Abstract
This study assessed whether preoperative maximum standardized uptake value (SUVmax) of metastatic lymph nodes (LNs) measured by 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) could improve the prediction of prognosis in gastric cancer.
One hundred fifty-one patients with gastric cancer and pathologically confirmed LN involvement who had undergone preoperative 18F-FDG PET/CT prior to curative surgical resection were retrospectively enrolled. To obtain nodal SUVmax, a transaxial image representing the highest 18F-FDG uptake was carefully selected, and a region of interest was manually drawn on the highest 18F-FDG accumulating LN. Conventional prognostic parameters and PET findings (primary tumor and nodal SUVmax) were analyzed for prediction of recurrence-free survival (RFS) and overall survival (OS). Furthermore, prognostic accuracy of survival models was assessed using c-statistics.
Of the 151 patients, 38 (25%) experienced recurrence and 34 (23%) died during follow-up (median follow-up, 48 months; range, 5–74 months). Twenty-seven patients (18%) showed positive 18F-FDG nodal uptake (range, 2.0–22.6). In these 27 patients, a receiver-operating characteristic curve demonstrated a nodal SUVmax of 2.8 to be the optimal cutoff for predicting RFS and OS. The univariate and multivariate analyses showed that nodal SUVmax (hazard ratio [HR] = 2.71, P < 0.0001), pathologic N (pN) stage (HR = 2.58, P = 0.0058), and pathologic T (pT) stage (HR = 1.77, P = 0.0191) were independent prognostic factors for RFS. Also, nodal SUVmax (HR = 2.80, P < 0.0001) and pN stage (HR = 2.28, P = 0.0222) were independent prognostic factors for OS. A predictive survival model incorporating conventional risk factors (pT/pN stage) gave a c-statistic of 0.833 for RFS and 0.827 for OS, whereas a model combination of nodal SUVmax with pT/pN stage gave a c-statistic of 0.871 for RFS (P = 0.0355) and 0.877 for OS (P = 0.0313).
Nodal SUVmax measured by preoperative 18F-FDG PET/CT is an independent prognostic factor for RFS and OS. Combining nodal SUVmax with pT/pN staging can improve survival prediction precision in patients with gastric cancer.
INTRODUCTION
Gastric cancer is the second leading cause of cancer mortality worldwide with higher incidence rates in Northeast Asia, Eastern Europe, and South America.1 Although the mortality associated with gastric cancer has steadily declined, a substantial number of advanced gastric cancer patients still have poor outcomes.1,2
To date, depth of tumor invasion, level of lymph node (LN) metastasis, and stage of disease are the most significant prognostic factors for predicting recurrence.3–5 However, preoperative assessment of the optimal method to predict prognosis in gastric cancer is not well established. Developing effective methods for the preoperative risk stratification of gastric cancer is necessary to select an optimal treatment for gastric cancer.
In recent decades, 18F-fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography (PET) has been widely used to assess various malignancies.6 Several studies have revealed that 18F-FDG PET can be important in staging, response evaluation, relapse monitoring, and tumor recurrence prediction in patients with gastric cancer.7–15 However, 18F-FDG uptake by the primary tumor may not be useful for survival prediction in gastric cancer patients. 18F-FDG is not a cancer-specific agent, and as a result, many benign lesions such as gastritis, polyps, and normal gastric mucosa can demonstrate moderate-to-intense 18F-FDG uptake.
LN metastasis is another important prognostic factor for gastric cancer.16,17 However, 18F-FDG PET for the diagnosis of LN involvement is limited in determining the extent of lymphadenectomy due to its low sensitivity.8 However, relatively high specificity could provide a clinical benefit in initial therapy selection. Nevertheless, the prognostic value of 18F-FDG uptake by metastatic LNs has not been well investigated in patients with gastric cancer.
The objective of the present study was to investigate the prognostic value of preoperative SUVmax of metastatic LNs measured by 18F-FDG PET/computed tomography (CT) for recurrence-free survival (RFS) and overall survival (OS) in patients with LN involvement gastric cancer prior to curative surgical resection.
