Abstract
There has not been a clear answer about the efficacy of intraoperative radiotherapy (IORT) for women with early-stage breast cancer.
The aim of this meta-analysis was to summarize the available evidence comparing the efficacy and safety of IORT with those of whole-breast external beam radiotherapy (EBRT) for women with early-stage breast cancer.
MEDLINE, EMBASE, the Web of Science, and the Cochrane Library were searched up to October 2014. Two authors independently conducted the literature selection and data extraction.
Studies that compared IORT with whole-breast EBRT were included in the systematic review. IORT was defined as a single dose of irradiation to the tumor bed during breast-conserving surgery rather than whole-breast irradiation.
Qualities of RCTs were evaluated according to the PEDro scale. Qualities of non-RCTs were evaluated according to the Methodological Index for Non-Randomized Studies (MINORS). The risk ratios (RRs) of ipsilateral breast tumor recurrence, overall mortality, breast cancer mortality, non-breast cancer mortality, and distant metastasis were pooled using a random-effects model.
Four studies with 5415 patients were included in this meta-analysis, including 2 randomized controlled trials (RCTs) and 2 non-RCTs. Ipsilateral breast tumor recurrence was significantly higher in patients with IORT compared to those with whole-breast EBRT (RR 2.83, 95% CI 1.23–6.51), but with significant heterogeneity (I2 = 58.5%, P = 0.065). Comparing IORT with whole-breast EBRT, the pooled RRs for overall mortality, breast cancer mortality, non-breast cancer mortality, and distant metastasis were 0.88 (95% CI: 0.66–1.17), 1.20 (95% CI: 0.77–1.86), 0.76 (95% CI: 0.44–1.31), and 0.95 (95% CI: 0.61–1.49), respectively.
IORT had a significantly higher risk of ipsilateral breast tumor recurrence than whole-breast EBRT. Overall mortality did not differ significantly. IORT should be used in conjunction with the prudent selection of suitable patients. It is imperative to identify women with a low risk of local recurrence.
INTRODUCTION
Breast-conserving therapy has been demonstrated to be an equivalent treatment strategy to mastectomy for patients with early-stage breast cancer.1–6 Radiotherapy has been an integral part of breast-conserving therapy. A meta-analysis7 performed by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) confirmed that whole-breast irradiation after breast-conserving surgery could reduce local recurrence and translated into a survival benefit compared to no radiotherapy (1:4 theory). However, traditional whole-breast irradiation usually requires a lengthy treatment time of approximately 5–6 weeks. Many patients do not receive subsequent radiotherapy after breast-conserving surgery or choose mastectomy instead for reasons including the long course of treatment, advanced age, a long travel distance to the hospital, and socioeconomic status.8,9
Based on the finding that most local recurrences occur at or near the tumor bed after breast-conserving surgery,10,11 accelerated partial-breast irradiation (APBI), a new radiation technique that targets partial breast tissue around the tumor cavity with fewer fractions has emerged.12–16 Intraoperative radiotherapy (IORT) is one pattern of APBI. IORT is defined as a single dose of irradiation delivered to the tumor bed at the time of surgery and can be substituted for whole-breast irradiation.17 The overwhelming advantage of IORT was that it could shorten the treatment time from approximately 5 to 6 weeks to only once concurrently with surgery, which was very convenient. In addition, IORT could reduce the cost of RT and improve the quality of life.18,19 Recently, 2 randomized clinical trials—the ELIOT trial and the TARGIT trial12,13—have reported their results of local control and survival outcomes when comparing IORT with whole-breast EBRT, and both reported promising results regarding IORT. However, the results were controversial because of the relatively high rates of ipsilateral breast tumor recurrence (IBTR) and because of the various limitations of each study.
To date, there has not been a corresponding meta-analysis to investigate this problem. Thus, we conducted this meta-analysis to evaluate the efficacy and safety of IORT compared with whole-breast EBRT for women with early-stage breast cancer and breast-conserving surgery.
METHODS
Literature Search
We searched the MEDLINE, EMBASE, the Web of Science, Cochrane Library (CENTRAL, Issue 8 of 12, Oct 2014) and ClinicalTrials.gov databases using the following phrases: “breast cancer,” “radiotherapy,” “intraoperative radiotherapy,” “IORT,” “intrabeam,” and “external beam radiotherapy.” MeSH terms and free terms were used for each electronic search. There were no restrictions based on language. The references of the included studies were also reviewed to identify potentially eligible articles. Two reviewers (L.Z. and Z.Z.) conducted the literature search independently.
