Abstract
While preoperative chemoradiation followed by surgery (pre-OP CRT) has been widely applied in the treatment of patients with esophageal cancer, some studies have shown a survival benefit of postoperative chemoradiation (post-OP CRT). The optimal combination of multimodality therapy and the sequence of surgery and chemoradiation for esophageal cancer remain to be investigated.
A total of 1385 patients with clinical stage II and III esophageal squamous cell carcinoma (ESCC) were included. On the basis of the sequence of surgery and chemoradiation, the patients were grouped as follows: preoperative chemoradiation followed by surgery (pre-OP CRT+S), surgery alone (S), and surgery followed by postoperative chemoradiation (S+post-OP CRT). Propensity score matching analysis was used to identify 78 well-balanced patients in each group for outcome comparison.
In all, 753, 339, and 293 patients were in the pre-OP CRT+S, S, and S+post-OP CRT groups, respectively. Before matching, no differences were observed in the overall survival among the patients in these 3 groups (P = 0.422). After matching, both the pre-OP CRT+S and S+post-OP CRT groups were significantly associated with a better survival compared with the S group (pre-OP CRT+S vs. S: P < 0.001; S+post-OP CRT vs. S: P = 0.005). In contrast, the survival was similar between the pre-OP CRT+S and S+post-OP CRT groups (P = 0.544). In the subgroup analysis, patients with clinical T3/4 stage tumors or those with a tumor size greater than 5 cm were more likely to demonstrate an overall survival benefit from pre-OP CRT compared with post-OP CRT.
Both pre-OP CRT and post-OP CRT demonstrated a survival benefit compared with surgery alone, which indicates the importance of trimodality therapy in patients with clinical stage II/III ESCC. However, no survival difference was observed among patients in the pre-OP CRT+S and S+post-OP CRT groups, which suggests that the sequence of surgery and chemoradiation may be irrelevant to the outcome.
INTRODUCTION
Esophageal cancer is one of the deadliest cancers and is associated with high rates of disease recurrence and distant metastasis.1–3 Even after curative resection, the 5-year survival rate is rarely greater than 30%.2–4 Given that surgery alone does not result in a complete cure for patients with advanced esophageal cancer, multimodality therapy becomes necessary. Strategies of preoperative chemoradiation (pre-OP CRT) have been applied in an attempt to improve patient survival through the treatment of systemic micrometastases and through the downstaging of the disease which both increase the curative resection rate after subsequent surgery.5–11 In the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) that compared surgery alone and chemotherapy concurrent with radiotherapy followed by surgery for T1N1 or T2-3N0-1 esophageal cancer, the median overall survival was 49.4 months in the pre-OP CRT plus surgery group vs. 24.0 months in the surgery alone group. A significant estimated 5-year overall survival benefit of 13% was noted in favor of the pre-OP CRT plus surgery arm.5
In contrast, Rice et al12 and Bedard et al13 both showed a survival advantage in patients with esophageal cancer who were treated with postoperative chemoradiation (post-OP CRT). In the propensity-matched analysis by Rice et al,12 the median survival and 4-year survival rate in the surgery plus post-OP CRT group versus the surgery alone group were 28.0 vs. 15.0 months and 44% versus 0%, respectively (P < 0.05). Bedard et al13 also showed that the median overall survival in the surgery plus post-OP CRT group was 47.5 months, which was significantly longer than that of the surgery alone group (14.1 months, P = 0.001). Our recent retrospective analysis also showed that post-OP CRT provided a survival benefit to patients with esophageal squamous cell carcinoma (ESCC), especially those with lymph node-positive disease after R1 resection.14 In the past, the strategy of post-OP CRT has been eschewed because patients who undergo esophagectomy are typically debilitated and unable to withstand any postoperative treatments; however, earlier recovery and less morbidity can be consistently achieved with the advent of minimally invasive esophagectomy, and this may change the concept with regard to the sequence of trimodality therapy.
Based on the literature, very few studies have ever compared pre- and postoperative chemoradiation in patients with esophageal cancer. The optimal combination and timing of therapy for esophageal cancer remains controversial. We hypothesize that trimodality therapy is necessary for patients with esophageal cancer but that the sequence may be irrelevant to the outcome. In this study, we compare the survival of patients with clinical stage II and III ESCC who received pre-OP CRT followed by surgery (pre-OP CRT+S), surgery followed by post-OP CRT (S+post-OP CRT), and surgery alone (S). We aim to investigate the survival benefit of trimodality therapy over surgery alone and compare the effects of pre- and postoperative chemoradiation to establish the optimal combination of multidisciplinary treatments for ESCC.
METHODS
This study was reviewed by Institutional Review Board of the Taipei-Veterans General Hospital and granted a waiver of the informed consent process. Patient data were obtained from the Taiwan Cancer Registry, which is a national population-based cancer registration database organized and funded by the Health Promotion Administration, Ministry of Health and Welfare of the executive branch of the central government. Hospitals with more than a 50-bed capacity that provide outpatient and hospitalized cancer care are recruited to participate and report all newly diagnosed malignant neoplasms to the registry. The database contains information including sex, date of birth, date of diagnosis, clinical stage, treatment modality, date of initial treatments, pathological stage, tumor location, differentiation grade, tumor length, and survival status. Specifically, we identified 6140 patients who were diagnosed with ESCC between 2008 and 2011, with the appropriate International Classification of Diseases for Oncology (ICD-O-3) site codes (C15.0, C15.1, C15.2, C15.3, C15.4, C15.5, C15.8, and C15.9) and morphology codes (8052, 8070, 8071, 8072, 8073, 8074, 8076, 8077, 8083, and 8084). On the basis of the sequence of chemoradiation and surgery, patients with resectable clinical stage II and III ESCC, and patients who received pre-OP CRT followed by surgery, surgery alone, and surgery followed by post-OP CRT were eligible for inclusion in this study.
