Abstract
Tibet makes up the majority of the Qinghai-Tibet Plateau, often referred to as the roof of the world. Its complex landforms, physiognomy, and climate create a special heterogeneous environment for mosses. Each moss species inhabits its own habitat and ecological niche. This, in combination with its sensitivity to environmental change, makes moss species distribution a useful indicator of vegetation alteration and climate change. This study aimed to characterize the diversity and distribution of Didymodon (Pottiaceae) in Tibet, and model the potential distribution of its species. A total of 221 sample plots, each with a size of 10 × 10 m and located at different altitudes, were investigated across all vegetation types. Of these, the 181 plots in which Didymodon species were found were used to conduct analyses and modeling. Three noteworthy results were obtained. First, a total of 22 species of Didymodon were identified. Among these, Didymodon rigidulus var. subulatus had not previously been recorded in China, and Didymodon constrictus var. constrictus was the dominant species. Second, analysis of the relationships between species distributions and environmental factors using canonical correspondence analysis revealed that vegetation cover and altitude were the main factors affecting the distribution of Didymodon in Tibet. Third, based on the environmental factors of bioclimate, topography and vegetation, the distribution of Didymodon was predicted throughout Tibet at a spatial resolution of 1 km, using the presence-only MaxEnt model. Climatic variables were the key factors in the model. We conclude that the environment plays a significant role in moss diversity and distribution. Based on our research findings, we recommend that future studies should focus on the impacts of climate change on the distribution and conservation of Didymodon.
Introduction
As an important component of ecosystems, mosses have a strong influence on the cycling of water, nutrients, energy, and carbon [1,2]. Given their sensitivity to environmental change, mosses can be used as bioindicators of forest integrity [3], water quality [4], air pollution [5], metal accumulation [6], and climate change [7,8]. They also play key roles in long-term processes such as peat accumulation, the formation of microtopography, and permafrost stability [1]. Although mosses are widely distributed on land, different types of moss have certain ranges of climate and environmental conditions within which they can survive and successfully reproduce. Topographic factors such as aspect, slope, and altitude [9,10]; climatic factors [11]; soil factors including type, moisture, and pH [12,13]; vegetation type and coverage; and the type of substrate that mosses grow on [11,12,14] are all important environmental factors affecting the distribution of mosses.
Didymodon is the largest genus in the Pottiaceae family and includes approximately 122 species that are distributed worldwide, with the greatest diversity found in temperate and mountainous regions, where they primarily grow on rocks or soil [15]. The species diversity varies greatly in regions with a wide variety of habitats and high level of environmental heterogeneity. Although studies of Didymodon date back to the year 1801, only recently has the exact phylogenetic position of this genus been established. Species of Didymodon are often distributed on calcareous soil or rocky areas [16]. In China, a total of 26 species of Didymodon have been reported [17]. Many of these species are widely distributed, whereas some are found only in specific areas; for example, Didymodon anserinocapitatus is found only in Tibet, and Didymodon rigidulus var. icmadophilus occurs only in Inner Mongolia [18]. Currently, researchers studying Didymodon are paying close attention to its taxonomic [19,20], systematic [21], and morphological characters [22,23], but few are conducting research on the distribution of Didymodon with a consideration of habits and environmental variables at large scales.
The Tibetan Plateau has been called the roof of the world because of its very high altitude [24,25]. Characterized by its extreme environments, Tibet is particularly at risk from vegetation changes due to climatic change. Given the relatively low intensity of human disturbance occurring in the region, and the unique vegetation and climate zones of the plateau, there have been many studies of the effects of climate change on ecosystems in Tibet [26]. The first moss specimen was collected by Thomson during 1847–1849 in western Tibet. In China, comprehensive contributions were primarily made by scientific expeditions to the Qinghai-Tibet Plateau in 1952–1979 [27]. In recent years, further studies on moss ecology have been carried out, considering subjects such as the accumulation of heavy metals, responses to changes in air humidity, and bryophyte communities in the valley areas of Tibet [28,29,30]. Because of geographical conditions and traffic restrictions, these surveys have been concentrated in southeastern Tibet, and the northwest regions have rarely been investigated. However, although previous studies have focused on moss taxonomy, the environmental factors that affect moss diversity and distribution at microhabitat and macro spatial scales are still unknown [27].
Given its resistance to cold and drought, Didymodon is the dominant genus of moss in Tibet. Didymodon exhibits apparent morphological, physiological, and genealogical adaptations to its particular environments [15]. The Tibetan Plateau is an important area for the study of climate change and its effect on vegetation at large scales. Thus, understanding the diversity of Didymodon species, as well as their spatial distribution patterns and related environmental factors, is helpful for protecting Tibetan ecosystems, monitoring changes in vegetation and climate, and guiding future field surveys of unexplored areas in Tibet.
In recent years, remote sensing, geographical information systems (GIS), and species distribution models have been introduced for the study of moss distributions at large spatial scales. Rapalee et al. [31] used advanced, very high resolution radiometer data to simulate the distribution of mosses in the boreal forest ecosystem of central Canada, then compared their different distributions at scales of 10 m, 30 m, and 1 km. Vanderpoorten et al. [32] studied the growth of the rare moss Aneura maxima using GIS, and then predicted its distribution. Jiang et al. [33] predicted the distribution of epiphyllous liverworts in China based on environmental variables, using the MaxEnt model. However, these studies did not focus on the spatial distribution of Didymodon or its relationship to environmental factors in Tibet.
The objectives of this study were as follows: (1) to survey the species richness and diversity of Didymodon in Tibet, (2) to analyze the relationship between species distribution and micro-habitat environments, and (3) to identify macro-habitat factors affecting the spatial distribution of Didymodon on a broad spatial scale.
Materials and Methods
Study area
Tibet is located on the highest and largest plateau on Earth. Located in southwestern China (E78°25′–99°06′, N26°50′–36°53′), it is known as the world's third pole and the roof of the world (Fig 1). Tibet covers 1.2 million km2, and makes up 12.5% of the total area of China. The Tibetan region, which has an average altitude in excess of 4000 m, slopes gently downward from the northwest to the southeast. The region is surrounded by the Himalayas and the Kunlun and Tanggula Mountains, with an average altitude of over 6000 m. Tibet is also the source region of many major rivers, such as the Mekong, Indus, and Brahmaputra [24].
