Supplemental digital content is available in the text
Abstract
The aim of this study was to analyze the clinical, computed tomography (CT), and positron emission tomography (PET) findings of sarcoidosis, sarcoid reaction, and malignant lymph nodes (LNs) to the results of transbronchial LN aspiration and biopsy (TBNA).
The TBNA results of mediastinal and hilar LNs of 152 patients in our hospital from July 2008 to March 2013 were retrospectively reviewed. Two independent radiologists measured the size and attenuation of LNs on CT and assessed the probability of the 3 categories: sarcoidosis (n = 36), sarcoid reaction (n = 25), or malignant LNs (n = 91). The total volume and attenuation of LNs were measured using Image J (NIH). The median maximum standardized uptake value (maxSUV) of the 3 mediastinal and hilar LNs on PET/CT was obtained.
There was no significantly different CT finding between sarcoidosis and sarcoid reaction. Multivariate analysis showed that the age, total volume of LNs, and number of enlarged LNs significantly differed between sarcoid reaction and malignant LNs. Sarcoid reaction tends to be occurred in young patients (P = 0.007), the total volume of LNs was smaller (P = 0.04) than that of malignant LNs, and there were significantly more LNs >1 cm (P = 0.005). The median maxSUV of the 3 highest SUVs of the LNs did not significantly differ between the 3 entities.
INTRODUCTION
Sarcoidosis is a systemic disease manifestation of chronic noncaseating granulomatous inflammation, with predominantly involving lung >90% of all sarcoidosis patients.1 Different from the idiopathic sarcoidosis, sarcoid reaction is considered as an immunologic abnormality related to a malignant condition, and can occur in 4.4% of carcinoma, 7.3% of non-Hodgkin lymphoma, and 13.8% of Hodgkin disease.2 A previous article on the mediastinoscopy series in patients with lung cancer has shown a 4.3% to 7% incidence of sarcoid-like lesions.3,4 Although the lack of systemic symptoms in patients with sarcoid reaction and some immunohistochemical studies may help to differentiate sarcoidosis and sarcoid reaction, imaging findings of these entities may not be informative enough for both radiologists and clinicians. Therefore, sarcoid reaction should always be considered as a differential diagnosis when a cancer patient develops lymphadenopathy. If a case with malignant lymph nodes (LNs) is misdiagnosed as sarcoid reaction, the underestimation of the malignancy can lead to insufficient treatment that will affect the patient's disease morbidity and mortality. On the contrary, sarcoidosis or sarcoid reaction can mimic metastasis or progression of malignancy,5–11 which may result in unnecessary systemic treatment. To make a differential diagnosis, thoughtful review of previous computed tomography (CT) and pathologic confirmation of the involved lesions including mediastinal or hilar LNs are necessary.12 Positron emission tomography (PET)/CT can be used to guide early and aggressive therapy in patients with suspected cancer recurrence. However, benign hypermetabolic lymphadenopathy can mimic malignant lymphadenopathy on PET/CT. Four cases of patients affected by gynaecological malignancies and coexisting sarcoidosis are reported.5 Thus, it is important to obtain a histological diagnosis before initiating antineoplastic therapy based on CT findings.
Recently, CT evaluation to differentiate benign and malignant mediastinal LNs was performed, and has been suggested the potential accuracy of CT to differentiate the entities.13 In this study, we try to differentiate the mediastinal and hilar LNs in the sarcoidosis, sarcoid reaction, and malignant LNs, and quantitatively compare the radiologic findings of the 3 entities using CT and 18F-fluorodeoxyglucose-PET (FDG-PET)/CT scans. The objective of the study was to compare clinical, CT, and PET/CT findings in sarcoidosis, sarcoid reaction, and malignant LNs in order to determine how to differentiate them in clinical settings.
MATERIALS AND METHODS
Study Population
This study was approved by our hospital's Institutional Review Board (Approval number: 2014-0880), and the requirement for the patient's informed consent was waived because of retrospective manner. From July 2008 to March 2013, 301 patients with mediastinal or hilar LNs on CT underwent transbronchial LN aspiration and biopsy (TBNA). Equivocal mediastinal or hilar LN was defined as a LN with measured size of > 7 to 11 mm on the short axis of the node depending on the nodal station.14,15 If the results of TBNA were nondiagnostic, rebiopsy or LN dissection was considered, and if not available, close follow-up of the patients was performed. After excluding 44 patients who did not undergo CT within 1 month from the pathologic diagnosis including TBNA, CT findings of 91 patients with malignant LNs, 36 idiopathic sarcoidosis, and 25 sarcoid reaction were compared (Figure 1). To compare the FDG-PET/CT findings, 69 patients who did not perform PET/CT within 1 month from the CT or pathologic diagnosis were excluded. All patients were followed up for at least 6 months, with the mean follow-up period 2.6 years.
FIGURE 1.
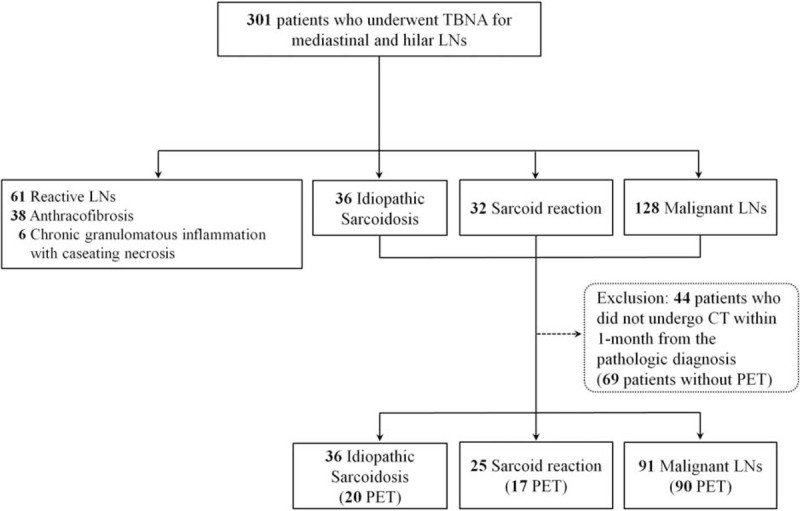
Patient population in this study. CT = computed tomography, LNs = lymph nodes, PET = positron emission tomography, TBNA = transbronchial lymph node aspiration and biopsy.
