Abstract
There are limited population-based studies on the progress of oseltamivir therapy for influenza infection.
Using insurance claims data of 2005, 2009, and 2010, the authors established an “in-time” cohort and a “lag-time” cohort representing influenza patients taking the medicine within and not within 1 week to examine the treatment progress. Incident outpatient visit, emergency care and hospitalization, and fatality were compared between the 2 cohorts in the first week and the second week of follow-up periods, after the oseltamivir therapy.
A total of 112,492 subjects diagnosed with influenza on oseltamivir therapy in 2005, 2009, and 2010 were identified. The multivariate logistic regression analysis showed that the in-time treatment was superior to the lag-time treatment with less repeat outpatient visits, hospitalizations, and fatality. The overall corresponding in-time treatment to lag-time treatment odds ratios (OR) were 0.50, 0.54, and 0.71 (all P value < 0.05), respectively. The in-time to lag-time ORs of all events were 0.50 in 2009 and 0.54 in 2010.
Our study demonstrates that the in-time oseltamivir therapy leads to significantly better treatment outcomes. Oseltamivir should be administered as early as the onset of influenza symptoms appears.
INTRODUCTION
Oseltamivir and zanamivir are drugs recommended by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (USCDC) are the first-line antiviral drug for patients infected with influenza A and B.1–3 The WHO guidelines recommended that patients with diagnosed or suspected influenza should be treated with antiviral medicine as soon as possible.2 The USCDC also recommended to treat patients with confirmed or suspected influenza with antiviral agents as soon as possible.3 Previous studies on the efficacy of antiviral medication used randomized controlled trials targeting patients with laboratory confirmed influenza infection.4–6 The study results consistently support that oseltamivir is clinically effective for the treatment of influenza and further for the prevention of pneumonia.4–6 Oseltamivir may provide symptomatic relief for patients with influenza infection, reduce viral excretion from the nose, and interrupt the household viral transmission. In addition, oseltamivir reduces the symptoms of flu, antibiotic uses, complications such as bronchitis and pneumonia, and death.7–13 The oseltamivir treatment may reduce nearly 60% of the secondary spread of influenza in family members who have contacted with the patient.14
An estimated 45 million patients have received the antiviral treatment worldwide by 2013, since 2005.15 A retrospective cohort study using a large health data of the US found that oseltamivir treatment twice daily could reduce pneumonia risk by 32%.14 A hospital-based study has proved that the oseltamivir treatment effectiveness for patients receiving treatment in time is superior than the patients with delayed antiviral administration.16
Although oseltamivir is known as an effective medicine for influenza treatment, comparing outcomes and adverse responses between prompt initiation and delayed antiviral administration of this medicine has not been well examined for Asian population. This study took the advantage of population data available in the Taiwan's National Health Insurance to perform a retrospective cohort study. We evaluated the efficacy of oseltamivir therapy for patients diagnosed with influenza infection. Patients with oseltamivir therapy within 1 week were compared to those with the therapy beyond 1 week for treatment progress, evaluated by additional outpatient visits, emergency uses, hospitalization, and mortality during a 2-week follow-up period.
METHODS
Database
This study used claims data of the National Health Insurance Research Dataset (NHIRD) from 2000 to 2010, provided by the National Health Research Institutes in Taiwan. This large and comprehensive population-based health data set covers over 23,275,000 people in Taiwan. The diagnoses were coded with the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) for insurance claims registered. This study was approved by the research ethics committee at China Medical University and Hospital.
