Supplemental digital content is available in the text
Abstract
A wide array of drugs are available for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (BPH), but the evidence for the comparative effectiveness is controversial.
The objective of this study is to evaluate the comparative effectiveness and safety of monodrug therapies for BPH.
Data sources are MEDLINE, EMBASE, and the Cochrane Library.
We included randomized controlled trials that compared α-blockers, 5-alpha reductase inhibitors (5ARIs), muscarinic receptor antagonists (MRAs), phosphodiesterase-5 inhibitor (PDE5-Is), or placebo for the treatment of BPH.
Comparative effectiveness and safety were pooled by both traditional meta-analysis and network meta-analysis. Summary effect size was calculated as mean difference (MD) and relative risk (RR), together with the 95% confidence intervals (CIs).
This study included 58,548 participants from 124 trials in total. When compared with placebo, α-blockers, 5ARIs, and PDE5-Is reduced International Prostate Symptom Score (IPSS) by −1.35 to −3.67 points and increased peak urinary flow rate (PUF) by −0.02 to 1.95 mL/s, with doxazosin (IPSS: MD, −3.67[−4.33 to −3.02]; PUF: MD, 1.95[1.61 to 2.30]) and terazosin (IPSS: MD, −3.37 [−4.24 to −2.50]; PUF: MD, 1.21[0.74 to 1.66]) showing the greatest improvement. The improvement in the IPSS was comparable among tamsulosin, alfuzosin, naftopidil, silodosin, dutasteride, sildenafil, vardenafil, and tadalafil. The incidence of total adverse events and withdraws due to adverse events were generally comparable among various agents.
In conclusion, α-blockers, 5ARIs, and PDE5-Is are effective for BPH, with doxazosin and terazosin appearing to be the most effective agents. Drug therapies for BPH are generally safe and well-tolerated, with no major difference regarding the overall safety profile.
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a nonmalignant enlargement of the prostate caused by cellular hyperplasia.1,2 It is a bothersome and potentially severe condition that may lead to lower urinary tract symptoms (LUTS) involving weak urinary stream, hesitancy, intermittency, frequent urination, and urgency. The prevalence of BPH increases markedly with age, ranging from about 8% in men aged 31 to 40 years to approximately 80% in those aged over 80 years.3,4 BPH is associated with great disease burden, and it is estimated that the direct costs of medical services for BPH management in the US exceed $1.1 billion annually.5 In the past 20 years, multiple treatment modalities for BPH have arisen, including watchful waiting, drug therapy, and surgical intervention.
Pharmacological treatment has become an accepted standard of care for BPH after reports of a series of randomized controlled trials (RCTs) showing the significant effectiveness of alpha-adrenergic blockers (α-blockers) (terazosin, doxazosin, tamsulosin, alfuzosin, and silodosin) and 5-alpha reductase inhibitors (5ARIs) (finasteride and dutasteride).6,7 These drugs are now widely recommended by clinical guidelines.1,2,8,9 For men with moderate to severe LUTS who predominantly have bladder storage symptoms, muscarinic receptor antagonists (MRAs) like tolterodine and fesoterodine might be considered.1,2 Phytotherapies such as cernilton and serenoa repens are also used for BPH but they are seldom recommended,1,2,8,9 because their clinical effects vary considerably even for a herbal drug from the same producer10 and their effectiveness is still controversial.11,12 In 2011, tadalafil, a phosphodiesterase-5 inhibitor (PDE5-I), was approved for the treatment of BPH by the US Food and Drug Administration, further adding to the treatment options for BPH.
As a wide array of drugs is now available for treatment of BPH, interest has been developed in investigating their comparative effectiveness and safety. Clinical guidelines have generally suggested that the various α-blockers are equally effective.1,2,8 However, many clinical trials13–17 and our previous overview of systematic reviews18 have indicated that certain α-blockers such as doxazosin may be superior to others. Some clinical trials comparing agents from different classes have indicated that α-blockers are likely to be more effective than 5ARIs.19–22 Although numerous clinical trials have been carried out to evaluate the effectiveness of drug therapies for BPH, direct comparisons among many agents are still lacking. Recently, a network meta-analysis has been performed to compare the efficacy of different drug therapies for LUTS/BPH.23 However, this study only included short-term trials and the literature search was not extensive. In addition, this study compared the effectiveness of different drug classes rather than individual agents; such an approach may be biased because the effectiveness of agents from the same class can be significantly different.13–17 The objective of this study was to evaluate the comparative effectiveness and safety of common monodrug treatments for BPH and to provide physicians with evidence for prescribing the optimal treatment.