Clinical Features
Patients’ demographics, smoking history, presence of malignancy, types of malignancy, history of sarcoidosis, coexisting granulomatous disease, and pulmonary function test (PFT) results were reviewed. The PFT parameters were recorded as percentages of the predicted values. The presence of systemic involvement of sarcoidosis such as skin, extrathoracic LNs, abdominal organ, cardiovascular system, nervous system, or endocrine system was thoroughly reviewed. The level of serum angiotensin converting enzyme in sarcoidosis or sarcoid reaction was also recorded.
CT Scanning Protocol
Using 2 CT scans, either SOMATOM Sensation 16 (Siemens Medical Solutions, Erlangen, Germany) or Lightspeed VCT (General Electric Medical Systems, Milwaukee, WI), chest CT examinations were performed. For the 16-detector row scanner, the scan parameters were 120 kV and 100 effective mA with dose modulation. In the standard algorithm, the reconstruction intervals were 3-/5-mm thickness without a gap, and 1-mm thickness with 5-mm gaps in the high-frequency algorithm. The scan parameters were 120 kV and 100 to 400 mA with dose modulation in the Lightspeed 64 detector row scanner. In the lung algorithm, the reconstruction intervals were 2.5-/5-mm thickness without a gap, and 1.25-mm thickness with 5-mm gaps in the bone algorithm. After the intravenous administration of 100 mL of iopromide 300 (300 mg I/mL; Ultravist, Bayer Pharma, Berlin, Germany) at a rate of 2.5 mL/s using a power injector, CT images were taken with a 50-second delay. All the images were viewed at the mediastinal (width, 450 HU, and level, 50 HU) and lung window (width, 1500 HU, and level, −700 HU) settings of the axial and coronal images on the picture archiving and communication system (PACS).
PET/CT SCANNING PROTOCOL
The FDG-PET/CT was obtained using a commercially available machine (Discovery PET/CT 690; GE Health Care, Mississauga, ON, Canada) (>2.5 times the maximum standardized uptake value [maxSUV]). After fasting for 6 hours, patients received an intravenous injection of 18F-FDG (5.2 MBq/kg body weight) followed by PET/CT scanning after 50 minutes. Three-dimensional PET acquisition and attenuation corrections for CT attenuation maps were carried out. Using the lean body mass-based SUV, the maxSUV was calculated. The mean interval between CT and PET/CT studies was 9.2 ± 8.6 days. On FDG PET/CT scans, the 3 highest maxSUVs of the mediastinal and hilar LNs were obtained, and the median value per patient was calculated.
Evaluation of the CT and FDG-PET/CT Findings
Subjective Assessment
Two chest radiologists who assessed the disease probability (MYK with a clinical experience of 18 years and SYS with a clinical experience of 2 years) were blinded to the patient information and all patients were assigned in random order. The disease probability was classified into 5 categories: probable sarcoidosis, possible sarcoidosis, indeterminate, possible metastasis, and probable metastasis. The probability was evaluated subjectively based on the bilaterality of the LNs, their general proportion, the hilar LN involvement, and the homogeneity of the LNs.16 The 2 chest radiologists independently measured the short diameter and attenuation of the LNs at the right lower paratracheal (4R), left lower paratracheal (4L), subaortic or paraaortic (5 or 6), subcarinal (7), right hilar (10R), and left hilar (10L) levels using PACS.17–20 Two above chest radiologists also investigated the number of LNs >1 cm, and the pulmonary infiltrates of sarcoidosis, the so-called “Stage 2 sarcoidosis,” which showed perilymphatic distributed micronodules predominantly in both upper lobes, in consensus.1
Objective Assessment
The total volume and attenuation of all mediastinal and hilar LNs on the CT images were quantitatively measured using the Image J (NIH), an open-source free-ware medical imaging software available at http://imagej.nih.gov/ij/, by multiplying the diameter of the LNs in all the axial images by the slice thickness of the CT images (Figure 2). The images stored in DICOM obtained from the CT were used for measuring the parameters.
FIGURE 2.
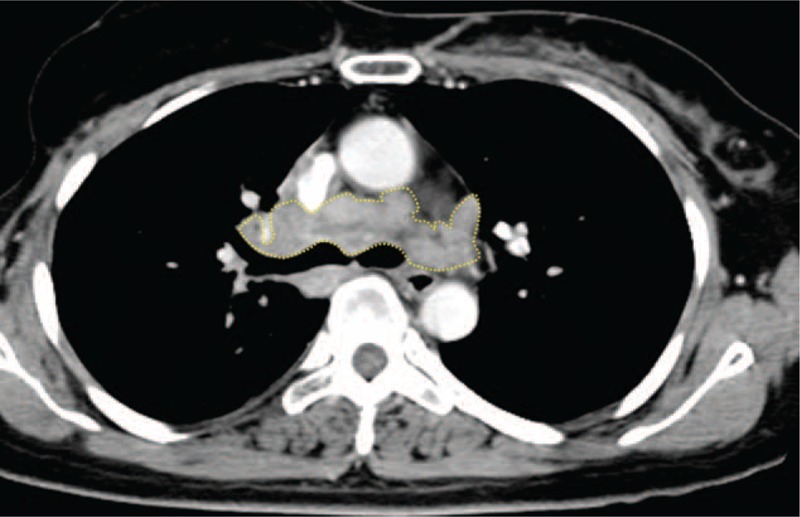
A 57-year-old female patient with breast cancer diagnosed as sarcoid reaction of mediastinal LNs. The total volume and attenuation of mediastinal and hilar LNs on CT images were quantitatively measured using the Image J program by multiplying the diameters of all LNs in all axial images by the slice thickness. An example of slices on Image J is shown. The measured total volume of LNs was 16903.5 mm3. CT = computed tomography, LNs = lymph nodes.
On PET/CT scans, the 3 highest maxSUVs of the mediastinal and hilar LNs were recorded, and the median value per patient was calculated. Then, the maxSUVs in the sarcoidosis versus sarcoid reaction and the sarcoid reaction versus malignant LNs were compared.