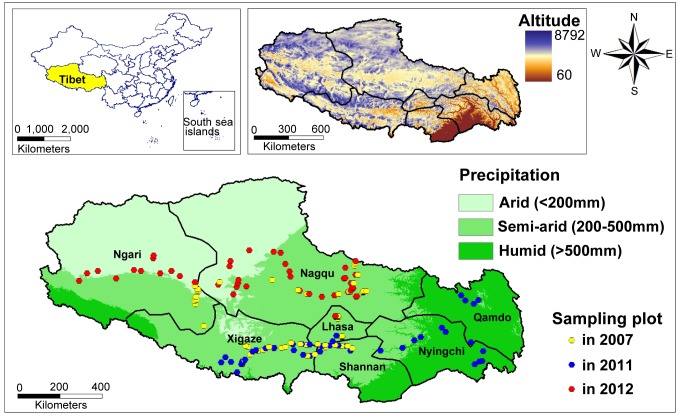
Fig 1. The study area containing 221 sampling plots, which was investigated in 2007, 2011, and 2012.
Pale green represents arid areas that receive an annual precipitation of less than 200 mm; medium green indicates semiarid regions, where the annual precipitation is between 200 and 500 mm; and dark green indicates humid areas, where the annual precipitation is greater than 500 mm.
Its complex landforms and physiognomy form a unique array of high altitude climates, characterized by strong solar radiation, low temperatures with high daily temperature variance, distinct rainy and dry seasons, low air pressure, low ambient oxygen levels, and high winds. The vegetation in this area is characterized by zonal perpendicularity and diversification. Annual rainfall varies greatly within the study area; most areas receive less than 500 mm, including northern Tibet, the entirety of Ngari and Nagqu, the midwestern region of Xigaze, and part of Shannan, Lhasa, and Qamdo. The elevation in this region ranges from 60 to 8792 m. The major vegetation types are saline land, mountain meadow, mountain steppe, desert steppe, swamp meadow, and alpine-cold meadow.
The national fundamental geographic data, which include spatial data concerning boundaries, rivers, roads, and other features, were obtained from the National Geomatics Center of China (http://www.cehui8.com/3S/GIS/20130702/205.html). We used ArcGIS 10.0 (Esri, Redlands, CA, USA) to select and export the shapefile of Tibet from China. The shapefiles were deposited in Dryad (http://datadryad.org/review?doi=doi:10.5061/dryad.m8b96).
Field sampling
Fieldwork was carried out from July to September in 2007, 2011, and 2012. The 221 sample plots were located at various altitudes in the arid, semi-arid, and humid regions of Tibet, in order to ensure that all vegetation types (11 groups of vegetation types, including alpine meadow, mountain steppe, pine forest, shrubbery, alpine tundra, deciduous broadleaved forest, desert steppe, swamp meadow, saline land, artificial woodland and alpine vegetation) were represented (Fig 1). The sample plots were situated at 100 m altitudinal intervals in the Himalayas, Tanggula Mountains, and Nyainqêntanglha Mountains, thus covering a significant elevation gradient. Finally, 181 plots in which Didymodon was found were used to conduct diversity analyses and distribution modeling. In each sample plot, three to seven quadrats (1 m2 area) were used; in total, 995 quadrats were used to investigate all ground-moss species [34]. Moss coverage was measured using a metallic quadrat divided into 100 grid cells and the number of grid cells that mosses covered was recorded. Moss specimens were collected from the sites, air dried, and identified to species level in the laboratory. Department of Science &Technology of Tibet Autonomous Region approved the field study, and none of the protected species was sampled during this study.
Species identification and diversity analysis
Using the characteristics of the gametophytes (plants, stems, leaves, specialized asexual reproduction, perichaetia, color reactions of leaf cells in 2% potassium hydroxide solution) and sporophytes (seta, capsule, calyptras, spores), each specimen was identified to genus and species in the laboratory, using a stereomicroscope and an optical microscope.
Species diversity was analyzed using relative frequency, relative coverage, and importance value. The following indices were used in this study [35].
| (1) |
| (2) |
where f is the number of sample plots in which a moss species appears, T is the total number of sample plots, and F i is the relative frequency of species i.
| (3) |
where C i is the coverage of species i, and C is the total coverage of species in each sampling plot.
| (4) |
The analyses were performed using the IBM SPSS Statistics (v19.0) software program, which was downloaded from the website http://emuch.net/html/f188.html.
Environmental variables
Detailed information about habitat characteristics were recorded during the collection of species data in the sampling plots. Altitude was measured by a GPSMap60 CSx (Garmin Corporation, Shijr, Taiwan). Vegetation cover was estimated visually as a projection of plant cover. The substrate that mosses grow on was divided into six types: land, stone, tree, water, rock cracks, and thin soil layer above the rock. Temperature and humidity were measured with a PH-II-C handheld weather station. Soil moisture was measured with a FieldScout TDR100 Soil Moisture Meter (Spectrum Technologies, Inc., Plainfield, IL, USA) at soil depths of 3.8 and 7.6 cm. The variables used in analyzing the effects of environmental heterogeneity are shown in Table 1.
Table 1. Environmental variables used for correlation analyses between environmental variables and Didymodon diversity in the study area.
| Category | Variables | Abbreviation | Units |
|---|---|---|---|
| Topographic | Altitude | Altitude | M |
| Vegetation | Vegetation type | Veg-type | dimensionless |
| Vegetation cover | Veg-cove | Degree | |
| Substrate | Substrate | Substrate | Degree |
| Bioclimatic | Temperature | Temp | °C |
| Humidity | Humidity | dimensionless | |
| Soil | Soil moisture 3.8 | Soil-mois | dimensionless |
| Soil moisture 7.6 | Soil-mois2 | dimensionless |
In this study, three categories of spatial data with a total of 18 GIS layers of large-scale environmental variables (Table 2) were collected in order to facilitate modeling of the distribution of Didymodon in Tibet, using the MaxEnt model.