PATIENTS AND METHODS
Patients
We retrospectively reviewed medical records of all gastric cancer patients who underwent 18F-FDG PET/CT for a staging work-up before treatment at our institution between January 2008 and December 2010. Of these cases, patients with microscopic or macroscopic residual disease after surgical treatment, distant metastases, or other cancers and patients who had received any neoadjuvant therapy prior to surgical treatment of gastric cancer were excluded. Finally, 151 patients with LN involvement gastric cancer who had undergone curative surgical resection were enrolled in this study. Curative surgical resection was defined by the absence of tumor macroscopically or microscopically after operation. In this study, all patients received radical gastrectomy along with D2 lymphadenectomy (advanced gastric cancer) and D1 + β or D2 lymphadenectomy (early gastric cancer). Patients had routinely been followed up every 3 months for the first year, every 6 months for the second year, and yearly thereafter. Recurrences were evaluated by physical examination, esophagogastroduodenoscopy, ultrasonography, contrast-enhanced CT, 18F-FDG PET/CT, magnetic resonance imaging, or histological biopsy. This retrospective study was approved by the institutional review board (2014-04-002), which waived the requirement of informed consent.
18F-FDG PET/CT Acquisition Protocol and Image Analysis
18F-FDG PET/CT scans were obtained using a Discovery STE PET/CT scanner (GE Healthcare, Milwaukee, WI) within 1 month before surgical resection. All patients fasted for at least 6 hours before the study. After the venous blood glucose level was managed to be <8.3 mmol/L (150 mg/dL), a dose of approximately 5.5 MBq/kg of 18F-FDG was intravenously injected, and imaging was performed 60 minutes later. Additionally, patients were requested to drink at least 500 mL of water prior to scanning to distend the stomach. Before PET, a low-dose CT scan was acquired from the skull vertex to proximal thighs (peak voltage of 120 kV, automated tube current ranging from 60 to 150 mA, and a slice thickness of 3.75 mm without contrast enhancement for attenuation correction). Immediately following the CT scan acquisition, the PET data were acquired with an acquisition time of 3 minutes per bed position in 3-dimensional mode. The CT data were used for attenuation correction and PET images were reconstructed using an ordered-subset expectation maximum iterative reconstruction algorithm.
Image display and analysis was performed using an Advantage Workstation 4.3 (GE Healthcare) providing multiplanar reformatted images. All of the 18F-FDG PET/CT images were retrospectively interpreted by 2 experienced nuclear medicine physicians and a consensus was reached. First, all of the 18F-FDG PET/CT images were visually evaluated and categorized as either positive or negative based on their 18F-FDG uptake findings. Primary tumors were characterized as positive for 18F-FDG uptake with abnormally increased 18F-FDG uptake exceeding the physiologic uptake by adjacent normal stomach wall and corresponding to lesions on esophagogastroduodenoscopy. Focally increased 18F-FDG uptake lesions that did not correspond to cancer lesions on esophagogastroduodenoscopy and histopathological findings were judged to be negative for 18F-FDG uptake. In addition, no visible significantly increased 18F-FDG uptake or diffusely increased 18F-FDG uptake indiscernible from physiological stomach wall uptake was defined as negative for 18F-FDG uptake. In the case of metastatic LNs, focally increased 18F-FDG avid LNs that correspond to metastatic LNs on histopathological results were regarded as positive for 18F-FDG uptake. However, in the case of suspicious 18F-FDG avid metastatic LNs with a low 18F-FDG uptake (SUVmax < 2), we decided that these metastatic LNs were negative for 18F-FDG uptake.