Statistics
Categorical and continuous variables were compared with the χ2 test and Student t test, respectively. The overall survival was calculated as the number of days between the date of initial treatment and that of death or December 31, 2012, whichever occurred first. Survival curves were plotted using the Kaplan–Meier method and were compared with the log-rank test. Differences in survival estimates were calculated using the Cox proportional hazards regression model. To minimize confounding effects due to nonrandomized assignment, propensity score matching, which is a statistical matching technique first introduced by Rosenbaum and Rubin, was used.15 This statistical method attempts to estimate the effect of a treatment by accounting for the covariates that predict receiving the treatment in a nonrandomized study. In brief, a propensity score for each patient was calculated by logistic regression using all possible confounding variables registered in the database. A 1:1 matched study group was created using the Greedy procedure. When matching the S+post-OP CRT and pre-OP CRT+S groups, a propensity score for each patient was calculated by logistic regression using the variables of age, sex, clinical stages, tumor location, differentiation grade, tumor size, and margin status. For matching patients who received S+post-OP CRT and those who underwent surgery alone, a propensity score was calculated using the variables of age, sex, clinical stages, tumor location, differentiation grade, pathological stages, tumor size, and margin status. All statistical calculations were performed by Chen HS with Statistical Analysis System version 9.3 (SAS Institute Inc, Cary, NC) and Statistical Product and Service Solutions version 20 (SPSS Inc, Chicago, IL). P values < 0.05 were considered statistically significant.
RESULTS
A total of 1385 patients met the inclusion criteria and were included in this study (Figure 1). In the pre-OP CRT+S group, the mean interval between the first day of preoperative chemoradiation and esophagectomy was 86.9 ± 35.8 days (25% Q1: 66; median: 78; 75% Q3: 97). In the S+post-OP CRT group, the mean interval between esophagectomy and the first day of postoperative chemoradiation was 47.6 ± 23.0 days (25% Q1: 34; median: 42; 75% Q3: 53). The clinical and pathological characteristics of the patients are summarized in Table 1 . Patients in the surgery alone group were older and had fewer advanced tumors in terms of clinical T stages and tumor length, whereas patients in the pre-OP CRT+S group were more likely to be clinically N-positive. As for the surgical results, the R1/2 resection rate was 8.6%, 8.3%, and 18.8% in the pre-OP CRT+S, surgery only, and S+post-OP CRT groups, respectively. In patients who underwent primary esophagectomy without preoperative chemoradiation (S and S+post-OP CRT groups), the pathological stage distribution was as follows: stage I, 17.9%; stage II, 38.0%; stage III, 39.2%, and stage IV, 3.6%.
FIGURE 1.
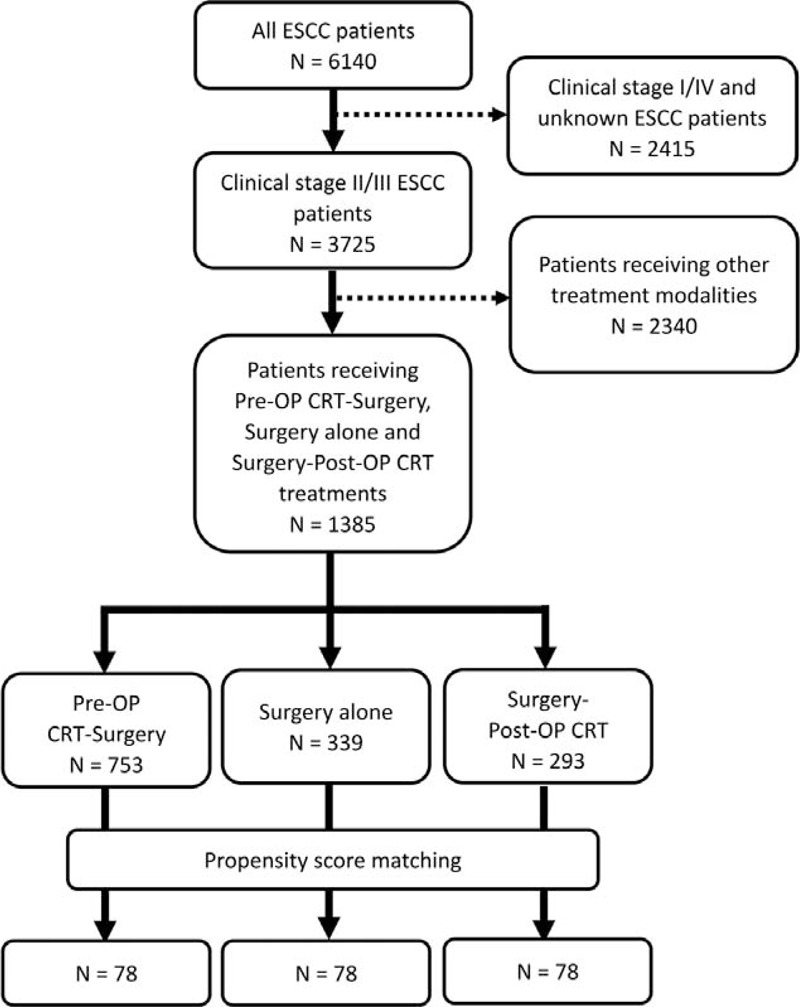
Flowchart with a summary of patient enrollment and propensity score matching.
TABLE 1.
Comparison of Patients in the Pre-OP CRT + Surgery, Surgery Alone, and Surgery + Post-OP CRT Groups

The mean ± SD and the median follow-up time for all patients were 20.95 ± 13.24 and 17.87 months, respectively. The 1-year and 3-year survival rates as well as the median survival time for all patients were 74.0%, 41.1%, and 24.8 months, respectively. The 1-year and 3-year survival rates and the median survival time for patients with clinical stage II disease were 80.1%, 52.3%, and 39.9 months, respectively, and 70.5%, 34.1%, 20.0 months, respectively, for patients with clinical stage III disease. The 1-year/3-year survival rates/median survival for the pre-OP CRT+S, surgery alone, and S+post-OP CRT groups were 75.3%/40.7%/23.9 months, 73.1%/43.9%/28.7 months, and 72.0%/39.0%/21.4 months, respectively. Kaplan–Meier curves showed no differences in the overall survival among patients in these 3 groups (P = 0.422, Figure 2A. Pre-OP CRT vs. surgery alone: P = 0.371; pre-OP CRT vs. post-OP CRT: P = 0.471; post-OP CRT vs. surgery alone: P = 0.219) and clinical stage II ESCC groups (Figure 2C). However, for patients with clinical stage III ESCC, significant differences were observed among these 3 treatment groups (P < 0.001, Figure 2E. Pre-OP CRT vs. surgery alone: P < 0.001; pre-OP CRT vs. post-OP CRT: P = 0.016; post-OP CRT vs. surgery alone: P = 0.023).