Study Selection
Studies assessing the efficacy and safety of IORT compared with whole-breast EBRT for the management of breast cancer were included. Eligibility criteria were as follows: population: women with early-stage breast cancer and breast-conserving therapy; intervention: IORT, which should have been delivered to the tumor bed using a single dose of irradiation during surgery and should have been regarded as a substitute for whole-breast external beam radiotherapy; control: whole-breast EBRT, consisting of conventional whole-breast irradiation using an external beam with or without a boost to the tumor bed; outcomes: ipsilateral breast tumor recurrence and survival outcomes and/or toxicity; and study design: randomized clinical trials (RCTs) and other comparative studies with control arms. The following studies were excluded: studies using IORT as a boost to whole-breast irradiation, single-arm studies, and reviews. For multiple publications from the same study, we used the publication with the most up-to-date data and applicable information.
Data Extraction and Quality Assessment
Two authors (L.Z. and Z.Z.) performed the data extraction and quality assessment independently. Disagreements were discussed and resolved by consensus (X.Y. and X.G.). Data were extracted from each study as follows: first author, dates of accrual, study design, study sample size, patient characteristics, study arms, study intervention, median follow-up time, and outcome. Outcomes included IBTR, disease-free survival, overall survival, breast-cancer deaths, non-breast-cancer deaths, toxicity, cosmetic outcome, and quality of life.
Qualities of RCTs were evaluated according to the PEDro scale.20 Qualities of non-RCTs were evaluated according to the Methodological Index for Non-Randomized Studies (MINORS).21
Statistical Analysis
Cochran Q-test was used to test heterogeneity between studies (considered significant for I2 > 50%, P ≤ 0.10), where statistical heterogeneity was greater than this between trials, possible explanations were investigated using subgroup analyses according to type of study design. To give a more conservative estimate of the efficacy and safety of IORT with those of EBRT for women with breast-conserving surgery, allowing for any heterogeneity between studies, a random-effects model was used for all meta-analyses.22 The pooled risk ratios (RRs) and 95% confidence intervals (CIs) for IBTR, overall mortality, breast cancer mortality, non-breast cancer mortality, and distant metastasis were calculated, Z test was adopted to detect the significance of pooled RRs (considered significant for P ≤ 0.05).22 Moreover, we performed a sensitivity analysis by exclude the studies one by one to detect the stability of our meta-analysis results. All statistical analyses were conducted using STATA 13.0 (College Station, TX).
RESULTS
Study Selection
We identified 2191 records. After excluding duplicated and irrelevant records by screening the titles and abstracts, 53 full-text articles were retrieved for further examination. Of these articles, 49 articles were excluded for the following reasons: IORT was utilized as a boost to whole-breast irradiation, or the study lacked a control arm, reviews, or comments. Finally, 4 studies12,13,23,24 met our eligibility criteria and were selected for the final analysis. The study selection process is shown in Figure 1.
FIGURE 1.
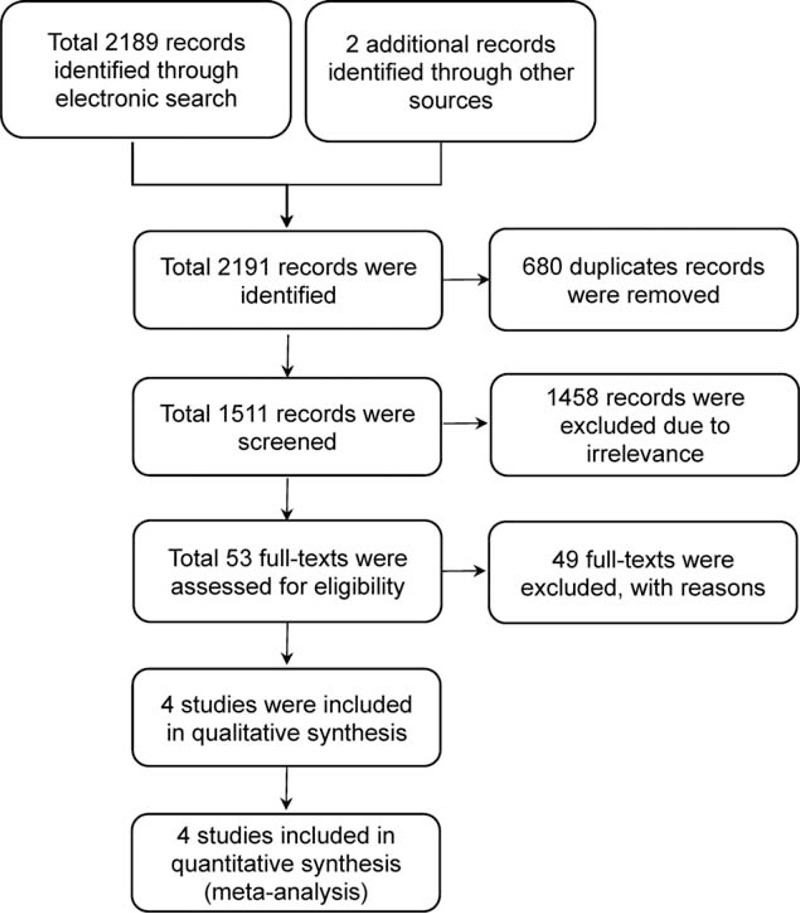
The process of the study selection.