Subsequently, the SUVmax was measured only in patients with positive primary tumors and 18F-FDG avid metastatic LNs for quantitative analysis. Circular regions of interest were manually drawn over the maximum 18F-FDG uptake lesions corresponding to the primary tumor and 18F-FDG avid metastatic LNs on the attenuation-corrected transaxial 18F-FDG PET images to obtain the SUVmax within these regions of interest. We assigned the SUVmax as 1.0 to patients with negative 18F-FDG uptake of the primary tumor or LNs. The SUVmax was calculated using the following formula:
 |
Clinicopathologic and Survival Data
Clinicopathologic data considered to be potentially important to prognosis were collected from the patients’ medical records. Data included age at surgery, sex, perineural invasion, histopathological subtypes, Lauren histotype, pathologic T (pT) stage, and pathologic N (pN) stage. pT and pN classifications were in accordance with the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer.18 In addition, the short-axis diameter of regional LNs identified with preoperative abdominal CT was included for survival analysis. RFS was defined as the interval from surgery to tumor recurrence or final medical examination for recurrence evaluation if recurrence did not occur. All patients with tumor recurrence were confirmed by pathology or at clinical follow-up. OS was calculated from the date of surgery to the date of death or last follow-up at our medical center.
Statistical Analysis
Numeric data are expressed as the mean ± standard deviation. The relationship between nodal SUVmax and N stage was evaluated using the analysis of variance test. The optimal cutoff LN size, primary tumor SUVmax, and nodal SUVmax for the prediction of recurrence and death were determined using receiver-operating characteristic (ROC) curve analysis. RFS and OS were calculated using the Kaplan–Meier method. Variables that significantly affected RFS and OS were investigated by the multivariate analysis using Cox regression model. The 95% confidence interval (CI) was determined for each parameter. The additional value of nodal SUVmax for prognostication was evaluated using c-statistics. Statistical analyses were performed using MedCalc for Windows, version 12.5 (MedCalc Software, Ostend, Belgium). P values <0.05 were considered statistically significant.
RESULTS
Patient Characteristics and 18F-FDG PET/CT Findings
A total of 151 patients with LN involvement gastric cancer treated with curative surgical resection were retrospectively analyzed. Overall, 38 of the 151 patients (25%) experienced recurrence during the clinical follow-up period (median follow-up, 48 months; range, 5–74 months). Of the 38 patients with recurrent disease, 34 (23%) died.
Characteristics of enrolled patients are summarized in Table 1. A total of 122 patients (81%) showed positive 18F-FDG uptake by primary tumors and 27 (18%) showed positive uptake by metastatic LNs. The mean nodal SUVmax of these 27 patients was 4.9 ± 4.8 (range, 2.0–22.6). Of the 38 patients with recurrent disease, 18 (47%) showed positive nodal 18F-FDG uptake and 20 (53%) showed negative nodal 18F-FDG uptake. Of the 20 patients who showed disease recurrence but negative nodal 18F-FDG uptake, 13 (65%) revealed small LN size ≤5 mm. Of the 34 patients who had died, 18 (53%) showed positive nodal 18F-FDG uptake and 16 (47%) showed negative nodal 18F-FDG uptake. Of the 16 patients who died but showed negative nodal 18F-FDG uptake, 11 (69%) had a LN size ≤5 mm.
TABLE 1.
Patient Characteristics

Among the 151 patients, 48 (32%) were N1 stage, 38 (25%) were N2 stage, and 65 (43%) were N3 stage. The mean nodal SUVmax was significantly different among the N stage groups (P = 0.006) and was increased by increases in the N stage. Mean nodal SUVmax was 1.14 ± 0.61 in N1 stage, 1.67 ± 2.36 in N2 stage, and 2.12 ± 3.24 in N3 stage, respectively. There were 15 patients (10%) in stage I, 50 (33%) in stage II, and 86 (57%) in stage III according to tumor node metastasis staging (Table 1).
Survival Analysis
At the time of analysis, 38 patients (25%) demonstrated recurrence and 34 (23%) died during the median follow-up of 48 months (range, 5–74 months). The median RFS and OS times were 36 and 48 months, respectively. Kaplan–Meier analysis revealed that positive nodal uptake was associated with significantly shorter RFS and OS compared with negative nodal SUVmax (Figure 1). In positive nodal uptake patients, an ROC curve demonstrated an optimal cutoff for nodal SUVmax of 2.8 to predict RFS and OS. Then, this LN-positive group was divided into 2 groups using this cutoff. Statistically significant differences of RFS and OS in a stepwise manner in accordance with groups with higher nodal SUVmax were observed between each grade by log-rank test (Figure 2).
FIGURE 1.