FIGURE 2.
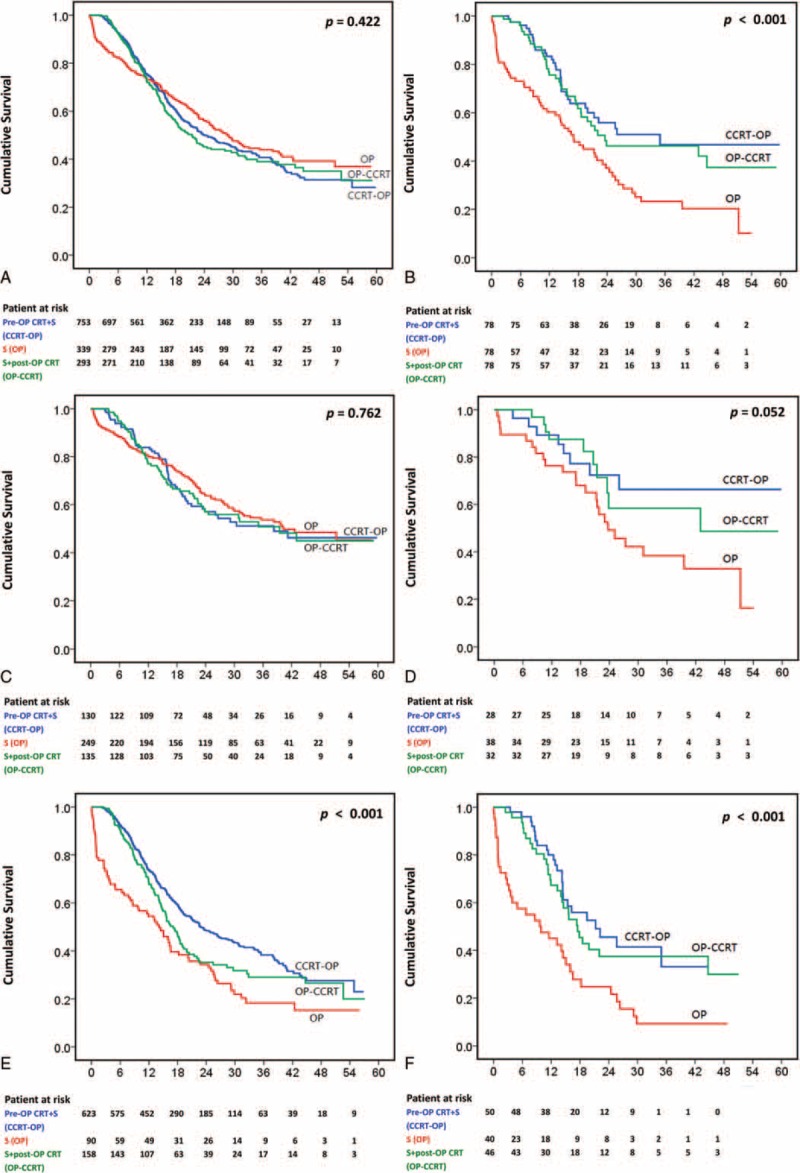
Kaplan–Meier curves. A, Patients with clinical stage II/III disease before matching (P = 0.422. Pre-OP CRT vs. surgery: P = 0.371; post-OP CRT vs. surgery: P = 0.219; pre-OP CRT vs. post-OP CRT: P = 0.471). B, Patients with clinical stage II/III disease after matching (P < 0.001. Pre-OP CRT vs. surgery: P < 0.001; post-OP CRT vs. surgery: P = 0.005; pre-OP CRT vs. post-OP CRT: P = 0.544). C, Patients with clinical stage II disease before matching (P = 0.762. Pre-OP CRT vs. surgery: P = 0.547; post-OP CRT vs. surgery: P = 0.518; pre-OP CRT vs. post-OP CRT: P = 0.912). D, Patients with clinical stage II disease after matching (P = 0.052. Pre-OP CRT vs. surgery: P = 0.038; post-OP CRT vs. surgery: P = 0.079; pre-OP CRT vs. post-OP CRT: P = 0.704). E, Patients with clinical stage III disease before matching (P < 0.001. Pre-OP CRT vs. surgery: P < 0.001; post-OP CRT vs. surgery: P = 0.023; pre-OP CRT vs. post-OP CRT: P = 0.016). F, Patients with clinical stage III disease after matching (P < 0.001. Pre-OP CRT vs. surgery: P < 0.001; post-OP CRT vs. surgery: P = 0.002; pre-OP CRT vs. post-OP CRT: P = 0.500).