Transbronchial LN Aspiration and Core Biopsy
TBNA was performed under endobronchial ultrasound (EBUS) guidance by 2 experienced bronchoscopists (C-MC and SWL with 12 and 11 years of experiences in thoracic oncology and pulmonology, respectively) using a flexible linear array video bronchoscope with a 7.5 MHZ linear ultrasound transducer probe (CP-EBUS, model XBF-UC260F; Olympus, Tokyo, Japan) and an ultrasound image processor (model EU-C2000; Olympus) on patients consciously sedated with midazolam. To determine the target LNs, CT findings were carefully reviewed. With a 22-gauge 4-cm cytology needle (NA-202C, Olympus), needle aspiration and core biopsies were performed approximately 4 to 5 times per targeted LN to obtain adequate specimens. Three or more nodal stations were targeted using real-time ultrasonic needle guidance for better diagnosis. In the 14 nondiagnostic cases, rebiopsy was performed in 2 patients, transbronchial lung biopsy in 3, and lobectomy with LN dissection in 5. Remaining 4 patients who refused to receive rebiopsy or surgical approach were closely followed up after 1 month, and every third months during 1 year.
Histopathology
Pathologists analyzed the core tissue of the targeted LN, which were fixed with 10% buffered formalin and hematoxylin-eosin stain. In all patients, the histologic diagnoses were made by an expert pathologist (JSJ, a nonauthor, with 23 years of experience in thoracic pathology). Acid-fast stain and tuberculosis polymerase chain reaction for distinction of the chronic granulomatous inflammation from tuberculosis, and washing cytology for the differentials of the malignant tumor cells were performed. Periodic acid-schiff and Gomori-Grocott methenamine silver stain were also performed if pathologically needed to differentiate the diagnosis from other infectious conditions. Sarcoidosis was diagnosed when there was no evidence of clinical and pathological metastasis or tuberculosis of LNs and when noncaseating granulomas were detected in the specimen.
Statistical Analysis
Statistical analysis was performed by 10 years experienced statistician (S-SK). For the statistical analysis, a statistical package (SPSS version 18.0; SPSS Inc, Chicago, IL) was used, and the results were expressed as means ± standard deviations or medians with ranges. A P value <0.05 was considered statistically significant. Although the CT findings of the 3 disease entities, sarcoidosis, sarcoid reaction, and malignant LNs are difficult to distinguish, we assumed there might be possible clues for differentiation. If the agreement of the disease probability in chest radiologists is high, that may suggest possible CT findings are exist to differentiate the diseases. Therefore, to assess the agreement for the disease probability in 2 independent chest radiologists, the κ value was obtained. Also, to overcome possible measurement error for the size and attenuation of the LNs, the interobserver correlation of the measurement of the size and attenuation of the LNs, the degree of agreement was determined using a reliability analysis. The presence of parenchymal infiltrates was assessed using Pearson χ2 test. The maxSUVs of the LNs were compared using a Mann–Whitney U test.
RESULTS
Patient Characteristics
In this study, clinical and radiologic findings of 91 patients with malignant LNs, 36 idiopathic sarcoidosis, and 25 sarcoid reaction were compared (Figure 1). The etiologies of the malignancy in the study patients are demonstrated in the Supplementary Table 1, http://links.lww.com/MD/A320.
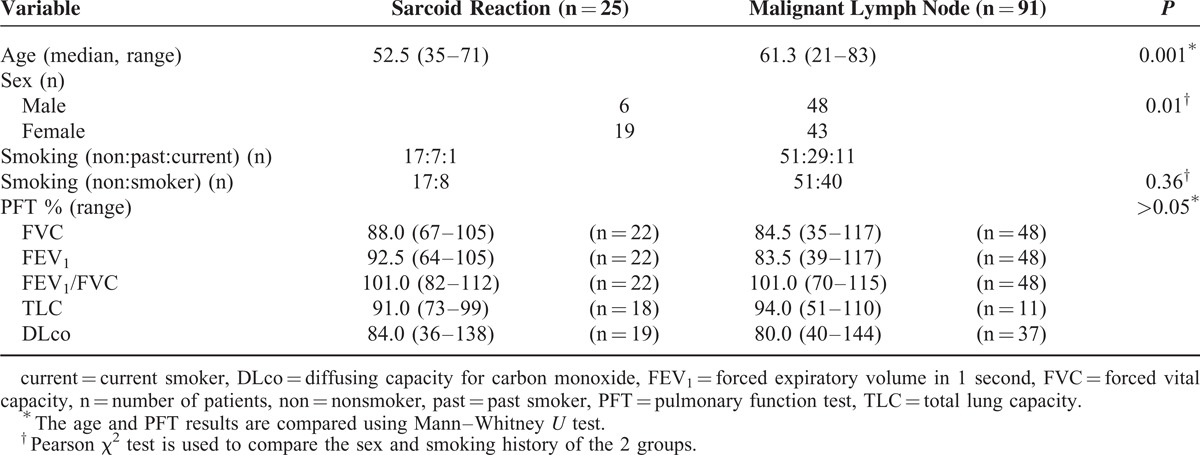
The clinical characteristics of the sarcoidosis and sarcoid reaction were not statistically different (Table 1). On the contrary, the median age of the patients in sarcoid reaction was significantly lower than that in the malignant LNs (P = 0.001), and those who had sarcoid reaction were mostly female (P = 0.01). However, smoking history and PFT results did not significantly differ between the 2 entities (Table 2).
TABLE 1.