Table 2. Environmental variables used in modeling the distribution of Didymodon in the study area.
| Category | Variables | Abbreviation | Units |
|---|---|---|---|
| Bioclimatic | Annual Mean Temperature | t_mean | °C |
| Temperature Seasonality | t_seas | dimensionless | |
| Max Temperature of Warmest Month | t_max | °C | |
| Min Temperature of Coldest Month | t_min | °C | |
| Annual Precipitation | p_ap | mm | |
| Precipitation in Wettest Month | p_max | mm | |
| Precipitation in Driest Month | p_min | mm | |
| Precipitation Seasonality | p_seas | dimensionless | |
| Potential evapotranspiration | PET | mm | |
| Aridity | AI | dimensionless | |
| Topographic | Altitude | Altitude | m |
| Aspect | Aspect | degree | |
| Slope | Slope | degree | |
| Vegetation | Annual minimum NDVI | NDVI_min | dimensionless |
| Annual mean NDVI | NDVI_mean | dimensionless | |
| Annual maximum NDVI | NDVI_max | dimensionless | |
| Amplitude NDVI | NDVI_amp | dimensionless | |
| Standard deviation NDVI | NDVI_std | Dimensionless |
Temperature and precipitation data were acquired from the database of the Chinese Meteorological Administration (http://www.cma.gov.cn/2011qxfw/2011qsjcx/). We selected records from 200 stations with less than 5.00% missing data between 2003 and 2012. Data concerning annual time series with annual means, seasonality, and extreme or limiting temperature and precipitation data were used [33,36]. The selected climate records were then interpolated to spatial climate datasets with a grain size of 1 × 1 km, using the thin-plate smoothing spline interpolation method of the ANUsplin software package [37]. Potential evapotranspiration (PET) and aridity index (AI) values were obtained from the CGIAR-CSI GeoPortal (http://csi.cgiar.org);
Digital elevation model data were obtained from the USGS GTOPO30 series (http://www1.gsi.go.jp/geowww/globalmap-gsi/gtopo30/gtopo30.html) and used to derive the aspect and slope data using ArcGIS 10.0;
Normalized difference vegetation index (NDVI) images at a 1-km resolution were obtained from Spot-Vegetation Programme (www.vgt.vito.be). The images were 10-day composites obtained by vegetation sensors located on SPOT4 and SPOT5 satellites. Using ERDAS IMAGINE software (Hexagon Geospatial, Norcross, GA, USA), we obtained NDVI indices from 2010 to 2012.
All of the environmental variables were projected as GIS raster layers in GCS_WGS_1984 coordinate system, and converted to ASCII format for using in the MaxEnt model, with a spatial resolution of 1 km. The data used in MaxEnt model was deposited in Dryad (http://datadryad.org/review?doi=doi:10.5061/dryad.m8b96).
Habitat heterogeneity analysis
At the microhabitat scale, habitat heterogeneity analysis was used to explore the relationships (1) between habitat properties and species richness, and (2) between habitat properties and species composition. Using canonical correspondence analysis (CCA) to analyze the relationship between Didymodon diversity and habitat properties required two data matrices (S1 File): one was the Didymodon matrix, which contained species names and coverages (Table A in S1 File); the other was an environmental data matrix, including all the information for quadrats in which Didymodon mosses were present (Table B in S1 File). Multiple environmental factors were analyzed together in the CCA, which was performed using CANOCO for Windows 4.5 (downloaded from http://download.csdn.net/detail/slowslap/1556879), and the relationships between species diversity and micro-environmental variables were displayed using CANODRAW.
In addition to CCA, CANOCO for Windows 4.5 software was also used to perform correlation analyses of different habitat properties, which necessitated the analysis of variables that were not normally distributed. We reported significant correlations at p < 0.05 or p < 0.01.
MaxEnt modeling
The MaxEnt algorithm in ecological niche modeling is a general-purpose machine-learning method that calculates probability distributions using incomplete information [38]. In this study, the MaxEnt (version 3.3.3e) was implemented to predict the probability of Didymodon distributions in Tibet.
The recommended default values were used for the convergence threshold (10−5), with the maximum number of iterations (500) and 10,000 background points. Suitable regularization values were automatically selected by the program. The selection of environmental variables or functions was carried out automatically under the default rules, which depend on the number of presence records. The default logistic output of MaxEnt is a set of continuous probability values ranging from 0 to 1, where high values indicate a higher relative suitability for a species distribution.
Executing the MaxEnt model involved two procedures: (1) the model was run on the full set of Didymodon occurrence data, taking advantage of all available data in order to provide the best estimation of the potential species distribution and the relative importance of the environmental variables; and (2) 10 random partitions of the occurrence data were created by randomly selecting 70% of the occurrence data for training and 30% for testing. The model was run based on each partition.
Model evaluation and statistical analysis
To evaluate the accuracy of the model predictions, we used both threshold-independent and threshold-dependent methods: (1) AUC (area under curve) is a threshold-independent method that is considered to be an effective indicator of modeling performance independent of the threshold probability [39,40]. The AUC method produces values between 0 and 1, where 1 indicates a perfect fit for the model, 0.9 or higher represents excellent model performance, and 0.5 suggests randomness [41,42]; and (2) TSS (true skill statistic) is a threshold-dependent method in which produced values ranging from -1 to 1, where 1 indicates a perfect fit and value of 0 or less indicates a performance no better than random [43]. The TSS was defined as:
| (5) |
The AUC and TSS were calculated and created using two output values that were extracted from the 10 random partitions. One value is the observed occurrence value (0 for pseudo-absence points or 1 for test presence points), the other is the predicted value from the logistic output of the MaxEnt model. The final AUC and TSSmax (the maximum TSS) values produced are the average values of the 10 replicates evaluated. The evaluation statistics were implemented in the MaxEnt model with an independent training dataset, using the presence-absence package in R 2.14.0 (Available from http://www.R-project.org). The jackknife test was applied to diagnose the relative importance of environmental variables that could potentially contribute to the species distribution model [44]. The environmental variable with the highest training gain when used in isolation is considered to contain the most predictive ability of any variables. Response curves were plotted to demonstrate how variables affect the probability of Didymodon presence in the study area, which used all point localities and the respective environmental variable in isolation. Both jackknife test and response curves are available options in MaxEnt.
Results
Species diversity
We found 983 Didymodon specimens in 181 sample plots. A total of 22 species were identified (Table 3), which comprises approximately 19.47% of the Pottiaceae species in the study area. Didymodon constrictus var. constrictus was the most dominant species in the study area, with an importance value of 27.909. D. rigidulus var. subulatus had not been previously recorded in China, whereas D. anserinocapitatus was on the first red list of endangered bryophytes in China.