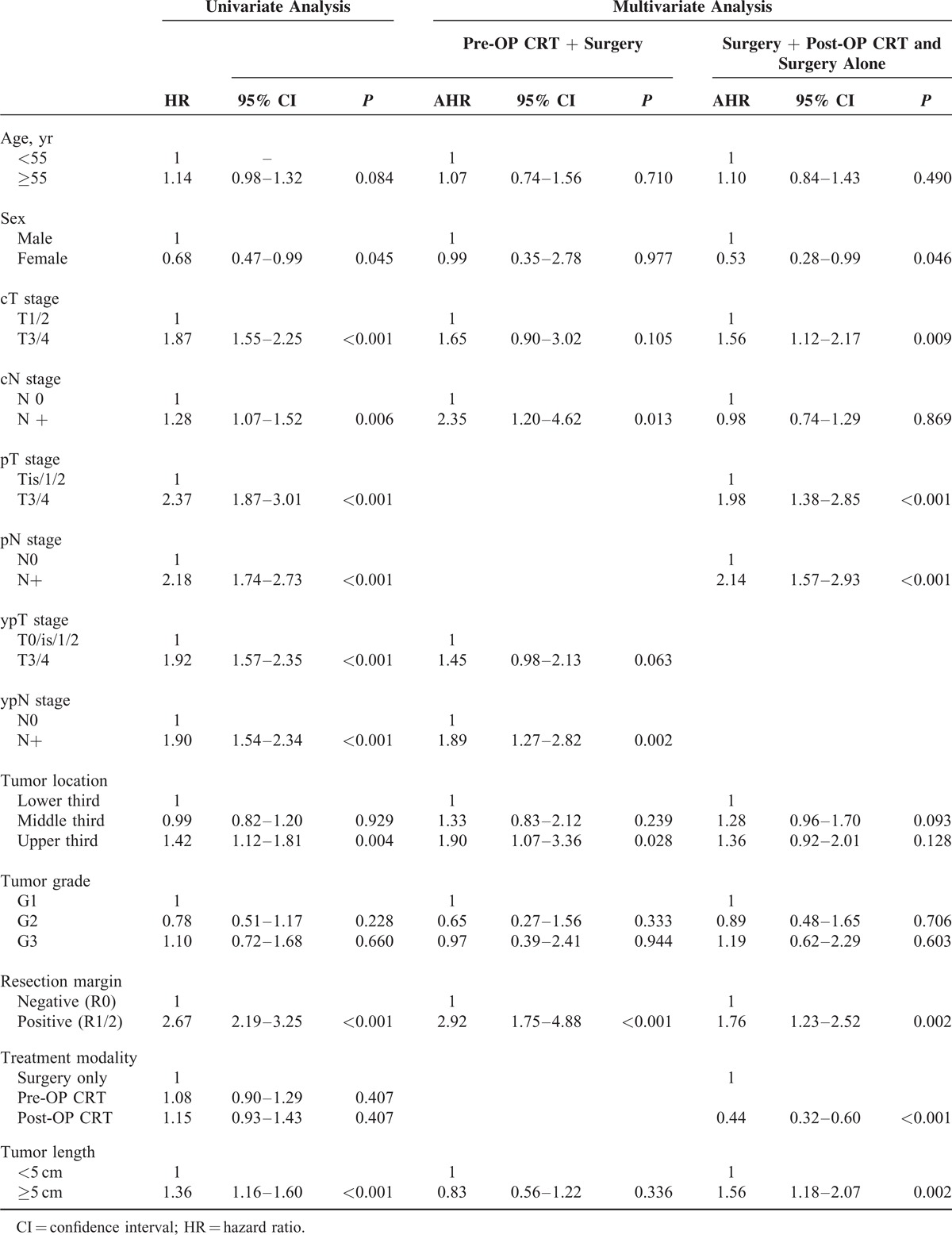
A survival analysis with a Cox proportional hazards regression model identified significant prognostic factors for poor outcome including clinical and pathological stages, a tumor length of more than 5 cm, tumors located in the upper third of the esophagus, and R1/2 resection, whereas female sex was associated with a better outcome (Table 2). According to the multivariate analysis, cN+, ypN+, tumors located in the upper third of the esophagus, and R1/2 resection remained significant independent prognostic factors for poor outcome in patients in the pre-OP CRT+S group. Clinical T3/4 stage, pT3/4 stage, pN+, a tumor length of more than 5 cm, tumors located in the upper third of the esophagus, and R1/2 resection remained significant independent prognostic factors for poor outcome in patients who received primary esophagectomy, whereas female sex and post-OP CRT were favorable prognostic factors. The addition of post-OP CRT was associated with a better outcome compared with surgery alone (hazard ratio (HR): 0.44, 95% confidence interval (CI): 0.32–0.60, P < 0.001).
TABLE 1 (Continued).
Comparison of Patients in the Pre-OP CRT + Surgery, Surgery Alone, and Surgery + Post-OP CRT Groups

Propensity Score Matching Analysis
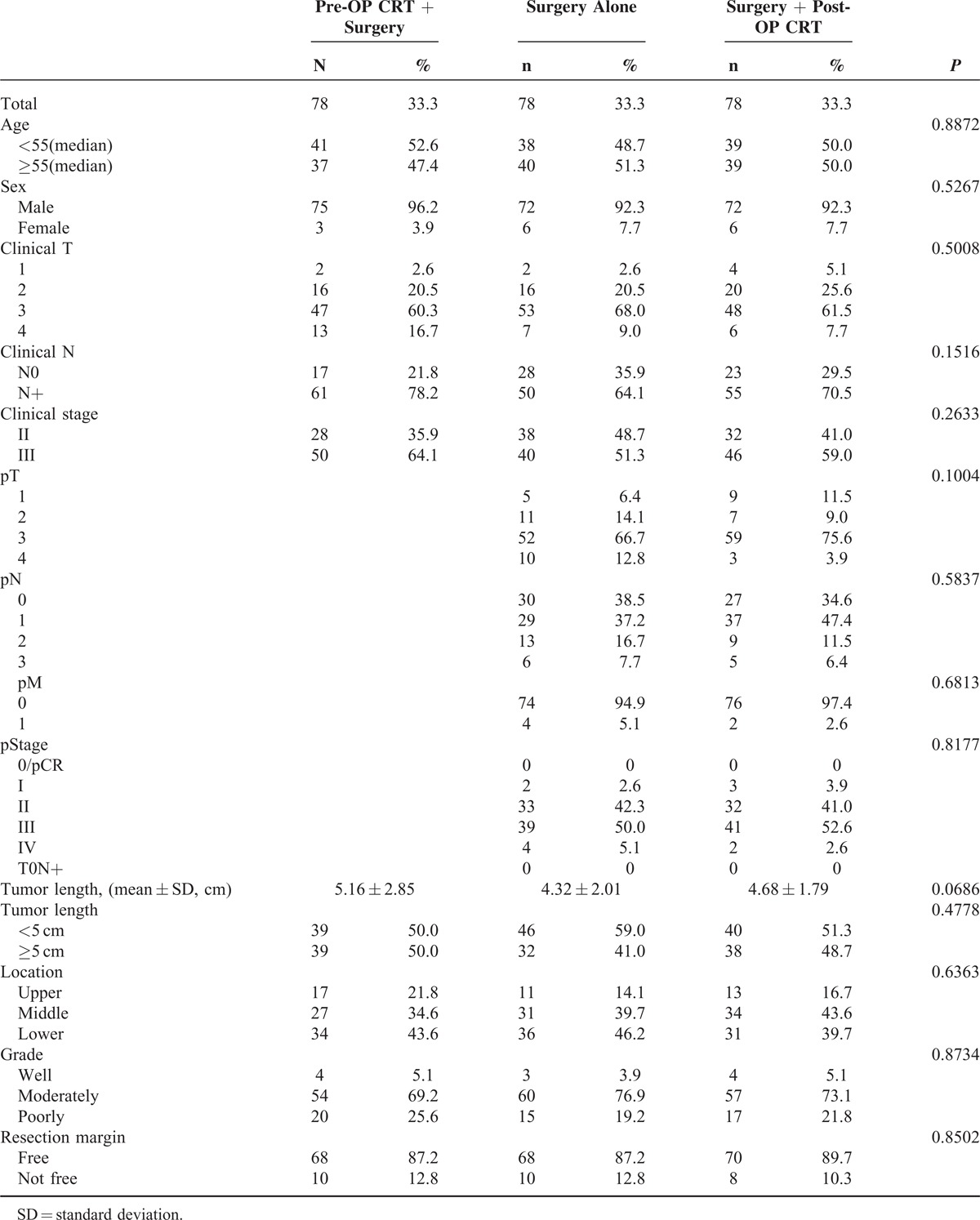
A propensity score matching analysis was performed in an attempt to reduce the bias due to the retrospective, nonrandomized nature of the analysis. After matching, 78 well-balanced patients in each treatment group were available for outcome comparison. No demographic difference was observed among these 3 groups (Table 3). The 1-year/3-year survival