Study Cohorts and Outcomes Observation
We identified all adult patients with the principal diagnosis of influenza (ICD-9-CM480–487) and prescription of oseltamivir during influenza seasons in 2005, 2009, and 2010 (Figure 1). This medicine was used in 2005 for an influenza prevention trial mainly for the elderly diagnosed with influenza, and has been implemented for all eligible patients since 2009. From the prescription records available in the claims data, we ascertained the usage of oseltamivir for the specific date, dose, and route of every prescription in years 2005, 2009, and 2010. The date a patient initiated with the oseltamivir prescription was designated as the index day. Patients clinically newly diagnosed with influenza by physician and initiated the oseltamivir prescription within 1 week were designated into the in-time cohort. Patients who were diagnosed with influenza and started taking oseltamivir beyond 1 week of the diagnosis were designated as the lag-time cohort. We traced all patients prescribed with oseltamivir for a 2-week follow-up period, to observe the treatment progress, using additional outpatient visit, emergency use, hospitalization, and death as study outcomes. The first period (week 1) was from the eighth day to the 14th day following the oseltamivir prescription. The week 2 period began immediately after the completion of the preceding week 1 follow-up (ie, from the 15th day to 21st day). A 2-week follow-up period was designed to observe a short-term progress of the treatments. Patients who had taken oseltamivir and sought further medical services, including outpatient visit, emergency use or hospitalization, or had encountered death were identified weekly from the claims data during the 2-week follow-up period. These events were designated as adverse progressions.
FIGURE 1.
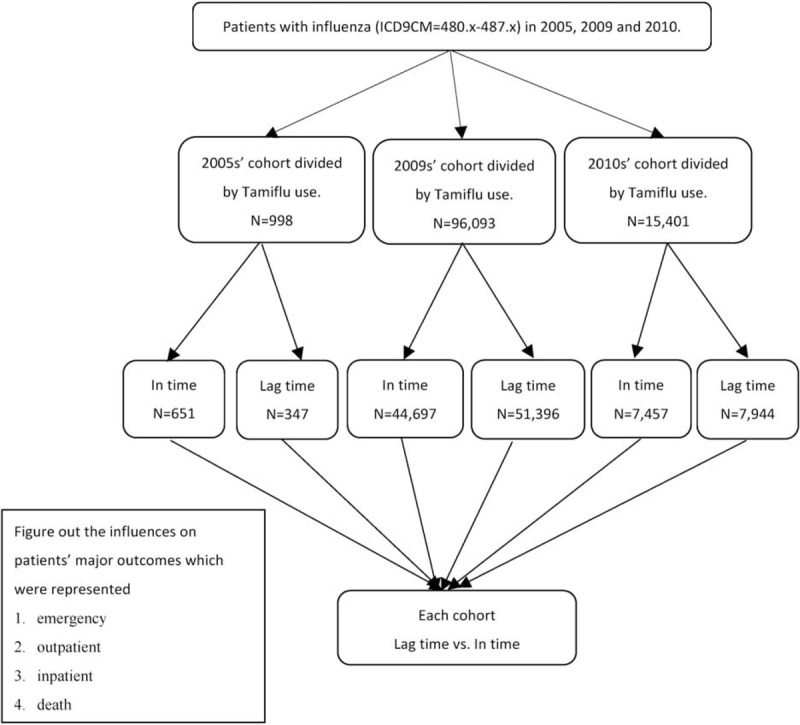
Flow chart of study design.
Statistical Analysis
Based on dates with influenza diagnosed and oseltamivir prescribed, patients were divided into “in-time” and “lag-time” cohorts, by the study year of 2005, 2009, and 2010. To determine whether patient characteristics were similar between the 2 prescription times for oseltamivir, we compared distributions of sex and age. The 2-week follow-up period-specific frequencies of outpatient visit, emergency use, hospitalization, and death were calculated for oseltamivir users in the in-time cohort and the lag-time cohort by study year. We used the Mantel-Haenszel method to calculate the in-time cohort to lag-time cohort odds ratio (OR) and 95% confidence interval (CI) of each event. We further used the multivariable logistic regression model to estimate the ORs of repeat outpatient visits, hospitalization, and death associated with in-time and lag-time treatments by age and sex in all 3 years combined. Similar data analysis method was also performed to estimate ORs of all events combined for 2009 and 2010 to evaluate whether the treatment effectiveness changed in 2010. The SAS software for Windows, Version 9.3 (SAS Institute Inc., Cary, NC) was used for data analyses with the 2-sided P value of 0.05 considered statistically significant.