MATERIALS AND METHODS
Literature Searches
An electronic search of MEDLINE, EMBASE, and the Cochrane Library, from their inception to October 2013, was conducted to identify eligible studies. The search strategy consisted of search items for BPH and clinical trials using the following: keywords “lower urinary tract symptoms,” “LUTS,” “benign prostatic hyperplasia,” “BPH,” “randomized controlled trial,” and “clinical trial.” All the searches were restricted to human studies and there was no limitation on publication status or language. The MetaRegister and WHO International Clinical Trials Registry Platform were searched for ongoing studies. Reference lists of the included studies were manually checked to identify additional studies. The literature search was updated on February 2015.
Study Selection
Trials were eligible for inclusion if they were parallel-design RCTs or cross-over studies; included patients diagnosed with BPH; compared any pair of the following drugs: terazosin, doxazosin, tamsulosin, alfuzosin, silodosin, naftopidil, finasteride, dutasteride, tolterodine, fesoterodine, solifenacin, or placebo; and treated patients for at least 2 weeks. The primary outcome for this study was the International Prostate Symptom Score (IPSS). Peak urinary flow rate (PUF), total adverse events (AE), serious or severe adverse events (SAE), withdrawal due to AE, and specific AEs were considered as the secondary outcomes. Trials were eligible if one or more of these outcomes were reported. The duplicated citations were initially removed using reference management software, and 2 authors then independently evaluated the eligibility of remaining studies by examining the titles, abstracts, and full articles sequentially. Discrepancies were resolved through discussion.
Data Extraction and Quality Assessment
Two investigators independently extracted the data using a standard form, with disagreement resolved by discussion. The following data were extracted: study information (ie, title, authors, country, publication time, patient number, and treatment duration), patient characteristics (i.e. age, weight, body mass index, BPH severity, and disease duration), intervention, control, methods (i.e. randomization, blinding, and loss to follow-up), and outcomes (i.e. estimated effects, standard error, P-value, and/or confidence interval [CI]). We consulted the authors of original studies to collect missing information as necessary.
The methodological quality of the included studies was appraised by 2 authors independently with the Cochrane Collaboration's tool for assessing risk of bias.24 Six domains were evaluated: sequence generation, concealment of allocation, blinding, incomplete outcome data, selective reporting, and other sources of bias.
Statistical Analysis
For the IPSS and PUF, we considered the mean changes from baseline to study end rather than the postintervention value as the effect measures. For studies that did not report the mean changes, we calculated the changes based on the baseline and final values according to the method reported in the Cochrane Handbook for Systematic Reviews of Interventions.24 Summary effect size was calculated as mean difference (MD) and risk ratio (RR), together with the 95% CIs.
The comparative effects were initially analyzed by conducting traditional pairwise meta-analysis using a random-effects model that accounts for both within and between-study variability. Heterogeneity among studies was assessed with the χ2 test and the I2-index statistic. Low level of heterogeneity was defined as I2 ≤ 25%, accompanied by P > 0.10 for the χ2 test.24 Publication bias was examined through visual inspection of funnel plot asymmetry, if more than 8 studies were involved in the meta-analysis.24
We then pooled the data including all of the drugs with a random-effects network meta-analysis model within a Bayesian framework.25 The underlying effects and relative rank of individual drugs were calculated to provide an overall evaluation of the efficacy and safety of all drugs. We considered placebo as the reference as the largest number of patients and studies used this comparator and it had the closest link with other drugs. The deviance information criterion was calculated to determine goodness of fit of the models.25
In addition to heterogeneity in direct comparisons, network meta-analysis also holds the assumption of similarity among trials as well as consistency between direct and indirect evidence.26–29 To verify similarities, we employed meta-regression analysis by adding covariates (i.e. dosage, average age, treatment duration, baseline IPSS and PUF, and prostate-specific antigen) to the network meta-analysis model.27,29 To eliminate the differences in common dosage ranges among various BPH drugs, we coded the covariate for dosage as a multiple of the standard dose, as reported in the previous study.30 The assumption of consistency was tested by comparing the residual deviance and deviance information criterion statistics between the consistency model and the unrelated mean effects model.31
We performed sensitivity analyses according to the dosage (including studies within the therapeutic range) and study quality (excluding studies with high risk of bias regarding randomization, allocation concealment, blinding, or incomplete outcome data). All data analyses were undertaken using Review Manager (RevMan 5.2.9) and WinBUGS 1.4. This study was reported according to the PRISMA statement.32
RESULTS
Study Characteristics
The literature search performed in 2013 yielded 4471 potentially relevant citations, and the electronic search updated in 2015 identified 547 additional citations. Four thousand nine hundred thirteen citations were excluded after reviewing the titles and abstracts, and the full texts of 205 remaining citations were screened, finally 124 studies with 58,548 participants were included (Figure 1, Supplemental Digital Content-Reference of included studies, http://links.lww.com/MD/A301). Most of the included trials had been conducted in Europe (40.3%), North America (27.4%), and East Asia (25.9%). The median sample-size of the included trials was 193 (range 26–4325). Figure 2 shows the network of pairwise comparisons from the included trials. Eighty five studies were placebo controlled trials and 45 studies involved 2 or more different active compounds. Medication dose in most of the included trials (117/124) were within the therapeutic range. Regarding the patient characteristics, the median age of the patients in the included studies was 65 years (range 53.9–74.4). The symptoms of patients in the included studies ranged from moderate to severe, with a median baseline IPSS of 17.85 (range 14.1–22.1) and a PUF of 10.05 mL/s (range 6.6–13.0 mL/s). The full characteristics of included studies are presented in the Supplemental Digital Content-Table 1, http://links.lww.com/MD/A301.
