistent, covering preclinical, pharmacokinetic (PK), and pharmacodynamic (PD) studies in humans and clinical areas; however, there are differences in emphasis. The EMA insulin-specific guidelines include detailed criteria on PK/PD studies, as do most other general biosimilar guidelines and, to a lesser extent, clinical studies. The U.S. Food and Drug Administration has general biosimilar guidelines, emphasizing consideration of the whole package of in vitro, biological, and human studies, rather than concentrating on any one aspect. In countries such as Mexico, guidelines are broad, leaving wide discretion to the regulatory authority. In conclusion, from a global perspective, this area of drug regulation is heterogeneous and evolving, and the authors call for an initiative aimed at harmonizing the requirements for biosimilar insulins.
Introduction
Abiosimilar is generally defined as a biological medicine developed to be similar to a biological medicine already approved for human use (the “reference” product). This widely accepted term is used throughout this article, although alternative terminology is sometimes used elsewhere.1
Biosimilars are regarded as different from generics, which are considered to be chemically identical to their reference product. Although a protein developed to be a biosimilar will have a nominal primary amino acid sequence the same as the reference product, the manufacturer of the biosimilar has to develop its own production methodology, having no access to the complex production and purification techniques used for the reference product. Accordingly, these techniques will differ from those of the reference product. Differences in chemistry and physical structure may lead to potential differences in the safety, efficacy, and immunogenicity of the biosimilar product.2 Although a biosimilar product is not expected to have clinically meaningful differences compared with the reference product, the different manufacturing techniques for biologics may lead to potential differences from the reference product. Thus, before it is approved as a biosimilar, a biological copy from a different manufacturer has to be shown, in an appropriate regulatory approval process, to have no clinically meaningful differences compared with the reference product.
Examples of possible differences that might result include the glycosylation pattern of the protein, batch-to-batch consistency, and product stability.2,3 Accordingly, it is generally accepted that the requirements for marketing authorization of a generic (identical chemical structure, dosage formulation, and route of administration, along with bioequivalence) will not generally be sufficient for a biosimilar.
The patent protection for several insulin analogs has expired or will shortly,2,4,5 paving the way for approval of biosimilar insulins. The availability of biosimilar insulins could offer benefits, including reduced treatment costs, increased access to insulin, and increased choice of treatment options.2 Copies of human and other insulins (often incorrectly referred to as “biosimilar,” but for which regulatory biosimilarity has not always been established) are already on the market in several countries. In such countries, regulations on marketing approval do not require proof of biosimilarity per se at the registration of such medicinal products, or the biosimilarity requirements are different from those in North America or Western Europe.
The aim of this article is to provide an overview of the current status of biosimilar regulatory requirements in different countries, but not the clinical profile of individual biologics. Healthcare providers increasingly interact across geographical boundaries, and people with diabetes everywhere have similar needs for medication efficacy and safety. Therefore, it would be helpful if regulatory standards worldwide were consistent or at least convergent. It is acknowledged that the development of biosimilar guidelines is ongoing in many countries and that changes are thus likely. This overview addresses general topics relevant to biosimilars and then focuses on guidelines for biosimilar insulins. The overview helps to highlight the need for harmonization of the respective guidelines in different countries.
Methodology
As regulatory guidelines are usually not published in the peer-reviewed scientific literature, a formal literature search as would be carried out for a “conventional” scientific review was not possible. Therefore, extensive Internet searches (using the broad search terms “biosimilar insulin guideline” and “biosimilar guideline” and other terms used for “biosimilars”) and hand-searching were required to identify relevant documents relating to both general and insulin-specific biosimilar guidelines.
From within the guidelines, for the purposes of comparison, details from common topics covered, such as requirements for pharmacokinetic (PK) and pharmacodynamic (PD) studies, were extracted, and any unique or unusual requirements or explicit exclusions were identified. Where references are made to any product attributes, it is in the context of how they may have led to regulatory considerations. A truly complete and comprehensive overview of the requirements in each existing biosimilar guideline is outside of the scope of this review.
Global Overview
In 2009, the World Health Organization (WHO) issued global guidelines for the evaluation of similar biotherapeutic products.6 However, no specific guideline for insulin is available from the WHO. As these are nonspecific guidelines, the WHO noted that preclinical studies will have to be defined on a case-by-case basis. Similarly, although PK studies are recommended, the requirements for PD studies are noted to be variable. Randomized controlled trials (RCTs) are recommended, designed to establish “equivalence” to the reference product. Some safety (exposure) studies are required, even if formal efficacy studies are not performed, including adequate immunogenicity studies.
The International Conference on Harmonisation (ICH),7 which aims to provide a consistent set of standards for worldwide regulatory authorities and pharmaceutical industries, has not published guidelines specifically addressing biosimilars/biosimilar insulins. However, not all countries adopt ICH guidance as law.8
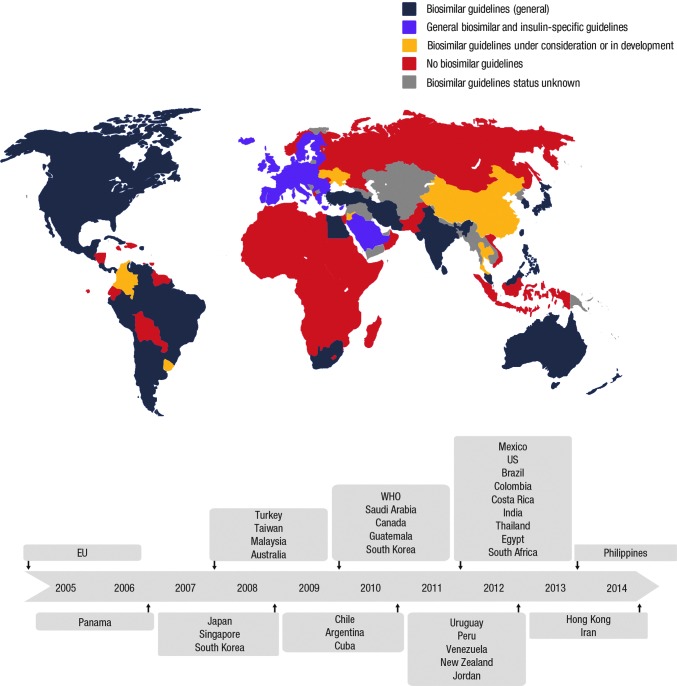
General guidelines for the approval of biosimilars have been developed or are being drafted by a large number of countries (Fig. 1),9 including all of North America, most of Europe, India and some other countries in Asia, Australia, and South Africa. The guidelines from Health Canada, the Jordan Food and Drug Administration (FDA), the Saudi Food and Drug Authority, the Australian Department of Health Therapeutic Goods Administration, the South African Department of Health, and the Ministry of Health Malaysia are informed by or have been adopted from the guidelines from the European Medicines Agency (EMA) (Table 1). As such, these guidelines have not been focused on in great detail in this overview, other than where there are differences (specifically in relation to insulins). A broader overview is provided for reference in Tables 2 and 3.6,10–26
FIG. 1.
Global overview of availability of general and insulin-specific biosimilar guidelines. EU, European Union; WHO, World Health Organization. After Scheinberg and Kay.9 © 2012 Macmillan Publishers Limited. All rights reserved.
Table 1.
Insulin-Specific Guidance in General Biosimilar Guidelines
| Country | Year | Insulin-specific guidance | Comments |
|---|---|---|---|
| Australia | 2013 | Crystal structure should be determined where this is necessary for action, such as for protamine zinc insulin; refer to product-specific guidelines from the EMA | Adapted from the 2005 EMA guidelines |
| Canada | 2008 | Specific nonclinical or clinical information (not specified) may be required for some products, including insulin; recombinant human soluble insulin products are an exception to the requirement for comparative clinical efficacy trials; only a comparative clinical safety study is required | Based on the WHO and EMA guidelines |
| Malaysia | 2008 | Requirements for drug classes such as insulins may vary (no specific guidance) | Reference to EU/EMA guidelines |
| India | 2012 | Recombinant human soluble insulin products are an exception to the requirement for comparative clinical efficacy trials; only a comparative clinical safety study is required | Based on the WHO and EMA guidelines |
| Egypt | 2012 | For clinical studies of insulin, the study population should consist of nonobese healthy volunteers or patients with type 1 diabetes, rather than insulin-resistant obese patients with type 2 diabetes | Based on the WHO, EMA, and ICH guidelines |
| South Africa | 2012 | Well-characterized, low-molecular-mass, medicinal biological compounds may be excluded by specific Council decision from biological medicine status | Based on the WHO and EMA guidelines |
| Jordan | Draft 2013 | Requirements for some drug classes such as insulins may vary; EMA guidelines (EMA/134217/2012) are presented | Apply EMA guidelines |
EMA, European Medicines Agency; EU, European Union; ICH, International Conference on Harmonisation; WHO, World Health Organization.
