Abstract
Insomnia is prevalent in patients with chronic obstructive pulmonary disease (COPD).
We conducted a population-based case-control study to evaluate the effects of hypnotics on the risk of adverse respiratory events in patients with COPD.
The case-control study was investigated using data retrieved from the Taiwan National Health Insurance Research Database. Patients with newly diagnosed adverse respiratory events (pneumonia, COPD with acute exacerbation, acute respiratory failure, and cardiopulmonary arrest) were included in the case group. Patients with COPD and no history of adverse respiratory events were randomly selected for the control group, which was frequency-matched with the case group according to index date, age (per 10 years), and sex. Patients who had used hypnotics within 1 month meant active users. The odds ratios (ORs) and 95% confidence intervals (CIs) of were calculated using univariable and multivariable logistic regression models.
Most of the study participants were male (71.6%), and the mean ages of the participants in the case and control groups were 69.2 (±12.4) and 67.5 (±12.3) years, respectively. After potential confounding factors were adjusting for, the adjusted ORs of adverse respiratory events were 12.0 for active users of benzodiazepines (95% CI, 8.11–17.6) and 10.5 for active users of nonbenzodiazepines (95% CI, 7.68–14.2) compared with the adjusted ORs of those who never used hypnotics.
The results of this epidemiological study suggested that hypnotics increased the risk of adverse respiratory events in patients with COPD.
INTRODUCTION
Insomnia, characterized by difficulty in falling and staying asleep and frequently waking up during the night, is a common complaint among the general population.1–3 Insomnia is evidently associated with comorbidities, such as prior psychiatric disorders, circulatory diseases, and gastrointestinal diseases.4
Chronic obstructive pulmonary disease (COPD), a common disease worldwide, can cause considerable disability and mortality. Studies have reported that 55% to 75% of patients with COPD exhibit sleep disturbances more frequently than does the general population.5–7 Patients with COPD experience sleep fragmentation with frequent arousals and reduced slow wave and rapid eye movement (REM) sleep, and a lower mean overnight oxygen saturation.8,9 Insomnia is a well-established factor that impairs the quality of life (QOL).10 Budhiraja et al6 reported that patients with COPD and insomnia have a low QOL. Patients with more severe COPD experience insomnia more frequently than patients with less severe COPD do.11 In addition, comorbidities such as anxiety and depression are commonly observed in patients with COPD.12
Patients with insomnia or sleep-related problems often seek medical help from general practitioners, who frequently prescribe hypnotic drugs.13 Despite improving sleep quality, these drugs involve detrimental risks, such as ataxia, falls, and cognitive impairment, in elderly patients.14 Patients with COPD may receive hypnotics for various reasons, including treatment for insomnia, depression, and anxiety.15 However, few epidemiological studies have been conducted to examine the relationship between hypnotics and adverse respiratory events in patients with COPD. Therefore, we conducted a case-control study to investigate the effects of hypnotics use, including benzodiazepines and nonbenzodiazepine hypnotics, on the adverse respiratory events experienced by patients with COPD.
METHODS
Data Source
Taiwan's National Health Insurance (NHI) program is a universal healthcare system established in 1995. This single-payer system covered approximately 99% of the 23 million inhabitants by 2009.16 The National Health Insurance Research Database (NHIRD), derived from the NHI reimbursement claims, was created for research purposes. In this study, we used a subset of the NHIRD, the Longitudinal Health Insurance Database 2000 (LHID2000), which comprises the patient data from 1996 to 2011. The LHID2000 includes the original claim data of 1,000,000 beneficiaries randomly sampled from the original NHIRD. The database contains encrypted personal information, such as patient identification numbers, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for diagnoses and procedures, prescription drug details, dates of admission and discharge, and basic sociodemographic information, including sex and birth date. Medications were classified according to the NHI drug codes and Anatomical Therapeutic Chemical (ATC) codes. Both codes are internationally accepted drug classification systems and are coordinated by the World Health Organization Collaborating Center for Drug Statistics Methodology. This study was approved by the Institutional Review Board of China Medical University, Taiwan (CMU-REC-101-012).