FIGURE 1.
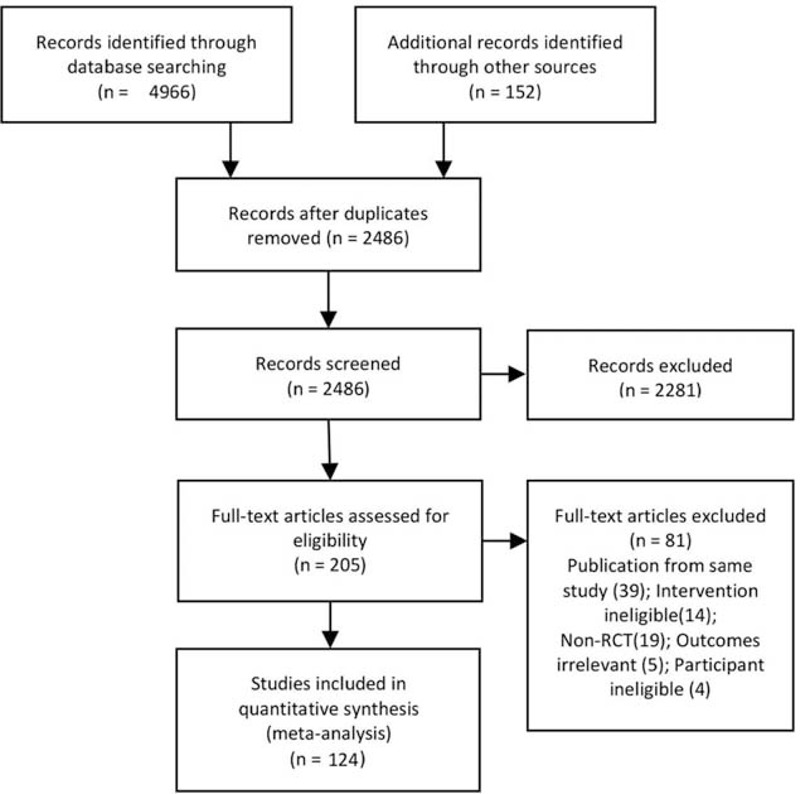
Flowchart of study selection.
FIGURE 2.
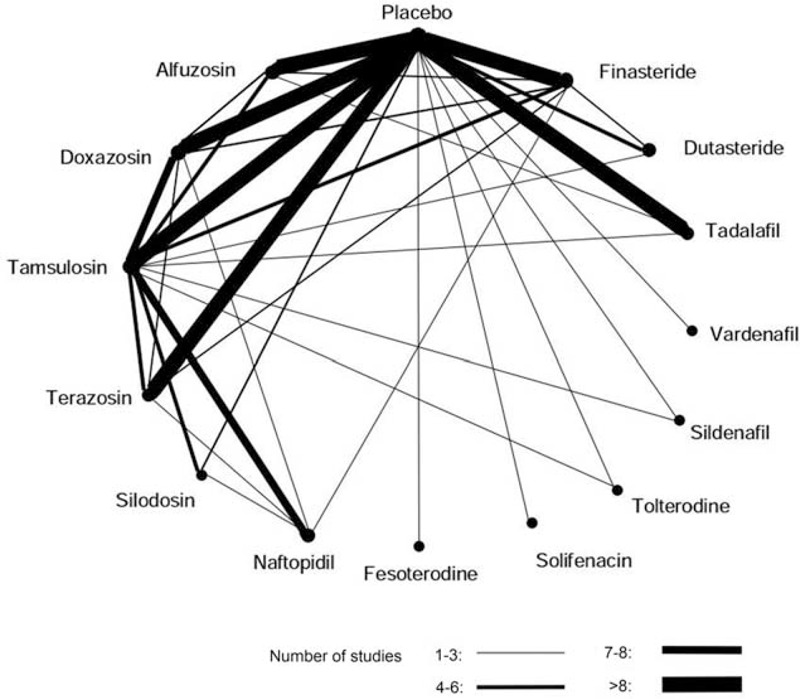
The network of pairwise comparisons from the included trials. The lines indicate available direct comparisons from included randomized controlled trials. The width of the lines is proportional to the number of studies for the comparisons.