Table 2.
Overview of Nonclinical and Pharmacokinetic/Pharmacodynamic Requirements for Biosimilars Across Global Regions
| Organization and/or country | Nonclinical evaluation | PK/PD studies |
|---|---|---|
| WHO6 | Studies should be defined on a case-by-case basis | PK studies should be performed; PD studies may be required |
| EU10–12 | ||
| General biosimilars | Comparability in terms of physiochemical properties and of biological and immunological parameters (where appropriate); comparative purity/impurities In vitro studies should normally be undertaken In vivo studies should be performed in a relevant species Detailed guidance is provided |
Comparative PK/PD studies designed to show clinical comparability are essential Such studies may be sufficient to demonstrate clinical comparability in some (defined) circumstances |
| Insulin-specific | Comparative studies such as in vitro bioassays for affinity, as well as insulin- and IGF-1-receptor-binding assays, in addition to tests for intrinsic activity should be performed In vivo studies normally not required |
Crossover, preferably double-blind, insulin clamp studies are considered suitable Detailed guidance is given |
| United States13–15 | Comparability in terms of physiochemical properties, as well as biological and immunological parameters (where appropriate); impurities In vitro studies where appropriate In vivo studies should be performed in a relevant species depending on product |
Human comparative PK and PD studies generally are fundamental components in supporting a demonstration of biosimilarity Expected unless sponsor can provide scientific justification that such studies are unnecessary |
| Canada16 | Comparability in terms of physiochemical properties, as well as biological and immunological parameters (where appropriate); impurities In vitro studies should normally be undertaken At least one repeat-dose toxicity study in a relevant species |
Comparative PK and PD studies should be conducted General guidance is given on study design |
| Mexico17 | Guidelines are limited Biocomparability studies required In vivo studies may not be required At least one repeat-dose toxicity study in a relevant species |
Comparative studies are required when requested |
| Saudi Arabia18 | ||
| General biosimilars | Tailored approach depending on specific product, including impurities, structure, and immunogenicity In vitro studies as required At least one repeat-dose toxicity study in a relevant species |
Comparative studies essential Biosimilar insulins, specific requirement: single-dose crossover study, subcutaneous administration, in people with T1DM |
| Insulin-specific | Comparative studies such as in vitro bioassays for affinity, as well as insulin- and IGF-1-receptor binding assays, in addition to tests for intrinsic activity should be performed In vivo studies not normally required At least one repeat-dose toxicity study in a relevant species |
Required General guidance is given on study design |
| Jordan19 | Comparability in terms of physiochemical properties, as well as biological and immunological parameters (where appropriate); impurities In vitro studies where appropriate At least one repeat-dose toxicity study in a relevant species |
Required Detailed and extensive guidance on study selection and design is given |
| Egypt20 | Comparability in terms of physiochemical properties, as well as biological and immunological parameters (where appropriate); impurities In vitro studies where appropriate At least one repeat-dose toxicity study in a relevant species |
Required; all absorption, distribution, metabolism, and excretion PK parameters should be investigated; PD markers selected according to relevance Basic guidance on study design and selection is given |
| India21 | Comparability in terms of physiochemical properties, as well as biological and immunological parameters (where appropriate); impurities In vitro studies should be performed At least one repeat-dose toxicity study in a relevant species |
Comparative PK/PD studies should be performed in the most relevant population Guidance is given on study design and selection |
| South Korea22 | Comparability in terms of physiochemical properties, as well as biological and immunological parameters (where appropriate); impurities In vitro studies where appropriate At least one repeat-dose toxicity study in a relevant species |
Comparative PK/PD studies required Guidance is given on study design and selection |
| Malaysia23 | Comparability in terms of physiochemical properties, as well as biological and immunological parameters (where appropriate); impurities In vitro studies where appropriate At least one repeat-dose toxicity study in a relevant species |
Comparative PK/PD studies should be conducted Limited guidance is given on study design and selection; study selection to be justified |
| Australia24 | Content, purity and impurity profile; structure; immunochemical properties (if appropriate) In vitro studies where appropriate No specific guidelines on animal studies |
No specific regional guidelines; studies specified by EU guidelines required |
| New Zealand25 | No specific regional guidelines but a position statement has been published; information from clinical studies should be included; refers to EU/Food and Drug Administration guidelines | |
| South Africa26 | Full quality dossier required In vitro studies normally required At least one repeat-dose toxicity study, including toxicokinetic measurement in a relevant species |
Comparative PK/PD studies required; refer to EU guidelines In certain cases, comparative PK/PD studies between the similar biological medicinal product and the reference medicinal product may be sufficient to demonstrate clinical comparability without the need for clinical trials |
Only guidelines available in English are described here.
EU, European Union; IGF-1, insulin-like growth factor-1; PD, pharmacodynamic; PK, pharmacokinetic; T1DM, type 1 diabetes; WHO, World Health Organization.
Table 3.
Overview of Clinical Study Requirements for Biosimilars Across Global Regions
| Organization and/or country | Efficacy studies | Safety studies | Immunogenicity studies |
|---|---|---|---|
| WHO6 | RCTs usually required; equivalence design preferred | Adequate safety studies required, including for products not undergoing efficacy studies | Required in humans |
| EU10–12 | |||
| General biosimilars | RCTs usually required | Adequate safety studies required | Required, normally in humans |
| Insulin-specific | No anticipated need for specific clinical efficacy studies | Confirm safety comparability | Required, including in people with T1DM, as well as for ≥12 months |
| United States13–15 | Comparative safety and effectiveness data required where there are residual uncertainties about the biosimilarity of the two products based on structural and functional characterization, animal testing, human PK and PD data, and clinical immunogenicity assessment | Required Guidance on study design and selection is provided Study details to be justified and agreed with the agency Comparative parallel study design (e.g., head-to-head) recommended |
|
| Canada16 | Crucially important to demonstrate similarity in efficacy and safety profiles with few exceptions Not required for recombinant human soluble insulin products (comparative clinical safety study only required) |
Comparative safety required (nature, severity, and frequency of adverse events) | Required; methods to be justified (detailed guidance not given) |
| Mexico17 | May be required | May be required | Required where an immune response may affect the endogenous protein or its biological function No specific guidance given |
| Saudi Arabia18 | |||
| General biosimilars | Comparative studies required for general biosimilars; advice on study design and selection given Not required for insulin biosimilars provided that clinical comparability can be concluded from PK and PD data |
Not specified | Rationale for proposed immunogenicity testing should be presented; guidance on study design and selection given Required for biosimilar insulins; basic guidance given on study design |
| Insulin-specific | No anticipated need for specific clinical efficacy studies | Confirm safety comparability | Comparative study (duration ≥12 months) required to evaluate immunogenicity |
| Jordan19 | Comparative studies required Advice regarding study design is limited for general biosimilars, but is more detailed for specific biologics (not including insulins) Insulin-specific EU guidelines are cited |
Required for general biosimilars Insulin-specific EU guidelines are cited |
Required; rationale for chosen studies required; specific advice for general biosimilars not given Insulin-specific EU guidelines are cited |
| Egypt20 | Required, basic guidance on suitable studies given | Required; basic guidance on suitable studies given | Should be conducted pre- and postauthorization; specific guidance not given |
| India21 | Required with few exceptions; equivalence trials preferred May be waived if strict criteria are met Comparative efficacy studies not required for recombinant human soluble insulin products (comparative clinical safety study only) |
Required for all biologics, even where clinical trials are waived; no specific guidance on study design given | |
| South Korea22 | Usually required, but PK/PD studies may be sufficient in some cases Safety data from clinical trials are usually sufficient |
Required | Required; in humans Guidance on considerations for immunological testing is given |
| Malaysia23 | Required; equivalence trials preferred, but no detailed guidance is given | Comparability should be demonstrated; specific guidance not given | Required; rationale for chosen studies required; specific advice not given |
| Australia24 | No specific regional guidelines; studies specified by EU guidelines required | ||
| New Zealand25 | No specific regional guidelines but a position statement has been published; information from clinical studies should be included; refers to EU/Food and Drug Administration guidelines | ||
| South Africa26 | Usually necessary; may not be required if comparative PK/PD studies sufficient; detailed guidance not provided | Required; specific guidance not given | Required; guidance provided |
Only guidelines available in English are described here.