RESULTS
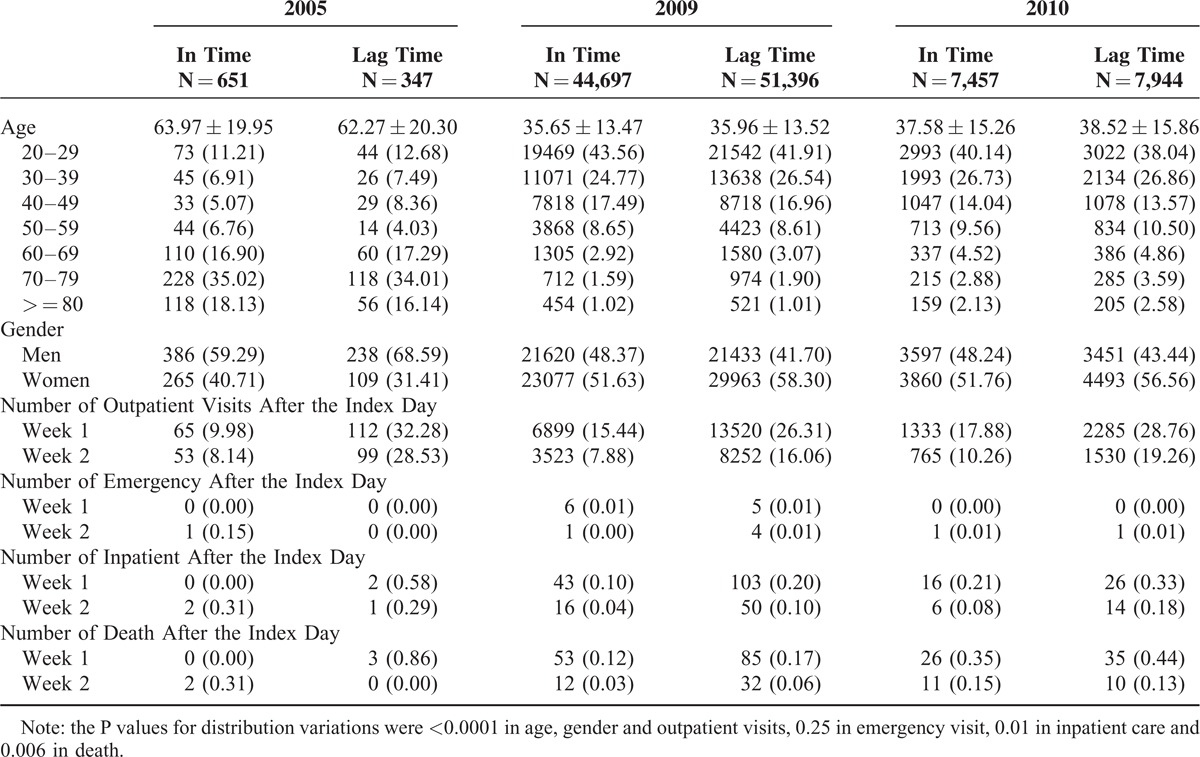
Among patients clinically diagnosed with influenza, there were 998 cases in 2005, 96,093 cases in 2009, and 15,401 cases in 2010 on the oseltamivir therapy (Table 1). Influenza patients in 2005 were much older than those in 2009 and 2010. There were more in-time therapy patients than lag-time therapy patients in 2005, but not in 2009 and 2010. After taking the medicine, outpatient visits declined weekly. The outpatient visits in week 1 follow-up were 3.2-fold more frequent in the lag-time cohort than in the in-time cohort (32.3 versus 9.98%) in 2005. The difference in outpatient visits between the lag-time cohort and the in-time cohort was reduced in 2009 (26.3 versus 15.4%, respectively) and in 2010 (28.8 versus 17.9%, respectively). Similar trends remained in week 2 follow-up. The incidences of hospitalization and the mortality were lower in the in-time cohort than in the lag-time cohort during the 2-week period. The hospitalization rate and mortality were greater in 2005.
TABLE 1.