Sampled Participants
Figure 1 illustrates the sampling scheme used in this retrospective case-control study. We extracted the claims data from the LHID2000 for the period from 1997 to 2011 for patients with COPD (ICD-9-CM codes 491, 492, and 496). Patients aged 20–85 years and newly diagnosed with adverse respiratory events (including pneumonia [ICD-9-CM codes 480–486], acute exacerbation [ICD-9-CM code 491.21], acute respiratory failure [acute respiratory failure (ARF), ICD-9-CM code 518.81], and cardiopulmonary arrest [ICD-9-CM codes 799.1, 798, 427.5]) between January 1, 2000 and December 31, 2011 were included in the case group. The date of initial adverse respiratory event diagnosis was set as the index date. The control group was randomly selected from the remaining patients with COPD and no history of adverse respiratory events and was frequency-matched with the case group according to index date, age (per 10 years), and sex. Patients treated with hypnotics (including benzodiazepines and nonbenzodiazepines) for more than 1 year before the index date and with incomplete age or sex information were excluded from the study. Finally, a total of 11,342 patients with a history of adverse respiratory events and 11,342 patients without a history of adverse respiratory events were included in the case and control groups, respectively.
FIGURE 1.
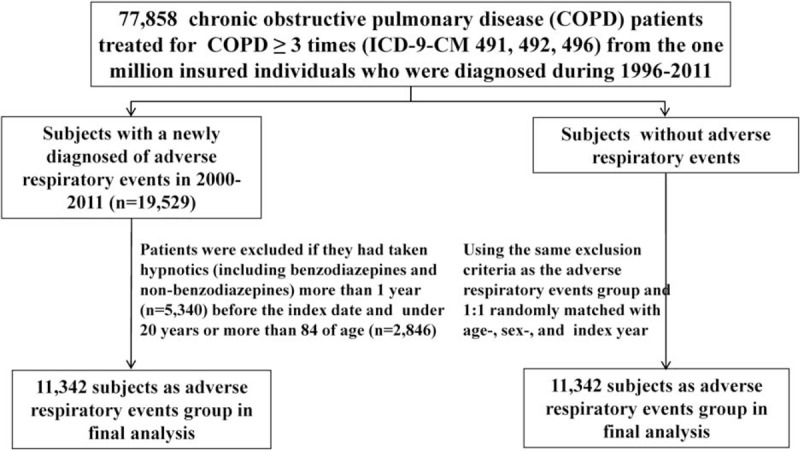
Sampling scheme used in this retrospective case-control study.
Definition of Chronic Obstructive Pulmonary Disease
In the present study, COPD was diagnosed by physicians, who referred to the history of participants regarding exposure to risk factors for COPD and respiratory symptoms such as shortness of breath, chronic cough, and sputum production. The physicians may examine the patients and perform a pulmonary function test and a bronchodilator test, which yield a postbronchodilator FEV1/FVC ratio of <0.7 (where FEV1 is forced expiratory volume in 1 second and FVC is forced vital capacity). The NHI Administration may audit the diagnosis and management codes used by the physicians and medical reimbursement specialists. Several Taiwan-based studies have demonstrated a high level of accuracy and validity associated with the diagnostic methods applied in this study.17,18
Definition of Exposure to Benzodiazepine and Nonbenzodiazepine Hypnotics
The primary exposures of interest were those to hypnotics, including benzodiazepines (flurazepam [ATC code N05CD01], nitrazepam [N05CD02], flunitrazepam [N05CD03], estazolam [N05CD04], triazolam [N05CD05], lormetazepam [N05CD06], midazolam [N05CD08], and brotizolam [N05CD09]) and nonbenzodiazepines (zopiclone [ATC code N05CF01], zolpidem [N05CF02], and zaleplon [N05CF03]). Only those participants who used hypnotics, including benzodiazepines and nonbenzodiazepines, within 1 year before the index date were included in the study. Participants who had never used hypnotics were defined as never used. Participants who had used hypnotics were further classified into 2 groups according to when they filled the hypnotics prescription before the index date: active users (≤1 m) and nonactive users (31–365 days).