rates/median survival rates of patients in the pre-OP CRT+S, surgery alone, and S+post-OP CRT groups were 83.3%/46.8%/35.0 months, 60.3%/23.3%/17.0 months, and 75.6%/46.3%/23.5 months, respectively (P < 0.001). Kaplan–Meier curves showed that both pre-OP CRT+S and S+post-OP CRT were significantly associated with a better overall survival than surgery alone (Figure 2B. Pre-OP CRT vs. surgery alone: P < 0.001; post-OP CRT vs. surgery alone: P = 0.005). In contrast, no survival difference was observed among patients in the pre-OP CRT+S and S+post-OP CRT groups (P = 0.544). After stratification by clinical stage, a significant survival difference was noted between patients with clinical stage II ESCC in the pre-OP CRT+S and those in the surgery alone group (P = 0.038, Figure 2D). In patients with clinical stage III ESCC, a significantly worse survival was observed in the surgery alone group (Figure 2F. Pre-OP CRT vs. surgery alone: P < 0.001; post-OP CRT vs. surgery alone: P = 0.002). However, no survival difference between patients in the pre-OP CRT+S and the S+post-OP CRT groups was found (P = 0.500).
TABLE 2.
Cox Regression Survival Analysis for All Patients
A univariate survival analysis in the matched patients identified significant prognostic factors including female sex, cT3/4 stage, pT3/4 stage, ypT3/4 stage, R1/2 resection, and the use of chemoradiation (pre-OP CRT vs. surgery alone: HR: 0.48, 95% CI: 0.32–0.74, P < 0.001; post-OP CRT vs. surgery alone: HR: 0.56, 95% CI: 0.37–0.84, P = 0.006, Table 4). A multivariate survival analysis in matched patients identified cN+ stage as independent prognostic factor in the pre-OP CRT+S group. Female sex, pN+ stage, tumor length ≥5 cm, and the use of postoperative chemoradiation (HR: 0.36, 95% CI: 0.23–0.58, P < 0.001) were independent prognostic factors in patients who underwent primary esophagectomy.
