Abstract
18F-FDG PET/CT is a promising tool in detecting aortic graft infection. Present study investigated the value of dual-time-point 18F-FDG PET/CT imaging (DTPI) with delayed imaging in assessing aortic graft infection.
Twenty-nine patients with suspected aortic graft infection were prospectively enrolled in this DTPI study. Two nuclear medicine physicians read all the images and achieved consensus about the measurement of maximal standardized uptake value (SUVmax) and grading of image quality. The percentages of SUVmax change between initial and delayed images were recorded as retention index (RI); sensitivity, specificity, and accuracy were calculated based on reference standard.
All the 5 infected aortic grafts had positive RIs, which were generally higher than that of noninfected grafts. Those noninfected grafts had variable RIs. Seven patients had improved image quality in delayed imaging. DTPI with delayed image detected all the infected grafts with improved specificity (88%) and accuracy (90%), providing conspicuous delineation of the infected graft extent.
In conclusion, noninfected aortic grafts had more variable RIs than infected ones. DTPI might be useful for detecting aortic graft infection, improving image quality, and enhancing delineation of the infected aortic grafts.
INTRODUCTION
Aortic graft infection is a severe medical problem associated with high mortality as well as morbidity. The reported incidence of aortic graft infection is 0.3% for open surgeries and 0.2% for percutaneous endovascular stent grafting.1 A punctual surgery to remove entire infected graft with extra-anatomic surgical bypass or in situ reconstruction can save life and improve outcomes of aortic graft infection; contrarily, unnecessary surgery for those noninfected grafts may be fatal. Therefore, making a prompt and precise diagnosis or exclusion of graft infection is essential to guide further treatment.
Accurate and timely diagnose of aortic graft infection is crucial, but sometimes graft infections are insidious with nonspecific symptoms and signs, which may not easy to detect and obtain definite diagnoses. Computed tomography (CT) is the first-line modality to detect aortic graft infection due to its accurate anatomical and morphological information as well as widely availability. Fast and accurate anatomic information also facilitates image-guided needle aspiration for prompt microbiological analysis.2 For a long time, CT has been used for imaging diagnosis of aortic graft infection.2 However, several reports showed that false negative rate of CT in detecting aortic graft infection remained high, especially in those aortic grafts with low-grade infection.3,4 The suboptimal sensitivity of CT in detecting low-grade aortic graft infection was a main problem in early detection of aortic graft infection, which might lead to delayed treatment and inferior prognosis.4 Many CT findings were not specific for infected aortic grafts or postoperative inflammation was another problem, which sometimes led to inconclusive results whether the febrile patients should receive another operation or not.2,5
18F-FDG is a promising tracer for detecting aortic graft infection, because activated leukocytes have high metabolic demand for 18F-FDG and aggregate on the infected aortic graft.5 Positron emission tomography/computed tomography (PET/CT) incorporated anatomic and metabolic information with improved resolution and lesion localization, which played an important role in differentiating aortic graft infection from adjacent soft-tissue infection, leading to optimized therapeutic strategy.5 PET/CT could separate aortic graft from physiological and abnormal 18F-FDG uptakes of esophagus, bowels, and lymph nodes, which were in the vicinity of implanted aortic grafts. 18F-FDG PET/CT improved the detection and diagnosis of aortic graft infection by reducing this kind of false positive and false negative results.2,5 However, Keidar et al6 demonstrated that most of the noninfected vascular grafts also had high and diffuse 18F-FDG uptake, which might persist for a long time. Those diffusely or heterogeneously increased 18F-FDG uptakes of aortic grafts were probably due to sterile inflammation, and were less specific for aortic graft infection. Several approaches of 18F-FDG PET/CT were developed to solve this problem, but the results were variable. Fukuchi et al7 demonstrated that infected grafts often had higher 18F-FDG uptake than noninfected grafts, and often presented as focal increased uptake on the 18F-FDG PET image. Spacek et al3 reported a 97% of accurate predict rate when both a focal 18F-FDG uptake and an irregular graft boundaries were noted on the integrated 18F-FDG PET/CT. Tokuda et al8 used a semiquantitative parameter, maximal standardized uptake value (SUVmax), to assess aortic graft infection, and reported that infected aortic grafts generally had higher SUVmax than noninfected ones. Sah et al9 adopted a 5-point scale with CT criteria to improve the accuracy of 18F-FDG PET/CT in diagnosing vascular graft infection. However, Saleem et al10 compared different interpretation methods in 18F-FDG PET and CT angiography, and found that the diagnostic accuracies of quantitative and visual 18F-FDG PET parameters for detecting aortic graft infection remained modest; the application of 18F-FDG PET/CT on aortic graft infection seemed limited by those false positive results.10 Since aortic neoplasm was rare, strategy to distinguish aortic graft infection from noninfectious graft inflammation was paramount, and might improve the accuracy of 18F-FDG PET/CT in assessing aortic graft infections. We hypothesized that infected aortic grafts might behave differently from noninfected grafts on the dual-time-point 18F-FDG PET/CT imaging (DTPI); with the progressive clearance of the blood pool 18F-FDG activities over time, the image quality might be improved, and the target-to-background ratio as well as the infected graft delineation might be enhanced on the delayed images. The aim of this study was to investigate the value of DTPI with delayed imaging in patients with suspected aortic graft infections.