Comorbid Medical Disorders and Respiratory Medicines
Information extracted from the claims data included sex, age, Charlson comorbidity index (CCI), and history of medication and comorbidities. The CCI was calculated for each participant according to the claims data for outpatient visits or hospitalizations before the index date. The CCI is a scoring system that includes weighting factors on critical concomitant diseases and has been validated for use with the ICD-9-CM coded administrative database.19,20 Baseline comorbidities and corresponding medications were considered as covariates, including sleep disorders (ICD-9-CM codes 370.4 and 780.5), coronary artery disease (ICD-9-CM codes 410–414), congestive heart failure (ICD-9-CM codes 428, 398.91, and 402.x1), depression (ICD-9-CM codes 296.2, 296.3, 300.4, and 311), anxiety (ICD-9-CM code 300.00), alcohol-related illness (ICD-9-CM codes 291, 303, 305, 571.0, 571.1, 571.2, 571.3, 790.3, A215, and V11.3), and dementia (ICD-9-CM codes 290, 294.1, and 331.0).
Statistical Analysis
Descriptive statistics were used to analyze demographic variables, hypnotics use, CCI, and the history of medications and comorbidities. The differences between the case and control groups were examined using the Chi-square test. Continuous variables were analyzed using the t test. The effects of hypnotics treatment, CCI, and the history of both medications and comorbidities on the risk of adverse respiratory events were estimated using univariable and multivariable unconditional logistic regression and were indicated as the odds ratio (OR) and 95% confidence interval (CI). The