Risk of Bias
The overall methodological quality was moderate. We only included randomized studies, but most did not report the techniques for randomization (83.9%) and concealment (89.5%). Ninety seven studies were double-blinded and 9 were single-blinded. The risk of bias from incomplete outcome data was assessed as low in 110 studies. The full assessment of the study quality is available in the Supplemental Digital Content-Table 2, http://links.lww.com/MD/A301.
International Prostate Symptom Score
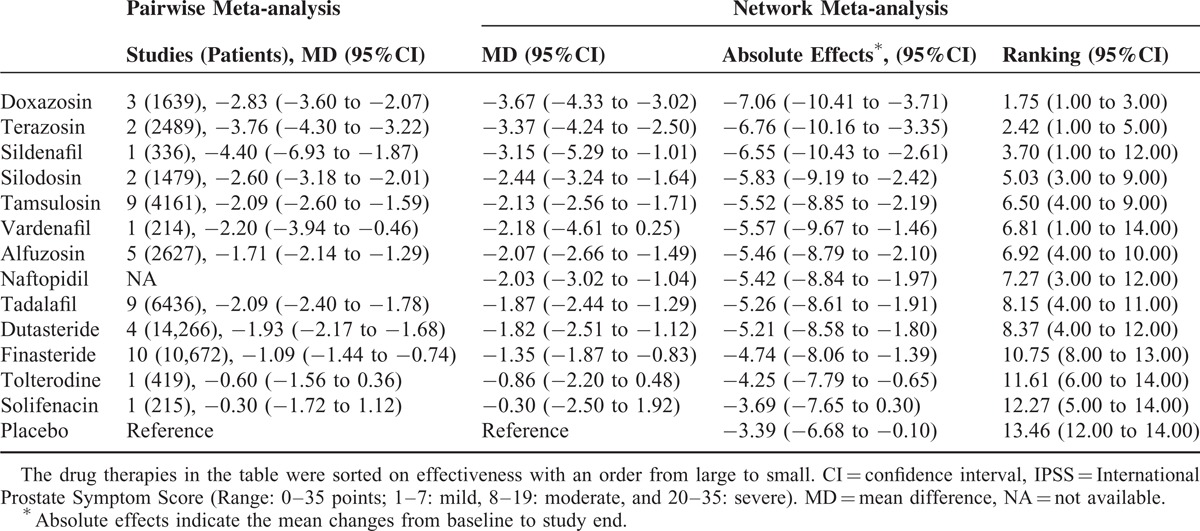
Eighty nine trials including 48,854 participants contributed to the analysis of the IPSS. Table 1 presents the relative effectiveness (compared with placebo), absolute effect, and ranking of drug therapies on IPSS. The pairwise meta-analyses were generally homogenous, with 3 of 30 comparisons showing a moderate to high degree of heterogeneity. We did not identify any major differences between the results from traditional pairwise meta-analysis and network meta-analysis. Network meta-analysis demonstrated that the reduction in the IPSS from baseline for various drug therapies ranged from −3.69 to −7.06 points, with doxazosin, and terazosin yielding the greatest improvement. All drug therapies except tolterodine and solifenacin significantly improved the IPSS compared with placebo. Regarding the comparative effectiveness of various drugs, network meta-analysis demonstrated that doxazosin and terazosin were significantly more effective than tamsulosin, alfuzosin, tadalafil, naftopidil, dutasteride, finasteride, tolterodine, and solifenacin. The improvement in the IPSS was comparable among silodosin, tamsulosin, alfuzosin, naftopidil, dutasteride, vardenafil, sildenafil, and tadalafil. The full results of comparative effectiveness are presented in the Supplemental Digital Content-Table 3, http://links.lww.com/MD/A301.
TABLE 1.