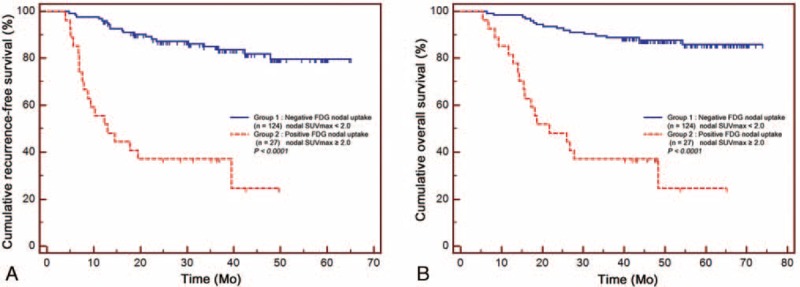
(A) Cumulative recurrence-free survival curves and (B) overall survival curves according to the 18F-FDG uptake by metastatic lymph node. 18F-FDG = 18F-fluoro-2-deoxy-D-glucose.
FIGURE 2.

(A) Cumulative recurrence-free survival curves and (B) overall survival curves according to the nodal SUVmax (<2.0, 2.0–2.8, >2.8). SUVmax = maximum standardized uptake value.
Univariate analysis revealed that pT stage (1, 2, 3, 4), pN stage (1, 2, 3), LN size (≤5 vs >5 mm), perineural invasion (− vs +), primary tumor SUVmax (≤4.5 vs >4.5), and nodal SUVmax (<2.0, 2.0–2.8, >2.8) were significantly associated with RFS and OS (Table 2). On the multivariate analysis using Cox proportional hazards models, nodal SUVmax (hazard ratio [HR] = 2.71; 95% CI, 1.76–4.17; P < 0.0001), pN stage (HR = 2.58; 95% CI, 1.32–5.05; P = 0.0058), and pT stage (HR = 1.77; 95% CI, 1.10–2.84; P = 0.0191) were independent prognostic factors for RFS, and nodal SUVmax (HR = 2.80; 95% CI, 1.80–4.36; P < 0.0001) and pN stage (HR = 2.28; 95% CI, 1.13–4.61; P = 0.0222) were independent prognostic factors for OS (Table 3). Although, LN size, perineural, and primary tumor SUVmax were significantly correlated with RFS and OS on univariate analysis, these parameters were not independent prognostic factors for RFS and OS on multivariate analysis.
TABLE 2.
Univariate Analysis of Prognostic Factors for RFS and OS

TABLE 3.
Multivariate Analysis of Prognostic Factors for RFS and OS
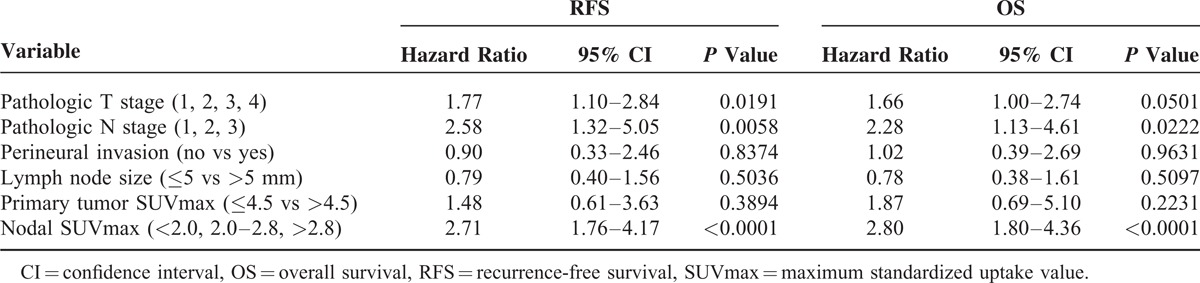
When analyzing pT and pN stage in all patients, c-statistics were 0.833 and 0.827 for RFS and OS, respectively. Combining nodal SUVmax with pT/pN stage gave a c-statistic of 0.871 for RFS (P = 0.0355) and 0.877 for OS (P = 0.0313) (Figure 3).
FIGURE 3.