multivariable analysis was performed to adjust for potential confounders (including age, sex, CCI, and history of comorbidities). All analyses were performed using SAS statistical software (Version 9.3; SAS Institute, Inc., Cary, NC), and results were considered statistically significant when 2-tailed P values were <0.05.
RESULTS
Comparison Between the Case and Control Group
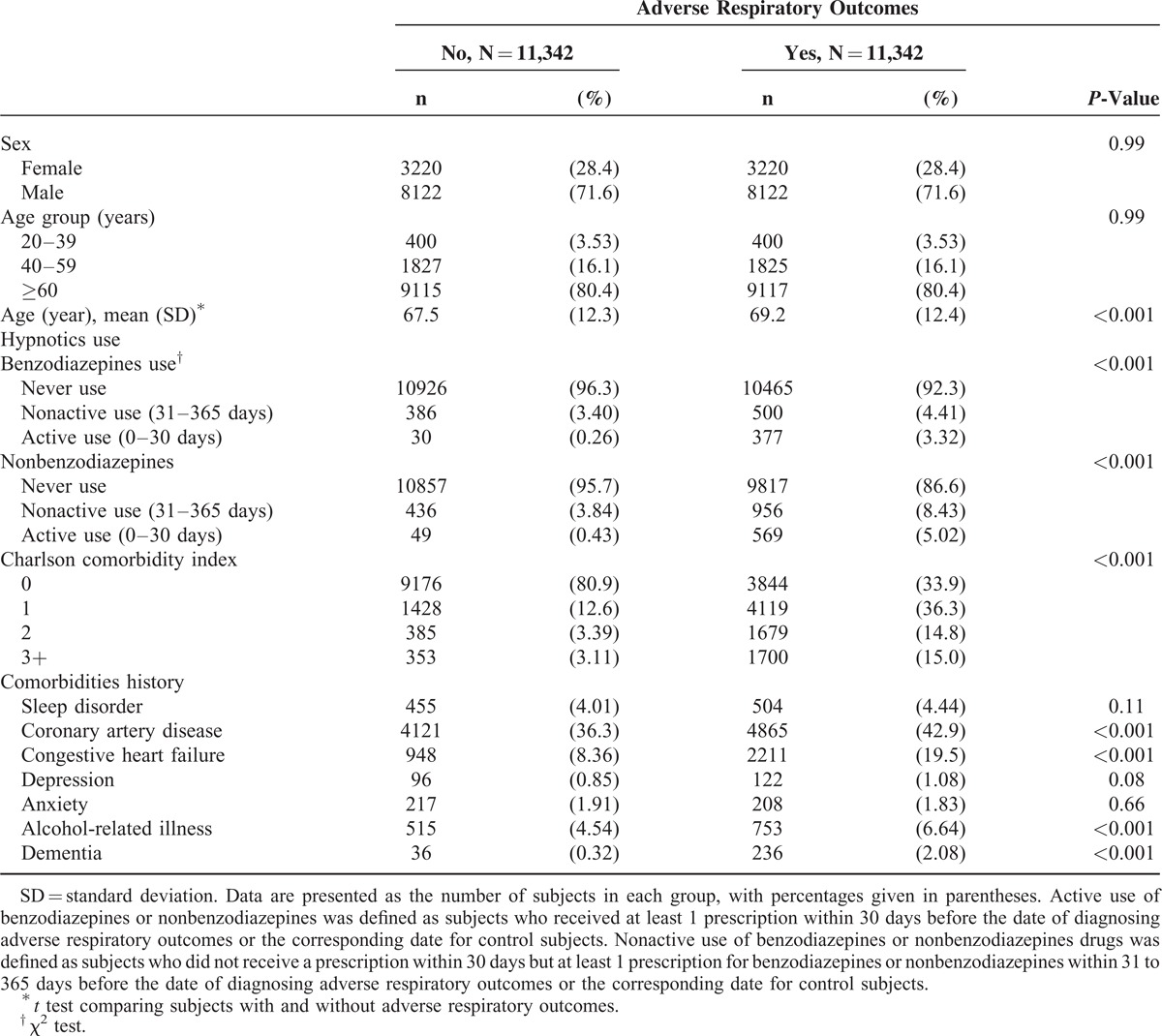
Table 1 shows a comparison between the case and control groups for the demographic variables, hypnotics use, CCI, and history of medications and comorbidities. No significant differences in distributions of age and sex were observed between the 2 groups. Of the 11,342 participants in the case group, 71.6% were male and 80.4% were older than 60 years. The mean ages of participants in the case and control groups were 69.2 (±12.4) and 67.5 (±12.3) years, respectively. The case group had a higher number of users of both benzodiazepines (7.73% vs. 3.66%) and nonbenzodiazepines (13.5% vs. 4.27%) than did the control group. The CCI score was higher in the case group than in the control group. The occurrences of comorbidities, such as coronary artery disease, congestive heart failure, alcohol-related illness, and dementia, in the case group were significantly higher than those in the control group.
TABLE 1.
Characteristics of the Case and Control Groups
Factors Associated With the Risk of Adverse Respiratory Events in the Patients With Chronic Obstructive Pulmonary Disease
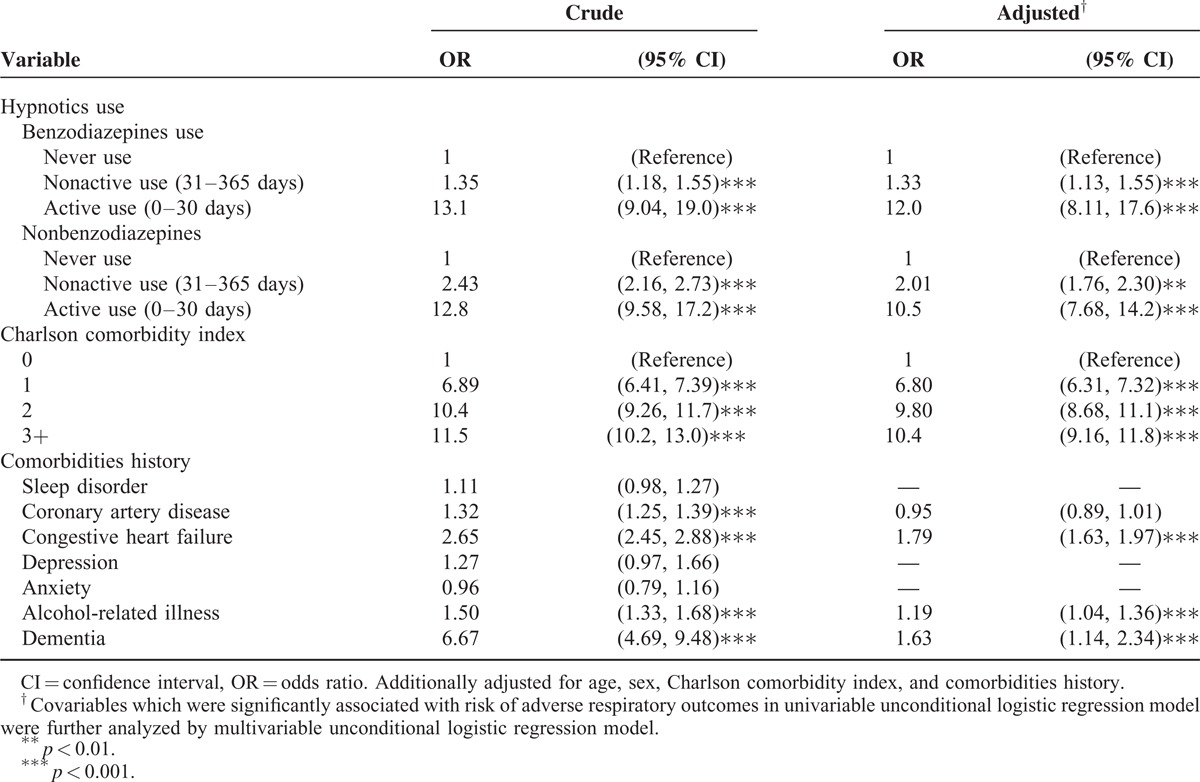