PATIENTS AND METHODS
Inclusion and Exclusion Criteria
From July 2012 to May 2014, 29 patients (19 male, 10 female) were prospectively referred for 18F-FDG PET/CT to detect aortic graft infection by cardiovascular surgeons. The suspicion of aortic graft infections was based on surgeons’ experiences and impressions, clinical symptoms and signs, and evidences such as unknown fever, malaise, radiological findings, elevated CRP levels, and WBC counts in patients who had ever received aortic grafts implantation without other evident infection sources. Nineteen patients had received endovascular stent grafting, 7 patients had received traditional open surgery, and 3 patients had received both types of surgeries. The locations of these aortic grafts were at thoracic aorta (n = 15), abdominal aorta (n = 8), or at both sites (n = 6). Patients with documented vasculitis were not included in present study. The study was approved by institutional review board of Taipei Veterans General Hospital, and informed consent was obtained from all individual participants included in this study.
Imaging Protocol
The patients were asked to fast for at least 6 h before the 18F-FDG PET/CT examination. We measured each patient's fasting blood glucose level before 18F-FDG PET/CT examination to ensure that the level was <8 mM. Patients received an intravenous injection of about 370 MBq of 18F-FDG adjusted for body weight; no intravenous or oral CT contrast medium was used in this study. Initial scans with 3-dimentional (3D) acquisition from head to upper thigh were obtained on a 64-slice PET/CT scanner (GE Healthcare, Waukesha, WI, USA) after resting for 45 to 60 min. Delayed scans with 3D acquisition targeted on the aortic grafts were obtained on each patient after a second rest for about 45 min. The slice thickness and axial field of view were 0.327 and 15.7 cm, respectively.11 The images were reconstructed iteratively using the ordered-subset reconstruction algorithm, and attenuation corrections of PET were calculated from CT transmission maps generated from CT data set.12
Image Interpretation
All images were reviewed on a workstation, which allowed 3D reconstruction of the PET and CT images as well as semiquantitative measurements. Two experienced nuclear medicine physicians reviewed all the initial and delayed images blindly and independently, and searched for foci of abnormal 18F-FDG uptakes. The shape, distribution, and the intensity of each abnormal 18F-FDG uptake on the initial and delayed images, particularly focusing on the grafts and the perigraft areas, were recorded. Maximal standardized uptake values (SUVmax) of these abnormal increased 18F-FDG uptakes on the initial (iSUV) and delayed (dSUV) images were determined and corrected by body weight. 18F-FDG retention indexes (RIs) were derived from differences of SUVmax between initial and delayed images (dSUV–iSUV) divided by SUVmax of the initial image (iSUV). On each pair of the initial and delayed images, two 3D spheres, 1.8 cm in size, were placed on the same site in the aortic lumen of the ungrafted abdominal or descending thoracic aorta with avoidance of any abnormal focal or atherosclerotic 18F-FDG uptake; the average 18F-FDG uptake inside each sphere was SUVmean. The blood pool 18F-FDG activities of the initial and the delayed scans were determined by averaging the 2 SUVmean values. Subjective criteria such as blood pool activity, target-to-background ratio, image contrast, extent of the infected grafts, misregistration between PET and CT images, and urinary as well as respiratory artifacts were reviewed. The image quality of both initial and delayed images was graded as poor (apparent influence on image interpretation), fair (minimal influence on image interpretation), and good (no influence on image interpretation) by the 2 nuclear medicine physicians. Discordances were resolved by consensus.