Graphs of receiver-operating characteristic curve analysis show an additional value of nodal SUVmax for predicting (A) recurrence-free survival and (B) overall survival rates. pN = pathologic N, pT = pathologic T, SUVmax = maximum standardized uptake value.
As positive nodal uptake was significantly associated with poor prognosis, an analysis of adjuvant chemotherapy effects within subgroup of 27 patients who showed positive nodal uptake on 18F-FDG PET/CT was performed. Of these 27 patients, 9 (33.3%) received adjuvant chemotherapy and 14 received regular checkups without adjuvant chemotherapy. There was no statistical difference of RFS and OS between the 2 groups. The estimated median RFS was 15 months (95% CI, 7–19) in the surgery-only group versus 12 months (95% CI, 9–18) in the group receiving adjuvant chemotherapy (P = 0.4495), and the estimated median OS was 22 months (95% CI, 16–27) in the surgery-only group versus 17 months (95% CI, 14–28) in the group receiving adjuvant chemotherapy (P = 0.2180).
DISCUSSION
In this study, we evaluated the value of preoperative nodal SUVmax on 18F-FDG PET/CT to predict outcomes in gastric cancer with LN involvement. Our results demonstrate that nodal SUVmax is an independent prognostic factor for recurrence and death after curative surgical resection. Although the detection rate of 18F-FDG PET/CT for LN involvement was only 18%, comparing nodal SUVmax to established prognostic factors by multivariate analysis demonstrated an independent association. Furthermore, incorporation of nodal SUVmax with conventional risk factors (pT/pN stage) improved survival prediction beyond conventional staging alone.
Yun et al8 reported that 18F-FDG PET had sensitivities and specificities of 34% and 96% for N1 and N2 disease and 50% and 99% for N3 disease, respectively. In our study, 27 of the 151 patients (18%) showed positive uptake by metastatic LNs. This difference could be due to patient selection. We excluded patients with residual disease, distant metastases, or those who received neoadjuvant therapy prior to resection.
Many studies have shown that SUVmax of primary lesions can assist in predicting recurrence. Most studies found that FDG uptake by primary gastric cancer lesion is an independent prognostic factor for tumor recurrence following resection.14,15,19 Kim et al14 demonstrated that pretreatment primary tumor SUVmax has a significant predictive value for progression-free survival in gastric cancer. However, nodal SUVmax was not investigated for prognostication and they included patients with stage IV disease who have significantly worse prognosis than those with lower stage disease. Lee et al15 revealed that positive 18F-FDG uptake by gastric cancer lesions is an independent and significant prognostic factor for recurrence after resection. However, they did not use nodal SUVmax for prognostication. Recently, Coupe et al19 have reported that 18F-FDG positive primary tumors and positive LNs were associated with inferior OS and remained an independent predictor on multivariate analysis. They included patients who received neoadjuvant chemotherapy; the pathologic results of primary tumor and metastatic LNs may be different after therapy. In subgroup analysis of patients following curative surgery, 18F-FDG positive primary tumor was not a significant prognostic factor on multivariate analysis whereas positive LNs had a significant negative impact on RFS and OS by multivariate analysis. Although positive LNs were only statistically significant prognostic factors in patients who underwent surgery, there was no definite cutoff value for nodal SUVmax in that study.
The results of our study showed that primary tumor SUVmax was significantly associated with RFS and OS on univariate analysis, which was not preserved on multivariate analysis. On the contrary, nodal SUVmax was the most significant predictive factor for RFS and OS on both analyses, and an optimal cutoff value for nodal SUVmax was determined as 2.8. Furthermore, analyzing differences in the area under the ROC curve showed a significant improvement in the accuracy of risk prediction for RFS and OS rates when nodal SUVmax incorporated established risk factors (pT stage and pN stage) in our study. These results suggest that FDG uptake by metastatic LNs could provide risk stratification in gastric cancer. It might be possible to differentiate aggressive phenotypes by preoperative 18F-FDG PET/CT. Conventional risk factors including tumor invasion depth and extent of LN involvement are sometimes difficult to evaluate before surgery; therefore, 18F-FDG PET/CT as a noninvasive evaluation method could provide additional value in predicting prognosis.