EU, European Union; PD, pharmacodynamic; PK, pharmacokinetic; RCT, randomized controlled trial; T1DM, type 1 diabetes; WHO, World Health Organization.
The U.S. FDA issued three draft guidance documents on biosimilar product development in February 2012 (Scientific Considerations in Demonstrating Biosimilarity to a Reference Product,13 Quality Considerations in Demonstrating Biosimilarity to a Reference Protein Product,14 and Biosimilars: Questions and Answers Regarding Implementation of the Biologics Price Competition and Innovation Act of 200915). A subsequent guideline from meetings between biosimilar sponsors and the U.S. FDA followed in 2013.27 Several biologics, including insulins, are currently regulated by the U.S. FDA as chemical drugs (under section 505 of the U.S. Food, Drug, and Cosmetic Act) and not as biological products. Certain “biological products” such as insulins will, however, be licensed as “biologics” starting in the year 2020. Meanwhile, Eli Lilly's LY2963016 (insulin glargine) was accepted as a New Drug Application rather than through the biosimilars pathway28 and received tentative approval by the U.S. FDA.29
The China State FDA does not have an established regulatory pathway for the development of copies of biological drugs, meaning that they have to go through a new drug-approval process. Domestic copies of multinational, branded biologicals have been approved without the need for clinical data to support comparability with previously registered branded products. Up to December 2011, 40 biological drugs have been approved in China, many of which were copies of biological drugs, and between 2011 and 2012, 41 companies were actively engaged in research relating to such copies in China.30 The Chinese Center for Drug Evaluation (part of the China State FDA) published draft guidelines for approval of biosimilars in October 2014 for consultation. Meanwhile, copies of biological drugs will continue to go through a new drug-approval process.31
It is worth noting that, although alternative regulatory pathways may be used (e.g., as a new drug [as in the United States for insulin] or as a copy of a biological drug), these do not preclude the application of comparable scientific principles and expectations to evaluate or demonstrate similarity of a biological product compared with a reference product to both pathways.
Aspects of General Biosimilar Regulations Relevant to Biosimilar Insulins
Selection of reference product
All guidelines identified, with the exception of those from South Africa, provide some degree of guidance for selection of the reference product (predominantly requiring the reference product to have been approved in the country), although those from Mexico are limited in this respect. The use of a single reference product for all comparative quality, safety, and efficacy studies is a consistent requirement across most guidelines. Most require that the reference product should have been approved in the same regulatory jurisdiction, although the use of reference products approved by regulatory authorities with similar scientific and regulatory standards would be considered by some authorities (in the European Union [EU], Saudi Arabia, Jordan, Egypt, South Korea, and India). In Australia, reference products manufactured and sourced outside of Australia can be used for comparison if a bridging study between the Australia-sourced product and the reference product is conducted.24 Similarly, draft U.S. FDA guidelines require the use of a single reference product previously licensed by the U.S. FDA, but under certain circumstances, a non–U.S.-licensed reference product may be used.13
Generally, the biosimilar to be studied is required to have the same dosage form, strength, and route of administration as the reference product. The use of another biosimilar or a reference standard (e.g., human insulin for an insulin analog) as the reference product is not permitted by the majority of guidelines.
Physicochemical characterization
Differences have been observed in the development of biosimilars in physicochemical characterization compared with reference products, for example, with biosimilar erythropoietins.32 These have included differences in composition, including products that did not meet self-declared specifications and batch-to-batch variation. The potential for such differences for new biosimilars based on equally or more complex production processes has driven requirements for characterization and preclinical studies. Accordingly, all available guidelines include detailed recommendations for nonclinical studies. Generally, these include assessment of structure, physiochemical properties, and biological and immunological properties where appropriate (Table 2). Such methods would vary by the type of biosimilar being developed, with only EU and Saudi Arabian guidelines providing details specific to insulin biosimilars (see below). The EMA provides very detailed general guidance regarding physicochemical and biological characterization, as well as quality attributes pertaining to a biosimilar and its reference product, including assessment of composition, its physical properties, and primary- and higher-order structures of a biosimilar together with qualitative and quantitative comparison of the purity and impurity profiles.33,34
The U.S. FDA guidelines highlight that the biological product should be highly similar to the reference product, notwithstanding minor differences in clinically inactive components, and that there should be no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product. Product- and process-related impurities should be characterized, identified, and quantified for the biosimilar and the reference product, and the potential impact of differences in the impurity profile on safety should be addressed and supported by appropriate data.14
The Korean FDA (now the Ministry of Food and Drug Safety) guidelines are as stringent as the EU guidelines, requesting a complete description of the manufacturing process for the biosimilar—including demonstration of consistent quality, compliance with good manufacturing practice requirements, and details of quality control/quality assurance, in-process controls, and process validation. They also request that if a change is introduced to the manufacturing process of the biosimilar product, comparability studies should be carried out, and the comparability of the biosimilar products manufactured before and after such a change should be evaluated.22 Like the EMA and draft U.S. FDA guidelines, the Korean FDA guidelines note that physicochemical characterization should include determination of composition, physicochemical properties, and primary- and higher-order structures of the active ingredient of the biosimilar, together with evaluation of any product- and process-related impurities and/or degradation products.22 A biological assay can be complementary to physicochemical analysis. The purity and impurity profiles of the biosimilar and the reference product should be assessed both qualitatively and quantitatively by a combination of analytical procedures. The guidelines also discuss the potential for degradation and the need for stability studies.
The Mexican guidelines state that if a biosimilar is well characterized, and the physicochemical comparability is proven, a lower level of clinical evidence is required.17
In vitro bioassays
Most guidelines include requirements for in vitro biological studies, which for insulin would imply receptor binding and postreceptor cellular bioactivity studies in relevant insulin-sensitive cultured tissues (see Table 2). Although not specified, the history of insulin analog development would also imply some insulin-like growth factor-1 receptor studies. The insulin-specific guidance of the EMA stresses that assays that have been demonstrated to have appropriate sensitivity to detect small differences should be used and that experiments need to be based on sufficient numbers of replicates, dilutions, or time points per curve to characterize the whole concentration–response or time–response relationship.12
The draft U.S. FDA guidelines provide guidance for comparative in vitro and/or in vivo functional assays, including, but not limited to, bioassays, biological assays, binding assays, and enzyme kinetics, which are recommended to support biosimilarity and may be used to justify a selective and targeted approach to animal and/or clinical testing.13 If animal toxicity studies are not warranted (see below), additional comparative in vitro testing, using human cells or tissues, may be warranted. The Korean FDA guidelines note that receptor-binding studies or cell-based assays should normally be undertaken to establish the comparability of the biological/PD activity of the biosimilar and the reference product.22 A requirement for in vitro studies (to be read as tissue biology studies) is usual in other guidelines (Table 2). In contrast, the Mexican guidelines state that in vitro studies may not always be required.17
Preclinical animal studies
Preclinical animal studies, such as PK/PD, toxicology, and immunology studies, tend to be required to a lesser extent in biosimilar guidelines than in standard new drug submissions. The U.S. FDA notes that under certain circumstances, a single-dose study in animals comparing the biosimilar and reference product using PK and PD measures may contribute to the totality of evidence.13 Although the general EMA guidelines note that in vivo studies may be required to provide complementary information, provided that a relevant model with regard to species or design is available,35 they are not required for insulin biosimilars (see below). Many of the country guidelines suggest in vivo repeated-dose toxicology studies, but the specification of “at least one” implies these would be limited compared with a new biological drug. Such studies are of limited value for insulin, where hypoglycemia limits the ability to give supraphysiological doses for toxicology purposes. The U.S. FDA guidelines note that the scope and extent of animal toxicity studies will depend on the body of information available on the reference product. In general, nonclinical safety pharmacology, reproductive and developmental toxicity, and carcinogenicity studies are not required when the biosimilar product has been demonstrated to be highly similar through extensive structural and functional characterization.13
The Korean FDA guidelines require that at least one repeat-dose study be performed in a relevant species, using state-of-the-art technology, and that, depending on route of administration of a biosimilar product, a local tolerance study may need to be performed. If comparability of the biosimilar product and reference product is verified through quality evaluation, other toxicological studies are not generally required, unless triggered by results of the repeat-dose toxicity study and/or by other known toxicological properties of the reference product.22
The Mexican guidelines provide significantly less detail than the other guidelines. However, they note that preclinical studies on animals, comparing the reference product and the biosimilar product, should be carried out in animal species relevant to the study model and must include a comparative study of the PD effect and activity relevant to the clinical application, as well as a comparative toxicology report in at least one toxicity study of repeated dosage, including toxicokinetic measurements. They also state that the study duration should allow the detection of relevant differences in toxicity and immune responses between the biosimilar and the reference product.17
The U.S. FDA guidelines note that animal immunogenicity assessments generally do not predict potential immunogenic response to protein products in humans, although they may be useful if there are differences in impurities or excipients or differences in manufacturing.13 Similarly, the Korean FDA guidelines acknowledge that the predictive value of animal models for immunogenicity in humans is generally low.22
Comparative human PK/PD studies
In general, biosimilar guidelines all require human PK/PD studies and, in particular, studies making comparisons with the reference product (Table 2). Typically, studies form part of a complete clinical studies package, but some guidelines (e.g., those from Saudi Arabia and South Africa) state that data from these studies may be sufficient to obviate the need for additional clinical trials.