Reference Standard
The reference criteria for presence of infected grafts were based on surgical findings and microbiological exams derived directly from surgical specimens and fluids, or image-guided drainage. When patients with suspicious infected grafts did not received surgery or image-guided drainage, they underwent radiological and clinical follow-up with series of blood cultures for >11 months. Operative findings such as purulence, turbid fluid, or infected-appearing thrombus were regarded as positive for infected aortic graft. Radiographic findings such as multiple foci of gas and adjacent fluid collection were suggestive of aortic graft infection. A graft was confirmed to be infected if the culture specimen obtained during surgery or image-guided drainage grew bacteria, or the disease progressed dramatically during the radiological and clinical follow-up with positive blood culture results and lack of other infection sources. For patients with negative blood culture results and improved or stable disease during the radiological and clinical follow-up, the aortic grafts were considered to be negative for infection.
Statistical Analysis
Those semiquantitative parameters of SUVmax and blood pool activity on the initial and delayed images were recorded as iSUV, dSUV, iBP, and dBP, respectively. Dichotomous results of infected and noninfected grafts were based on the reference standard, and the iSUV, dSUV, and RI of the 2 patient groups were compared by Mann–Whitney rank sum test. Performance of the conventional 18F-FDG PET/CT and DTPI were tested against the reference standard using receiver operating characteristic (ROC) curve analysis, and the optimal sensitivity, specificity, and accuracy for detecting infected grafts were derived at optimal cut off point from ROC curve (Sigmaplot 12.3, Systat Software Inc., San Jose, CA, USA). A P value <0.05 was considered significant.
RESULT
Of the 29 patients, 3 of them went on to have surgery due to suspected infected grafts, 3 had image-guided drainage, and the rest 23 patients received conservative management as well as radiological and clinical follow-up with series of blood examination. There were 5 patients with infected aortic grafts and 24 patients with noninfected grafts based on the reference standard. 18F-FDG PET/CT detected extra-prosthetic sites of infection in 6 patients with noninfected grafts (4 pneumonias, 1 cellulitis on the back, and 1 pancreatitis). The patients’ characteristic and geographic data were shown in Table 1. Four patients had surgical specimen or drained fluid confirmed to have infected grafts; 1 patient was diagnosed as infected graft based on positive blood culture and deteriorated follow-up images; the rest 24 patients, who lacked evidences of infected grafts and had uneventful follow-up, were diagnosed to have noninfected grafts.
TABLE 1.
Geographic Data of Study Population
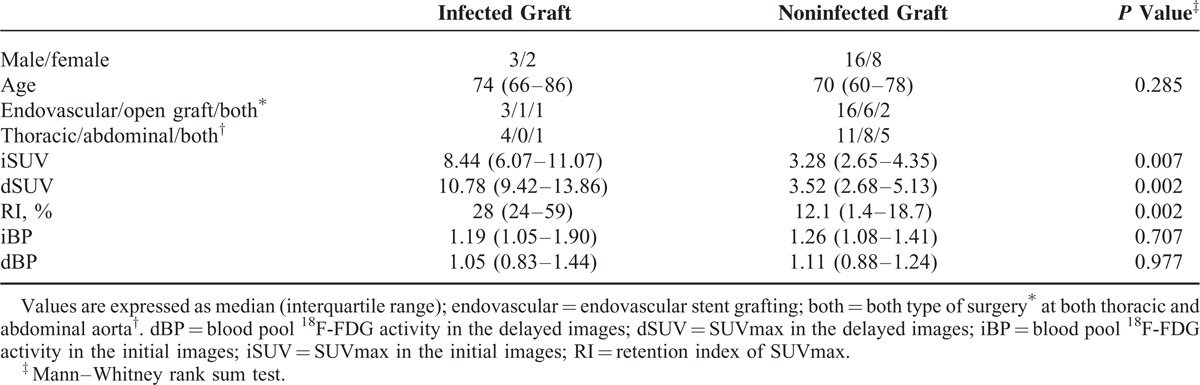
The median iSUV of infected and noninfected grafts were 8.44 and 3.28, respectively, and infected grafts generally had higher iSUV than noninfected grafts (Table 1). Similarly, the median dSUV of infected grafts was higher than that of noninfected ones (Figure 1). All the 5 infected grafts had positive RIs ranging from 0.20 to 0.89 (Figure 2), whereas great variations of RIs were noted among the noninfected aortic grafts, ranging from −0.36 to 0.31 (Figure 1). The median RI of infected grafts was higher than that of noninfected ones (Table 1). Among the 24 noninfected grafts, RIs were negative in 4, positive but <20% in 17 (Figure 3), and >20% in 3. Progressive clearance of 18F-FDG from blood was evident in both patients with infected and noninfected grafts; 7 patients had improved image quality grading on the delayed images due to low dBP and high dSUV of the grafts, leading to altered extent of an infected graft (Figure 2; Table 2). DTPI with 20% increment as the optimal cut off point of RI detected all the infected aortic grafts, with a specificity and accuracy superior to SUVmax using 5 as the optimal cut off point in the conventional 18F-FDG PET/CT imaging (Table 3). However, 3 noninfected grafts with active inflammation had RIs >20%, which were indistinguishable from that of infected aortic grafts.