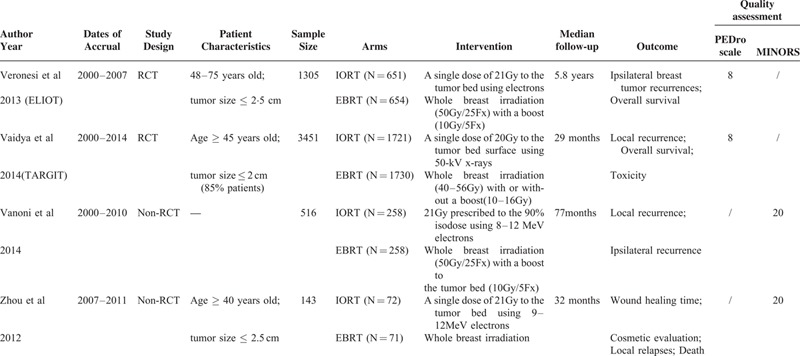
Study Characteristics
The 4 included studies, consisting of 2 RCTs12,13,25 (the ELIOT and TARGIT trials) and 2 non-RCTs,23,24 included 5415 patients: 2702 patients in the IORT arm and 2713 in the EBRT arm. The baseline characteristics of each study were balanced between both arms. All studies were initiated in 2000 or later and were published between 2012 and 2014. The sample size varied from 143 to 3451 cases. Except for the study by Vanoni et al, patients were at least 40 years old, with a tumor size of 2.5 cm or smaller.
Quality Assessment
Overall, the methodological quality of the 2 RCTs was good. Both the 2 RCTs had a score of 8 points. Points were lost because the patients and investigators were not blinded. The MINORS’ scores of the other 2 non-RCTs were both 20 and were acceptable.
The main characteristics and the quality assessment results of the 4 included studies are shown in Table 1.
TABLE 1.
The Main Characteristics and the Quality Assessment Results of the Included Studies
Ipsilateral Breast Tumor Recurrence (IBTR)
The IBTR was defined as all recurrences in any quadrant of the treated breast. The RRs for the IBTR data were available for all of the 4 studies (5415 patients in total).12,13,23,24 There was significant heterogeneity for IBTR (I2 = 58.5%, P = 0.065). In the 2 RCTs, high heterogeneity existed (I2 = 80.8%, P = 0.022). In the other 2 non-RCTs, no significant heterogeneity was observed (I2 = 0%, P = 0.862). The pooled RR for IBTR showed that there was a significant difference between the IORT group and the whole-breast EBRT group (RR = 2.83, 95% CI: 1.23–6.51). Ipsilateral breast tumor recurrence was significantly higher in patients with IORT. According to the subgroup analysis, the pooled RR for IBTR was 4.11 (95% CI: 0.99–17.13) in the RCTs and was 1.62 (95% CI: 0.68–3.86) in the non-RCTs (Figure 2).
FIGURE 2.
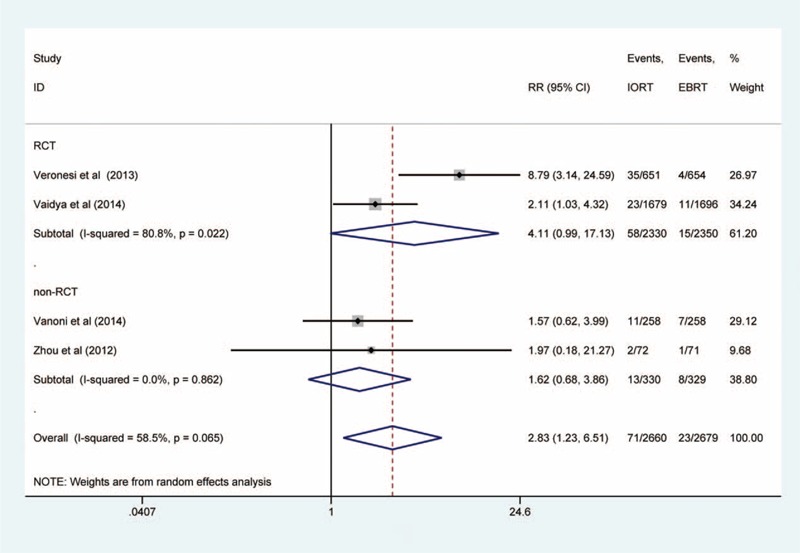
Forest plot of ipsilateral breast tumor recurrence.