The draft guidance from the U.S. FDA states that scientific justification for the selection of the human PK/PD study population and parameters, taking into consideration the relevance of these choices, should be provided. Guidance on the type of studies required, considerations for study design, and selecting the study population is also provided.13 The Korean FDA guidelines state that PK studies should generally be performed for all proposed routes of administration and using doses within the therapeutic dose range of the reference product. The choice of studies should be “justified,” and guidance on the type and design of suitable studies is provided.22 Similarly, the Egyptian guidelines provide basic guidance on study design and selection.20 The Mexican guidelines note that a comparative PK study report may be required in order to demonstrate the PK biocomparability between the biocomparable biotechnological medication and the reference biotechnological medication with regard to the key parameters, but specific detailed guidance is not given.17
The EMA has provided detailed biosimilar insulin-specific guidelines (discussed below), which appear to be more stringent, perhaps because of their level of clarity and detail.
Clinical studies: efficacy
Clinical studies address four main areas, namely, efficacy, tolerability, safety, and immunogenicity, generally all within a single clinical trial. Requirements for comparative clinical efficacy studies vary across the guidelines from different countries, with a general trend toward no specific requirement for demonstration of efficacy per se (Table 3) and rather greater focus on safety assessment.
The U.S. FDA has retained the discretion to determine whether certain requirements are included in a biosimilar application and recommends a stepwise approach to demonstrating biosimilarity and consideration of the totality of the evidence when reviewing applications.13 Therefore, the type and extent of the comparative clinical safety and effectiveness data required can be influenced by the extent of, and findings from, structural and functional characterization studies and findings from preclinical and PK/PD analyses. Additional factors can influence clinical safety and effectiveness requirements, including the degree of understanding of the mechanism of action of the reference product and disease pathology, together with the extent of clinical experience with the reference product and its therapeutic class.13
If required, clinical studies should be designed such that they can demonstrate that the proposed product has neither decreased nor increased activity compared with the reference product, both of which would affect licensure. The guidelines state that a study using a two-sided test, in which the null hypothesis is that either that the proposed product is inferior to the reference product or the proposed product is superior to the reference product based on a prespecified equivalence margin, is the most straightforward design. Margins should be scientifically justified and enable detection of clinically meaningful differences in effectiveness and safety between the proposed product and the reference product. This has implications for study power, and although no details are provided, the standard Phase 3 minimum duration of 26 weeks would seem to apply as a general standard.
The U.S. FDA recommends that end points and study populations that will be clinically relevant and sensitive in detecting clinically meaningful differences in effectiveness between the proposed product and reference product should be used.13 End points different from those in the reference product's clinical trials can be used if they are scientifically justified.
In the Canadian guidelines, it is noted that comparative clinical trials are crucial for demonstrating the similarity in efficacy between the biosimilar and the reference product, with few exceptions. The exception noted is recombinant unmodified human insulin products, for which no efficacy study is required.16
In South Korea, the efficacy of the biosimilar product and the reference product should be demonstrated in an adequately powered, randomized, and parallel-group clinical trial.22 Such studies should preferably be double-blinded or observer-blinded at a minimum. The South Korean guidelines state that, usually, clinical trials are required to demonstrate similar efficacy between the biosimilar product and the reference product. However, comparative PK/PD studies alone may be appropriate if the PK/PD properties of the reference product are well characterized, if at least one PD marker is an accepted surrogate marker for efficacy, or if the relationship between dose/exposure, the relevant PD marker(s), and response/efficacy of the reference product is well established. The study population and dosage should be sensitive enough to detect potential differences. Otherwise, it will be necessary to investigate a relevant dose range to demonstrate that the test system is discriminatory.
As with the Canadian guidelines, the Egyptian guidelines require a confirmatory clinical study to demonstrate biosimilarity, but note that the study can be waived if certain conditions are met.20 Similarly, in India, confirmatory efficacy studies can be waived if specified criteria are met.21
Clinical safety, tolerability, and immunogenicity
Comparative safety studies are generally required for biosimilars in all guidelines (Table 3), although the population exposure in terms of both duration and numbers tends to be smaller than for a new product. For perspective, using a new glucose-lowering entity as an example, the Phase 3 development program for liraglutide comprised six RCTs involving >4,400 participants.36
The Korean FDA guidelines require that preauthorization safety data should be obtained in a sufficient number of participants to characterize the safety profile of the biosimilar product, and comparison with the reference product should include type, frequency, and severity of adverse reactions.22 Such safety data obtained from clinical trials are usually sufficient, but further close monitoring of clinical safety of the biosimilar product is usually needed in the postmarketing period.
As noted above, the U.S. FDA guidelines state that a clinical study is performed for efficacy and safety.13 In the Mexican guidelines, although guidance on study design and selection is not provided, comparative clinical safety studies compliant with good clinical research practice are required to demonstrate the clinical similarity between the biocomparable biotechnological medication and the reference biotechnological medication.17
Many of the guidelines pay closer attention to immunogenicity, perhaps reflecting the general awareness of this aspect of biological drugs, rather than an insulin-specific issue. The U.S. FDA guidelines provide requirements for the type and design of appropriate studies, but note that the extent and timing of a clinical immunogenicity program can vary depending on the extent of analytical similarity and the incidence and clinical consequences of immune responses to the reference product. If the clinical consequence of an immune reaction is severe, more extensive immunogenicity assessments will likely be needed. However, if the immune response to the reference product is relatively rare, as in diabetes, two separate studies may be sufficient to evaluate immunogenicity—a premarket study powered to detect major differences and a postmarket study to detect more subtle differences.13 Use of a comparative parallel design (head-to-head) is recommended, and the study population should be justified and agreed to by the U.S. FDA. Similar to criteria for general efficacy and safety assessment, a one-sided test may also be adequate in a clinical study evaluating immunogenicity. The follow-up period should be determined based on the time course for the generation of immune responses and expected clinical sequelae, the time course of disappearance of the immune responses and clinical sequelae following cessation of therapy, and the length of administration of the product.13 Guidance on expected end points for clinical immunogenicity studies is provided.
The EMA has similarly detailed guidelines on the clinical assessment of immunogenicity (discussed below for insulin-specific guidelines). The Korean FDA noted that the frequency and type of antibodies induced as well as possible clinical consequences of an immune response should be compared for a biosimilar product and a reference product before authorization, having been investigated in people, including all participants in any clinical studies; these studies need to be of sufficient duration to allow for observation of clinically significant antibody formation.22
Mexican guidelines require immunological testing and reporting, including for hypersensitivity, infusion reactions, and effects on efficacy for any product where an immune response may affect the endogenous protein or its biological function.17 The Egyptian guidelines note that immunogenicity studies are required but provide limited guidance other than that immunogenicity studies should be conducted both pre- and postauthorization.20 In India, pre- and postapproval assessments of safety including immunogenicity data are recommended, but only basic guidance and requirements are provided.21
Pharmacovigilance and risk management
Risk management plans (RMPs) and pharmacovigilance (PV) plans are required in the majority of available guidelines but are not in general further specified with respect to biosimilars. Often, there is reference to the ICH or EU/U.S. guidelines on postmarketing safety monitoring instead. For example, in the EU, an RMP in accordance with current EU legislation and PV guidelines should be presented. The European Commission introduced a PV directive in 2010, which is a legal requirement for a member state in the EU to take all necessary measures to identify biological medicines that are prescribed, dispensed, or sold in its country.37 The directive notes that all medicinal products with a new active substance and biological medicinal products, including biosimilars, are priorities for PV.