FIGURE 1.
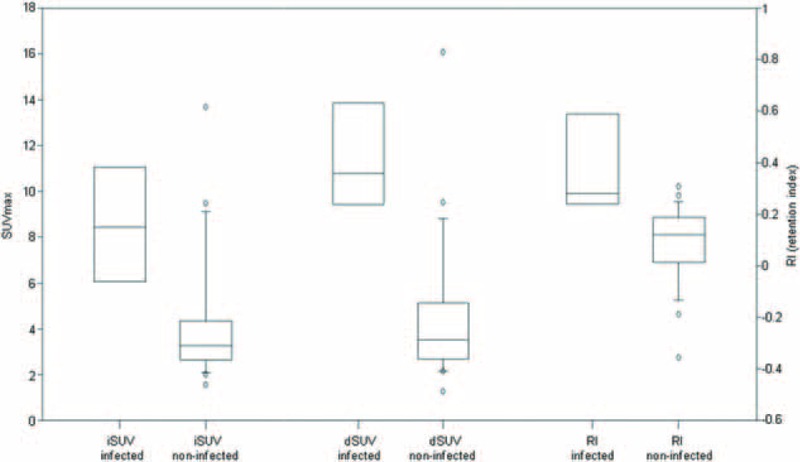
Box plots of iSUV, dSUV, and retention index (RI) of the infected and noninfected aortic grafts were shown. The infected grafts generally had higher iSUV, dSUV, and RI than noninfected grafts (P < 0.01, Mann–Whitney rank sum test). dSUV = SUVmax of the aortic graft on delayed image; iSUV = SUVmax of the aortic graft on initial image.
FIGURE 2.
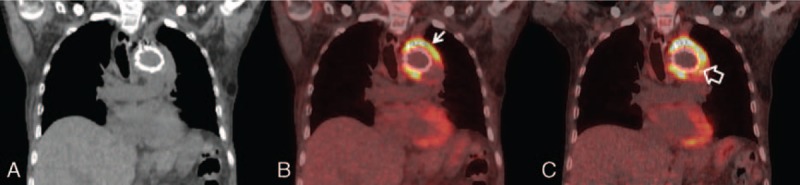
Coronal images (A: CT, B: PET/CT, C: delayed PET/CT; 61 min and 112 min after 18F-FDG injection) of a 60-year-old male with aortic arch stent graft revealed heterogeneously and progressively increased 18F-FDG uptake (arrow, SUVmax raised from 12.63 to 16.28) on and around the graft with widespread involvement of aortic graft infection (open arrow) and enhanced target-to-background ratio on the delayed images, improving the image quality grading from fair to good. Subsequent surgery confirmed the extent of graft infection from Klebsiella pneumoniae. CT = computed tomography; PET/CT = positron emission tomography/computed tomography.
FIGURE 3.