Overall Mortality
The RR for overall mortality was available for all of the 4 studies (5415 patients in total).12,13,23,24 There was no significant heterogeneity for overall mortality (I2 = 0%, P = 0.530). The pooled RR for overall mortality indicated that there was no significant difference between the IORT and the whole-breast EBRT groups (RR = 0.88, 95% CI: 0.66–1.17). In the 2 RCTs, the pooled RR was 0.88 (95% CI: 0.59–1.32); in the other 2 non-RCTs, the pooled RR was 0.90 (95% CI: 0.41–2.00) (Figure 3A).
FIGURE 3.
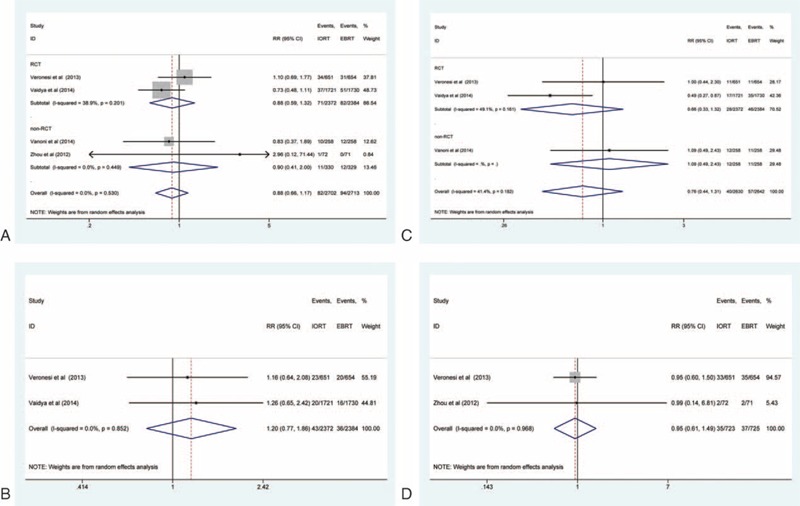
Forest plots of overall mortality, breast cancer mortality, non-breast cancer mortality, and distant metastasis. A, overall mortality; B, breast cancer mortality; C, non-breast cancer mortality; D, distant metastasis.
Breast Cancer Mortality
The RR for breast cancer mortality was available for the 2 RCTs (4756 patients in total).12,13 There was no significant overall heterogeneity for breast cancer mortality (I2 = 0%, P = 0.852). The pooled RR for breast cancer mortality indicated that there was no significant difference between the IORT and the whole-breast EBRT groups (RR = 1.20, 95% CI: 0.77–1.86) (Figure 3B).
Non-Breast Cancer Mortality
The RR for non-breast cancer mortality was available for 3 of the studies12,13,23 (2 RCTs and 1 non-RCT, 5272 patients in total). There was no significant heterogeneity for non-breast cancer mortality (I2 = 41.4%, P = 0.182). The pooled RR showed that there was no significant difference between the IORT and the EBRT groups (RR = 0.76, 95% CI: 0.44–1.31). In the 2 RCTs, the pooled RR was 0.66 (95% CI: 0.33–1.32) (Figure 3C).
Distant Metastasis
The pooled RR for distant metastasis was available for 2 of the studies12,24 (1 RCT and 1 non-RCT, 1448 patients in total). There was no significant heterogeneity for distant metastasis (I2 = 0%, P = 0.968). The pooled RR indicated that there was no significant difference between the IORT and the EBRT groups (RR = 0.95, 95% CI: 0.61–1.49) (Figure 3D).
Cosmetic Outcome
Cosmetic outcome was evaluated in 2 of the studies. Keshtgar et al26 assessed the cosmetic outcome of 342 patients using frontal digital photographs objectively in the TARGIT trial. The result showed that the odds of having an outcome of excellent or good was significantly higher for patients in the IORT group than in the EBRT group at both the first year (OR 2.07, 95% CI 1.12–3.85, P = 0.021) and the second year after the operation (OR 2.11, 95% CI 1.0–4.45, P = 0.05). Zhou et al24 evaluated overall cosmetic outcomes at 1 year after the operation and also found that the ratios of excellent or good outcomes were better in the IORT group than in the EBRT group (53 of 59 cases versus 42 of 56 cases, P = 0.032).