Table 2 shows the crude and adjusted ORs for the model fitted to examine the association between potential factors and the risk of adverse respiratory events. After adjustment for potential confounding factors, the adjusted ORs of adverse respiratory events were 12.0 among the active users of benzodiazepines (95% CI, 8.11–17.6) and 10.5 among the active users of nonbenzodiazepines (95% CI, 7.68–14.2), compared with those for the patients without use of hypnotics The adjusted ORs gradually decreased to 1.33 (95% CI, 1.13–1.55) among the nonactive users of benzodiazepines and to 2.01 (95% CI, 1.76–2.30) among the nonactive users of nonbenzodiazepines. In addition, a higher CCI score and history of comorbidities, including congestive heart failure, alcohol-related illness, and dementia, were the independent risk factors of adverse respiratory events in the patients with COPD.
TABLE 2.
Factors Associated With the Risk of Adverse Respiratory Events in the Patients With COPD
The Relationship Between Hypnotics Use and Various Adverse Respiratory Events
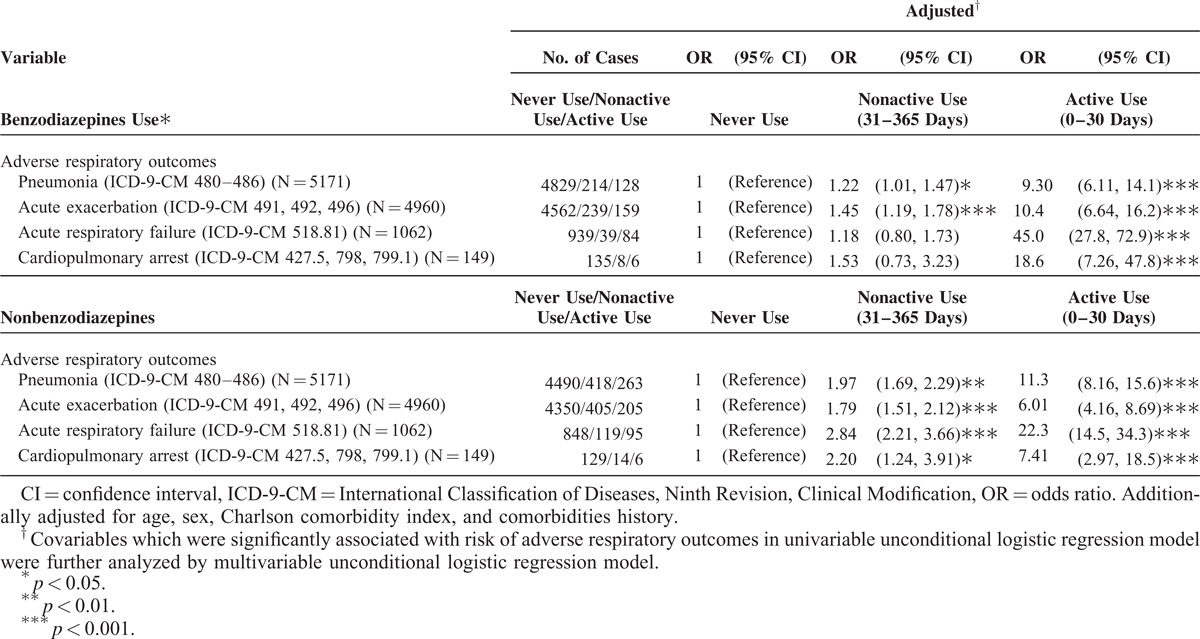
Table 3 shows the relationship between various adverse respiratory events associated with hypnotics use. The active users of both benzodiazepines and nonbenzodiazepines exhibited a greater risk of various adverse respiratory events than the participants in the never used group did. The nonactive users of benzodiazepines showed a higher risk of pneumonia and COPD with acute exacerbation than the participants in the never used group did. The nonactive users of nonbenzodiazepines were associated with an increased risk of pneumonia, COPD with acute exacerbation, ARF, and cardiopulmonary arrest.