Clinical Characteristics of the Patients With Sarcoid Reaction and Idiopathic Sarcoidosis
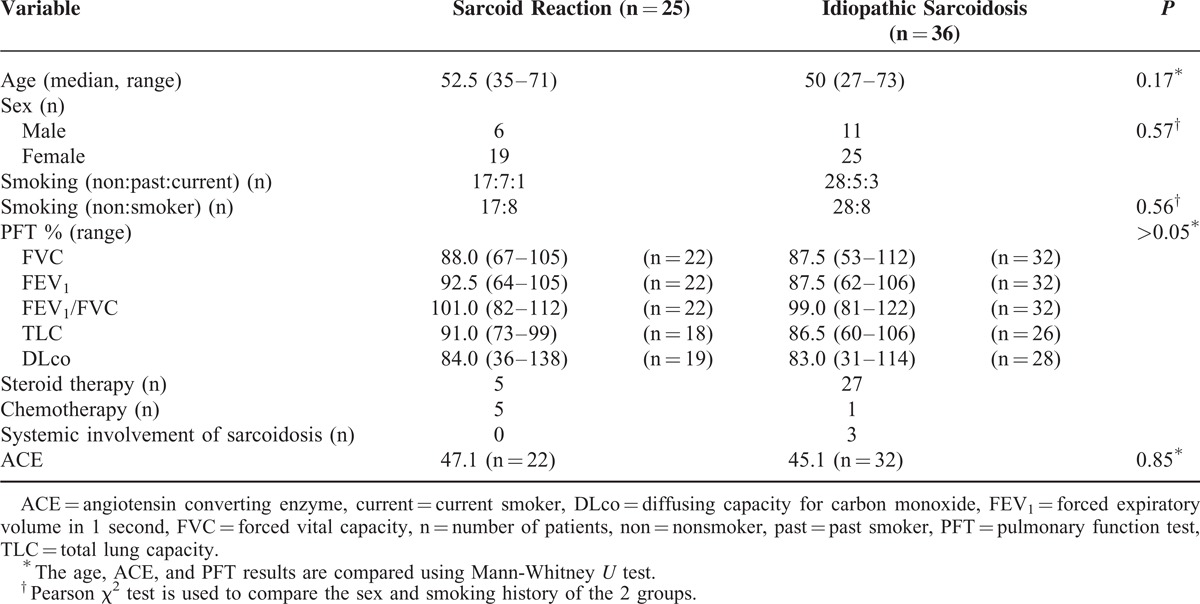
TABLE 2.
Clinical Characteristics of the Patients With Sarcoid Reaction and Malignant Lymph Nodes
CT and FDG-PET/CT Findings
Idiopathic Sarcoidosis Versus Sarcoid Reaction
Kappa index regarding size of LNs between the 2 radiologists was 0.68 to 0.94, and the result supported the good reliability of imaging assessment. However, the agreement of sarcoidosis between the 2 radiologists was poor with κ value of 0.366 (P = 0.002) (Supplementary Table 2, http://links.lww.com/MD/A320). Parenchymal infiltrates of sarcoidosis was detected in 16 (64%) patients in the sarcoid reaction. In univariate and multivariate analyses, there were no variables which showed statistical difference (Tables 3 and 4). The median maxSUV of LNs in sarcoidosis was 8.2 (range, 2.2–16.5), and the median maxSUV of LNs in sarcoid reaction was 7.5 (range, 2.5–23.3). PET/CT results were not statistically different between the 2 groups.
TABLE 3.
Comparison of the Radiologic Findings of the Sarcoid Reaction and Idiopathic Sarcoidosis
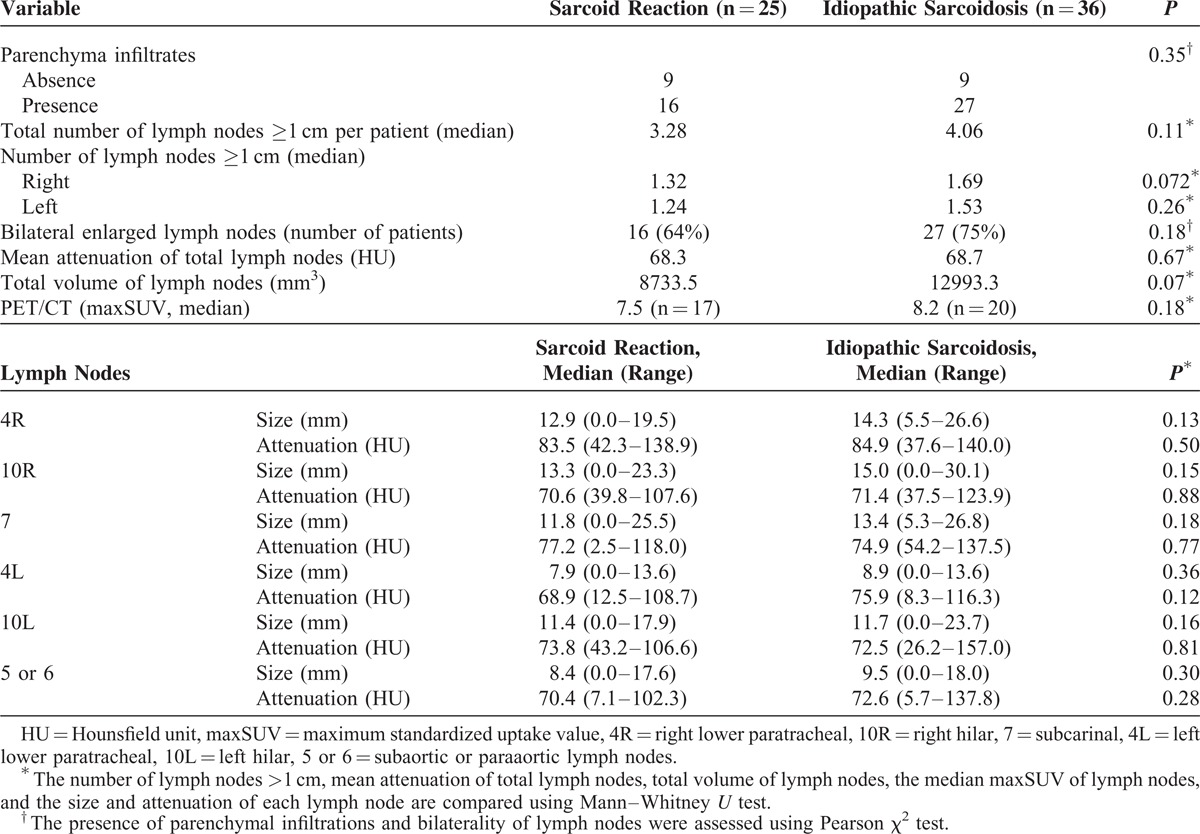
TABLE 4.
Multivariate Analysis of the Clinical Factors in the Idiopathic Sarcoidosis and Sarcoid Reaction
Sarcoid Reaction Versus Malignant LNs
The intraclass correlation coefficient of the probability of sarcoid reaction or metastasis was moderate (P = 0.64). The measured sizes of the thoracic LNs were highly reliable. The measured attenuations of the LNs were also very reliable (P < 0.001), except for left hilar area (10L) (Supplementary Table 3, http://links.lww.com/MD/A320).