Table 3. Didymodon species identified in Tibet, and their relative frequency, coverage, and importance value.
| No. | Species | Relative Frequency | Relative Coverage | Importance value |
|---|---|---|---|---|
| S1 | Didymodon constrictus var. constrictus | 25.969 | 29.848 | 27.909 |
| S2 | Didymodon tectorus | 9.948 | 6.797 | 8.372 |
| S3 | Didymodon constrictus var. flexicuspis | 7.539 | 3.913 | 5.726 |
| S4 | Didymodon perobtusus | 4.817 | 1.969 | 3.393 |
| S5 | Didymodon rigidulus var. rigidulus | 4.712 | 3.542 | 4.127 |
| S6 | Didymodon tophaceus | 4.712 | 2.935 | 3.824 |
| S7 | Didymodon rigidulus var. icmadophilus | 4.084 | 2.551 | 3.318 |
| S8 | Didymodon nigrescens | 3.874 | 2.423 | 3.149 |
| S9 | Didymodon michiganensis | 3.351 | 1.726 | 2.539 |
| S10 | Didymodon rigidulus var. ditrichoides | 2.827 | 1.375 | 2.101 |
| S11 | Didymodon vinealis | 2.094 | 1.701 | 1.898 |
| S12 | Didymodon rigidulus var. gracilis | 1.780 | 1.100 | 1.440 |
| S13 | Didymodon asperifolius | 1.152 | 0.729 | 0.940 |
| S14 | Didymodon rigidulus var. subulatus | 1.152 | 0.492 | 0.822 |
| S15 | Didymodon ferrugineus | 0.942 | 1.061 | 1.002 |
| S16 | Didymodon fallax | 0.628 | 0.345 | 0.487 |
| S17 | Didymodon giganteus | 0.524 | 0.480 | 0.502 |
| S18 | Didymodon rivicolus | 0.524 | 0.301 | 0.412 |
| S19 | Didymodon rufidulus | 0.209 | 0.850 | 0.530 |
| S20 | Didymodon anserinocapitatus | 0.105 | 0.026 | 0.065 |
| S21 | Didymodon japonicus | 0.105 | 0.032 | 0.068 |
| S22 | Didymodon johansenii | 0.105 | 0.115 | 0.110 |
In order to understand the overall influence of environmental conditions on moss species distribution, we divided the aforementioned 181 sample plots by altitude into six groups, then compared the diversity of species under the different classes of precipitation and altitude (Table 4). Moss species richness differed significantly according elevation and precipitation pattern. Semi-arid areas exhibited the greatest species richness (22 species). Among the different elevation classes, the greatest species diversity was found in the class between 4500 and 5000 m (21 species).
Table 4. Number of Didymodon species along the altitude gradient and under different precipitation regimes in Tibet.
| Altitude (m) | Arid zone | Semi-arid zone | Humid zone | Total |
|---|---|---|---|---|
| 2800–3000 | 0 | 4 | 5 | 7 |
| 3000–3500 | 0 | 7 | 1 | 7 |
| 3500–4000 | 0 | 17 | 2 | 19 |
| 4000–4500 | 8 | 13 | 7 | 18 |
| 4500–5000 | 16 | 21 | 0 | 21 |
| 5000–5600 | 10 | 17 | 0 | 17 |
| Total | 17 | 22 | 9 | 22 |
Analysis of environmental heterogeneity
The correlations between species diversity and micro-environmental factors showed that vegetation cover, and altitude were the main environmental factors affecting Didymodon diversity in the study area (Table 5). The correlations among micro-environmental factors were generally weak, except for easily interpreted cases such as the correlation between soil moisture at soil depths of 3.8 cm and 7.6 cm (positive), humidity and soil moisture (positive), and the correlation between temperature and altitude (negative), temperature and vegetation type (negative). Humidity and soil moisture at soil depths of 3.8 cm, soil moisture a soil depth of 3.8 cm and soil moisture a soil depth of 7.6 cm were significantly autocorrelated, and thus we included only soil moisture at a soil depth of 3.8 cm below ground in the following analysis of species and habitat micro-environmental factors.
Table 5. Correlation of species diversity and environmental factors affecting Didymodon in the study area.
| Species diversity | Altitude | Veg-type | Veg-cove | Substrate | Temp | Humidity | Soil-mois | |
|---|---|---|---|---|---|---|---|---|
| Altitude | 0.25 | |||||||
| Veg-type | 0.02 | 0.28** | ||||||
| Veg-cove | -0.17* | -0.13 | 0.15 | |||||
| Substrate | 0.14 | -0.01 | -0.01 | -0.19* | ||||
| Temp | 0.18* | -0.34** | -0.29** | -0.13 | -0.07 | |||
| Humidity | 0.47 | -0.01 | -0.14 | -0.01 | -0.01 | 0.40 | ||
| Soil-mois | 0.27 | -0.10 | -0.15 | 0.02 | -0.03 | 0.48 | 0.67** | |
| Soil-mois2 | 0.31 | -0.02 | -0.06 | 0.01 | -0.04 | 0.37 | 0.72** | 0.98** |
Note:
* and ** represent statistically significant correlations.
*: p < 0.05
**: p < 0.01
Veg-type represents vegetation type, Veg-cove represents vegetation cover, Temp represents temperature, Soil-mois represents soil moisture at a depth of 3.8 cm, and Soil-mois2 represents soil moisture at a depth of 7.6 cm.