In Australia, a comprehensive RMP must be developed and submitted with the biosimilar application, outlining the PV procedures to be implemented as detailed in the Australian and adopted EU guidelines. A range of mandatory postregistration requirements for the sponsor are provided, including notification of the Therapeutic Goods Administration (TGA) of the person responsible for fulfilling the sponsor's obligations, submitting Periodic Safety Update Reports and Adverse Events Reports, notifying the TGA of any significant safety issues, and ensuring that requests for additional information from the TGA are answered fully and within the requested time frame.24 Importantly, conventions for naming biosimilars are provided, in terms of both Australian approved names and trade names, with the aim of ensuring prescribers are able to identify the reference product and clearly distinguish between it and different biosimilars (see additional details below).
The guidance from the U.S. FDA states that postmarketing safety monitoring should take into consideration any particular safety or effectiveness concerns associated with the use of the reference product and that adequate mechanisms should be in place to differentiate between the adverse events (AEs) associated with the proposed biosimilar product and its reference medication.13
Health Canada requires that an RMP be developed prior to authorization and notes that a PV plan should be provided. Postmarketing requirements (in terms of adverse drug reaction reporting and period-safety update reports) are also provided.16 In Mexico, for new drug molecules, including biological and biotechnological products, an RMP must be presented to the National Pharmacovigilance Centre. Where a specific risk is identified following approval, an RMP is required, which may include an intensive PV study.17
The guidelines from Egypt stipulate that a PV plan in accordance with Egyptian Pharmacovigilance Center guidelines must be submitted and should include a protocol for a postmarketing immunogenicity study at the time of submission of the marketing application.20 RMPs are required in the Indian guidelines, and these should include a PV plan. In India, which is the largest market for biosimilars worldwide with longstanding use and clinical experience of a range of biosimilars, there have been examples of concerns or withdrawals that have led to a call for more stringent PV.38 Postmarketing requirements (in terms of adverse drug reaction reporting and period-safety update reports) are also provided.21
Interchangeability and substitution
Although there is no standard definition worldwide, interchangeability generally refers to the practice of switching one medicine for another, provided that the medicines have been determined to be equivalent in a given clinical setting. Substitution generally refers to a national administrative rule that requires or permits the switch from one medicine to another medicine proven to have the same quality, safety, and efficacy, usually taking place at the retail pharmacy level or at hospital pharmacies.39
Issues of interchangeability and substitution are not generally discussed by available guidelines, and only the U.S., Saudi Arabian, and Jordanian guidelines provide any guidance.13,18,19 In the EU, substitution decisions are the responsibility of the national drug-regulatory and -prescribing agencies in each country.
Although interchangeability is not specifically discussed in the U.S. FDA biosimilar guidelines, interchangeability is clearly defined in U.S. law in the Biologics Price Competition and Innovation Act of 2009.40 This amendment to Section 351(k) of the Public Health Service Act creates an abbreviated licensure pathway for biological products shown to be biosimilar to, or interchangeable with, a U.S. FDA-licensed biological reference product. To meet the higher standard of “interchangeability,” sufficient information must be provided to demonstrate biosimilarity and also to demonstrate that the biological product can be expected to produce the same clinical result as the reference product in any given patient; as well, if the biological product is administered more than once to an individual, the risk in terms of safety or diminished efficacy of alternating or switching between the use of the biological product and the reference product is not greater than the risk of using the reference product without such alternation or switch. “Interchangeable” products may be substituted for the reference product without the intervention of the prescribing healthcare provider. Labeling should indicate if the product has or has not been determined to be interchangeable with the reference product.
Guidelines from Saudi Arabia state that pharmacists cannot substitute biosimilars without consultation with treating physicians.18 The guidelines recommend that, following mandatory physician and patient discussion, substitution of an innovator drug for a biosimilar that used the innovator drug as a reference product for comparability (or vice versa) is acceptable. Changing from a biosimilar from one manufacturer to a biosimilar from another manufacturer is also allowed if they used the same reference product for comparability, but patients should be closely monitored. The Jordanian FDA guidelines stipulate that automatic substitution cannot apply to biologicals, including biosimilars.19
Extrapolation of indication
Extrapolation of indication refers to the regulatory principle that a product approved as a biosimilar for one indication (i.e., in one particular patient population) will be approved for use in all patient populations and disease states in which the originator product had been licensed. For example, an insulin biosimilar could be approved based on a study in people with type 1 diabetes, but upon approval, it would receive approval for use in all patient populations for which the reference product has been approved (e.g., type 2 diabetes, pediatrics).
Overall, guidance on extrapolation of indication is not provided or specified by many of the biosimilar guidelines. Where the issue is addressed, in guidelines from the United States, Canada, Saudi Arabia, Jordan, Egypt, South Korea, Malaysia, and Australia, it is stated that extrapolation may be possible if a range of conditions are met.13,16,18–20,22–24 For example, in the United States, sufficient scientific justification for extrapolating clinical data to support a determination of biosimilarity for each condition of use will need to be provided; guidance on such requirements is given.13 In South Korea, if similar efficacy and safety have been demonstrated with the biosimilar product and the reference product for a particular clinical indication, extrapolation of these data to other indications of the reference product may be possible if a sensitive clinical test model has been used that is able to detect potential differences between the biosimilar product and the reference product, if the clinically relevant mechanism of action and/or involved receptor(s) is the same, and if safety and immunogenicity have been sufficiently characterized.22
Insulin-Specific Biosimilar Guidelines
Only two guidelines specific to biosimilar insulins have been issued. The EMA has issued several product-specific annexes, including a specific guideline covering recombinant human insulin issued in 2006 and revised in 2012 and in 2014.12,41,42 The guidelines from Saudi Arabia have a separate section dedicated to insulin, seemingly following the EMA guidelines, cited in the guideline document.18
Limited references to specific considerations for insulin biosimilars are given in other general biosimilar guidelines from seven countries, but these are often historic, such as the Australian reference to crystal structure or the Canadian/Indian exemption for unmodified human insulin products (Table 1). In some guidelines, reference is often made to insulin having specific requirements, without those being listed (e.g., Jordan, Malaysia).
With regard to preclinical studies, the insulin-specific guidelines from the EMA call for comparative and head-to-head tissue studies, including in vitro bioassays for insulin and insulin-like growth factor-1 receptor affinity and insulin postreceptor biological activity.12 The EMA guidelines note that comparative studies of PD effects in animals would not be anticipated to be sensitive enough to detect differences not identified by in vitro assays and are, therefore, normally not required.12 Separate repeated-dose toxicity studies are also not generally required (although they should be considered if novel or less well-studied excipients are to be used), and safety pharmacology and reproduction toxicology studies are not required.
For clinical PK/PD studies, the draft EMA insulin-specific guidelines provide particularly detailed guidance on what is required.12 Crossover, double-blind (preferably), glucose clamp studies using single subcutaneous doses of the test and reference agents performed at an interval of a few days to a few weeks are considered suitable, with a wash-out phase between study periods to avoid carryover effects. Time–concentration and time–action profiles should be studied simultaneously (in the same clamp study). Intravenous studies are not required. The study population should be homogeneous and insulin-sensitive to best detect potential product-related differences and may consist of normal-weight healthy volunteers or people with type 1 diabetes (established by measurement of serum C-peptide concentrations). For long-acting insulins, the guidelines note that it may be difficult to determine the duration of action, particularly where production of endogenous insulin is present. Therefore, people with type 1 diabetes are judged to be most suitable for determining the time–action profile of long-acting insulins. Recommendations are not given on repeat-dose clamps where insulin duration is in excess of 24 h. As insulin sensitivity in women may vary during the menstrual cycle, restriction of studies to men would be justified. Detailed information on clamp methodology and the PK/PD end points and statistical analyses is given. Clamp biosimilarity is established when the results are within certain confidence intervals, namely, 80–125%.12
Although the general EMA biosimilar guidelines state that RCTs for efficacy are usually required, the EMA insulin-specific guideline states that there is no anticipated need for specific efficacy RCTs because end points used in such studies, usually level of hemoglobin A1c (HbA1c), are not considered sensitive enough for the purpose of showing biosimilarity of two insulins. There is no discussion of other possible end points, such as prebreakfast (fasting, basal) or postprandial plasma glucose.12
In the insulin-specific EMA guidance, the main focus of any clinical study gathering safety data is, as might be expected, the AE profile, but this includes experience of hypoglycemia and local (injection-site) tolerability. These are expected to remain within statistically defined limits of being indistinguishable for the biosimilar and the reference product.12
For immunogenicity studies, the EMA makes the specific requirement that studies should always include a reasonable number of people with type 1 diabetes, reflecting the propensity of this population to immunological responses. If a mixed population is included, stratification for type of diabetes and preexisting anti-insulin antibodies is necessary. It is acknowledged that blinding of study participants is likely unfeasible, but, at minimum, anti-drug antibodies should be determined in a blinded fashion.