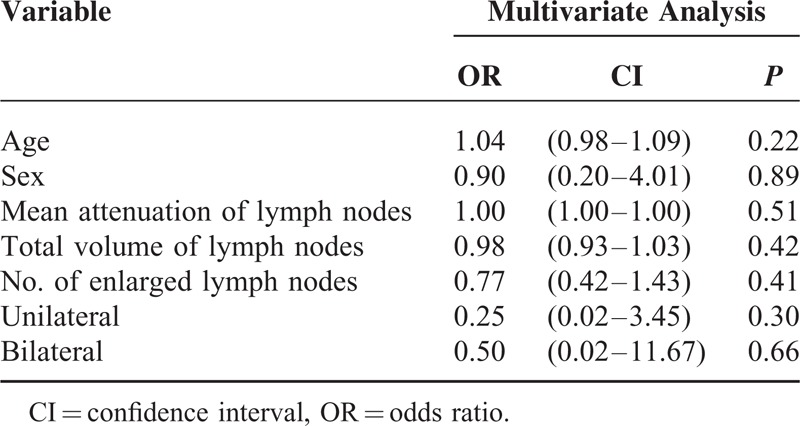
The measured sizes of the LNs showed that the LNs of sarcoid reaction tended to be larger than the malignant LNs, and that the LNs at the 4R, 7, 4L, 10L, and 5 or 6 levels were significantly larger (P < 0.05). However, the attenuations of LNs did not significantly differ between the 2 entities. There were significantly more LNs >1 cm per patient in the sarcoid reaction (P = 0.005). Although bilaterality of the thoracic LNs was observed in 17.6% of the patients with malignant LNs, 64% of the patients in the sarcoid reaction showed bilateral LNs (P = 0.004). However, the total volume of LNs was significantly lower in the sarcoid reaction than in the malignant LNs (P = 0.04). The median maxSUV of malignant LNs was 6.6 (range, 1–23). The median maxSUV of the 3 highest SUVs of the LNs did not significantly differ between the sarcoid reaction and malignant LNs (P = 0.38) (Table 5 and Figure 3). In the multivariate analysis, the age (P = 0.007), total volume of the LNs (P = 0.03), and the number of LNs (P = 0.04) significantly differed (Table 6 and Figure 4).
TABLE 5.
Comparison of the Radiologic Findings of the Sarcoid Reaction and Malignant Lymph Nodes
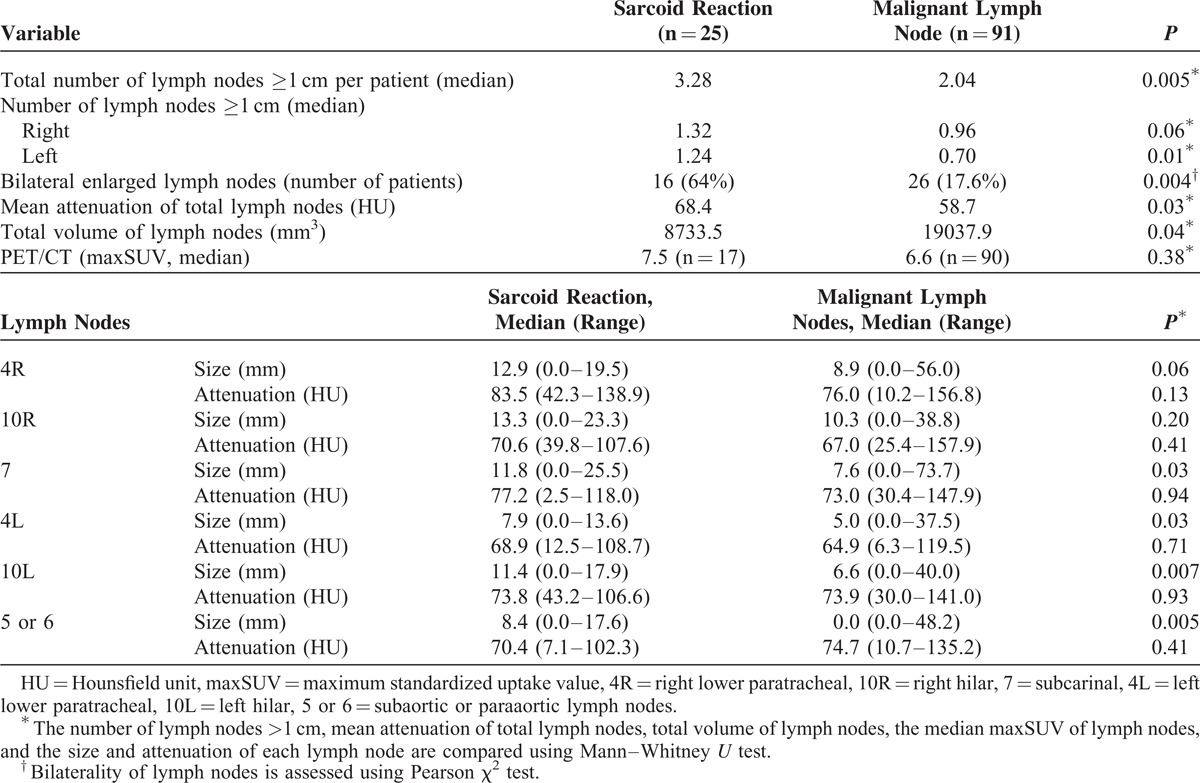
FIGURE 3.