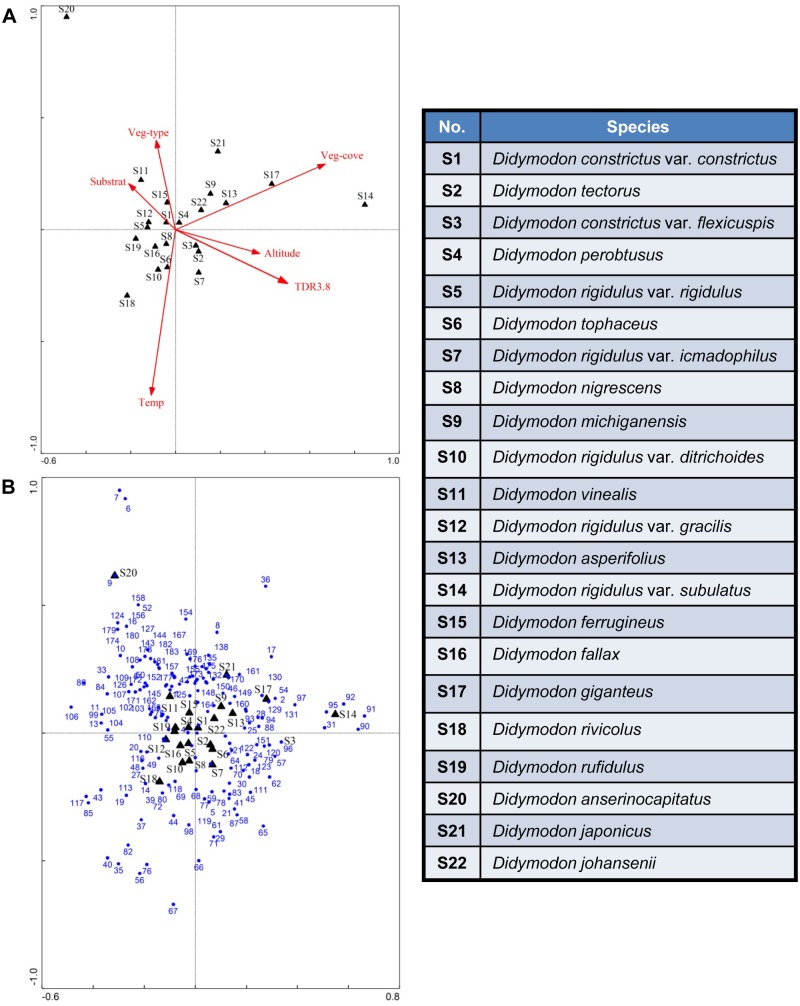
Analysis of the bryophytes and habitat micro-environmental factors using the 181 plots in CCA showed that altitude, vegetation coverage, and temperature were the key factors influencing the distribution of Didymodon (Fig 2). The effects of soil moisture at a depth of 3.8 cm and vegetation type on the distribution of Didymodon were minor in comparison to the effects of the other micro-environmental factors. The substrate had the greatest impact on D. constrictus var. constrictus (S1), Didymodon tectorus (S2), and Didymodon constrictus var. flexicuspis (S3) (Fig 2A); further, D. constrictus var. constrictus, Didymodon perobtusus (S4), Didymodon asperifolius (S13), Didymodon giganteus (S17) were widely distributed in plots where vegetation cover was 30–65%. D. anserinocapitatus (S20) appeared only in shrub vegetation at an elevation of 2748 m. Didymodon japonicus (S21), and Didymodon johansenii (S22) were found in areas of swamp meadow vegetation at 4787 m and 4800 m altitudes, respectively. Didymodon rigidulus var. subulatus (S14) was restricted to the alpine meadows of the Nyainqentanglha Mountains and Dagze Mountain at 4800–5200 m altitudes. Other species were primarily distributed in alpine meadows from 3500 to 5600 m (Fig 2B).
Fig 2. CCA ordination of 22 Didymodon species, environmental factors, and sampling plots in the study area.
A: CCA ordination of 22 Didymodon species and environmental factors; B: CCA ordination of 22 Didymodon species and the 181 sampling plots where they were found to grow. The black triangles represent 22 species of Didymodon; the blue circles represent the 181 sampling plots where Didymodon was found. The red arrows depict environmental factors: Temp represents temperature, Veg-cove represents vegetation cover, Veg-type represents vegetation type, and TDR 3.8 represents soil moisture soil depth of 3.8 cm. S1–S22 refers to Didymodon species listed in Table 3.
Potential distribution of Didymodon
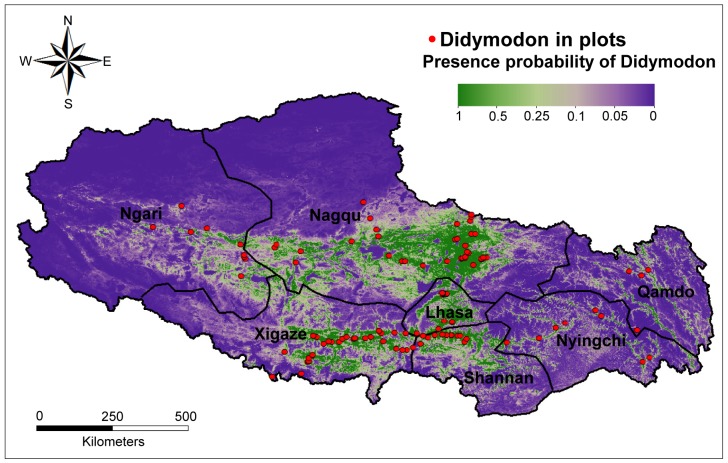
Using the species localities, environmental variables, and the MaxEnt model, we generated maps of the spatial distribution of Didymodon in Tibet (Fig 3). The distribution probability was divided into three classes according to the fractional predicted area: most areas are blue, indicating a rare probability (less than 0.05), violet to light green indicates a low to medium probability (0.05–0.25), and dark green indicates a high probability of distribution (0.5–1.0). Fig 3 also clearly shows that the semi-arid regions of Tibet, mainly comprising Nagqu, Xigaze, and Lhasa, had higher distribution probabilities than the other two regions, with Nagqu exhibiting a larger distribution area than the other regions. The AUC value of the threshold-independent method was 0.90, and the TSSmax value of the threshold-dependent method was 0.66. These values indicate exceptionally high discrimination of the variation in environmental variables across the confirmed Didymodon habitats.
Fig 3. The presence probability of Didymodon spatial distributions in Tibet.
The red circles represent the Didymodon species in the plots that were investigated.