Toxicity
Veronesi et al12 reported the skin toxicity of 876 patients (464 patients in the IORT group; 412 patients in the EBRT group) in the ELIOT trial. They demonstrated that there were significantly fewer overall skin side effects in the IORT group than in the EBRT group (P = 0.0002). In particular, except for fat necrosis, the rates of erythema (P < 0.0001), dryness (P = 0.04), hyper-pigmentation (P = 0.0004), pruritus (P = 0.002), and pulmonary fibrosis (P < 0.0001) were significantly lower in the IORT group versus the EBRT group. Vaidya et al25 also reported the complications of the 2232 patients (1113 patients in the IORT group and 1119 in the EBRT group) in the TARGIT A trial. They demonstrated that the frequency of all complications and of major toxicity was similar in the IORT and the EBRT groups [for major toxicity, 3.3% vs. 3.9%, P = 0.443; for haematoma needing surgical evacuation, 1.0% vs. 0.6%, P = 0.338; for infection needing intravenous antibiotics or surgical intervention, 1.8% vs. 1.3%, P = 0.292; for skin breakdown or delayed wound healing, 2.8% vs. 1.9%, P = 0.155]. Radiotherapy-related toxicity (RTOG grade 3 or 4) for dermatitis, telangiectasia, and pain in the irradiated field was significantly lower in the IORT group than in the EBRT group (0.5% vs. 2.1%, P = 0. 002), although the risk of seroma needing aspiration was significantly higher in the IORT group than in the EBRT group (2.1% vs. 0.8%, P = 0.012). Zhou et al reported the average wound healing time, in IORT group is 13–22 days and 9–14 days in EBRT group, respectively.24
Sensitivity Analysis
The results of the sensitivity analysis indicated that the IBTR results of this meta-analysis were roughly stable and acceptable (Figure 4).
FIGURE 4.
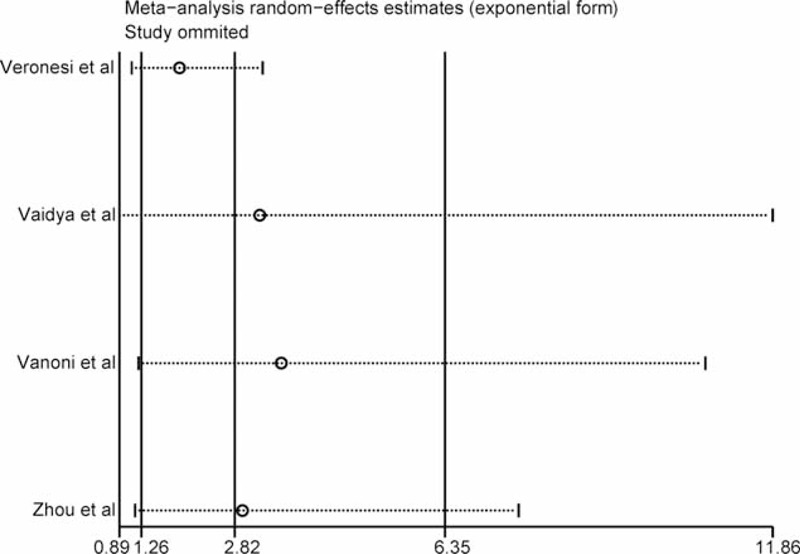
Sensitivity analysis.
DISCUSSION
In this meta-analysis of 5415 patients, pooled results demonstrated that the risk of IBTR was significantly higher with IORT than with whole-breast EBRT for women with early-stage breast cancer and breast-conserving surgery, due to moderate heterogeneity, the results should be interpreted with caution. Overall mortality, breast cancer mortality, non-breast cancer mortality, and distant metastases did not differ significantly between the groups. The cosmetic effects of IORT were better, and the overall side effects were significantly less than those of whole-breast EBRT.
As far as we know, this is the first meta-analysis summarizing current trials that focused on the newer radiation strategy—a single dose of IORT to the tumor bed at the time of surgery. Our results indicate that IORT should be used with caution for women with early-stage breast cancer and breast-conserving surgery because of the higher risk of IBTR.