Baseline Characteristics of Patients Among 3 Cohorts
Among patients with oseltamivir therapy in the 3 years combined, 9502 (18.0%) in the in-time cohort and 18,174 (30.4%) in the lag-time cohort had repeat outpatient visits (Table 2). The Cochran-Mantel-Haenszel analysis estimated overall OR of repeat outpatient visits in patients with in-time therapy was 0.50 (95% CI = 0.49–0.52, P < 0.0001), comparing to lag-time therapy. The corresponding ORs of hospitalization and death for patients with in-time therapy were 0.54 (95% CI = 0.37–0.62, P < 0.0001) and 0.71(95% CI = 0.56–0.91, P < 0.0001), respectively. The oseltamivir therapy was consistently superior in the in-time cohort than in the lag-time cohort among study years, age groups, and both sexes. The age-specific results showed that the OR decreased as age increased, from 0.60 (95% CI = 0.58–0.63, P < 0.0001) in 20 to 29 years old to 0.34 (95% CI = 0.29–0.41, P < 0.0001) in 70 to 79 years old. The age-specific OR pattern of repeat outpatient visits, however, did not appear in hospitalization and death. The reduction in hospitalization risk remained significant for most age groups. Reductions in outpatient visits and hospitalization were similar in men and women, while the reduction in mortality risk was significant for men, but not for women.
TABLE 2.
Cochran-Mantel-Haenszel Analysis Estimated Odds Ratio of Events for 3-Year Combined In-Time Therapy Cohort Compared with Lag-Time Therapy
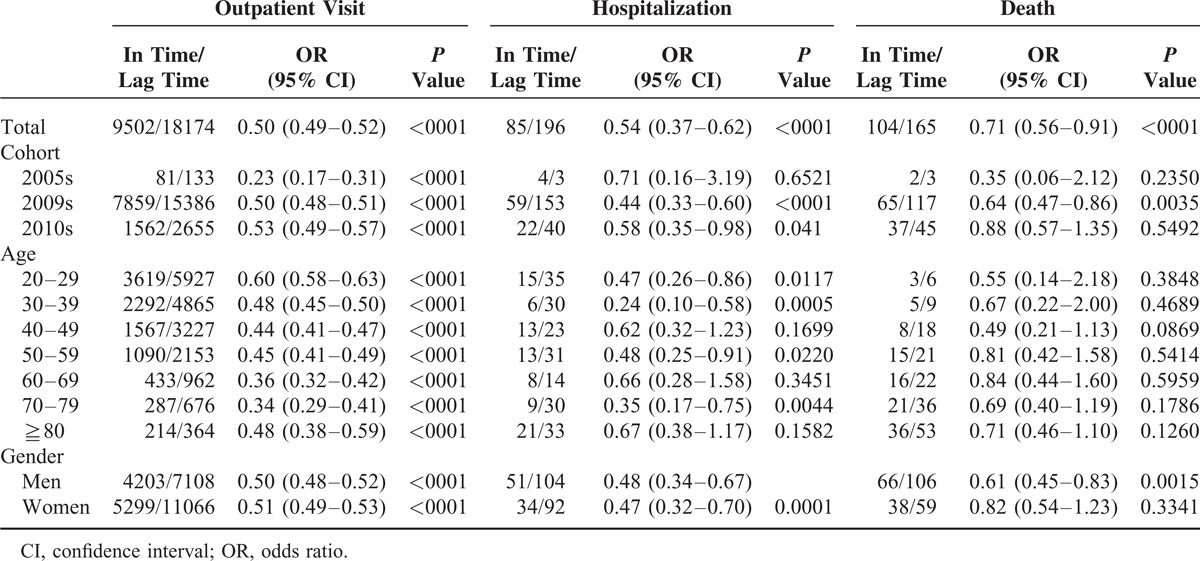
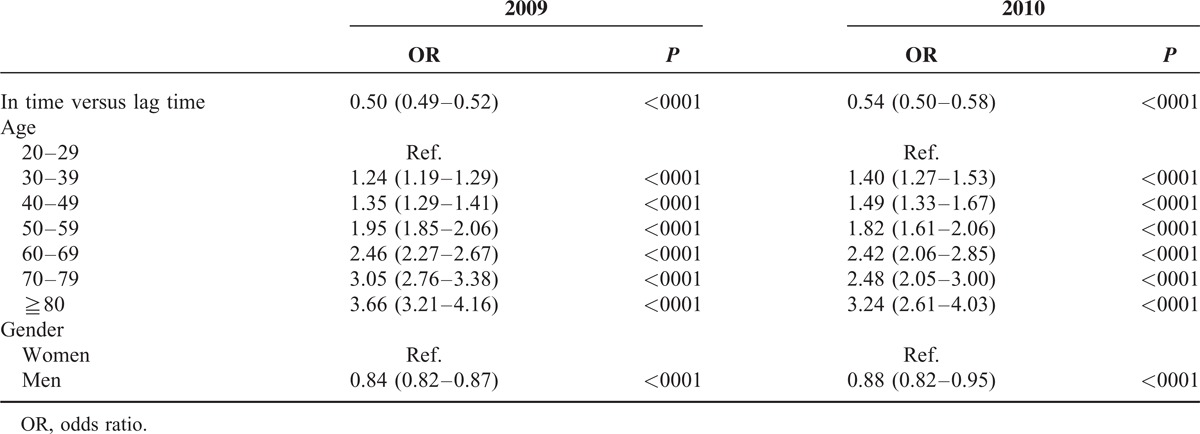
Table 3 shows in-time relative to lag-time ORs of repeat outpatient visits, hospitalization and death associated with age and sex. The risk of repeat outpatient visits, hospitalization or death increased with age. Compared with patients 20 to 29 years old, the OR of death was increased to 307 (95% CI = 154–611, P < 0.0001) in patients aged 80 years and above. Men were at lower risk of repeat outpatient visits (OR = 0.84, 95% CI = 0.82–0.86) but at higher risk of hospitalization (OR = 1.39, 95% CI = 1.1–1.77) or death (OR = 1.83, 95% CI = 1.41–2.36). Table 4 shows that the superior effectiveness of the in-time oseltamivir therapy relative to lag-time for all events changed slightly between 2009 and 2010, with overall ORs of 0.50 (95% CI = 0.49–0.52) and 0.54(95% CI = 0.50–0.58), respectively.
TABLE 3.
Logistic Regression Analysis Estimated In-Time to Lag-Time ORs of Repeat Outpatient Visit, Hospitalization and Death, and ORs Among Age Groups and Between Men and Women

TABLE 4.