Environmental variables affecting the distribution of Didymodon at macro spatial scales
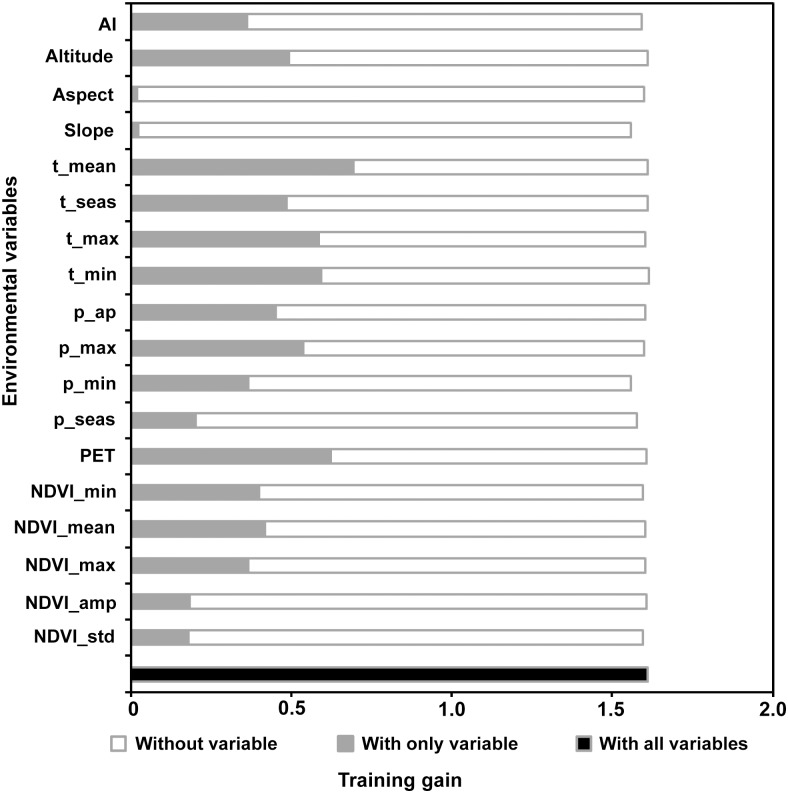
The contributions of environmental variables to Didymodon distributions are shown in Fig 4. The analysis revealed that the climatic variables (the annual mean temperature) exhibited the greatest gains (at 0.70) in the MaxEnt model. The variables of potential evapotranspiration (0.62), minimum temperature of the coldest month (0.60), maximum temperature of the warmest month (0.59), and precipitation of the wettest month (0.54) exhibited the high gains in defining Didymodon distribution. The topographic variables of slope and aspect exhibited the lowest gains (both at 0.02), indicating almost no contribution in the model.
Fig 4. The importance of 22 environmental variables in modeling the distribution of Didymodon in Tibet.
The training gain describes how much better the MaxEnt distribution fits the presence data compared to a uniform distribution. The names and descriptions of environmental variables are listed in Table 2. The white squares represent the effect of removing a single variable from the full model. The gray squares represent the training gains when using only one environmental variable in MaxEnt. The black square represents the training gains when all variables were run in MaxEnt (1.61).
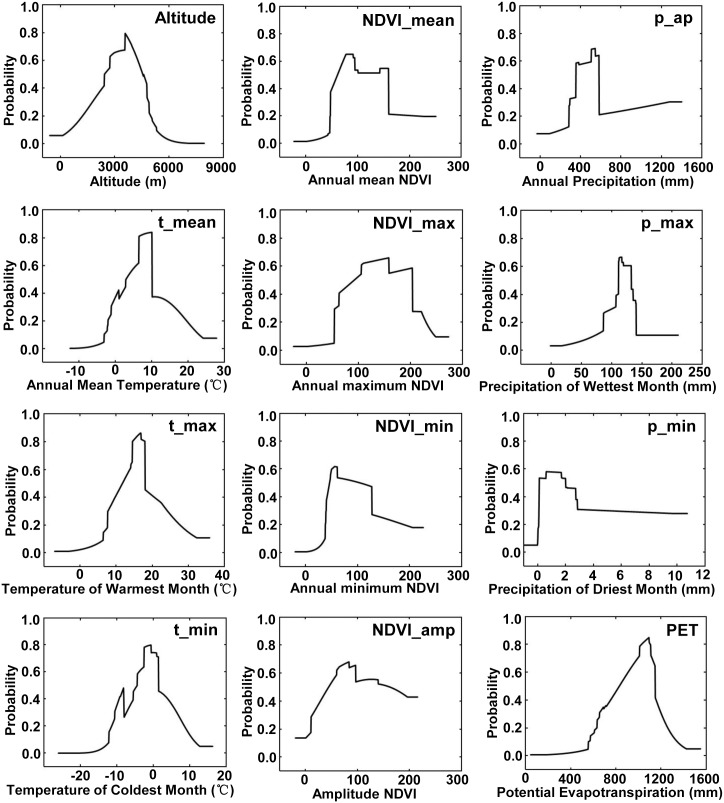
We examined 12 of the important environmental variables affecting the presence probability of Didymodon in the study area (Fig 5). According to the response curves, the presence probability responded positively to altitude, climatic variables, and vegetation variables when a certain value was reached. For example, Didymodon exhibited a high probability (over 0.65) of occurrence in plots with altitudes between 3500 and 4200 m, the annual mean temperature was 6.5–10.0°C, and the potential evapotranspiration was 951–1148 mm. When precipitation during the driest month was 0–2 mm, and the annual mean NDVI was 60–160, the possibility of species occurrence was greater than 0.50.
Fig 5. Response curves for the relationship between the probability distributions of Didymodon and environmental variables.
The curves show the change in the response of Didymodon distribution to specific environmental variables.
Discussion
Because of rapid tectonic uplift, Tibet, as the highest plateau in the world, has distinct topography and atmospheric circulation patterns, which in turn greatly affect global climate. The complex climate types found in Tibet create habitat heterogeneity and well-known highland vegetation patterns [45]. In our study, nearly all moss species were collected from the alpine zone. The results showed that species of the genus Didymodon are frequently pioneer mosses, able to colonize extreme habitats because of adaptations that enable survival in very harsh environments. This is consistent with the findings of other researchers [15,27].
Studies on moss diversity in northwest Tibet have been almost nonexistent, mainly because of the area’s difficult terrain and traffic restrictions. We accessed these remote areas, particularly northwest Tibet, and conducted our investigation, which involved the exhaustive collection of species data and the discovery of a new record of Didymodon species for China. Claudine et al. [9] pointed out that moss diversity changes with altitude and is influenced by microhabitat conditions. Li et al. [13] and Tian et al. [46] also pointed out that the diversity of both specialists and generalists, and the distribution of mosses are positively associated with local habitat and its heterogeneity. Mosses are sensitive to changes in environmental conditions, but are more adaptable to arid desert environments than vascular plants [29].
We illustrated the potential spatial distribution of Didymodon in Tibet using the MaxEnt model, which has several advantages over other methods. For example, this model can perform modeling with spatially biased data and limited species occurrence records [47,48]; in addition, it can perform modeling analyses with presence-only points and conduct a built-in jackknife test, which allows for the estimation of the significance of individual environmental variables when computing species distribution [47,49]. The model in this study performed very well, generating high values of AUC and TSSmax.