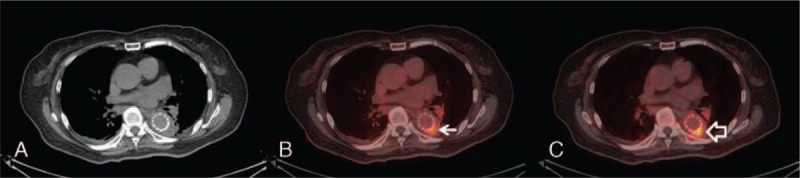
Trans-axial images (A: CT, B: PET/CT, C: delayed PET/CT; 59 min and 102 min after 18F-FDG injection) of a 79-year-old female with thoracic aortic stent graft demonstrated a persistent focus of increased 18F-FDG uptake in the aortic graft (arrow, SUVmax: 6.03/7.03, initial/delayed image). She was found to have urinary tract infection but no evidence of graft infection during the clinical and radiological follow-up. CT = computed tomography; PET/CT = positron emission tomography/computed tomography; SUVmax = maximal standardized uptake value.
TABLE 2.
Image Qualities of Initial and Delayed 18F-FDG PET/CT

TABLE 3.
Conventional and Dual-Time-Point 18F-FDG PET/CT for Detecting Aortic Graft Infection

DISCUSSION
Our study demonstrated that infected aortic grafts tended to have high RIs, but noninfected aortic grafts had variable RIs. For the 4 noninfected grafts with negative RI, it was possible that chronic sterile inflammation existed in the aortic grafts. Gheng et al demonstrated that acute infection and granulomatous inflammation were distinct from chronic inflammation on delayed 18F-FDG PET/CT imaging due to different patterns of 18F-FDG retention and clearance,13 and implied that there might be differences in inflammatory cells and cytokines involved.13,14 Sanz-Viedma et al speculated that some inflammatory cells had an increased ratio of glucose-6-phospatase to hexokinase leading to washout of 18F-FDG from the cells in delayed image,15 which might explain the negative RIs of the 4 noninfected aortic grafts. Mamede et al demonstrated that neutrophils and macrophages had an overproduction of the hexokinase during the respiratory burst, which provided clues for 18F-FDG retention on delayed image.16,17 The variable positive RIs of the rest 20 noninfected grafts in our study might represent variable severities and degrees of inflammation, and was compatible with previous observation on active inflammation. Although the mechanism of high RIs in those infected aortic grafts was not well understood, we implied that aortic graft infections were possibly accompanied with hyperemia and active noninfectious inflammation. The high 18F-FDG uptake and RI on infected aortic grafts might be caused by a combination of leukocytes attracted by both graft infection and active inflammatory process.
A complete and en-block resection of the infected grafts is crucial for eradication of infection, and depends on accurate perception of the extent of the infected grafts. Delayed 18F-FDG PET/CT images had low blood pool activities, increased target-to-background ratio, and enhanced image contrast for assessing aortic grafts infection, which was useful for delineating the detailed extent of the infected aortic graft to facilitate a precise and complete resection (Figure 2). For the patients with noninfected grafts, 6 of them were found to have other extra-prosthetic infections by 18F-FDG PET/CT, leading to targeted cultures and guided antibiotic treatments in these patients. As several studies demonstrated the usefulness of 18F-FDG PET/CT in searching undiscovered infectious foci,17,18 DTPI might play a pivotal role in management of patients with suspected aortic graft infection, particularly in diagnosing or excluding aortic graft infection, and discovering extra-prosthetic infectious sources, serving as a promising 1-stop-shop examination in febrile patients with suspected infected aortic grafts.
Our study might have clinical implications in the diagnosis of aortic graft infection. First, DTPI could provide additional information for assessing aortic graft infection. Interpreting high 18F-FDG uptakes on the aortic grafts was challenging since several studies noticed the overlapping of SUVmax between infected and noninfected aortic grafts.8–10 Keidar et al6 reported 92% of the noninfected grafts had diffusely high 18F-FDG uptake due to foreign body reaction and sterile inflammation. Controversies existed on whether the patterns of the abnormal uptake on the aortic grafts could help to differentiate aortic graft infection from noninfectious graft inflammation.7,10 Other studies suggested combined interpretation of the co-registered CT findings to improve the performance of 18F-FDG PET/CT.3,9 Several reports suggested gallium and leukocyte scans in detecting vascular graft infection.4,19 Erba et al4 used 99mTc-HMPAO-WBC SPECT/CT to reduce 37% of the false positive results with a specificity superior to that in our study, which was useful in detecting late and low-grade vascular prosthesis infections. Yilmaz et al20 used 18F-FDG-labelled leukocytes, instead of 99mTc- and 111In-labled WBCs, successfully to identify aortic graft infection from aseptic graft inflammation. Although limited by small sample population and referral nature, our preliminary result suggested that infected and noninfected aortic grafts might behave differently on the DTPI, and aortic grafts with RIs >20% were at high risk of graft infection.