As anti-drug antibodies, if any, usually develop early on, a 6-month study investigating incidence and titers of antibodies to the test and reference medicinal products should be performed. The primary outcome measure should be the incidence and titers of antibodies to the test and reference products. However, there is no need to power the study to demonstrate formally noninferiority.
The guidelines also state that the potential impact of antibodies (notably insulin-neutralizing antibodies on glycemic control and insulin dose requirement, and safety measures), in particular, local and systemic hypersensitivity reactions, should be investigated, and the necessity for further characterization (for example, with regard to neutralizing potential) should be considered.12 In certain cases, a prelicensing safety study including immunogenicity assessment may be waived, if the specific prerequisites apply. First, biosimilarity between the biosimilar and the reference insulin can be convincingly concluded from the physicochemical and functional characterization, from comparison using sensitive, orthogonal, and state-of-the-art analytical methods, and from the comparison of the PK and PD profiles. These data should provide sufficient reassurance that adverse drug reactions that are related to exaggerated pharmacological effects (e.g., hypoglycemia) can be expected at similar frequencies. Second, the impurity profile and the nature of excipients of the biosimilar do not give rise to concerns. Appropriate scientific justification for this should be provided.
Discussion
Guidelines on the registration and marketing authorization of biosimilars reflect an increased level of activity and development in this area. This is very much the case in the insulin field, with the expiry of patents for insulin analogs such as insulin lispro, insulin aspart, and insulin glargine in the first half of the current decade. The submission of copies of human insulin or insulin analogs for marketing approval is not new and includes experience with human insulin in earlier years in Europe43–46 and, more recently, the positive opinion from the Committee for Medicinal Products for Human Use for Eli Lilly's LY296301647 and its approval by the European Commission.
The global experience with erythropoietin and human growth hormone has suggested that despite important benefits of biologic copies, potential, unexpected immunogenicity or safety findings can occur, which may require greater postmarketing surveillance.48–50 With erythropoietin, such findings occurred after a change in the production process. This underlines the importance of (and the requirement for) postmarketing surveillance for all biological products; this should be at least as great as existing surveillance programs for the introduction of a new reference product.
Based on a broad search, biosimilar guidelines now exist in many countries, although in most cases these are general rather than insulin-specific guidelines. Often they do seem to have, or acknowledge, allegiance to earlier EMA biosimilar guidelines. Nevertheless, some differences do exist between guidelines, notably in the availability of insulin-specific guidelines, and, perhaps in parallel to that, the amount of detail given varies—notably from the methodological advice on glucose clamp studies in the current draft of the EMA guideline to the relatively indeterminate advice in the Mexico guidelines, or the complete absence of guidelines in China.12,17 In some countries, such as the United States, biosimilar insulins are currently reviewed as new medications, even in cases where the same product may have been approved as a biosimilar in other countries or regions. It is worth noting that the focus of our article is on written guidelines; however, in the actual submission package for all newly developed medicines, the results from specific regulatory consultation and feedback during the review process are introduced. In other words, guidelines are only the starting point of the approval process.
Perhaps not surprisingly, there is considerable consistency in the requirement for demonstration of physicochemical identity as far as is possible, but detail as to what that means for insulin is absent. Similarly, although in vitro receptor binding and biological action studies are mandated, the nature of these is seemingly left for the most part to the applicant. In general, it is recognized that PK/PD studies and immunogenicity studies in animals are less informative, although this is not universal. Similarly, general guidelines often suggest the performance of a single animal toxicity study, but the problems of this in regard to insulins are not usually addressed.
PK/PD studies with humans are generally regarded as core requirements, and the insulin-specific guidelines from the EMA put great emphasis on them. However, some limitations of this technique bear acknowledgment, such as the challenge of conducting glucose clamp procedures for at least 24 h in order to compare long-acting basal insulins, with extended periods of no food intake by subjects being a key issue.51 Indeed, although the EMA puts great emphasis on the PK/PD studies, the suggested confidence intervals for bioequivalence are 80–125% for PK, which appear rather wide for clinical reliability, where small changes in dose could impact glycemic control or hypoglycemia. The rationale for a smaller confidence interval range is based on several clinical and biological aspects related to insulin therapy. We believe that a potential 45% variation in the PK profile of any two products would be considered rather large, particularly if they are expected to be considered “similar.”
This suggests that the more general approach of the U.S. FDA, that the data package needs to be looked at in its entirety, is more appropriate. The U.S. FDA guidelines note that margins should be scientifically justified and enable detection of clinically meaningful differences in effectiveness and safety between the proposed product and the reference product. This would imply for HbA1c that the criterion used for noninferiority in new insulin development studies might be used, namely, within a confidence interval of 0.30% or 0.40% (3.3 or 4.4 mmol/mol). The EMA might be right that HbA1c is not a sensitive discriminator in clinical efficacy studies, even in people with type 1 diabetes, but other measures in combination can also be used—notably, pre-injection glucose level, prebreakfast glucose level (basal insulins), 2-h postprandial glucose (prandial insulins), various measures of variability, and hypoglycemia at different times of day can all contribute to the comparability assessment. However, biosimilar guidelines do not usually refer to the clinical end points just mentioned for comparison or establishment of biosimilarity. This is illustrated by the recent package of clinical and PK/PD studies on their insulin glargine presented by Eli Lilly at the 2014 American Diabetes Association meeting,52–57 where, in addition to HbA1c, other glycemic measures provide confidence of biosimilarity.
In general, the safety and immunogenicity requirements in the guidelines are fairly consistent (a clinical study of limited duration and limited size with insulin antibody and related measurements), but the kind of exposures likely to be acceptable (a maximum of 500 person-years) may not be able to fully assess uncommon AEs. A potential problem here is that although postmarketing surveillance is also mandated in different ways by most guidelines, this approach, too, is also not best suited to detect rare or uncommon events and would not, for example, detect an immune-related event (including neutralizing activity) at 1:1,000 people with diabetes. Furthermore, if prescription by nonproprietary (scientific) name rather than the proprietary (brand/manufacturer) name becomes the norm, and there is not a unique naming system in place for a biosimilar and its reference product, traceability and identification of new AEs will be problematic, a point not specifically addressed by these guidelines. This is an important consideration that is possible to implement in practice and that is within jurisdiction pathways that can be used by the agencies. Given the range of RMP and PV plan requirements across countries and pharmaceutical companies, the variability in reporting by healthcare providers, and the absence of any “centralized” worldwide reporting registry, establishing any long-term trends or findings of safety or immunogenicity signals may be a challenge. The lack of a common worldwide standard reporting infrastructure could also create difficulty in consolidating or detecting any patterns of safety findings, or determining whether an AE signal identified with one copy is necessarily detected by other marketed copies or the reference product.
Monitoring immunogenicity may have limitations. Immunological problems with insulin mostly disappeared in the 1970s with the introduction of chromatographic methods of purification in manufacturing, including for insulin analogs (meaning analogs of human insulin), human insulin, and animal insulins. Current methods could detect differences in immunogenicity potentially related to manufacturing process differences, although this tends to be more applicable to noninsulin biologics, such as monoclonal antibodies and glucagon-like peptide-1 receptor agonists.58–60 To our knowledge, insulin antibodies are rarely measured in clinical practice, posing difficulties for postmarketing surveillance of antibody-related problems.
There is one additional potential challenge, which is that guidelines cannot easily address or monitor manufacturing consistency over time (such as batch-to-batch variability, impurity patterns), which can be a source of potential immunogenicity.
Our global overview has several limitations. Searching for national guidelines is not as simple as searching for scientific publications, and indeed several national guidelines were not available in full online or in English. In some cases, the guideline versions will not have been final, and indeed this is a fluid field, so revisions may be under way without our being aware of them. Given the number of countries involved and the lack of consistent structure between guidelines, an attempt has been made to select and highlight what might be of interest to the reader, accepting that needs do vary. However, it is hoped that Tables 1–3 go some way toward redress of that issue.