TABLE 3.
The Relationship Between Hypnotics Use and Various Adverse Respiratory Events
DISCUSSION
This case-control study indicated that the active users of benzodiazepines exhibited a 9.30-fold adjusted OR of pneumonia, 10.40-fold adjusted OR of acute exacerbation, 45.00-fold adjusted OR of ARF, and 18.60-fold adjusted OR of cardiopulmonary arrest compared with the participants in the control group. Vozoris et al21 used Ontario provincial health administrative databases, showing that elderly patients with COPD receiving benzodiazepine therapy had a 1.45-fold risk of respiratory exacerbation and a 1.92-fold risk of emergency room visits for COPD or pneumonia compared with nonusers. The discrepancy regarding the adverse respiratory risk between the present study and the Canadian study may be associated with racial differences.
The respiratory suppression by the nonbenzodiazepine hypnotics remained inconsistent.22–24 Therefore, we further analyzed the effect of nonbenzodiazepine hypnotics on the adverse respiratory events in the patients with COPD. In the active users, a considerable effect of the nonbenzodiazepine hypnotics on adverse respiratory events was observed: an 11.30-fold adjusted OR of pneumonia, 6.01-fold adjusted OR of acute exacerbation, 22.30-fold adjusted OR of ARF, and 7.41-fold adjusted OR of cardiopulmonary arrest, compared with the participants in the control group. The patients with COPD and adverse respiratory events had higher occurrences of comorbid medical diseases than the participants in the control group did. After adjusting for the potential covariates, use of benzodiazepine and nonbenzodiazepine hypnotics remained an independent risk factor for adverse respiratory events.
Sleep disturbances in the patients with COPD may be associated with coughing, excessive mucus production, dyspnea, and nocturnal oxygen desaturation.25,26 Hypoventilation causes gas-exchange alteration in patients with COPD, leading to hypercapnia and hypoxemia, particularly during REM sleep when marked respiratory muscle atonia occurs. The hypoxemia can lead to more arousals and sleep disruption.27 Many patients with COPD may have concomitant obstructive sleep apnea syndrome, which aggravates hypoxemia and hypercapnia during sleep. Therefore, to eliminate coexisting sleep-related breathing disorders, polysomnography should be considered for patients with COPD and considerable daytime hypercapnia.27
Although benzodiazepines and nonbenzodiazepine hypnotics effectively improve sleep quality, they may suppress respiratory function and decrease oxygen saturation.28,29 Previous studies have found that hypnotics suppress respiratory function by depressing the central respiratory drive, decreasing respiratory muscle strength, and increasing upper airway resistance.28,30
Insomnia is highly prevalent in patients with COPD.6 Thus, managing sleep-related problems for these patients is crucial. Nocturnal oxygen supplement may improve sleep quality in patients with COPD.31 However, the American Thoracic Society does not recommend nocturnal oxygen therapy for isolated nocturnal hypoxemia, except in cases of hypoxemia complications, such as polycythemia or cor pulmonale.32 Although theophyllines may improve gas exchange and arterial oxygen saturation (SaO2), sleep quality may be impaired.33 Anticholinergic agents such as ipratropium bromide improve sleep SaO2 and sleep quality in patients with moderate-to-severe COPD.34 Clinicians may also consider nonpharmacological strategies, such as sleep hygiene, relaxation techniques, biofeedback, and cognitive behavior therapy, for patients with COPD experiencing insomnia.28,35,36
The risk of adverse respiratory events increased with the increase in CCI scores, a finding that is consistent with those of previous studies.37,38 The CCI may be a valid tool for predicting patient outcomes. In addition, congestive heart failure, alcohol-related illness, and dementia were the independent risk factors of adverse respiratory events in the patients with COPD. These findings emphasize the importance of reducing the potential risk in patients with COPD by involving a multidisciplinary team and an integrated approach to intervention.