The higher risk of IBTR with IORT might be attributed to the selection of patients who were unsuitable for this technique. Except for the TARGIT trial,13 patients in the other 3 studies12,23,24 were assigned to the IORT group or the EBRT group without the consideration of adverse clinical and pathologic prognostic factors. In the ELIOT trial,12 approximately half of the patients were less than 60 years old. Thirteen percent (13%) of the patients had a tumor size of more than 2 cm, and about one third of patients had positive nodes, which exceeds the “suitable” criteria of ASTRO's guidelines for APBI.27 Leonardi et al28 assigned 1822 patients treated with IORT at the European Institute of Oncology to suitable, cautionary and unsuitable groups. The results showed that the 5-year rate of IBTR increased from the suitable to the cautionary to the unsuitable group (1.5% vs. 4.4% vs. 8.8%, respectively; P = 0.0003). Similarly, when patients were assigned to good, possible, and contraindication groups according to the GEC-ESTRO recommendations for APBI, the 5-year rate of IBTR was 1.9%, 7.4%, and 7.7%, respectively (P = 0.001).29 In the ELIOT trial, when patients were divided into subgroups according to unfavorable factors, the 5-year rate of IBTR for women who did not have any unfavorable factors was far lower than for women who did have unfavorable factors (1.5% vs. 11.3%, P < 0·0001).12 The TARGIT trial had a risk-adapted design.13 This study predefined several adverse features before randomization, and additional whole-breast irradiation would be prescribed after IORT if adverse features appeared. This indicates that the IORT technique should be applied in an individualized way, and the prudent selection of patients who are at low risk of recurrence and suitable for IORT is essential.
To date, criteria have not been identified to determine which patients could receive single-dose IORT safely. Multivariate analysis in the ELIOT trial12 found that some factors doubled the risk of IBTR, including a tumor size greater than 2 cm, the presence of 4 or more positive lymph nodes, a poorly differentiated tumor and a triple-negative subtype. The adverse features in the TARGIT trial13 included a tumor-free margin smaller than 1 mm, an extensive in-situ component, an unexpected invasive lobular carcinoma, close margins, and other adverse prognostic factors (several positive nodes, extensive lymph-vascular invasion, etc.). The ASTRO and ESTRO guidelines for APBI could be used as references when selecting suitable patients for IORT.27–30 Sperk et al demonstrated that the selection of patients for IORT should be more restrictive.31 Excluding clinical and pathological factors, patients’ preference should also be taken into consideration when making decisions.32
Our study had some limitations. First, our meta-analysis only included 4 studies of 5415 patients. Two of these 4 studies were non-RCTs with a limited sample size, suggesting that our study was prone to biases, such as selection bias. Second, significant heterogeneity was observed for IBTR. Actually, a great deal of heterogeneity existed among the 4 included studies, in various dimensions. First, the patient characteristics were different. Some studies limited the patients’ age and tumor size, but with different threshold values. Some studies did not. Second, the study designs were different. The TARGIT trial had a risk-adapted design. In the IORT group, 15.2% patients received both IORT and EBRT because of the appearance of predefined adverse features. The other 3 trials did not take any clinical or pathological prognostic factors into consideration. Third, the techniques used to deliver IORT were different. The TARGIT trial adopted a prescription of 20 Gy using 50-kV x-rays, but the other 3 studies delivered 21 Gy using electrons. It was also hard to assess and compare the real target area irradiated. Fourth, the follow-up time was different in each study and might have been insufficient. All of these studies started in the year 2000 or later. The median follow-up time varied from 29 months to 5.8 years. This might be insufficient to observe differences in local control, survival outcomes, and adverse effects, especially late complications.33,34 Darby et al34 demonstrated that ischemic heart disease started within a few years and continued for at least 20 years after radiotherapy in breast cancer patients.
In conclusion, there was a significantly higher risk of IBTR with IORT compared to whole-breast EBRT for women with early-stage breast cancer and breast-conserving surgery, due to moderate heterogeneity, the results should be interpreted with caution. Overall mortality did not differ significantly between the groups. Identifying appropriate patients with a low risk of local recurrence is very important. For carefully selected patients who have a low risk of local recurrence, IORT should be a feasible choice.