In conclusion, global regulatory guidelines demonstrate considerable variation, although there are some important consistent elements in establishing standards for review and approval of biosimilar products, including physicochemical characterization, precise PK/PD assessment, and postmarketing surveillance. As the availability of biosimilar products offers the increasing benefits of access and reduced cost to patients worldwide, the presence of more uniform global standards may also become more important clinically. Similar to other global pharmaceutical standards such ICH or Good Clinical Practice, it might be beneficial to clinicians if regulatory bodies were to establish a common evaluation criterion for biological products available globally.
Acknowledgments
The contents of this article and the opinions expressed within are those of the authors, and it was the decision of the authors to submit the manuscript for publication. The authors took responsibility for the writing of this manuscript, including critical review and editing of each draft, and approval of the submitted version. The authors received writing/editorial support in the preparation of this manuscript provided by Katherine Roberts, PhD, of Excerpta Medica, funded by Sanofi US, Inc. The authors have not received any funding from Sanofi for writing this manuscript.
Author Disclosure Statement
L.H. is an employee of Science & Co. He receives, or has received, funding from companies manufacturing insulin or developing novel insulins, including Biodel, Eli Lilly, Halozyme, Novo Nordisk, and Sanofi. He is a member of a global biosimilar insulin advisory board of Sanofi. He is a partner of Profil Institut für Stoffwechselforschung, Neuss, Germany, and Profil Institut for Clinical Research, San Diego, CA. H.K. is an employee of Sanofi. R.M. provides consultancy services in relation to biosimilars on a periodic basis to AbbVie, Sanofi Aventis, Medscape, and the not-for-profit entity Therapeutic Innovation Australia. In the past 3 years, he has received research funding from the National Health and Medical Research Council (Australia), Australian Research Council, the Ramaciotti Foundations, Flinders Medical Centre Foundation, Flinders University, Cancer Council South Australia, Bio SA, the Heart Foundation, and the Australian Federal Government Department of Industry. P.H. receives, or has received, funding from most manufacturers of glucose-lowering products in diabetes care including insulins, and specifically from insulin manufacturers of potential reference or biosimilar products, including Biocon, Eli Lilly, Merck, Mylan, Novo Nordisk, and Sanofi.
References
- 1.Thorpe R, Wadhwa M: Terminology for biosimilars—a confusing minefield. GaBI J 2012;1:132–134 [Google Scholar]
- 2.Rotenstein LS, Ran N, Shivers JP, et al. : Opportunities and challenges for biosimilars: what's on the horizon in the global insulin market? Clin Diabetes 2012;30:138–150 [Google Scholar]
- 3.Owens DR, Landgraf W, Schmidt A, et al. : The emergence of biosimilar insulin preparations: a cause for concern? Diabetes Technol Ther 2012;14:989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinemann L: Biosimilar insulins. Expert Opin Biol Ther 2012;12:1009–1016 [DOI] [PubMed] [Google Scholar]
- 5.Gough S: Biosimilar insulins: opportunities and challenges. Pract Diabetes 2013;30:146–148 [Google Scholar]
- 6.World Health Organization: Guidelines on Evaluation of Similar Biotherapeutic Products (SBPs). Expert Committee on Biological Standardization, Geneva, 19–23 October 2009. www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf (accessed January27, 2014)
- 7.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: ICH Guidelines. www.ich.org/products/guidelines.html (accessed August13, 2014) [DOI] [PMC free article] [PubMed]
- 8.Parker GE, Witwer S, Miller Bowker G: Regulatory Approval of Biosimilars: A Global Perspective. RAPS Regulatory Focus January 2014. www.raps.org/regulatoryDetail.aspx?id=9837 (accessed August13, 2014)
- 9.Scheinberg MA, Kay J: The advent of biosimilar therapies in rheumatology—“O brave new world.” Nat Rev Rheumatol 2012;8:430–436 [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency: EMA Guideline on Similar Biological Medicinal Products. 2005. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003517.pdf (accessed August13, 2014)
- 11.European Medicines Agency: EMA Guideline on Similar Biological Medicinal Products. Draft. 2013. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/05/WC500142978.pdf (accessed August13, 2014)
- 12.European Medicines Agency: EMA Guideline on Non-Clinical and Clinical Development of Similar Biological Medicinal Products Containing Recombinant Human Insulin and Insulin Analogues. Draft. 2014. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/04/WC500165988.pdf (accessed August13, 2014)
- 13.U.S. Food and Drug Administration: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. 2012. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf (accessed August13, 2014)
- 14.U.S. Food and Drug Administration: Quality Considerations in Demonstrating Biosimilarity to a Reference Protein Product. 2012. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291134.pdf (accessed August13, 2014)
- 15.U.S. Food and Drug Administration: Biosimilars: Questions and Answers Regarding Implementation of the Biologics Price Competition and Innovation Act of 2009. 2012. www.fda.gov/downloads/Drugs/Guidances/UCM273001.pdf (accessed August13, 2014)
- 16.Minister of Public Works and Government Services Canada: Guidance for Sponsors: Information and Submission Requirements for Subsequent Entry Biologics (SEBs). 2010. www.hc-sc.gc.ca/dhp-mps/brgtherap/applic-demande/guides/seb-pbu/seb-pbu_2010-eng.php (accessed August13, 2014)
- 17.Official Journal of the Federation: Decreto por el que se Reforman y Adicionan Diversas Disposiciones del Reglamento de Insumos Para la Salud. 2011. http://dof.gob.mx/nota_detalle.php?codigo=5214882&fecha=19/10/2011 (accessed August13, 2014)
- 18.Saudi Food & Drug Authority: Guidelines on Biosimilars. Version 1.1. 2010. http://old.sfda.gov.sa/NR/rdonlyres/CB28C6AF-79CD-4412-8801-35AEDEED0183/0/Guidelinesonbiosimilars_v11.pdf (accessed August13, 2014)
- 19.Jordan Food & Drug Administration: Guidance for Registration of Biosimilars. 2013. www.jfda.jo/Download/News/239_530.pdf (accessed August13, 2014)
- 20.Egyptian Ministry of Health, Central Administration for Pharmaceutical Affairs, General Registration Department, Department of Biological Products Registration: Draft Guideline for Registration of Biosimilar Products. 2012. www.eda.mohp.gov.eg/Download/Docs/Final%20biosimilar%20guideline.pdf (accessed August13, 2014)
- 21.Government of India, Department of Biotechnology, Ministry of Science and Technology, Central Drugs Standard Control Organization, Ministry of Health and Family Welfare: Guidelines on Similar Biologics: Regulatory Requirements for Marketing Authorization in India. 2012. http://cdsco.nic.in/writereaddata/Bio%20Similar%20Guideline.pdf (accessed August13, 2014) [DOI] [PubMed]
- 22.Korean Food and Drug Administration: Guidelines on the Evaluation of Biosimilar Products. 2010. www.mfds.go.kr/jsp/common/download.jsp?fileinfo=S*1*%B5%BF%B5%EE%BB%FD%B9%B0%C0%C7%BE%E0%C7%B0%20%C6%F2%B0%A1%20%B0%A1%C0%CC%B5%E5%B6%F3%C0%CE%20(%BF%B5%B9%AE).pdf*e9a03e5980ec4520b888e54dbf6ee908*pdf*/files/upload/1/TB_F_INFODATA/13325/e9a03e5980ec4520b888e54dbf6ee908*265678*2012:08:29%2016:10:23 (accessed August13, 2014)
- 23.Ministry of Health Malaysia National Pharmaceutical Control Bureau: Guidance Document and Guidelines for Registration of Biosimilars in Malaysia. 2008. http://portal.bpfk.gov.my/view_file.cfm?fileid=302 (accessed August13, 2014)
- 24.Therapeutic Goods Administration, Department of Health, Australian Government: Evaluation of Biosimilars. Version 1.0, July 2013. www.tga.gov.au/pdf/pm-argpm-biosimilars.pdf (accessed August13, 2014)
- 25.MEDSAFE New Zealand Medicines and Medical Devices Safety Authority: Medicines: Biosimilars. 2013. www.medsafe.govt.nz/profs/RIss/Biosimilars.asp (accessed August13, 2014)