The Effectiveness of Drug Therapies in Improving International Prostate Symptom Score
Peak Urinary Flow Rate
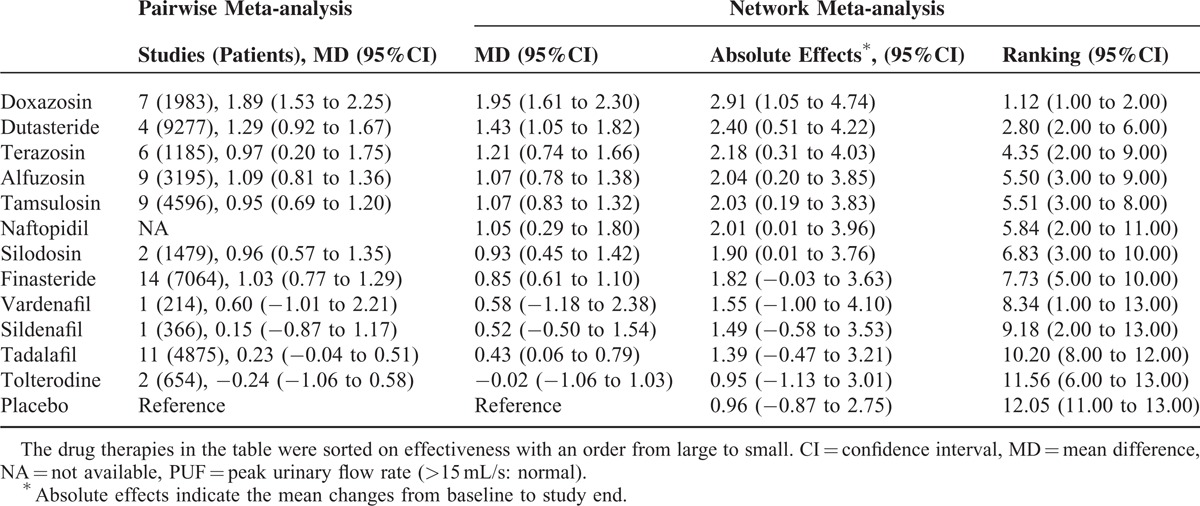
A total of 105 trials, including 45,955 participants, contributed to the analysis of PUF. Table 2 presents the relative effectiveness (compared with placebo), absolute effect, and ranking of drug therapies on PUF. The traditional pairwise meta-analyses were homogenous across included trials (except for the comparison between finasteride and doxazosin: P = 0.03; I2 = 80%) and consistent with network meta-analysis. Network meta-analysis demonstrated that the increase in the PUF from baseline for drug therapies ranged from 0.95 to 2.91 mL/s. Doxazosin and dutasteride showed the greatest improvements. When compared with placebo, doxazosin, dutasteride, terazosin, alfuzosin, tamsulosin, naftopidil, and silodosin significantly increased the PUF. Regarding the comparative effectiveness among different drugs, network meta-analysis demonstrated that doxazosin was significantly more effective than all other drug therapies. The effectiveness of dutasteride, terazosin, alfuzosin, tamsulosin, naftopidil, and silodosin was comparable. The effectiveness of different classes of PDE5-Is was comparable in improving PUF. The full results of comparative effectiveness on PUF were presented in the Supplemental Digital Content-Table 3, http://links.lww.com/MD/A301.
TABLE 2.
The Effectiveness of Drug Therapies in Improving Peak Urinary Flow Rate
Safety
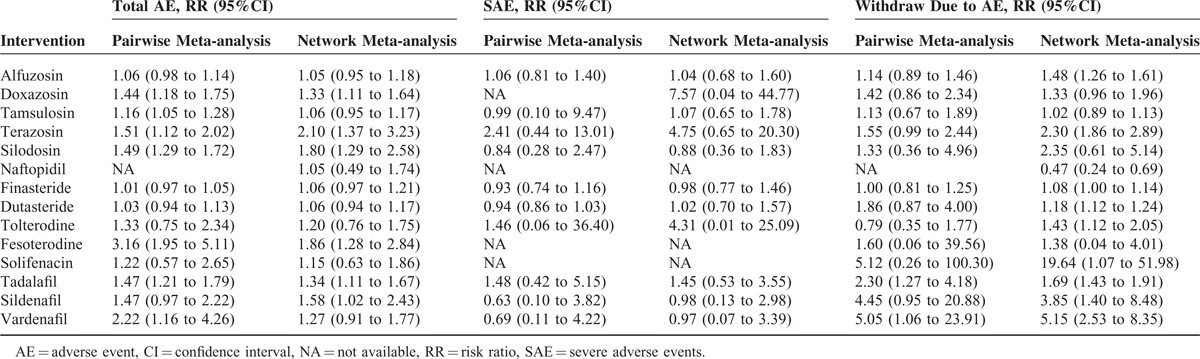
Drug therapies were typically safe and well tolerated. When compared with placebo, doxazosin (RR, 1.33; 95%CI, 1.11–1.64), terazosin (RR, 2.10; 95%CI, 1.37–3.23), silodosin (RR, 1.80; 95%CI, 1.29–2.58), fesoterodine (RR,1.86; 95%CI, 1.28–2.84), and tadalafil (RR, 1.34; 95%CI, 1.11–1.67) were associated with significantly higher incidence of total AEs; alfuzosin (RR, 1.48; 95%CI, 1.26–1.61) terazosin (RR, 2.30; 95%CI, 1.86–2.89), dutasteride (RR, 1.18; 95%CI, 1.12–1.24), tolterodine (RR, 1.43; 95%CI, 1.12–2.05), tadalafil (RR, 2.66; 95%CI, 1.52–4.47), sildenafil (RR, 3.85; 95%CI, 1.40–8.48), and vardenafil (RR, 5.15; 95%CI, 2.53–8.35) had more AE-related withdrawals. Doxazosin (RR: 7.57; 95%CI, 0.04–44.77), terazosin (RR: 4.75; 95%CI, 0.65–1.78), and tolterodine (RR: 4.31; 95%CI, 0.01–25.09) were associated with insignificantly but high risk of severe adverse events (Table 3). The primary specific AEs reported for individual drugs varied among α-blockers (ie, dizziness, headache, and asthenia), 5ARIs (ie, impotence and decreased libido), MRAs (ie, dry mouth, constipation, and dizziness), and PDE5-Is (ie, headache and back pain).