Appendix
Search terms (PubMed)
#17 Search (((((((breast tumour) OR breast carcinoma) OR breast neoplasms) OR breast cancer) OR “Breast Neoplasms”[Mesh])) AND ((((intraoperative radiotherapy) OR TARGIT) OR ELIOT) OR INTRABEAM)) AND ((((radiotherapy) OR EBRT) OR irradiation) OR radiation)
#16 Search (((radiotherapy) OR EBRT) OR irradiation) OR radiation
#15 Search radiation
#14Search irradiation
#13 Search EBRT
#12Search radiotherapy
#11Search (((intraoperative radiotherapy) OR TARGIT) OR ELIOT) OR INTRABEAM
#10 Search INTRABEAM
#9 Search ELIOT
#8 Search TARGIT
#7 Search intraoperative radiotherapy
#6 Search ((((breast tumour) OR breast carcinoma) OR breast neoplasms) OR breast cancer) OR “Breast Neoplasms”[Mesh]
#5 Search “Breast Neoplasms”[Mesh]
#4 Search breast neoplasms
#3 Search breast carcinoma
#2 Search breast tumour
#1 Search breast cancer
Search terms (EMBASE)
#16. ’breast cancer’ OR ’breast tumor’/exp OR ’breast tumor’ OR ’breast carcinoma’/exp OR ’breast carcinoma’ OR ’breast neoplasms’/exp OR ’breast neoplasms’ OR ’breast cancer’/exp AND (intraoperative AND (’radiotherapy’/exp OR radiotherapy) OR targit OR intrabeam OR eiot) AND (’radiotherapy’/exp OR ’radiotherapy’ OR ebrt OR ’irradiation’/exp OR ’irradiation’)
#15. ’radiotherapy’/exp OR ’radiotherapy’ OR ebrt OR ’irradiation’/exp OR ’irradiation’
#14. ’irradiation’/exp OR ’irradiation’
#13. ebrt
#12. ’radiotherapy’/exp OR ’radiotherapy’
#11. intraoperative AND (’radiotherapy’/exp OR radiotherapy) OR targit OR intrabeam OR eiot
#10. eiot
#9. intrabeam
#8. targit
#7. intraoperative AND (’radiotherapy’/exp OR radiotherapy)
#6. ’breast cancer’ OR ’breast tumor’/exp OR ’breast tumor’ OR ’breast carcinoma’/exp OR ’breast carcinoma’ OR ’breast neoplasms’/exp OR ’breast neoplasms’ OR ’breast cancer’/exp
#5. ’breast cancer’/exp
#4. ’breast neoplasms’/exp OR ’breast neoplasms’
#3. ’breast carcinoma’/exp OR ’breast carcinoma’
#2. ’breast tumor’/exp OR ’breast tumor’
#1. ’breast cancer’/exp OR ’breast cancer’
Footnotes
Abbreviations: APBI = accelerated partial breast irradiation, CI = confidence interval, EBRT = external beam radiotherapy, IBTR = ipsilateral breast tumor recurrence, IORT = intraoperative radiotherapy, RCT = randomized controlled trials, RR = risk ratio.
L.Z. and Z.Z. have contributed equally to this work.
Conception and design: L.Z., Z.Z., X.Y., X.G.
Collection and assembly of data: L.Z., Z.Z., X.Y., X.G.
Data analysis and interpretation: Z.Z. and L.Z. completed the statistical analysis. All authors participated in the interpretation of pooled results.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
Financial support: X.Y., X.G.
This work was partly supported by the National Natural Science Foundation of China (Grant Numbers 81372430, 81402525).
The authors indicated no potential conflicts of interest.
REFERENCES
- 1.van Dongen JA, Bartelink H, Fentiman IS, et al. Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. J Natl Cancer Instit Monographs 1992; 11:15–18. [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347:1233–1241. [DOI] [PubMed] [Google Scholar]
- 3.Lichter AS, Lippman ME, Danforth DN, Jr, et al. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: a randomized trial at the National Cancer Institute. J Clin Oncol 1992; 10:976–983. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002; 347:1227–1232. [DOI] [PubMed] [Google Scholar]
- 5.Arriagada R, Le MG, Guinebretiere JM, et al. Late local recurrences in a randomised trial comparing conservative treatment with total mastectomy in early breast cancer patients. Ann Oncol 2003; 14:1617–1622. [DOI] [PubMed] [Google Scholar]
- 6.Blichert-Toft M, Rose C, Andersen JA, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Instit Monographs 1992; 11:19–25. [PubMed] [Google Scholar]
- 7.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366:2087–2106. [DOI] [PubMed] [Google Scholar]
- 8.Athas WF, Adams-Cameron M, Hunt WC, et al. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Instit 2000; 92:269–271. [DOI] [PubMed] [Google Scholar]
- 9.Du Xianglin L, Gor BJ. Racial disparities and trends in radiation therapy after breast-conserving surgery for early-stage breast cancer in women 1992 to 2002. Ethnicity Dis 2007; 17:122–128. [PMC free article] [PubMed] [Google Scholar]