18F-FDG uptake reflects both tumor biology and size for lesions <2 cm.20 LN size would influence 18F-FDG uptake and could be a potential prognostic factor. Tokunaga et al21 revealed that patients with enlarged LNs (a short-axis diameter measuring ≥15 mm) conferred worse outcomes. In the present study, an ROC curve demonstrated a LN size of 5 mm to be the optimal cutoff. Differences in LN size cutoff values might reflect patient selection. They excluded patients who received adjuvant chemotherapy. Although, large LN size patients showed poorer prognosis than small LN size patients on univariate analysis, LN size lost prognostic significance on multivariate analysis in our study. Also, Coupe et al19 reported that lymphadenopathy on CT scans did not result in a significant difference in OS. These results suggest that metabolic information measured by 18F-FDG PET/CT is more important than enlargement of metastatic LNs. However, underestimation of nodal SUVmax might have happened in 13 patients with disease recurrence and 11 patients who died because of absent nodal 18F-FDG uptake within small-sized LNs.
In this study, we show that metabolic information of metastatic LNs represented by nodal SUVmax is more important than primary tumor lesion for predicting survival in gastric cancer. Malignant cells are well known to exhibit increased glucose uptake and consumption.22 Glucose transporter 1 (GLUT-1) plays an important role in response for basal glucose uptake and its expression correlates with the rate of glucose metabolism. Overexpression of GLUT-1 has been observed in many cancers including gastric cancer.23–25 Moreover, Kawamura et al26 identified positive GLUT-1 expression of primary tumors was associated with depth of invasion, lymphatic permeation, vascular invasion, LN metastases, hepatic metastases, and peritoneal dissemination in gastric cancer. In addition, GLUT-1 expression levels of primary tumor were correlated with poor prognosis. Recently, Kim et al27 reported that GLUT-1 expression of metastatic LNs is the most important predictive factor for 18F-FDG uptake by metastatic LNs, but they did not mention an association between GLUT-1 expression of metastatic LNs and prognosis. The ability of metastasized tumor cells to invade lymphatic vessels is a more powerful prognostic factor than primary tumor features. Several authors emphasize that 18F-FDG uptake by LNs is an important prognostic factor in patients with invasive ductal breast,28 oral squamous cell,29 cervical,30 and endometrial cancers.31 In line with these investigations, we presently report that high nodal SUVmax is significantly associated with poor prognosis in gastric cancer.
Despite many studies have demonstrated that postoperative adjuvant chemotherapy was associated with a significant improvement in OS compared with surgery alone,32–34 there is no individualized adjuvant therapy approach according to 18F-FDG PET/CT findings. Although part of patients with an expected poor prognosis received adjuvant chemotherapy in our study, the results of subgroup analysis on positive nodal uptake patients did not support the benefit of adjuvant chemotherapy. However, because the results of our study are a retrospective single-center study, further studies are needed to compare the prognosis between treatments in patients with positive nodal uptake on 18F-FDG PET/CT. On the contrary, the results of the British MAGIC trial revealed that perioperative chemotherapy improved OS in patients who received perioperative chemotherapy compared with patients treated by surgery only.35 Furthermore, a large clinical PRODIGY trial that evaluated the docetaxel + oxaliplatin + S-1 regimen as neoadjuvant chemotherapy in advanced gastric cancer is currently ongoing.36 However, neoadjuvant chemotherapy benefit represented different outcome according to tumor location and Lauren histotype.37,38 Considering prognostic significance of nodal SUVmax in gastric cancer, further controlled prospective studies with more patients are required to clarify nodal SUVmax to be accepted as a treatment personalization technique for prediction of those patients requiring neoadjuvant therapy in gastric cancer.
There are several limitations to this study. First, only node-positive patients who underwent curative surgical resection of gastric cancer were enrolled in this study. This may limit the generalizability and preclude application of risk stratification to patients without LNs metastases. Second, partial-volume effects could affect 18F-FDG uptake of small-sized LNs in patients with LN sizes of <2.0 cm by underestimating nodal SUVmax. Finally, the results of this study might be subject to selection bias stemming from its retrospective design. In this study, medical insurance did not cover adjuvant chemotherapy therapy in gastric patients with LN involvement. Consequently, the rate of adjuvant chemotherapy is low. This lower rate of chemotherapy could influence outcomes.