Based on species diversity and environmental heterogeneity, we predicted the spatial distribution of Didymodon in Tibet. However, there is the potential for deviation when using the MaxEnt model for this purpose, given that our sampling plots were mainly in the semi-arid regions and thus the environmental variables may not be sufficient to accurately describe the habits relevant to species distribution; this may lead to an imprecise prediction of habitat suitability for Didymodon [38].
In this study, we measured species diversity, predicted the spatial distribution of Didymodon, and analyzed its relationship to environmental heterogeneity in Tibet for the first time. We obtained three primary results. First, a total of 22 species of Didymodon was identified in Tibet. Of these, D. rigidulus var. subulatus is a new record for China, while D. constrictus var. constrictus is the dominant species. Second, Didymodon had higher distribution probabilities in the semi-arid regions than in the arid and humid regions of Tibet. Third, climatic variables were the main impact factors affecting the distribution of Didymodon. These findings are essential for the effective conservation of mosses in Tibet, not only with respect to estimating the species distribution ranges of Didymodon, but also for identifying the environmental factors limiting moss distribution, and even monitoring climate change. Our findings also indicate that climate change in Tibet should be given further attention.
Supporting Information
Didymodon matrix contains species names and their coverage in the sample plots (Table A). Environmental data matrix includes all relevant environmental information for quadrats in which Didymodon mosses were present (Table B).
(PDF)
Acknowledgments
We thank Prof. Youfang Wang (East China Normal University) for the help in identifying moss specimens. We also thank Jianshuang Wu, Pengfeng Wu, Yihua Zhang, Wenhong Cui and Chu Lun for their assistance in the field.
Data Availability
Didymodon species, community-site matrix, and environment-site matrix used in canonical correspondence analysis are within the paper and its Supporting Information files. The other data and related metadata such as environmental variables used in MaxEnt model (ASC format), Tibet shapefile, Didymodon location (DBF format, Shape format, and ASC format) and so on are available from the Dryad database (Please refer to http://datadryad.org/review?doi=doi:10.5061/dryad.m8b96).
Funding Statement
This work was supported by the grant entitled "Research and demonstration on key techniques of safety growing and efficient use of forage grass in the One River, Two Streams area" from the Tibet Autonomous Region (NO:Z2013C02N02, URL: http://tibetsti.gov.cn/xzkjtportal/index.aspx). XS received the funding. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Turetsk MR, Bond-Lamberty B, Euskirchen E, Talbot J, Frolking S, McGuire AD, et al. The resilience and functional role of moss in boreal and arctic ecosystems. New Phytol. 2012;196: 49–67. doi: 10.1111/j.1469-8137.2012.04254.x [DOI] [PubMed] [Google Scholar]
- 2. Imada Y, Kawakita A, Kato M. Allopatric distribution and diversification without niche shift in a bryophyte-feeding basal moth lineage (Lepidoptera: Micropterigidae). Proc Biol Sci. 2011;278: 3026–3033. doi: 10.1098/rspb.2011.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frego KA. Bryophytes as potential indicators of forest integrity. Forest Ecol Manag. 2007;242: 65–75. [Google Scholar]
- 4. Ceschin S, Aleffi M, Bisceglie S, Savo V, Zuccarello V. Aquatic bryophytes as ecological indicators of the water quality status in the Tiber River basin (Italy). Ecol Indic. 2012;14: 74–81. [Google Scholar]
- 5. Ermakova EV, Frontasyeva MV, Steinnes E. Air pollution studies in Central Russia (Tula Region) using the moss biomonitoring technique, INAA and AAS. J Radioanal Nucl Ch. 2004;259: 51–58. [Google Scholar]
- 6. Pesch R, Schroeder W. Mosses as bioindicators for metal accumulation: Statistical aggregation of measurement data to exposure indices. Ecol Indic. 2006;6: 137–152. [Google Scholar]
- 7. Karger DN, Kluge J, Abrahamczyk S, Salazar L, Homeier J, Lehnert M, et al. Bryophyte cover on trees as proxy for air humidity in the tropics. Ecol Indic. 2012;20: 277–281. [Google Scholar]
- 8. Frahm JP. Bryophytes as indicators of climate change. Gefahrst Reinhalt L. 2007;67: 269–273. [Google Scholar]
- 9. Ah-Peng C, Chuah-Petiot M, Descamps-Julien B, Bardat J, Stamenoff P, Strasberg D. Bryophyte diversity and distribution along an altitudinal gradient on a lava flow in La Réunion. Divers Distrib. 2007;13: 654–662. [Google Scholar]
- 10. Wu PC. [Bryophytes Biology]. Beijing: Science Press; 1998. Chinese. [Google Scholar]
- 11. Weibull H, Rydin H. Bryophyte species richness on boulders: relationship to area, habitat diversity and canopy tree species. Biol Conserv. 2005;122: 71–79. [Google Scholar]
- 12. Ingerpuu N, Vellak K, Kukk T, Päartel M. Bryophyte and vascular plant species richness in boreo-nemoral moist forests and mires. Biodivers Conserv. 2001;10: 2153–2166. [Google Scholar]
- 13. Li FX, Wang YF, Zhan QF, Xu B, Zhai DC, Dang GD. Species diversity of floor bryophyte communities in Foping Nature Reserve. Chinese J Plant Ecol. 2006;6: 919–923. [Google Scholar]
- 14. Belland RJ. A multivariate study of moss distributions in relation to environment in the Gulf of St. Lawrence region, Canada. Can J Bot. 2005;83: 243–263. [Google Scholar]
- 15. Zander RH. Genera of the Pottiaceae: Mosses of harsh environments. Bulletin of the Buffalo Society of Natural Sciences. 1993;32: 1–162. [Google Scholar]
- 16. Jiménez JA, Ros RM, Cano MJ, Guerra J. A revision of Didymodon section fallaces (Musci, Pottiaceae) in Europe, North Africa, Macaronesia, and Southwest and Central Asia. Ann Mo Bot Gard. 2005;92: 225–247. [Google Scholar]
- 17.Ren DM. Studies on taxonomy and flora of Pottiaceae in China [dissertation] Graduate School of Inner Mongolia University. 2012.