- 26.Medicines Control Council, Republic of South Africa Department of Health: Guidelines for Similar Biological Medicines (Biosimilar Medicines): Quality, Non-Clinical and Clinical Requirements. 2012. www.mccza.com/genericDocuments/2.30_Biosimilars_Mar2012_v2.pdf (accessed August13, 2014)
- 27.U.S. Food and Drug Administration: Formal Meetings Between the FDA and Biosimilar Biological Product Sponsors or Applicants. 2013. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM345649.pdf (accessed August12, 2014)
- 28.Lilly: Eli Lilly and Company and Boehringer Ingelheim Announce New Drug Application Filing in the U.S. for New Insulin Glargine Product [press release]. 2013. https://investor.lilly.com/releasedetail.cfm?releaseid=815459 (accessed August13, 2014)
- 29.Boehringer Ingelheim: FDA Grants Tentative Approval for Lilly and Boehringer Ingelheim's Basaglar™ (Insulin Glargine Injection) [press release]. August 18, 2014. http://us.boehringer-ingelheim.com/news_events/press_releases/press_release_archive/2014/08-18-14-fda-grants-tentative-approval-lilly-boehringer-ingelheim-basaglar-insulin-glargine-injection.html (accessed August31, 2014)
- 30.Reddy S, Balamuralidhara V, Pramod Kumar TM, et al. : Regulatory stratergies for biosimilars in regulated and emerging markets. Pharma Times 2013;45:11–14 [Google Scholar]
- 31.GaBI Online: China Releases Draft Biosimilars Guidance. November 14, 2014. www.gabionline.net/Guidelines/China-releases-draft-biosimilars-guidance (accessed January8, 2015)
- 32.Combe C, Tredree RL, Schellekens H: Biosimilar epoetins: an analysis based on recently implemented European Medicines Evaluation Agency guidelines on comparability of biopharmaceutical proteins. Pharmacotherapy 2005;25:954–962 [DOI] [PubMed] [Google Scholar]
- 33.European Medicines Agency: EMA Guideline on Similar Biological Medicinal Products Containing Biotechnology-Derived Proteins as Active Substance: Quality Issues (Revision 1). 2012. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/05/WC500127960.pdf (accessed August13, 2014)
- 34.European Medicines Agency: EMA ICH Guideline S6 (R1)—Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals. 2011. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002828.pdf (accessed August13, 2014)
- 35.European Medicines Agency: EMA Guideline on Similar Biological Medicinal Products Containing Biotechnology-Derived Proteins as Active Substance: Non-Clinical and Clinical Issues. Draft. 2013. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/06/WC500144124.pdf (accessed August13, 2014)
- 36.McGill JB: Insights from the Liraglutide Clinical Development Program—the Liraglutide Effect and Action in Diabetes (LEAD) studies. Postgrad Med 2009;121:16–25 [DOI] [PubMed] [Google Scholar]
- 37.Official Journal of the European Union: Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010 Amending, as Regards Pharmacovigilance, Directive 2001/83/EC on the Community Code Relating to Medicinal Products for Human Use. 2010. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:348:0074:0099:EN:PDF (accessed August13, 2014)
- 38.Joshi SR: Biosimilar peptides: need for pharmacovigilance. J Assoc Physicians India 2011;59(Suppl):44–47 [PubMed] [Google Scholar]
- 39.European Generic Medicines Association: EGA Handbook on Biosimilar Medicines. Brussels: European Generic Medicines Association, 2008 [Google Scholar]
- 40.U.S. Food and Drug Administration: Biologics Price Competition and Innovation (BPCI) Act. 2009. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/ucm216146.pdf (accessed August13, 2014)
- 41.European Medicines Agency: EMA Guideline on Similar Medicinal Products Containing Recombinant Human Soluble Insulin. 2006. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003957.pdf (accessed August13, 2014)
- 42.European Medicines Agency: EMA Guideline on Non-Clinical and Clinical Development of Similar Biological Medicinal Products Containing Recombinant Human Insulin and Insulin Analogues. Draft. 2012. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/12/WC500136392.pdf (accessed August13, 2014)
- 43.European Medicines Agency: Marvel LifeSciences Ltd Withdraws Its Marketing-Authorisation Applications for Solumarv, Isomarv and Combimarv (Human Insulin) [press release]. 2012. www.ema.europa.eu/docs/en_GB/document_library/Press_release/2012/11/WC500135156.pdf (accessed August13, 2014)
- 44.European Medicines Agency: Withdrawal Assessment Report for Insulin Human Rapid Marvel. 2008. www.ema.europa.eu/docs/en_GB/document_library/Application_withdrawal_assessment_report/2010/01/WC500067086.pdf (accessed August13, 2014)
- 45.European Medicines Agency: Withdrawal Assessment Report for Insulin Human Long Marvel. 2008. www.ema.europa.eu/docs/en_GB/document_library/Application_withdrawal_assessment_report/2010/01/WC500067170.pdf (accessed August13, 2014)
- 46.European Medicines Agency: Withdrawal Assessment Report for Insulin Human 30/70 Mix Marvel. 2008. www.ema.europa.eu/docs/en_GB/document_library/Application_withdrawal_assessment_report/2010/01/WC500067169.pdf (accessed August13, 2014)
- 47.European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP) Press Release. Meeting Highlights from the Committee for Medicinal Products for Human Use (CHMP) 23–26 June 2014. www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2014/06/news_detail_002129.jsp&mid=WC0b01ac058004d5c1 (accessed August31, 2014)
- 48.McKoy JM, Stonecash RE, Cournoyer D, et al. : Epoetin-associated pure red cell aplasia: past, present, and future considerations. Transfusion 2008;48:1754–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schellekens H: Biosimilar therapeutics—what do we need to consider? NDT Plus 2009;2(Suppl 1):i27–i36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lispi M, Datola A, Bierau H, et al. : Heterogeneity of commercial recombinant human growth hormone (r-hGH) preparations containing a thioether variant. J Pharm Sci 2009;98:4511–4524 [DOI] [PubMed] [Google Scholar]
- 51.Swinnen SG, Holleman F, DeVries JH: The interpretation of glucose clamp studies of long-acting insulin analogues: from physiology to marketing and back. Diabetologia 2008;51:1790–1795 [DOI] [PubMed] [Google Scholar]
- 52.Blevins T, Dahl D, Rosenstock J, et al. : Similar efficacy and safety with LY2963016 insulin glargine compared with Lantus® insulin glargine in patients with T1DM: the ELEMENT 1 study [abstract 69-OR]. Diabetes 2014;63(Suppl 1):A19 [Google Scholar]
- 53.Deeg M, Ilag L, Huster WJ, et al. : Evaluation of immunogenicity of LY2963016 insulin glargine compared with Lantus® insulin glargine in patients with T1DM or T2DM [abstract 70-OR]. Diabetes 2014;63(Suppl 1):A19 [Google Scholar]
- 54.Linnebjerg H, Chen Quin Lam E, Seger ME, et al. : Comparative pharmacokinetics (PK) and pharmacodynamics (PD) of LY2963016 insulin glargine and EU- and U.S.-approved versions of Lantus® insulin glargine in healthy subjects [abstract 889-P]. Diabetes 2014;63(Suppl 1):A227 [Google Scholar]
- 55.Heise T, Zhang X, Chen Quin Lam E, et al. : Duration of action of 2 insulin glargine products, LY2963016 and Lantus®, in subjects with type 1 diabetes mellitus (T1DM) [abstract 891-P]. Diabetes 2014;63(Suppl 1):A228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenstock J, Hollander P, Bhargava A, et al. : Similar efficacy and safety with LY2963016 insulin glargine compared with Lantus® insulin glargine in patients with T2DM: the ELEMENT 2 study [abstract 64-OR]. Diabetes 2014;63(Suppl 1):A17 [Google Scholar]
- 57.Zhang X, Chen Quin Lam E, Seger ME, et al. : Comparative pharmacokinetics and pharmacodynamics of two insulin glargine products, LY2963016 and Lantus®, in healthy subjects at two dose levels [abstract 890-P]. Diabetes 2014;63(Suppl 1):A227 [Google Scholar]
- 58.Nanda KS, Cheifetz AS, Moss AC: Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol 2013;108:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drucker DJ, Buse JB, Taylor K, et al. : Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 [DOI] [PubMed] [Google Scholar]
- 60.Fineman MS, Mace KF, Diamant M, et al. : Clinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes Metab 2012;14:546–554 [DOI] [PubMed] [Google Scholar]