Second, our study determined the optimal timing of 18F-FDG PET/CT imaging for aortic graft infection—90 min post-18F-FDG injection. Since the optimal protocols of 18F-FDG PET/CT for infection and inflammation imaging varied on different clinical indications,18 we proposed that the 18F-FDG uptake of the infected aortic grafts did not reach a plateau at 1 h imaging, and the dynamic 18F-FDG uptake relative to time might be different between infected and noninfected aortic grafts. The time intervals between initial and delayed images in our study were not restrictively fixed for practical reasons. The critical patients with mechanical ventilation and unstable vital signs usually could not tolerate a long stay at PET room for the delayed imaging. The busy schedule of PET examination sometimes postponed the delayed scans. Although the delay time between the scans might affect the values of dSUV and RI, this pilot study suggested that those infected aortic grafts did not reach peak 18F-FDG uptake before 90 min post-18F-FDG injection, providing clues for the optimal timing of 18F-FDG PET/CT imaging in patients with suspicious aortic graft infection. Further large-scaled and detailed investigations on the dynamic 18F-FDG uptake of infected aortic grafts are needed.
STUDY LIMITATIONS AND CONCLUSION
Present study had several limitations. First, only 5 patients had infected aortic grafts according to the reference standard, and 24 patients received conservative management. Only patients with high suspicion of aortic graft infection and acceptable physical condition were candidate for surgery in our hospital. This might reflect the rare incidence of aortic graft infection not only in our study but also in general population, and could bias the result. Second, the effects of antibiotics on the 18F-FDG uptake were not involved,11 although most of the patients received empiric antibiotic treatment at 18F-FDG PET/CT examination. Bias would be inevitable too due to the small sample size and single-institution referral nature of this study. We believed that further studies with large sample sizes are needed to clarify the potential benefits of DTPI in assessing aortic grafts infection.
In conclusion, noninfected aortic grafts had variable RIs, and infected aortic grafts tended to have high RIs. Aortic grafts with negative RIs and low 18F-FDG uptakes were less likely to be infected. 18F-FDG PET/CT was also helpful in discovering extra-prosthetic infection in this situation. The DTPI might be useful for detecting aortic graft infection by improving image quality as well as conspicuity of the infected graft extent in this study, but active noninfectious inflammation might degrade the performance of the DTPI. Further validation study should be performed.
ACKNOWLEDGMENT
The authors thank Chih-Hao Chen, Ming-Chu Wu, Jyh-Wei Lin, and Chia-Wen Huang for their technical assistance.
Footnotes
Abbreviations: CT = computed tomography, dBP = blood pool activity of delayed image, dSUV = SUVmax of the aortic graft on delayed image, DTPI = dual-time-point 18F-FDG PET/CT imaging, iBP = blood pool activity of initial image, iSUV = SUVmax of the aortic graft on initial image, PET/CT = positron emission tomography/computed tomography, RI = retention index, ROC curve = receiver operating characteristic curve, SUVmax = maximal standardized uptake value, SUVmean = mean standardized uptake value.