TABLE 3.
Comparison of Propensity Score Matched Patients in the Pre-OP CRT + Surgery, Surgery Alone, and Surgery + Post-OP CRT Groups
Pre-OP CRT or Post-OP CRT?
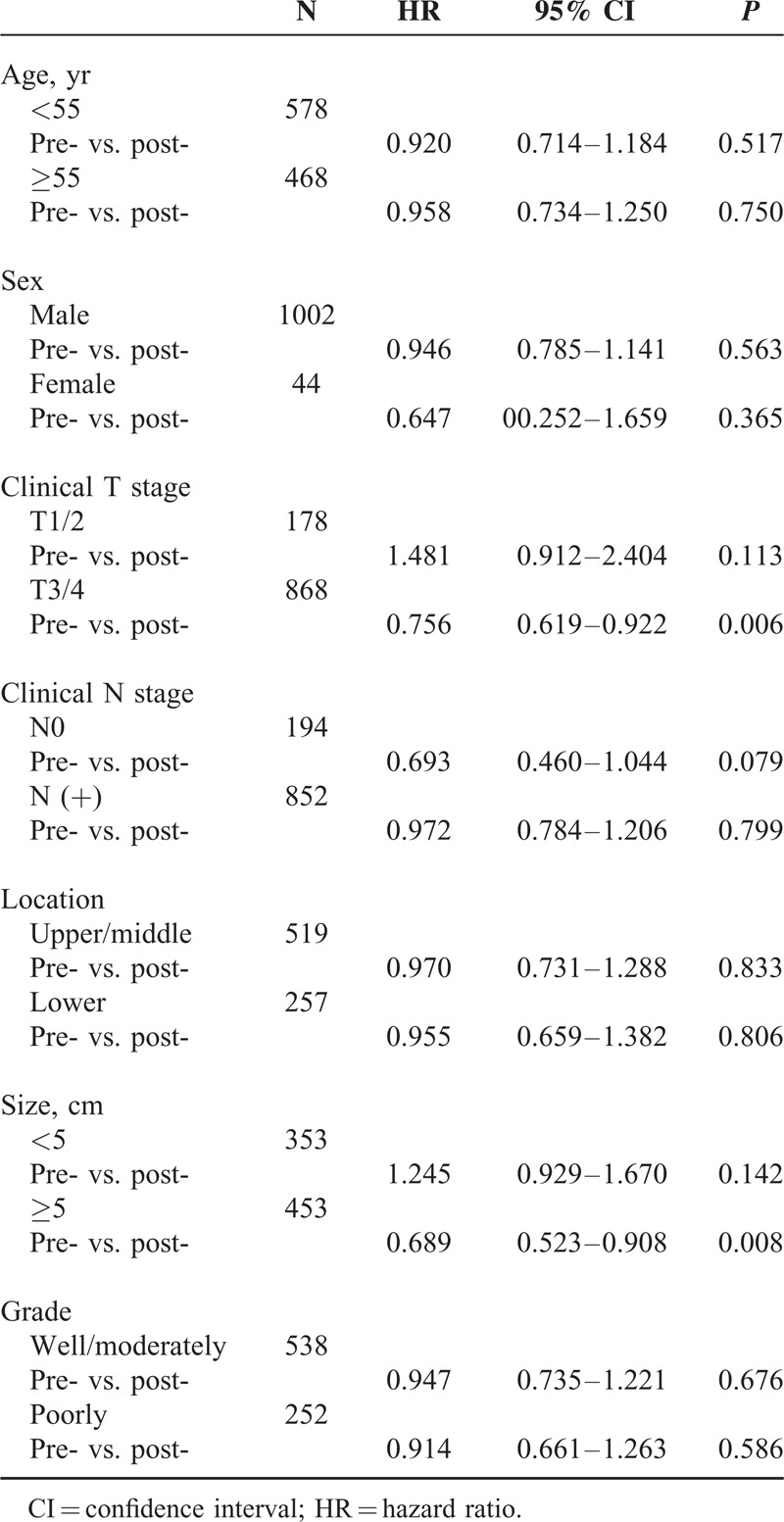
To identify patients who will benefit from pre-OP CRT or post-CRT in addition to surgery, we also performed a survival comparison between the pre-OP CRT+S and the S+post-OP CRT in subgroups of patients with different characteristics (Table 5). Patients with clinical T3/4 stage tumors and those with a tumor size greater than 5 cm were more likely to demonstrate an overall survival benefit from pre-OP CRT compared with post-OP CRT. In patients with cT3/4 stage tumors, the 1-year and 3-year survival rates as well as the median survival in the pre-OP CRT +S group were 74.5%, 40.0%, and 23.6 months, respectively, while in the S+post-OP CRT group, those values were 66.8%, 29.1%, and 17.5 months, respectively (HR: 0.756, 95% CI: 0.619–0.922, P = 0.006). In patients with tumors longer than 5 cm, the 1-year and 3-year survival rates as well as the median survival in the pre-OP CRT+S and S+post-OP CRT groups were 76.0%, 40.9%, 24.8 months and 62.2%, 28.0%, 15.8 months, respectively (HR: 0.689, 95% CI: 0.523–0.908, P = 0.008). These results suggest that both clinical T stage and tumor length are indications for pre-OP CRT followed by surgery.
TABLE 4.
Cox Regression Survival Analysis in Matched Patients
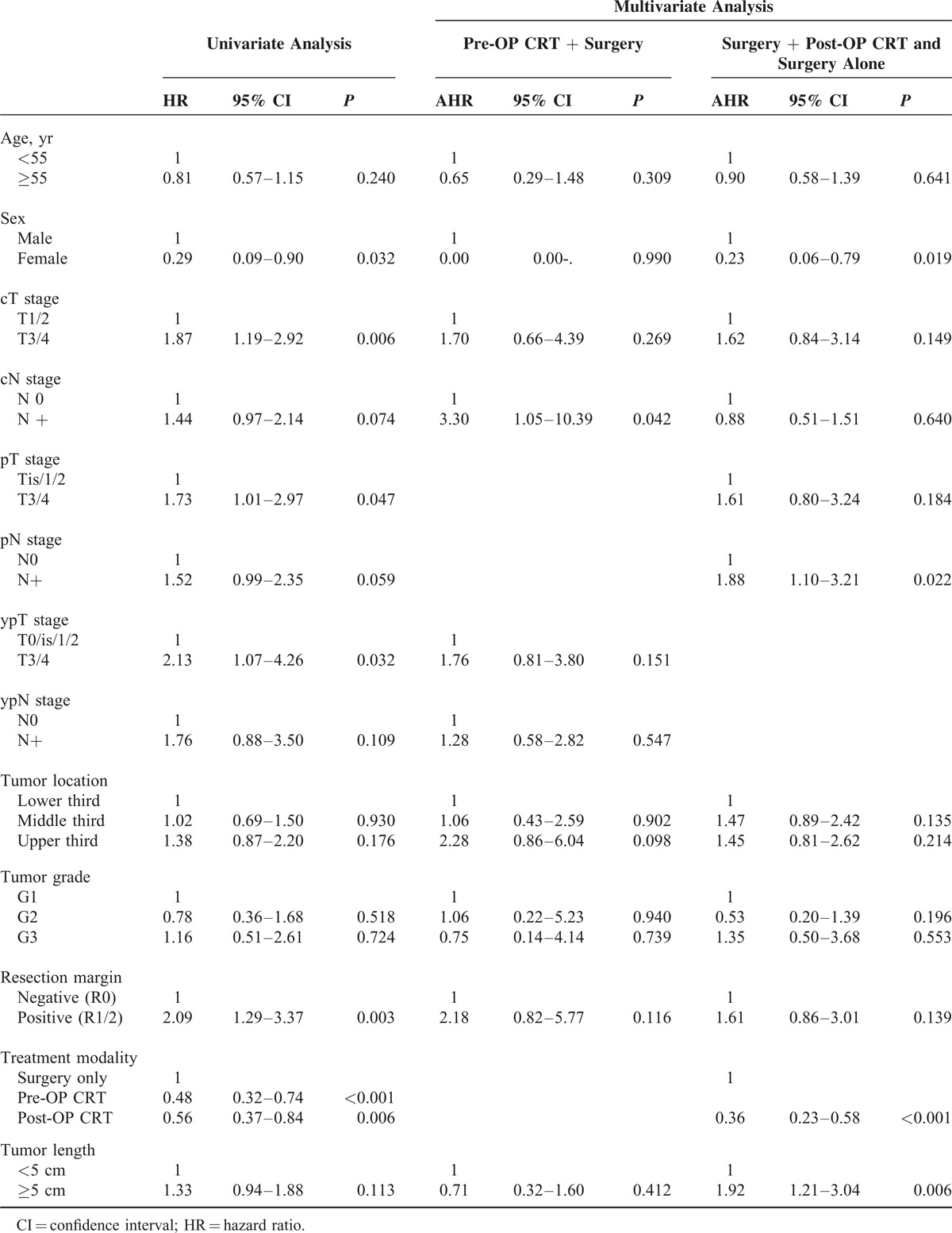
TABLE 5.