The population-based study’ strength is to analyze the association between hypnotics and an increased risk of adverse respiratory events in patients with COPD. In Taiwan, NHI is unique and obligatory. Therefore, every Taiwanese has the medical insurance to pay the prescriptions’ cost. We can have the detailed data for every study participant's medications. In addition, each insurant has own identification number. Therefore, we can trace and follow-up every study subject in the database. We think the analyses should be trusted due to many multivariable models were done to assure the risk to develop adverse respiratory events. Of course, there are some study limitations when we interpret these results. In the NHIRD, there are no available detailed data about the patients’ symptoms (ie, evaluation of breathless and COPD). Therefore, we cannot provide COPD's severity according to the Global Initiative for COPD.39
In conclusion, we found that benzodiazepine and nonbenzodiazepine hypnotics, especially in active users (0–30 days), is associated with an increased risk of adverse respiratory events (including adverse respiratory events, including pneumonia, COPD with acute exacerbation, acute respiratory failure, and cardiopulmonary arrest) in these COPD patients. This increased risk of adverse respiratory events emphasizes that evaluating the current insomnia treatments with hypnotics for these COPD patients is necessary and important. Clinicians should keep alert of the potential risk of adverse respiratory events in these COPD patients before prescribing hypnotics. However, further studies remain necessary to prove the causal relationship.
Acknowledgments
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Footnotes
Abbreviations: ATC = Anatomical Therapeutic Chemical, CCI score = Charlson comorbidity index score, CI = confidence interval, COPD = chronic obstructive pulmonary disease, ICD-9-CM = the International Classification of Diseases, Ninth Revision, Clinical Modification, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, OR = odds ratio, QOL = quality of life, REM = rapid eye movement.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
W-SC and C-YL contributed equally to this work.
Author contributions: All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript—Conception/design: W-SC, C-HK; Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript: all authors.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ohayon MM, Hong SC. Prevalence of insomnia and associated factors in South Korea. J Psychosom Res 2002; 53:593–600. [DOI] [PubMed] [Google Scholar]
- 2.Mai E, Buysse DJ. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin 2008; 3:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung WS, Lin CL, Chen YF, et al. Sleep disorders and increased risk of subsequent acute coronary syndrome in individuals without sleep apnea: a nationwide population-based cohort study. Sleep 2013; 36:1963–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallander MA, Johansson S, Ruigomez A, et al. Morbidity associated with sleep disorders in primary care: a longitudinal cohort study. Prim Care Companion J Clin Psychiatry 2007; 9:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klink M, Quan SF. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseases. Chest 1987; 91:540–546. [DOI] [PubMed] [Google Scholar]
- 6.Budhiraja R, Parthasarathy S, Budhiraja P, et al. Insomnia in patients with COPD. Sleep 2012; 35:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellia V, Catalano F, Scichilone N, et al. Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA study. Sleep 2003; 26:318–323. [DOI] [PubMed] [Google Scholar]
- 8.Cormick W, Olson LG, Hensley MJ, et al. Nocturnal hypoxaemia and quality of sleep in patients with chronic obstructive lung disease. Thorax 1986; 41:846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valipour A, Lavie P, Lothaller H, et al. Sleep profile and symptoms of sleep disorders in patients with stable mild to moderate chronic obstructive pulmonary disease. Sleep Med 2011; 12:367–372. [DOI] [PubMed] [Google Scholar]
- 10.Leger D, Morin CM, Uchiyama M, et al. Chronic insomnia, quality-of-life, and utility scores: comparison with good sleepers in a cross-sectional international survey. Sleep med 2012; 13:43–51. [DOI] [PubMed] [Google Scholar]
- 11.Hynninen MJ, Pallesen S, Hardie J, et al. Insomnia symptoms, objectively measured sleep, and disease severity in chronic obstructive pulmonary disease outpatients. Sleep Med 2013; 14:1328–1333. [DOI] [PubMed] [Google Scholar]