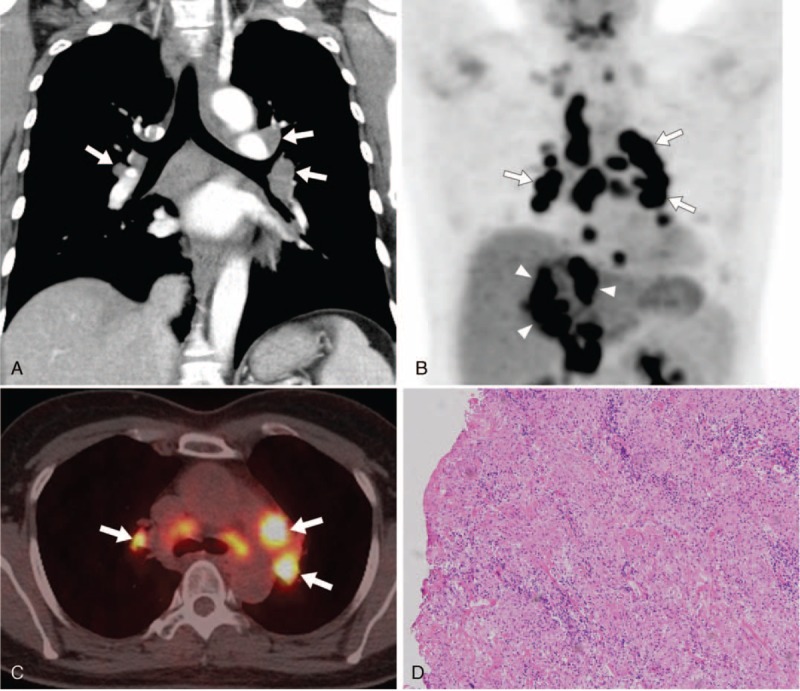
A 44-year-old female with hepatocellular carcinoma (HCC) and papillary thyroid cancer. (A) Chest CT coronal image shows bilateral enlarged LNs (arrows) in the mediastinum and hilar areas. (B, C) FDG-PET/CT scan shows multiple hypermetabolic activity in the mediastinal and hilar LNs (arrows) and large masses (arrowheads) in the hepatic segment IV and upper abdomen. Initially, the patients suspected as HCC with multiple LNs metastasis in the thorax and upper abdomen. The maxSUV of thoracic LNs measured up to 6.8, 9.0, and 13.9. (D) The pathologic findings of the LNs obtained from transbronchial LN biopsy are revealed chronic noncaseating granulomatous inflammation (hematoxylin-eosin stain, original magnification × 100). CT = computed tomography, LNs = lymph nodes, FDG-PET/CT = 18F-fluorodeoxyglucose-positron emission tomography/CT, maxSUV = maximum standardized uptake value.
TABLE 6.
Multivariate Analysis of the Clinical Factors in the Sarcoid Reaction and Malignant Lymph Nodes
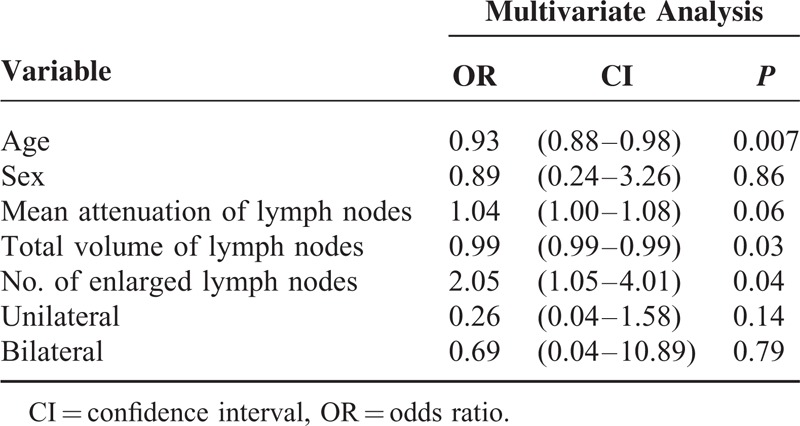
FIGURE 4.
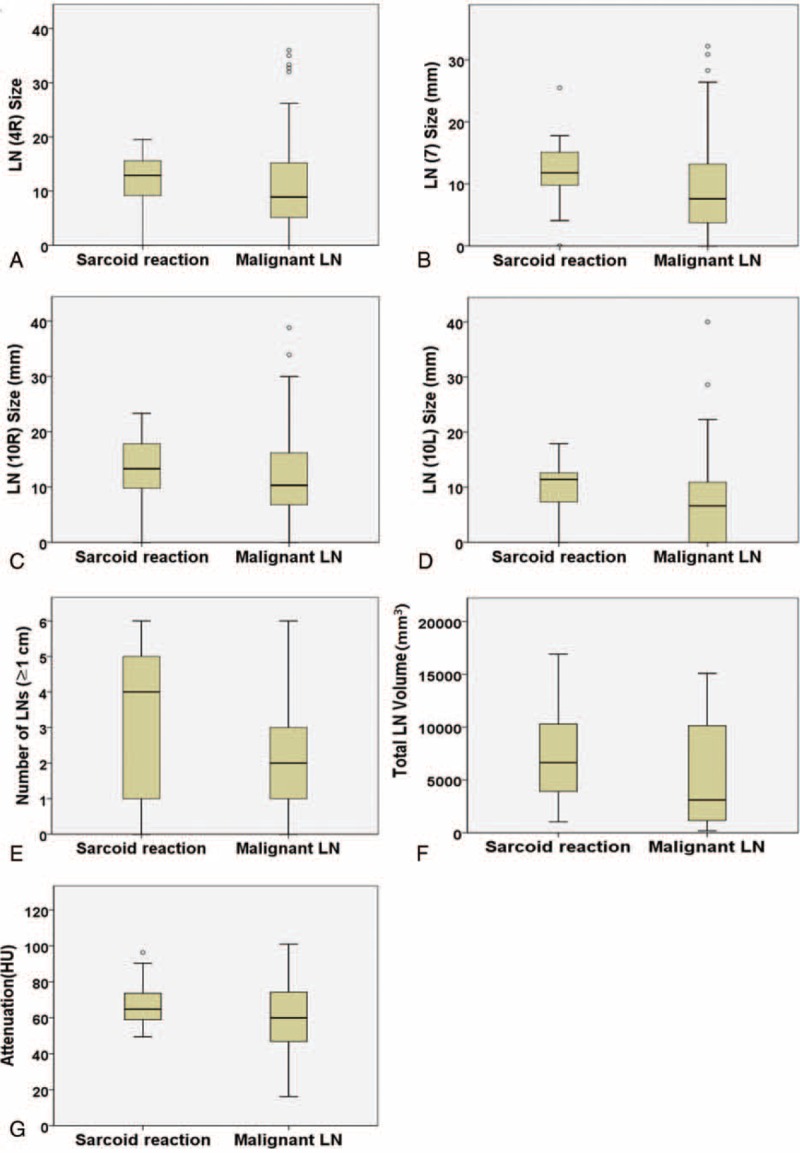
Box plots to show the distributions of the sizes of each nodal station, the number of LNs >1 cm, total volume, and attenuation of LNs in the sarcoid reaction and malignant LNs. (A–D) The LNs of sarcoid reaction tend to be larger with narrow spectrum than the malignant LNs (right lower paratracheal, 4R; subcarinal, 7; right hilar, 10R; left hilar, 10L). (E) There are more LNs >1 cm per patient in sarcoid reaction compared with the malignant LNs. (F) The total volume of LN is lower in sarcoid reaction than in malignant LNs. (G) The attenuation of all LNs is not significantly different between the 2 groups. LNs = lymph nodes.