CONCLUSIONS
The present study revealed that nodal SUVmax on preoperative 18F-FDG PET/CT is a significant independent predictor of RFS and OS after curative resection in gastric cancer patients with LN involvement. Combining nodal SUVmax with pT/pN stage might provide a more precise prognostic prediction. Thus, 18F-FDG PET/CT might be useful for risk stratification before surgery and possibly aid in treatment selection.
Footnotes
Abbreviations: 18F-FDG PET/CT = 18F-fluro-2-deoxy-D-glucosepositronemissiontomography/computedtomography, HR = hazard ratio, LN = lymph node, OS = overall survival, pN = pathologic N, pT = pathologic T, RFS = recurrence-free survival, SUVmax = maximum standardized uptake value.
This research was supported by the Bisa Research Grant of Keimyung University in 2013 (2013–0315), and by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MSIP) (No. 2014R1A5A2010008).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Bertuccio P, Chatenoud L, Levi F, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer 2009; 125:666–673. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Wan F, Pan J, et al. Tumor size: a non-neglectable independent prognostic factor for gastric cancer. J Surg Oncol 2008; 97:236–240. [DOI] [PubMed] [Google Scholar]
- 4.Shiraishi N, Inomata M, Osawa N, et al. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer 2000; 89:255–261. [DOI] [PubMed] [Google Scholar]
- 5.Adachi Y, Oshiro T, Mori M, et al. Prediction of early and late recurrence after curative resection for gastric carcinoma. Cancer 1996; 77:2445–2448. [DOI] [PubMed] [Google Scholar]
- 6.Kostakoglu L, Agress H, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radiographics 2003; 23:315–340. [DOI] [PubMed] [Google Scholar]
- 7.Chen-Xi Wu, Zhao-Hui Zhu. Diagnosis and evaluation of gastric cancer by positron emission tomography. World J Gastroenterol 2014; 20:4574–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yun M, Lim JS, Noh SH, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med 2005; 46:1582–1588. [PubMed] [Google Scholar]
- 9.Chung HW, Lee EJ, Cho Y-H, et al. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res Clin Oncol 2010; 136:1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshioka T, Yamaguchi K, Kubota K, et al. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med 2003; 44:690–699. [PubMed] [Google Scholar]
- 11.Ott K, Herrmann K, Lordick F, et al. Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long-term results of a prospective study. Clin Cancer Res 2008; 14:2012–2018. [DOI] [PubMed] [Google Scholar]
- 12.Di Fabio F, Pinto C, Rojas Llimpe FL, et al. The predictive value of 18F-FDG-PET early evaluation in patients with metastatic gastric adenocarcinoma treated with chemotherapy plus cetuximab. Gastric Cancer 2007; 10:221–227. [DOI] [PubMed] [Google Scholar]
- 13.Graziosi L, Bugiantella W, Cavazzoni E, et al. Role of FDG-PET/CT in follow-up of patients treated with resective gastric surgery for tumour. Ann Ital Chir 2011; 82:125–129. [PubMed] [Google Scholar]
- 14.Kim J, Lim ST, Na CJ, et al. Pretreatment F-18 FDG PET/CT parameters to evaluate progression-free survival in gastric cancer. Nucl Med Mol Imaging 2014; 48:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JW, Lee SM, Lee M-S, et al. Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging 2012; 39:1425–1434. [DOI] [PubMed] [Google Scholar]
- 16.Jaehne J, Meyer HJ, Maschek H, et al. Lymphadenectomy in gastric carcinoma. A prospective and prognostic study. Arch Surg 1992; 127:290–294. [DOI] [PubMed] [Google Scholar]