- 12.Putman-Casdorph H, McCrone S. Chronic obstructive pulmonary disease, anxiety, and depression: state of the science. Heart Lung 2009; 38:34–47. [DOI] [PubMed] [Google Scholar]
- 13.Siriwardena AN, Apekey T, Tilling M, et al. General practitioners’ preferences for managing insomnia and opportunities for reducing hypnotic prescribing. J Eval Clin Pract 2010; 16:731–737. [DOI] [PubMed] [Google Scholar]
- 14.Glass J, Lanctot KL, Herrmann N, et al. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ 2005; 331:1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine use among older adults with chronic obstructive pulmonary disease: a population-based cohort study. Drugs Aging 2013; 30:183–192. [DOI] [PubMed] [Google Scholar]
- 16.National Health Insurance Administration, Ministry of Health and Welfare. National Health Insurance Administration. 2014. http://www.nhi.gov.tw/english/index.aspx Accessed October 21, 2014 [Google Scholar]
- 17.Chung WS, Lin CL, Ho FM, et al. Asthma increases pulmonary thromboembolism risk: a nationwide population cohort study. Eur Respir J 2014; 43:801–807. [DOI] [PubMed] [Google Scholar]
- 18.Shen TC, Chung WS, Lin CL, et al. Does chronic obstructive pulmonary disease with or without type 2 diabetes mellitus influence the risk of lung cancer? Result from a population-based cohort study. PLoS One 2014; 9:e98290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–619. [DOI] [PubMed] [Google Scholar]
- 21.Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2014; 44:332–340. [DOI] [PubMed] [Google Scholar]
- 22.Mendelson WB, Martin JV, Rapoport DM. Effects of buspirone on sleep and respiration. Am Rev Respir Dis 1990; 141:1527–1530. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XJ, Li QY, Wang Y, et al. The effect of non-benzodiazepine hypnotics on sleep quality and severity in patients with OSA: a meta-analysis. Sleep Breath 2014; 18:781–789. [DOI] [PubMed] [Google Scholar]
- 24.Wagner J, Wagner ML. Non-benzodiazepines for the treatment of insomnia. Sleep Medicine Rev 2000; 4:551–581. [DOI] [PubMed] [Google Scholar]
- 25.Roth T. Hypnotic use for insomnia management in chronic obstructive pulmonary disease. Sleep Med 2009; 10:19–25. [DOI] [PubMed] [Google Scholar]
- 26.McNicholas WT, Verbraecken J, Marin JM. Sleep disorders in COPD: the forgotten dimension. Eur Respir Rev 2013; 22:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gay PC. Chronic obstructive pulmonary disease and sleep. Respir Care 2004; 49:39–51.discussion 2.. [PubMed] [Google Scholar]
- 28.Stege G, Vos PJ, van den Elshout FJ, et al. Sleep, hypnotics and chronic obstructive pulmonary disease. Respir Med 2008; 102:801–814. [DOI] [PubMed] [Google Scholar]
- 29.Cohn MA, Morris DD, Juan D. Effects of estazolam and flurazepam on cardiopulmonary function in patients with chronic obstructive pulmonary disease. Drug Saf 1992; 7:152–158. [DOI] [PubMed] [Google Scholar]
- 30.Beaupre A, Soucy R, Phillips R, et al. Respiratory center output following zopiclone or diazepam administration in patients with pulmonary disease. Respiration 1988; 54:235–240. [DOI] [PubMed] [Google Scholar]
- 31.Calverley PM, Brezinova V, Douglas NJ, et al. The effect of oxygenation on sleep quality in chronic bronchitis and emphysema. Am Rev Respir Dis 1982; 126:206–210. [DOI] [PubMed] [Google Scholar]
- 32.Sleep problems in COPD. American Thoracic Society. 2014. Accessed September 25, 2014. [Google Scholar]
- 33.Mulloy E, McNicholas WT. Theophylline improves gas exchange during rest, exercise, and sleep in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1993; 148:1030–1036. [DOI] [PubMed] [Google Scholar]
- 34.Martin RJ, Bartelson BL, Smith P, et al. Effect of ipratropium bromide treatment on oxygen saturation and sleep quality in COPD. Chest 1999; 115:1338–1345. [DOI] [PubMed] [Google Scholar]
- 35.Morin CM, Colecchi C, Stone J, et al. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA 1999; 281:991–999. [DOI] [PubMed] [Google Scholar]
- 36.Kapella MC, Herdegen JJ, Perlis ML, et al. Cognitive behavioral therapy for insomnia comorbid with COPD is feasible with preliminary evidence of positive sleep and fatigue effects. Int J Chron Obstruct Pulmon Dis 2011; 6:625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis 2001; 37:337–342. [DOI] [PubMed] [Google Scholar]
- 38.Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg 2004; 8:1061–1067. [DOI] [PubMed] [Google Scholar]
- 39.Global strategy for the diagnosis, management, and prevention of COPD updated 2014. GOLD committees. 2014. Accessed October 17, 2014. [Google Scholar]