Logistic Regression Analysis Estimated In-Time to Lag-Time ORs of Repeat Outpatient Visit, Hospitalization and Death, and ORs Among Age Groups and Between Men and Women in 2009 and 2010
DISCUSSION
Oral oseltamivir is a well-tolerated and effective antiviral drug for influenza patients of all ages, even for those with comorbidities of respiratory diseases and/or chronic cardiac disease.17 Population-based study on antiviral efficacy has been limited to retrospective cohort study because it is difficult to perform a randomized population trial.14–16 Our study used insurance claims data to perform another retrospective cohort study. The results showed that oseltamivir treatment was also effective for Asian patients with influenza infection of all ages in 2005, 2009, or 2010. Previous studies emphasized in evaluating whether the oseltamivir treatment reduced lower respiratory tract complications, antibiotic use and/or hospitalization, alleviated symptoms, or reduced mortality.8,18–21 An earlier double blind prospective trial involving 3564 flu patients concluded that the oseltamivir treatment could reduce lower respiratory tract complications, antibiotic use, and hospitalization.8
Studies recommended early treatment with oseltamivir for patients with influenza A (H1N1 and H5N1).21–23 Delayed oseltamivir treatment increases the lung involvement.24 An UK study found that in influenza patients treated with oseltamivir 75 mg within 24 hours of symptom onset, the duration of symptoms was 43 hours shorter than placebo controls.18 A Japanese study found that pediatric patients with 2009 H1N1 infection treated within 48 hours of symptom onset with neuraminidase inhibitors, primarily oseltamivir, could reduce case fatality to 0.1%.21 Nursing home and hospital studies on oseltamivir therapy also showed that the in-time treatment reduced the disease spread, duration of illness, level of viral shedding, and respiratory failure.25–30
Our study defined oseltamivir treatment within 1 week of symptom onset as the prompt in-time treatment. The in-time treatment was consistently superior to the lag-time treatment in the reduction of adverse events; there were overall 50% (OR = 0.50) reduction in repeat outpatient visits, 46% (OR = 0.54) reduction in hospitalization, and 29% (OR = 0.71) reduction in mortality within the 2-week follow-up period (Table 2). The superior effects of the in-time treatment appeared in each study year, each age group, males, and females. The stratified analysis, however, showed that the fatality reduction was significant only in 2009 and for men. We were unable to observe a significant trend for the emergency admission due to small sample size.