- 10.Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol 2001; 12:997–1003. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz JM, Amalric R, Brandone H, et al. Local recurrence after breast-conserving surgery and radiotherapy. Frequency, time course, and prognosis. Cancer 1989; 63:1912–1917. [DOI] [PubMed] [Google Scholar]
- 12.Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013; 14:1269–1277. [DOI] [PubMed] [Google Scholar]
- 13.Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014; 383:603–613. [DOI] [PubMed] [Google Scholar]
- 14.Polgar C, Fodor J, Major T, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma: 5-year results of a randomized trial. Int J Radiat Oncol Biol Phys 2007; 69:694–702. [DOI] [PubMed] [Google Scholar]
- 15.Vicini F, Winter K, Wong J, et al. Initial efficacy results of RTOG 0319: three-dimensional conformal radiation therapy (3D-CRT) confined to the region of the lumpectomy cavity for stage I/II breast carcinoma. Int J Radiat Oncol Biol Phys 2010; 77:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson JC, Beitsch PD, Vicini FA, et al. Four-year clinical update from the American Society of Breast Surgeons MammoSite brachytherapy trial. Am J Surg 2009; 198:83–91. [DOI] [PubMed] [Google Scholar]
- 17.Vaidya JS, Tobias JS, Baum M, et al. Intraoperative radiotherapy for breast cancer. Lancet Oncol 2004; 5:165–173. [DOI] [PubMed] [Google Scholar]
- 18.Alvarado MD, Mohan AJ, Esserman LJ, et al. Cost-effectiveness analysis of intraoperative radiation therapy for early-stage breast cancer. Ann Surg Oncol 2013; 20:2873–2880. [DOI] [PubMed] [Google Scholar]
- 19.Holmes DR, Pulicharamveettil J, Sutter GL, et al. Cost comparison of radiation treatment options after lumpectomy for breast cancer: intraoperative radiotherapy versus alternatives. J Clin Oncol 2012; 30 (suppl 27):abstr 187.Available from http://meeting.ascopubs.org/cgi/content/abstract/30/27_suppl/187. 2012. [Google Scholar]
- 20.PEDro scale. http://www.pedro.org.au/english/downloads/pedro-scale/ 1999 [Google Scholar]
- 21.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003; 73:712–716. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane-handbook.org 2011. [Google Scholar]
- 23.Vanoni V, Bou Selman S, Mussari S, et al. External beam radiation therapy versus intraoperative radiation therapy for breast-conserving therapy: a large single-institution matched-pair evaluation. Int J Radiat Oncol Biol Phys 2014; 90:S261. [DOI] [PubMed] [Google Scholar]
- 24.Zhou SF, Shi WF, Meng D, et al. Interoperative radiotherapy of seventy-two cases of early breast cancer patients during breast-conserving surgery. Asian Pacific J Cancer Prevention 2012; 13:1131–1135. [DOI] [PubMed] [Google Scholar]
- 25.Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010; 376:91–102. [DOI] [PubMed] [Google Scholar]
- 26.Keshtgar MR, Williams NR, Bulsara M, et al. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treatment 2013; 140:519–525. [DOI] [PubMed] [Google Scholar]
- 27.Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009; 74:987–1001. [DOI] [PubMed] [Google Scholar]
- 28.Leonardi MC, Maisonneuve P, Mastropasqua MG, et al. How do the ASTRO consensus statement guidelines for the application of accelerated partial breast irradiation fit intraoperative radiotherapy? A retrospective analysis of patients treated at the European Institute of Oncology. Int J Radiat Oncol Biol Phys 2012; 83:806–813. [DOI] [PubMed] [Google Scholar]
- 29.Leonardi MC, Maisonneuve P, Mastropasqua MG, et al. Accelerated partial breast irradiation with intraoperative electrons: using GEC-ESTRO recommendations as guidance for patient selection. Radiother Oncol 2013; 106:21–27. [DOI] [PubMed] [Google Scholar]
- 30.Polgar C, Van Limbergen E, Potter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence. Radiother Oncol 2010; 94:264–273. [DOI] [PubMed] [Google Scholar]
- 31.Sperk E, Astor D, Keller A, et al. A cohort analysis to identify eligible patients for intraoperative radiotherapy (IORT) of early breast cancer. Radiat Oncol (London, England) 2014; 9:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarado MD, Conolly J, Park C, et al. Patient preferences regarding intraoperative versus external beam radiotherapy following breast-conserving surgery. Breast Cancer Res Treatment 2014; 143:135–140. [DOI] [PubMed] [Google Scholar]
- 33.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011; 378:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368:987–998. [DOI] [PubMed] [Google Scholar]