This work was supported by grant MOST 104-2623-E-075-002-NU and NSC 102-2314-B-075-056-MY2 from Ministry of Science and Technology, Taiwan, and grant V103B-031 from Taipei Veterans General Hospital.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Chaikof EL, Brewster DC, Dalman RL, et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009; 50:S2–S49. [DOI] [PubMed] [Google Scholar]
- 2.Bruggink JL, Slart RH, Pol JA, et al. Current role of imaging in diagnosing aortic graft infections. Semin Vasc Surg 2011; 24:182–190. [DOI] [PubMed] [Google Scholar]
- 3.Spacek M, Belohlavek O, Votrubova J, et al. Diagnostics of “non-acute” vascular prosthesis infection using 18F-FDG PET/CT: our experience with 96 prostheses. Eur J Nucl Med Mol Imaging 2009; 36:850–858. [DOI] [PubMed] [Google Scholar]
- 4.Erba PA, Leo G, Sollini M, et al. Radiolabelled leucocyte scintigraphy versus conventional radiological imaging for the management of late, low-grade vascular prosthesis infections. Eur J Nucl Med Mol Imaging 2014; 41:357–368. [DOI] [PubMed] [Google Scholar]
- 5.Keidar Z, Nitecki S. FDG-PET in prosthetic graft infections. Semin Nucl Med 2013; 43:396–402. [DOI] [PubMed] [Google Scholar]
- 6.Keidar Z, Pirmisashvili N, Leiderman M, et al. 18F-FDG uptake in noninfected prosthetic vascular grafts: incidence, patterns, and changes over time. J Nucl Med 2014; 55:392–395. [DOI] [PubMed] [Google Scholar]
- 7.Fukuchi K, Ishida Y, Higashi M, et al. Detection of aortic graft infection by fluorodeoxyglucose positron emission tomography: comparison with computed tomographic findings. J Vasc Surg 2005; 42:919–925. [DOI] [PubMed] [Google Scholar]
- 8.Tokuda Y, Oshima H, Araki Y, et al. Detection of thoracic aortic prosthetic graft infection with 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Eur J Cardiothorac Surg 2013; 43:1183–1187. [DOI] [PubMed] [Google Scholar]
- 9.Sah BR, Husmann L, Mayer D, et al. Diagnostic performance of 18F-FDG-PET/CT in vascular graft infections. Eur J Vasc Endovasc Surg 2015; 49:455–464. [DOI] [PubMed] [Google Scholar]
- 10.Saleem BR, Berger P, Vaartjes I, et al. Modest utility of quantitative measures in (18)F-fluorodeoxyglucose positron emission tomography scanning for the diagnosis of aortic prosthetic graft infection. J Vasc Surg 2015; 61:965–971. [DOI] [PubMed] [Google Scholar]
- 11.Chang CY, Yang BH, Lin KH, et al. Feasibility and incremental benefit of puffed-cheek 18F-FDG PET/CT on oral cancer patients. Clin Nucl Med 2013; 38:e374–e378. [DOI] [PubMed] [Google Scholar]
- 12.Chang CY, Tzao C, Lee SC, et al. Incremental value of integrated FDG-PET/CT in evaluating indeterminate solitary pulmonary nodule for malignancy. Mol Imaging Biol 2010; 12:204–209. [DOI] [PubMed] [Google Scholar]
- 13.Cheng G, Alavi A, Del Bello CV, et al. Differential washout of FDG activity in two different inflammatory lesions: implications for delayed imaging. Clin Nucl Med 2013; 38:576–579. [DOI] [PubMed] [Google Scholar]
- 14.Cheng G, Torigian DA, Zhuang H, et al. When should we recommend use of dual time-point and delayed time-point imaging techniques in FDG PET? Eur J Nucl Med Mol Imaging 2013; 40:779–787. [DOI] [PubMed] [Google Scholar]
- 15.Sanz-Viedma S, Torigian DA, Parsons M, et al. Potential clinical utility of dual time point FDG-PET for distinguishing benign from malignant lesions: implications for oncological imaging. Rev Esp Med Nucl 2009; 28:159–166. [PubMed] [Google Scholar]
- 16.Mamede M, Higashi T, Kitaichi M, et al. [18F]FDG uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia 2005; 7:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meller J, Sahlmann CO, Scheel AK. 18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med 2007; 48:35–45. [PubMed] [Google Scholar]
- 18.Jamar F, Buscombe J, Chiti A, et al. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J Nucl Med 2013; 54:647–658. [DOI] [PubMed] [Google Scholar]
- 19.Alonso-Bartolome P, Martinez-Taboada VM, Pina T, et al. Hypertrophic osteoarthropathy secondary to vascular prosthesis infection: report of 3 cases and review of the literature. Medicine (Baltimore) 2006; 85:183–191. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz S, Asa S, Ozhan M, et al. Graft infection imaging with FDG and FDG-labeled leukocytes. Intern Med 2013; 52:1009–1010. [DOI] [PubMed] [Google Scholar]