Survival Difference Between Patients With Different Characteristics in the Pre-OP CRT and Post-OP CRT Subgroups
DISCUSSION
Surgical resection has historically been the standard of care in the treatment of esophageal cancer. However, esophagectomy alone has demonstrated modest efficacy, and thus treatment regimens that include multimodal therapy have become necessary. The current guidelines of the National Comprehensive Cancer Network (NCCN)16 recommend neoadjuvant chemoradiation or definitive chemoradiation for all patients if the stage of disease exceeds T1N0. Although this is now widely used, many studies that have investigated preoperative chemoradiation have presented conflicting findings.5–11 Randomized trials by Urba et al (3-year survival rate/median survival for pre-OP CRT + surgery vs. surgery alone: 30%/16.9 vs. 16%/17.6 months, P = 0.15), Burmeister et al (pre-OP CRT + surgery vs. surgery alone in progression-free survival and overall survival: HR: 0.82 (95% CI: 0.61–1.10) and 0.89 (95% CI: 0.67–1.19)), and Lee et al (median survival for pre-OP CRT + surgery vs. surgery alone: 28.2 vs. 27.3 months, P = 0.67) all failed to demonstrate a survival benefit of preoperative chemoradiation.6–8 The recent FFCD9901 trial also showed that pre-OP CRT with cisplatin plus fluorouracil did not enhance survival for stage I or II esophageal cancer compared with surgery alone.9 In contrast, in the CALGB9781 trial, Tepper et al compared pre-OP CRT + surgery with surgery alone and demonstrated that the median survival times were 4.48 and 1.79 years, respectively, and that the 5-year survival rates were 39% and 16%, respectively, in favor of trimodality therapy.11 In the CROSS trial, the median overall survival and 5-year survival rates were 49.4 months and 47%, respectively, in the pre-OP CRT + surgery group, versus 24.0 months and 34%, respectively, in the surgery alone group. A significant survival benefit was observed in favor of the pre-OP CRT + surgery arm.5 Our results were similar to those of the FFCD9901 trial. No survival difference was noted between the pre-OP CRT+S and surgery alone groups in patients with clinical stage II disease. Even after a propensity score match analysis, the survival impact of pre-OP CRT was more evident in patients with clinical stage III disease than in patients with clinical stage II disease. Indeed, our propensity score match analysis showed significant survival benefits in favor of pre-OP CRT+S compared with surgery alone in patients with clinical stage II/III disease, which was compatible with the results from the CROSS trial.
On the contrary, the role of postoperative chemoradiation was understudied, and some studies suggested a survival benefit compared with surgery alone.12,13 Our recent study that compared surgery followed by post-OP CRT with surgery alone in patients with ESCC also demonstrated a survival benefit of post-OP CRT.14 After propensity score matching, the median overall survival/3-year survival rates were and 33.0 months/57.0% and 19.0 months/26.0%, respectively (P = 0.016) in favor of the S+post-OP CRT group. The survival impact was more evident in patients with N+ tumors. For patients with lymph node involvement in the S+post-OP CRT group the median overall survival was 29.0 months (95% CI: 21.0–37.0) versus 16.0 months (95% CI: 6.6–25.4) in the surgery alone group. The 1-year and 3-year overall survival rates in the S+post-OP CRT group and the surgery alone group were 86.0% versus 44.0% and 48.6% versus 16.8%, respectively (P = 0.003). The rationale of postoperative treatments included an indication for chemoradiation which was based on accurate pathological staging; thus, the risk of overtreatment was minimized. For example, the current study showed that 18.5% of patients with clinical stage II/III disease who underwent primary esophagectomy had stage 0/I based on the pathological staging. Patients such as these may be overtreated if we apply pre-OP CRT to all patients with clinical stage II/III ESCC. However, this strategy was understudied because patients who undergo esophagectomy are typically debilitated and unable to withstand any postoperative treatments. With the advent of minimally invasive esophagectomy, an earlier return of physical function may render patients better able to tolerate adjuvant therapy. For example, Parameswaran et al have compared the recovery of patients who underwent open and minimally invasive esophagectomy.17 They found that after surgery, the fatigue level of the patients gradually recovered to baseline following minimally invasive surgery within 6 months, but remained elevated in the open esophagectomy group. The early restoration of health-related quality of life after minimally invasive esophagectomy gives us an opportunity to re-evaluate the sequence of trimodality therapy.
In the literature, direct comparisons between pre-OP CRT and post-OP CRT are limited. Hong et al analyzed the SEER-Medicare database and compared the survival of 126 patients after pre-OP CRT+S and 40 patients after S+post-OP CRT.18 No difference in overall survival (P = 0.06) or cause-specific survival (P = 0.17) was found. Another single-center study also showed no significant difference in survival rates after a comparison of the pre-OP CRT and post-OP CRT arms.19 The 1-year survival rate/median survival was 91.3%/53 months in the pre-OP CRT+S group and 91.0%/48 months in the S+post-OP CRT group (P = 0.498). The current study includes 1385 patients with clinical stage II/III ESCC. Before propensity matching, both pre- and post-OP CRT demonstrated a survival benefit compared with surgery alone in patients with clinical stage III disease, which indicates that trimodality therapy is important for patients with esophageal cancer. After matching, both pre- and post-OP CRT demonstrated a survival benefit compared with surgery alone in patients with clinical stage II/ III disease. Additionally, no difference in overall survival was observed between the pre-OP CRT+S and S+post-OP CRT groups, which suggests that the sequence may be irrelevant to the outcome. Moreover, our subgroup analysis showed that patients with clinical T3/4 stage tumors and a tumor size greater than 5 cm are more likely to demonstrate an overall survival benefit from pre-OP CRT compared with post-OP CRT, which suggests clinical T stage and tumor length are indications for pre-OP CRT followed by surgical management.