- 17.Adachi Y, Kamakura T, Mori M, et al. Prognostic significance of the number of positive lymph nodes in gastric carcinoma. Br J Surg 1994; 81:414–416. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 19.Coupe NA, Karikios D, Chong S, et al. Metabolic information on staging FDG-PET-CT as a prognostic tool in the evaluation of 97 patients with gastric cancer. Ann Nucl Med 2014; 28:128–135. [DOI] [PubMed] [Google Scholar]
- 20.Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med 2007; 48:932–945. [DOI] [PubMed] [Google Scholar]
- 21.Tokunaga M, Sugisawa N, Tanizawa Y, et al. The impact of preoperative lymph node size on long-term outcome following curative gastrectomy for gastric cancer. Ann Surg Oncol 2013; 20:1598–1603. [DOI] [PubMed] [Google Scholar]
- 22.Isselbacher KJ. Sugar and amino acid transport by cells in culture: differences between normal and malignant cells. N Engl J Med 1972; 286:929–933. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho KC, Cunha IW, Rocha RM, et al. GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics (Sao Paulo) 2011; 66:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada A, Oguchi K, Fukushima M, et al. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med 2006; 20:597–604. [DOI] [PubMed] [Google Scholar]
- 25.Alakus H, Batur M, Schmidt M, et al. Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun 2010; 31:532–538. [DOI] [PubMed] [Google Scholar]
- 26.Kawamura T, Kusakabe T, Sugino T, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer 2001; 92:634–641. [DOI] [PubMed] [Google Scholar]
- 27.Kim YH, Choi JY, Do I-G, et al. Factors affecting 18F-FDG uptake by metastatic lymph nodes in gastric cancer. J Comput Assist Tomogr 2013; 37:815–819. [DOI] [PubMed] [Google Scholar]
- 28.Song B-I, Lee S-W, Jeong SY, et al. 18F-FDG uptake by metastatic axillary lymph nodes on pretreatment PET/CT as a prognostic factor for recurrence in patients with invasive ductal breast cancer. J Nucl Med 2012; 53:1337–1344. [DOI] [PubMed] [Google Scholar]
- 29.Liao C-T, Chang JT-C, Wang H-M, et al. Preoperative [18F]-fluorodeoxyglucose positron emission tomography standardized uptake value of neck lymph nodes may aid in selecting patients with oral cavity squamous cell carcinoma for salvage therapy after relapse. Eur J Nucl Med Mol Imaging 2009; 36:1783–1793. [DOI] [PubMed] [Google Scholar]
- 30.Kidd EA, Siegel BA, Dehdashti F, et al. Pelvic lymph node F-18 fluorodeoxyglucose uptake as a prognostic biomarker in newly diagnosed patients with locally advanced cervical cancer. Cancer 2010; 116:1469–1475. [DOI] [PubMed] [Google Scholar]
- 31.Chung HH, Cheon GJ, Kim HS, et al. Preoperative PET/CT standardized FDG uptake values of pelvic lymph nodes as a significant prognostic factor in patients with endometrial cancer. Eur J Nucl Med Mol Imaging 2014; 41:1793–1799. [DOI] [PubMed] [Google Scholar]
- 32.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007; 357:1810–1820. [DOI] [PubMed] [Google Scholar]
- 33.Paoletti X, Oba K, Burzykowski T, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. J Am Med Assoc 2010; 303:1729–1737. [DOI] [PubMed] [Google Scholar]
- 34.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012; 379:315–321. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham D, Allum W, Stenning S, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355:11–20. [DOI] [PubMed] [Google Scholar]
- 36.Docetaxel + oxaliplatin + S-1 (DOS) regimen as neoadjuvant chemotherapy in advanced gastric cancer (PRODIGY). https://clinicaltrials.gov/ct2/show/NCT01515748. [Google Scholar]
- 37.Reim D, Gertler R, Novotny A, et al. Adenocarcinomas of the esophagogastric junction are more likely to respond to preoperative chemotherapy than distal gastric cancer. Ann Surg Oncol 2012; 19:2108–2118. [DOI] [PubMed] [Google Scholar]
- 38.Messager M, Lefevre JH, Pichot-Delahaye V, et al. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg 2011; 254:684–693. [DOI] [PubMed] [Google Scholar]