DISCUSSION
In this study, we compared the radiologic findings of the sarcoidosis, sarcoid reaction, and malignant LNs. Idiopathic sarcoidosis and sarcoid reaction were undistinguishable. However, in comparison to malignant LNs, sarcoid reaction had more LNs >1 cm and tended to be bilateral, and their total volume was smaller.
Sarcoid reaction is considered as a diagnosis of exclusion by the detection of noncaseating granulomas in a patient with a history of malignancy, and who has not fulfilled the criteria for idiopathic sarcoidosis.21 However, because of the various temporal relationships of the appearance of sarcoid reaction with the time of diagnosis of a primary tumor, the diagnosis for each case seems complex.21 There has been a question if sarcoid reaction and idiopathic sarcoidosis are really 2 different diseases, or in 1 spectrum with 2 different clinical history.16 Similar pathologic findings of sarcoidosis and sarcoid reaction support this theory that these 2 might be in the same disease entity. From our study, the CT findings of sarcoidosis and sarcoid reaction were similar, and those were hardly distinguishable from each other just by CT imaging itself. On FDG-PET/CT, the mediastinal and hilar lymphadenopathy may show hypermetabolic activity in both sarcoidosis and sarcoid reaction, making it difficult to differentiate, even though PET/CT may predict disease activity indirectly.22,23 The FDG-PET/CT finding was not helpful, and previous studies also showed that hyper-metabolic uptake can be observed in benign lesions such as a sarcoid reaction, mimicking malignant lesions.7,8,22–24
In comparison to the malignant LNs, sarcoid reaction was observed more commonly in young females. The concordance of the probability of sarcoidosis was moderate, and this suggests there might be clues for differentiation. Sarcoid reaction tends to be distributed bilaterally rather than malignant LNs. Although the total volume of LNs was lower, there were more LNs >1 cm in a sarcoid reaction than in malignant LNs. As for the attenuation of the LNs, although there was borderline significance, sarcoid reaction showed narrower attenuation spectra and higher attenuation values, and was more homogenous than the malignant LNs regardless of the location of the LNs. In 64% of the patients in the sarcoid reaction parenchymal infiltrates of sarcoidosis was detected, one of the typical features of idiopathic sarcoidosis. From our experience, this might be an ancillary CT finding that suggests a sarcoid reaction rather than malignant LNs. The FDG-PET/CT finding was not helpful, and previous studies also showed that hypermetabolic uptake can be observed in benign lesions such as a sarcoid reaction, mimicking malignant lesions.7,8,22–24 In our study, the LNs in sarcoidosis and sarcoid reaction showed diverse metabolic uptake up to 23.3, and the maxSUV of LNs on PET/CT could not provide evidence to differentiate those from metastatic LNs. A histological diagnosis can be acquired through TBNA, which is safe, minimally invasive, and reportedly 75% to 90% sensitive, and up to 100% specific.25–30 In our hospital, this procedure has been carried out routinely since 2008, without critical complications. However, we must remember that the diagnosis of a sarcoidosis or sarcoid reaction via TBNA does not definitively rule out the possibility of coexisting malignancy. Especially when there is an accompanying persistent or progressive disease, or suspicious findings such as asymmetric thoracic lymphadenopathy, coexisting extrathoracic lymphadenopathy, or a newly developed lung nodule, pathological reevaluation should be strongly recommended.
This study had several limitations. First, there might be a selection bias in this retrospective study. The criterion of inclusion of only equivocal LNs suggestive of malignant lymphadenopathy could be a bias. However, we have performed TBNA for all patients with mediastinal and hilar lymphadenopathy consecutively within the certain period. Second, noncaseating granulomas could be seen not only in a sarcoid reaction, but also in other conditions such as tuberculosis, or even in malignant LNs, and there is a possibility of coexisting malignant cells in the LNs.31 To minimize the possibility of misdiagnosis, we followed up the patients using CT and found no case of clinical or radiological features that indicated other possible differentials. Third, in this study, the types of malignancy and the stage of the disease that caused equivocal mediastinal or hilar LNs varied. In addition, the diagnosis of sarcoid reaction is not permanent considering the potential progression of certain malignancy can occur. However, this study aimed to find the reliable radiologic findings useful to differentiate the mediastinal and hilar LNs.
The suggestions by radiologists of the possibility of sarcoidosis, sarcoid reaction, or malignant LNs depending on imaging findings, together with pathological confirmation, may greatly help decide the appropriate management approach. Eventually, it is expected to give patients a chance for curative resection or definitive therapy for their underlying malignancy or help prevent needless systemic chemotherapy.
CONCLUSIONS
CT and PET/CT evaluations of the mediastinal and hilar LNs between sarcoidosis and sarcoid reaction show no difference. In comparison to the malignant LNs, sarcoid reaction occurs more in young females. The LNs in a sarcoid reaction are larger and more bilateral than malignant LNs on CT. The median maxSUV of the 3 highest SUVs of the mediastinal and hilar LNs did not significantly differ between the 3 entities.
Footnotes
Abbreviations: CT = computed tomography, EBUS = endobronchial ultrasound, FDG-PET/CT = 18F-fluorodeoxyglucose-positron emission tomography/CT, maxSUV = maximum standardized uptake value, PACS = picture archiving and communication system, TBNA = transbronchial lymph node aspiration and biopsy.
The guarantor of this article is MYK, taking responsibility for the integrity of the work as a whole, from inception to published article. MYK contributed to conceiving the idea, data analysis and interpretation, and drafting and revising the manuscript for intellectual content; HJK contributed to data collection, data analysis, and drafting and revising the manuscript; SYS and SS contributed to data collection, data analysis, and revising the manuscript; S-SK has significant statistical expertise and contributed to the statistical analysis. SWL and C-MC contributed to patient management, data collection, and revising the manuscript.