Studies have reported that the 2008 to 2009 influenza A (H1N1) viruses exhibited decreased susceptibility to oseltamivir.30–32 Using influenza surveillance network laboratories study of the Taiwan Centers for Disease Control, Yang et al found that the oseltamivir-resistant influenza virus had developed in Taiwan during the pandemic outbreak.30,32 The trend of oseltamivir resistance also appeared noticeable in our study results of 2009 and 2010. Our data showed that the outpatient visit, hospitalization, and mortality were higher in 2010, especially for the patients receiving delayed oseltamivir treatment. We conducted a further analysis to compare all the events using the study year as a covariate. Compared with 2005, the adjusted ORs of having adverse events were 1.81 (95% CI = 1.55–2.11) in 2009 and 2.04 (95% CI = 1.74–2.38) in 2010 (data not shown). The overall in-time to lag-time treatment OR of all events, however, changed slightly from 0.50 in 2009 to 0.54 in 2010, indicating that the early oseltamivir treatment remains an effective choice for interrupting seasonal influenza. The oseltamivir-resistant virus might be slightly happening in 2010 in Taiwan's population.
This study is an example using population-based insurance claims data to evaluate the treatment progress of prompt initiation of oseltamivir therapy for reducing repeat outpatient visits, hospitalizations, and mortality in an Asian population. In addition to a large number of study population, we examined a wide range of age groups, including the elderly. Unlike clinical trials, this natural history study was able to demonstrate that an earlier antiretroviral administration reduced near half of odds for outpatient visits and hospitalization.
Our study has a number of limitations. First, the nature of retrospective study has an inherent limitation of selection bias. Patients administered with oseltamivir usually suffered from severe influenza symptoms. Thus, our study results may not be applicable to patients with mild symptoms. Second, Taiwan government provided free oseltamivir to patients only in 2005, 2009, and 2010, we therefore constrained the current study using data available for these 3 years. Nevertheless, our study results show that oseltamivir is effective and useful to relieve influenza symptoms, especially for the in-time cohort. The clinical features show that patients in the in-time cohort usually suffer from more severe symptoms at the onset of influenza than those who are in the lag-time cohort. If early oseltamivir treatment is more effective for patients with severe symptoms than those with mild symptoms, such treatment should be more significant for patients with severe symptoms. Third, some personal factors such as body mass index, lifestyle, and family medical history were not considered in our study because of lack of related information. Finally, we only identified the patients infected with influenza, but we did not analyze the subtype to which the influenza belonged to. Future studies can be conducted to examine the treatment effectiveness of oseltamivir on the subtypes of influenza.
CONCLUSIONS
Oseltamivir therapy was initiated with a small size trial for mainly elderly patients in 2005 in Taiwan, which resulted successful effectiveness on reducing outpatient visit, hospitalization, and mortality. Oseltamivir also exhibits superior effectiveness in reducing outpatient visit, hospitalization, and mortality when much larger sizes of patients were treated promptly in 2009 and 2010, although minor variant in drug resistance was noted from 2009 to 2010. This national data with large sample size obviously shows that oseltamivir is an effective medicine until now, especially in reducing the reoutpatient visit rates for patients of all ages.
Acknowledgments
All authors would like to thank the Taiwan National Health Research Institutes for providing the NHI database.
Footnotes
Abbreviations: ICD-9-CM = International Classification of Disease, Ninth Revision, Clinical Modification, OR and 95% CI = odds ratio and 95% confidence interval, USCDC = US Centers for Disease Control and Prevention, WHO = World Health Organization.
Author Contributions: Sung FC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Wang CB, Chiu ML, Lin PC, Chang YJ, Sung FC.
Acquisition of data: Chen CY, Wu TN, Sung FC.
Analysis and interpretation of data: Wang CB, Chiu ML, Lin PC, Liang WM, Wang JH, Sung FC.
Drafting of the manuscript: Wang CB, Chiu ML, Lin PC, Sung FC.