TABLE 3.
The Safety of Drug Therapies for Benign Prostatic Hyperplasia Compared With Placebo
Tests for Network Meta-analysis Assumptions, Sensitivity Analysis, and Publication Bias
Meta-regression analysis testing for similarity revealed that dose, age, the baseline IPSS, the baseline PUF, treatment duration, prostate volume, and prostate-specific antigen did not significantly contribute to the change estimates for IPSS or PUF (see Supplemental Digital Content-Table 4, http://links.lww.com/MD/A301). We did not identify any major inconsistency in models as the residual deviance and deviance information criterion statistics between consistency model and unrelated mean effects model were similar, and plots of the posterior mean deviance of individual data showed favorable linearity (see Supplemental Digital Content-Table 5, Figure 1, http://links.lww.com/MD/A301).
Sensitivity analysis according to the drug dosage and methodological quality did not reveal any major influence on the IPSS and PUF (see Supplemental Digital Content-Table 6, http://links.lww.com/MD/A301). Funnel plots evaluating the risk of publication bias were carried out in 9 of the 62 direct comparisons, and a visual inspection of these funnel plots did not show any asymmetry (see Supplemental Digital Content-Figure 2, http://links.lww.com/MD/A301).
DISCUSSION
This systematic review provides a comprehensive evaluation of the comparative effectiveness and safety of monodrug therapies for BPH. The primary findings are as follows:
When compared with placebo, α-blockers, 5ARIs, and tadalafil are more effective in improving the IPSS and PUF. Sildenafil and vardenafil may significantly improve the symptoms but their effect on the PUF is limited; MRA monotherapy can neither improve the IPSS nor PUF as compared with placebo.
In recommended doses, doxazosin was observed to exhibit highest effectiveness among all monotherapies, followed by terazosin.
Drug therapies for BPH are generally mild and well tolerated. Although the primary specific AEs vary among drugs from different classes, there is no major difference in terms of total AE, SAE, and AE-related withdrawals.
α-Blockers are currently recommended as first-line therapies for BPH. In addition to a high efficacy, α-blockers are also the least costly and the most well-tolerated drugs for relieving LUTS.33 Our study demonstrated that doxazosin and terazosin show relatively better effectiveness which is consistent with original trials.13–17 Importantly, these 2 agents may be least expensive among all current BPH drugs. According to an evaluation by Consumer Reports Best Buy Drugs, the lowest average monthly cost is about US$7 for doxazosin and US$24 for terazosin, compared with approximately US$82 for alfuzosin and US$75 for finasteride.34
The effectiveness of 5ARIs has been confirmed in this study and this was consistent with past systematic reviews.35,36 Of all of the drugs analyzed, the effectiveness of 5ARIs, especially of finasteride, tends to be inferior to α-blockers. It should be noted that 5ARIs act by reducing the size of the prostate gland and usually need long treatment duration, from 6 to 12 months, to improve symptoms. In this meta-analysis, we extracted data with maximum treatment duration for analysis to ensure the treatment duration was long enough for 5ARIs to achieve sufficient effectiveness. Of all the included 5ARIs related trials, over two thirds had treatment durations longer than 1 year. In the largest RCT of finasteride,37 IPSS was improved by approximately 0.7 point at year 1, 1.2 points at year 2, and 2.1 points at year 4. Even at year 4, the effectiveness was still smaller than for most α-blockers where IPSS improvement ranged from about 2 to 3.7 points in our meta-analysis. However, strong evidence has indicated that 5ARIs have significant advantages in reducing the prostate volume, the risk of surgery, acute urinary retention, and prostate cancer.35–39 Combination of α-blockers and 5ARIs may provide a strategy to bring the advantages of different classes of BPH drugs together.
Our study found MRAs can improve neither the IPSS nor the PUF, which was consistent with the findings of clinical trials.40,41 In clinical practice, MRAs are recommended in men with moderate to severe LUTS who predominantly have bladder storage symptoms. This study also indicated that PDE5-Is may significantly alleviate symptoms, but the improvement of the PUF is limited; this is consistent with the findings of a recently published systematic review.42 However, PDE5-Is are currently the most effective drug therapy for treating erectile dysfunction,43 which is another common condition in aged men. For patients with both BPH and erectile dysfunction, PDE5-Is present as a favorable choice.
We did not perform data analysis according to drug classes because direct comparison of α-blocker/5ARIs as compared to placebo showed significant heterogeneity and significant subgroup differences according to individual agents. The basic assumption for network meta-analysis is therefore not fulfilled. In addition, as the effectiveness of selective α-blockers and nonselective α-blockers are different, grouping them together may conceal these different effects.