The impact of our study includes the reappraisal of the role of postoperative treatment in esophageal cancer. The NCCN guidelines for ESCC recommended no additional treatments after surgery unless the margins are positive, irrespective of lymph node status.16 However, our results did suggest the survival benefits provided by post-OP CRT. It is also necessary to re-evaluate the significance of previously reported clinical trials with regard to preoperative chemoradiation. In the literature, the role of preoperative chemoradiation was established based on comparisons between pre-OP CRT+S and surgery alone.5–11 Adjuvant therapy was not given to the surgery alone groups, which means that patients with metastatic disease to the lymph nodes did not receive any systemic therapy. However, based on our findings, surgery alone is not enough for these patients with locally advanced disease. Those previous studies might have actually compared pre-OP CRT+S with incomplete treatment, that is, surgery alone. We highlight the need for a randomized trial to directly compare one trimodality therapy (pre-OP CRT) with another trimodality therapy (post-OP CRT) to establish the optimal combination and timing of multidisciplinary treatments for ESCC. The strengths of our study included a large database of patients, and the impact of different management approaches was based on the sequence of chemoradiation and surgery. However, the retrospective population-based nature of our database is a limitation of our study. The indication of pre-OP CRT or primary esophagectomy was not randomized. Some surgeons may perform primary esophagectomy for smaller, less advanced tumors and arrange pre-OP CRT only for more advanced tumors. Despite this possible bias, the survival of patients after surgery alone was still worse than that of patients in the pre-OP CRT+S group. Moreover, the indication for post-OP CRT was not randomized. Some surgeons may suggest post-OP CRT for patients with N+, T4 diseases and tumor infiltration close to the surgical margins, while they may suggest observation only for patients with less advanced tumors. Even so, the survival in S+post-OP CRT group was still better than surgery alone. Second, this population-based database lacks detailed information about staging workup, chemoradiation regimens, surgical techniques, and follow-up protocols, which is a common limitation to all population-based studies. However, this lacking information has little impact on the results. The data used for analysis is very solid and enough to reach conclusive results. Third, although we used propensity score matching to compensate for the unequal distribution of patients, which was due to retrospective nature of the study, some hidden factors may still influence the results. Our conclusions should be carefully interpreted and validated by prospective randomized trials in the future.
In conclusion, both pre- and post-OP CRT demonstrated survival benefits compared with surgery alone, which indicates that trimodality therapy is important for patients with stage II/III ESCC. However, no survival difference was observed between patients who receive pre-OP CRT and post-OP CRT, which suggests that the sequence may be irrelevant to the outcome. Although not recommended by the current NCCN guidelines, the strategy of surgery followed by post-OP CRT guided by pathologic findings did not negatively impact survival. Furthermore, accurate pathological staging could improve prognosis prediction and minimize overtreatment of patients with early-stage diseases. In the subgroup analysis, patients with clinical T3/4 stage tumors or those with a tumor size greater than 5 cm were more likely to demonstrate an overall survival benefit from pre-OP CRT compared with post-OP CRT. Based on the findings of this retrospective study, future prospective trials are needed to assess the optimal sequence of trimodality therapy.
Footnotes
Abbreviations: CI = confidence interval, CROSS = Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study, ESCC = esophageal squamous cell carcinoma, HR = hazard ratio, NCCN = National Comprehensive Cancer Network, post-OP CRT = postoperative chemoradiation, pre-OP CRT = preoperative chemoradiation, pre-OP CRT+S = preoperative chemoradiation followed by surgery, S = surgery alone, S+post-OP CRT = surgery followed by postoperative chemoradiation.
Funding: None.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hsu PK, Wang BY, Huang CS, et al. Prognostic factors for post-recurrence survival in esophageal squamous cell carcinoma patients with recurrence after resection. J Gastrointest Surg 2011; 15:558–565. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013; 381:400–412. [DOI] [PubMed] [Google Scholar]
- 3.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349:2241–2252. [DOI] [PubMed] [Google Scholar]
- 4.Hsu PK, Wu YC, Chou TY, et al. Comparison of the 6th and 7th editions of the American Joint Committee on Cancer tumor-node-metastasis staging system in patients with resected esophageal carcinoma. Ann Thorac Surg 2010; 89:1024–1031. [DOI] [PubMed] [Google Scholar]
- 5.Van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional Cancer. N Engl J Med 2012; 366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 6.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001; 19:305–313. [DOI] [PubMed] [Google Scholar]
- 7.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomized controlled phase III trial. Lancet Oncol 2005; 6:659–668. [DOI] [PubMed] [Google Scholar]
- 8.Lee JL, Park SI, Kim S-B, et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol 2004; 15:947–954. [DOI] [PubMed] [Google Scholar]
- 9.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled Pphase III trial FFCD 9901. J Clin Oncol 2014; 32:2416–2422. [DOI] [PubMed] [Google Scholar]
- 10.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous cell cancer of the esophagus. N Engl J Med 1997; 337:161–167. [DOI] [PubMed] [Google Scholar]
- 11.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodalityity therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008; 26:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice TW, Adelstein DJ, Chidel MA, et al. Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. J Thorac Cardiovasc Surg 2003; 126:1590–1596. [DOI] [PubMed] [Google Scholar]
- 13.Bedard ELR, Inculet RI, Malthaner RA, et al. The role of surgery and postoperative chemoradiation therapy in patients with lymph node positive esophageal carcinoma. Cancer 2001; 91:2423–2430. [PubMed] [Google Scholar]
- 14.Hsu PK, Huang CS, Wang BY, et al. Survival benefits of postoperative chemoradiation in lymph node-positive esophageal squamous cell carcinoma. Ann Thorac Surg 2014; 97:1734–1741. [DOI] [PubMed] [Google Scholar]
- 15.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997; 127:757–763. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. Esophageal cancer clinical practice guidelines in oncology. Available at: www.nccn.org Accessed Dec. 2014 [Google Scholar]
- 17.Parameswaran R, Titcomb DR, Blencowe NS, et al. Assessment and comparison of recovery after open and minimally invasive esophagectomy for cancer: an exploratory study in two centers. Ann Surg Oncol 2013; 20:1970–1977. [DOI] [PubMed] [Google Scholar]
- 18.Hong JC, Murphy JD, Wang SJ, et al. Chemoradiotherapy before and after surgery for locally advanced esophageal cancer: a SEER-Medicare analysis. Ann Surg Oncol 2013; 20:3999–4007. [DOI] [PubMed] [Google Scholar]
- 19.Lv J, Cao XF, Zhu B, et al. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol 2010; 16:1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]