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Criado E, Sanchez M, Ramirez J, et al. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics 2010; 30:1567–1586. [DOI] [PubMed] [Google Scholar]
- 2.Brincker H. Sarcoid reactions in malignant tumours. Cancer Treat Rev 1986; 13:147–156. [DOI] [PubMed] [Google Scholar]
- 3.Steinfort DP, Irving LB. Sarcoidal reactions in regional lymph nodes of patients with non-small cell lung cancer: incidence and implications for minimally invasive staging with endobronchial ultrasound. Lung Cancer 2009; 66:305–308. [DOI] [PubMed] [Google Scholar]
- 4.Hunt BM, Vallieres E, Buduhan G, et al. Sarcoidosis as a benign cause of lymphadenopathy in cancer patients. Am J Surg 2009; 197:629–632.discussion 632. [DOI] [PubMed] [Google Scholar]
- 5.Mapelli P, Mangili G, Picchio M, et al. Sarcoidosis mimicking metastatic gynaecological malignancies: a diagnostic and therapeutic challenge? Rev Esp Med Nucl Imagen Mol 2013; 32:314–317. [DOI] [PubMed] [Google Scholar]
- 6.Bush E, Lamonica D, O’Connor T. Sarcoidosis mimicking metastatic breast cancer. Breast J 2011; 17:533–535. [DOI] [PubMed] [Google Scholar]
- 7.Chida M, Inoue T, Honma K, et al. Sarcoid-like reaction mimics progression of disease after induction chemotherapy for lung cancer. Ann Thorac Surg 2010; 90:2031–2033. [DOI] [PubMed] [Google Scholar]
- 8.Haluska P, Luetmer PH, Inwards CY, et al. Complications of therapy and a diagnostic dilemma case. Case 3. Diagnostic dilemma: sarcoidosis simulating metastatic malignancy. J Clin Oncol 2003; 21:4653–4654. [DOI] [PubMed] [Google Scholar]
- 9.Lequoy M, Coriat R, Rouquette A, et al. Sarcoidosis lung nodules in colorectal cancer follow-up: sarcoidosis or not? Am J Med 2013; 126:642–645. [DOI] [PubMed] [Google Scholar]
- 10.Martella S, Lohsiriwat V, Barbalho DM, et al. Sarcoid-like reaction in breast cancer: a long-term follow-up series of eight patients. Surg Today 2012; 42:259–263. [DOI] [PubMed] [Google Scholar]
- 11.Tolaney SM, Colson YL, Gill RR, et al. Sarcoidosis mimicking metastatic breast cancer. Clin Breast Cancer 2007; 7:804–810. [DOI] [PubMed] [Google Scholar]
- 12.Navani N, Nankivell M, Woolhouse I, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrathoracic lymphadenopathy in patients with extrathoracic malignancy: a multicenter study. J Thorac Oncol 2011; 6:1505–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayanati H, R. ET, Souza CA, et al. Quantitative CT texture and shape analysis: can it differentiate benign and malignant mediastinal lymph nodes in patients with primary lung cancer? Eur Radiol 2014. [DOI] [PubMed] [Google Scholar]
- 14.Glazer GM, Gross BH, Quint LE, et al. Normal mediastinal lymph nodes: number and size according to American Thoracic Society mapping. AJR Am J Roentgenol 1985; 144:261–265. [DOI] [PubMed] [Google Scholar]
- 15.Kirchner J, Mueller P, Broll M, et al. Chest CT findings in EBUS-TBNA-proven anthracosis in enlarged mediastinal lymph nodes. Rofo 2014; 186:1122–1126. [DOI] [PubMed] [Google Scholar]
- 16.Hunsaker AR, Munden RF, Pugatch RD, et al. Sarcoidlike reaction in patients with malignancy. Radiology 1996; 200:255–261. [DOI] [PubMed] [Google Scholar]
- 17.UyBico SJ, Wu CC, Suh RD, et al. Lung cancer staging essentials: the new TNM staging system and potential imaging pitfalls. Radiographics 2010; 30:1163–1181. [DOI] [PubMed] [Google Scholar]
- 18.Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009; 4:568–577. [DOI] [PubMed] [Google Scholar]
- 19.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 2009; 15:4–9. [PubMed] [Google Scholar]
- 20.Teran MD, Brock MV. Staging lymph node metastases from lung cancer in the mediastinum. J Thorac Dis 2014; 6:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol 2007; 25:326–333. [DOI] [PubMed] [Google Scholar]
- 22.Craun JB, Banks KP, Clemenshaw MN, et al. Sarcoidlike reaction of neoplasia causing hypermetabolic thoracic adenopathy in setting of extrathoracic malignancy: report of two cases and a review of the differential diagnostic considerations. J Nucl Med Technol 2012; 40:231–235. [DOI] [PubMed] [Google Scholar]
- 23.Sobic-Saranovic D, Grozdic I, Videnovic-Ivanov J, et al. The utility of 18F-FDG PET/CT for diagnosis and adjustment of therapy in patients with active chronic sarcoidosis. J Nucl Med 2012; 53:1543–1549. [DOI] [PubMed] [Google Scholar]
- 24.Chowdhury FU, Sheerin F, Bradley KM, et al. Sarcoid-like reaction to malignancy on whole-body integrated (18)F-FDG PET/CT: prevalence and disease pattern. Clin Radiol 2009; 64:675–681. [DOI] [PubMed] [Google Scholar]
- 25.Gindesgaard CB, Schousboe LP, Christensen RK. Endobronchial ultrasound-guided transbronchial needle aspiration in an unselected cohort. J Bronchology Interv Pulmonol 2013; 20:140–146. [DOI] [PubMed] [Google Scholar]
- 26.Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest 2014; 146:547–556. [DOI] [PubMed] [Google Scholar]
- 27.Hong G, Lee KJ, Jeon K, et al. Usefulness of endobronchial ultrasound-guided transbronchial needle aspiration for diagnosis of sarcoidosis. Yonsei Med J 2013; 54:1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parmaksiz ET, Caglayan B, Salepci B, et al. The utility of endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal or hilar lymph node evaluation in extrathoracic malignancy: benign or malignant? Ann Thorac Med 2012; 7:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremblay A, Stather DR, Maceachern P, et al. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009; 136:340–346. [DOI] [PubMed] [Google Scholar]
- 30.von Bartheld MB, Dekkers OM, Szlubowski A, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA 2013; 309:2457–2464. [DOI] [PubMed] [Google Scholar]
- 31.Trisolini R, Cancellieri A, Patelli M. May sarcoidal reaction and malignant features coexist in regional lymph nodes of non-small cell lung cancer patients? Lung Cancer 2009; 66:272–273.author reply 273. [DOI] [PubMed] [Google Scholar]