To the best of our knowledge, this study is the most comprehensive study of monodrug therapies for BPH. An exhaustive and contemporaneous search strategy was undertaken to ensure that most eligible clinical trials were included, resulting in 124 studies and 58,548 participants being included. In addition, the data were synthesized by both traditional meta-analysis and network meta-analysis. Indirect effect estimates were obtained where direct comparisons were unavailable. Lastly, low risk of publication bias, stable sensitivity analysis, and the high consistency between the results from direct comparisons and network meta-analysis further strengthen our confidence in findings.
The limitations of this network meta-analysis arose primarily from the quality of the reviewed original trials. Because approximately 90% of the included studies did not report the techniques for the randomization and concealment, a risk of selection bias may exist. Furthermore, a risk of information bias may have to be present as 17 studies were open or did not report blinding. Adequate blinding is particularly critical for this study because the primary outcome, IPSS, is a score index based completely on patients’ reports. However, the influence from study quality was probably minor because the sensitivity analysis that excluded studies with a high risk of bias did not reveal any major change in the results.
In conclusion, α-blockers, 5ARIs, and PDE5Is are effective for BPH in recommended doses, with doxazosin and terazosin appearing to be the most effective agents. Drug therapies for BPH are generally safe and well-tolerated, with no major difference regarding the overall safety profile.
Acknowledgments
We thank Dr Diane Erin Threapleton for her assistance in editing the final report.
Footnotes
Abbreviations: 5ARI = 5-alpha reductase inhibitor, AE = adverse event, α-blocker = alpha-adrenergic blocker, BPH = benign prostatic hyperplasia, CI = confidence Interval, IPSS = international prostate symptom score, LUTS = lower urinary tract symptoms, MD = mean difference, MRA = muscarinic receptor antagonist, PDE5-I = phosphodiesterase-5 inhibitor, PUF = peak urinary flow rate, RR = risk ratio, SAE = severe adverse event.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Nickel JC, Mendez-Probst CE, Whelan TF, et al. 2010 Update: guidelines for the management of benign prostatic hyperplasia. Can Urol Assoc J 2010; 4:310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stohrer M, Blok B, Castro-Diaz D, et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol 2009; 56:81–88. [DOI] [PubMed] [Google Scholar]
- 3.McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care 2006; 12:S122–S128. [PubMed] [Google Scholar]
- 4.Parsons JK, Bergstrom J, Silberstein J, et al. Prevalence and characteristics of lower urinary tract symptoms in men aged > or = 80 years. Urology 2008; 72:318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in america project: benign prostatic hyperplasia. J Urol 2008; 179:S75–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lepor H. Medical treatment of benign prostatic hyperplasia. Rev Urol 2011; 13:20–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Graham DY. Endoscopic ulcers are neither meaningful nor validated as a surrogate for clinically significant upper gastrointestinal harm. Chin J Gastroenterol Hepatol 2009; 7:1147–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185:1793–1803. [DOI] [PubMed] [Google Scholar]
- 9.Speakman MJ, Kirby RS, Joyce A, et al. British Association of Urological S. Guideline for the primary care management of male lower urinary tract symptoms. BJU Int 2004; 93:985–990. [DOI] [PubMed] [Google Scholar]
- 10.Scaglione F, Lucini V, Pannacci M, et al. Comparison of the potency of different brands of Serenoa repens extract on 5alpha-reductase types I and II in prostatic co-cultured epithelial and fibroblast cells. Pharmacology 2008; 82:270–275. [DOI] [PubMed] [Google Scholar]
- 11.Tacklind J, Macdonald R, Rutks I, et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 2012; 12:CD001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilt T, Mac Donald R, Ishani A, et al. Cernilton for benign prostatic hyperplasia. Cochrane Database Syst Rev 2000; CD001042. [DOI] [PubMed] [Google Scholar]
- 13.Chung MS, Lee SH, Lee DH, et al. Comparative rapid onset of efficacy between doxazosin gastrointestinal therapeutic system and tamsulosin in patients with lower urinary tract symptoms from benign prostatic hyperplasia: A Multicentre, Prospective, Randomised Study. J Urol 2012; 187:1193–1199. [DOI] [PubMed] [Google Scholar]
- 14.Zhang K, Yu W, Jin J, et al. Effect of doxazosin gastrointestinal therapeutic system 4 mg vs tamsulosin 0.2 mg on nocturia in Chinese men with lower urinary tract symptoms: A Prospective, Multicenter, Randomized, Open, Parallel Study. Urology 2011; 78:636–640. [DOI] [PubMed] [Google Scholar]