- 18. Jia Y, He S. Species catalogue of China Volume 1 Plants: Bryophytes. Beijing: Science Press; 2013. [Google Scholar]
- 19. Ochyra R, Zander RH. The genera Didymodon and Bryoerythrophyllum (Pottiaceae) in Antarctica. J Bryol. 2002;24: 33–44. [Google Scholar]
- 20. Jiménez JA. Taxonomic revision of the genus Didymodon Hedw, (Pottiaceae, Bryophyta) in Europe, North Africa and Southwest and Central Asia. J Hattori Bot Lab. 2006;100: 211–292. [Google Scholar]
- 21. Werner O, Jiménez JA, Ros RM, Cano MJ, Guerra J. Preliminary investigation of the systematics of Didymodon (Pottiaceae, Musci) based on nrITS sequence data. Syst Bot. 2005;30: 461–470. [Google Scholar]
- 22. Werner O, Köckinger H, Jiménez JA, Ros RM. Molecular and morphological studies on the Didymodon tophaceus complex. Plant Biosyst. 2009;143 Suppl 1: S136–145. [Google Scholar]
- 23. Jimenez JA. The identity of Barbula purpurea Müll.Hal. with Didymodon bistratosus Hebr. and RB Pierrot. J Bryol. 2009;31: 49–50. [Google Scholar]
- 24. Immerzeel W, Stoorvogel J, Antle J. Can payments for ecosystem services secure the water tower of Tibet? Agric Syst. 2008;96: 52–63. [Google Scholar]
- 25. Gao QZ, Wan YF, Xu HM, Li Y, Jiangcun WZ, Borjigidai A. Alpine grassland degradation index and its response to recent climate variability in Northern Tibet, China. Quatern Int. 2010;226: 143–150. [Google Scholar]
- 26. Piao SL, Fang JY, He JS. Variations in vegetation net primary production in the Qinghai-Xizang Plateau, China, from 1982 to 1999. Climatic Change. 2006;74: 253–267. [Google Scholar]
- 27. Li XJ. [Moss Flora of Tibet]. Beijing: Science Press; 1985. Chinese. [Google Scholar]
- 28. Li R, Yu CQ, Jiang YB, Liu XC, Shao XM. Study on bryophyte communities in planted pastures of valley area of Tibet. Agricultural Research in the Arid Areas. 2010;28: 228–32. Chinese. [Google Scholar]
- 29. Cui XY, Gu S, Wu J, Tang YH. Photosynthetic response to dynamic changes of light and air humidity in two moss species from the Tibetan Plateau. Ecol Res. 2009;24: 645–653. [Google Scholar]
- 30.Shao JJ, Fu JJ, Shi JB, Jiang GB. Investigation of Heavy Metals in Moss Collected from Tibet. Abstracts, Section 2, The 28th Chinese Chemical Society Congress. 2012.
- 31. Rapalee G, Steyaert LT, Hall FG. Moss and lichen cover mapping at local and regional scales in the boreal forest ecosystem of central Canada. J Geophys Res. 2001;106: 33551–33563. [Google Scholar]
- 32. Vanderpoorten A, Sotiaux A, Engels P. A GIS-based model of the distribution of the rare liverwort Aneura maxima at the landscape scale for an improved assessment of its conservation status. Biodivers Conserv. 2006;15: 829–838. [Google Scholar]
- 33. Jiang YB, Wang TJ, de Bie CAJM, Skidmore AK, Liu XH, Song SS, et al. Satellite-derived vegetation indices contribute significantly to the prediction of epiphyllous liverworts. Ecol Indic. 2014;38: 72–80. [Google Scholar]
- 34. Jiang YB, Liu XH, Tian RX, Shao XM. Field-sampling methods for investigating ground-bryophyte populations in forest vegetation. Pol J Ecol. 2011;59: 317–327. [Google Scholar]
- 35. Zhang JT. [Quantitative Ecology]. Beijing: Science Press; 2011. Chinese. [Google Scholar]
- 36. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Quatern Int. 2005;25: 1965–1978. [Google Scholar]
- 37. Hutchinson MF. ANUSPLIN Version 4.3 user guide Centre for Resource and Environmental Studies. Canberra: The Australian National University; 2004. [Google Scholar]
- 38. Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006;190: 231–259. [Google Scholar]
- 39. Fielding AH, Bell JF. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv. 1997;24: 38–49. [Google Scholar]
- 40. Jiménez-Valverde A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Global Ecol Biogeogr. 2012;21: 498–507. [Google Scholar]
- 41. Pearce J, Ferrier S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol Model. 2000;133: 225–245. [Google Scholar]
- 42. Vilar del Hoyo L, Martin Isabel MP, Martinez Vega FJ. Logistic regression models for human-caused wildfire risk estimation: analysing the effect of the spatial accuracy in fire occurrence data. Eur J Forest Res. 2011;130: 983–996. [Google Scholar]
- 43. Allouche O, Tsoar A, Kadmon R. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol. 2006;43: 1223–1232. [Google Scholar]
- 44. Prates-Clark CD, Saatchi SS, Agosti D. Predicting geographical distribution models of high-value timber trees in the Amazon Basin using remotely sensed data. Ecol Model. 2008;211: 309–323. [Google Scholar]
- 45. Baniya CB. Vascular and cryptogam richness in the world's highest alpine zone, Tibet. Mt Res Dev. 2010;30: 275–281. [Google Scholar]
- 46.Tian WL. The research that the response of mountain ecosystem bryophytes affect on global climate. M.Sc. Thesis, Graduate School of Sichuan Normal University. 2011.
- 47. Pearson RG, Raxworthy CJ, Nakamura M, Townsend Peterson A. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J Biogeogr. 2007;34: 102–117. [Google Scholar]
- 48. Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography. 2006;29: 129–151. [Google Scholar]
- 49. Loiselle BA, Jørgensen PM, Consiglio T, Jiménez I, Blake JG, Lohmann LG, et al. Predicting species distributions from herbarium collections: does climate bias in collection sampling influence model outcomes? J Biogeogr. 2008;35: 105–116. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Didymodon matrix contains species names and their coverage in the sample plots (Table A). Environmental data matrix includes all relevant environmental information for quadrats in which Didymodon mosses were present (Table B).
(PDF)
Data Availability Statement
Didymodon species, community-site matrix, and environment-site matrix used in canonical correspondence analysis are within the paper and its Supporting Information files. The other data and related metadata such as environmental variables used in MaxEnt model (ASC format), Tibet shapefile, Didymodon location (DBF format, Shape format, and ASC format) and so on are available from the Dryad database (Please refer to http://datadryad.org/review?doi=doi:10.5061/dryad.m8b96).