- 15.Pompeo AC, Rosenblatt C, Bertero E, et al. A randomised, double-blind study comparing the efficacy and tolerability of controlled-release doxazosin and tamsulosin in the treatment of benign prostatic hyperplasia in Brazil. Int J Clin Pract 2006; 60:1172–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirby RS, Quinn S, Mallen S, et al. Doxazosin controlled release vs tamsulosin in the management of benign prostatic hyperplasia: an efficacy analysis. Int J Clin Pract 2004; 58:6–10. [DOI] [PubMed] [Google Scholar]
- 17.Tsujii T. Comparison of prazosin, terazosin and tamsulosin in the treatment of symptomatic benign prostatic hyperplasia: A short-term open, randomized multicenter study. Int J Urol 2000; 7:199–205. [DOI] [PubMed] [Google Scholar]
- 18.Yuan J, Liu Y, Yang Z, et al. The efficacy and safety of alpha-1 blockers for benign prostatic hyperplasia: an overview of 15 systematic reviews. Curr Med Res Opin 2013; 29:279–287. [DOI] [PubMed] [Google Scholar]
- 19.Kirby RS, Roehrborn C, Boyle P, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology 2003; 61:119–126. [DOI] [PubMed] [Google Scholar]
- 20.Debruyne FM, Jardin A, Colloi D, et al. Sustained-release alfuzosin, finasteride and the combination of both in the treatment of benign prostatic hyperplasia. European ALFIN Study Group. Eur Urol 1998; 34:169–175. [DOI] [PubMed] [Google Scholar]
- 21.Lee E. Comparison of tamsulosin and finasteride for lower urinary tract symptoms associated with benign prostatic hyperplasia in Korean patients. J Int Med Res 2002; 30:584–590. [DOI] [PubMed] [Google Scholar]
- 22.Rigatti P, Brausi M, Scarpa RM, et al. A comparison of the efficacy and tolerability of tamsulosin and finasteride in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis 2003; 6:315–323. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Wang X, Li S, et al. Comparative effectiveness of oral drug therapies for lower urinary tract symptoms due to benign prostatic hyperplasia: a systematic review and network meta-analysis. PLoS One 2014; 9:e107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011: Available at: www.cochrane-handbook.org. Accessed: June 1, 2015. [Google Scholar]
- 25.Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2:A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomized Controlled Trials (last updated April 2012). 2011. Available at: http://www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%2015April2014.pdf. Accessed: June 1, 2015. [PubMed] [Google Scholar]
- 26.Caldwell DM, Welton NJ, Ades AE. Mixed treatment comparison analysis provides internally coherent treatment effect estimates based on overviews of reviews and can reveal inconsistency. J Clin Epidemiol 2010; 63:875–882. [DOI] [PubMed] [Google Scholar]
- 27.Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU Technical Support Document 3: Heterogeneity: subgroups, meta-regression, bias and bias-adjustment (last updated April 2012). 2011. Available at:http://www.nicedsu.org.uk/TSD3%20Heterogeneity.final%20report.08.05.12.pdf. Accessed: June 1, 2015. [PubMed] [Google Scholar]
- 28.Dias S, Welton NJ, Sutton AJ, et al. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials (last updated April 2012). 2011. Available at: http://www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%2015April2014.pdfhttp://www.nicedsu.org.uk/TSD4%20Inconsistency.final.15April2014.pdf. Accessed: June 1, 2015. [PubMed] [Google Scholar]
- 29.Song F, Loke YK, Walsh T, et al. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ 2009; 338:b1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med 2009; 122:290–300. [DOI] [PubMed] [Google Scholar]
- 31.Dias S, Welton NJ, Sutton AJ, et al. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 2013; 33:641–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lepor H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol 2007; 9:181–190. [PMC free article] [PubMed] [Google Scholar]
- 34.Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 35.Wu XJ, Zhi Y, Zheng J, et al. Dutasteride on benign prostatic hyperplasia: a meta-analysis on randomized clinical trials in 6460 patients. Urology 2014; 83:539–543. [DOI] [PubMed] [Google Scholar]
- 36.Edwards JE, Moore RA. Finasteride in the treatment of clinical benign prostatic hyperplasia: a systematic review of randomised trials. BMC Urol 2002; 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med 1998; 338:557–563. [DOI] [PubMed] [Google Scholar]
- 38.Thompson IM, Jr, Goodman PJ, Tangen CM, et al. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med 2013; 369:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med 2010; 362:1192–1202. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan SA, Roehrborn CG, Rovner ES, et al. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA 2006; 296:2319–2328. [DOI] [PubMed] [Google Scholar]
- 41.Abrams P, Kaplan S, De Koning Gans HJ, et al. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol 2006; 175:999–1004.discussion 1004. [DOI] [PubMed] [Google Scholar]
- 42.Dong Y, Hao L, Shi Z, et al. Efficacy and safety of tadalafil monotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a meta-analysis. Urol Int 2013; 91:10–18. [DOI] [PubMed] [Google Scholar]
- 43.Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol 2013; 63:902–912. [DOI] [PubMed] [Google Scholar]