Critical revision of the manuscript for important intellectual content: Wang CB, Chiu ML, Lin PC, Sung FC.
Statistical analysis: Wang CB, Liang WM, Chang YJ, Sung FC.
Administrative, technical, or material support: Wang CB, Chiu ML, Lin PC, Liang WM, Chang YJ, Sung FC.
Study supervision: Wang CB, Lin PC, Liang WM, Chen CY, Wu TN, Wang JH, Sung FC.
Wang CB and Chiu ML have equal contribution.
Role of the Sponsor: None of the funders had a role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding/Support: The current study was supported by the National Sciences Council, Executive Yuan (grant numbers NSC 100–2621-M-039–001), China Medical University Hospital (grant number 1MS1), Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212–113002). China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103–2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212–124–002, Taiwan)
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Centers for Disease Control and Prevention. Influenza Antiviral Drug Resistance 2013. http://www.cdc.gov/flu/about/qa/antiviralresistance.html Accessed 17 June, 2013 [Google Scholar]
- 2.WHO Considerations for assessing the severity of an influenza pandemic. Wkly Epidemiol Rec 2009; 84:197–202. [PubMed] [Google Scholar]
- 3.Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep 2011; 60:1–24. [PubMed] [Google Scholar]
- 4.Cooper NJ, Sutton AJ, Abrams KR, et al. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. Brit Med J 2003; 326:1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner D, Wailoo A, Nicholson K, et al. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technol Assess 2003; 7:1–170.xi-xiii. [DOI] [PubMed] [Google Scholar]
- 6.Yu HJ, Liao QH, Yuan YA, et al. Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. Brit Med J 2010; 341:c4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee VJ, Yap J, Cook AR, et al. Oseltamivir ring prophylaxis for containment of 2009 H1N1 influenza outbreaks. New Engl J Med 2010; 362:2166–2174. [DOI] [PubMed] [Google Scholar]
- 8.Kaiser L, Wat C, Mills T, et al. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med 2003; 163:1667–1672. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence. Influenza-zanamivir, amantadine and oseltamivir (review) (TA168). Technology appraisals TA168 2009 http://guidance.nice.org.uk/TA168/ Accessed 17 June 2013 [Google Scholar]
- 10.Jefferson T, Demicheli V, Rivetti D, et al. Antivirals for influenza in healthy adults: systematic review. Lancet 2006; 367:303–313. [DOI] [PubMed] [Google Scholar]
- 11.Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza-A randomized controlled trial. J Am Med Assoc 2000; 283:1016–1024. [DOI] [PubMed] [Google Scholar]
- 12.Hayden FG, Treanor JJ, Fritz RS, et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza - Randomized controlled trials for prevention and treatment. J Am Med Assoc 1999; 282:1240–1246. [DOI] [PubMed] [Google Scholar]
- 13.Welliver R, Monto AS, Carewicz O, et al. Effectiveness of oseltamivir in preventing influenza in household contacts - A randomized controlled trial. J Am Med Assoc 2001; 285:748–754. [DOI] [PubMed] [Google Scholar]
- 14.Smith JR, Dutkowski R. Stockpiling oseltamivir: Roche clarifies data for improved mortality with oseltamivir. Brit Med J 2005; 331:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parry RL. Japan issues Tamiflu warning after child deaths. 2007; http://www.infowars.net/articles/march2007/210307_b_Tamiflu.htm Accessed 17 June 2013 [Google Scholar]
- 16.Ebell MH, Call M, Shinholser J. Effectiveness of oseltamivir in adults: a meta-analysis of published and unpublished clinical trials. Fam Pract 2013; 30:125–133. [DOI] [PubMed] [Google Scholar]
- 17.Singh S, Barghoorn J, Bagdonas A, et al. Clinical benefits with oseltamivir in treating influenza in adult populations: results of a pooled and subgroup analysis. Clin Drug Investig 2003; 23:561–569. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355:1845–1850. [DOI] [PubMed] [Google Scholar]
- 19.Orzeck EA, Shi N, Blumentals WA. Oseltamivir and the risk of influenza-related complications and hospitalizations in patients with diabetes. Clinical therapeutics 2007; 29:2246–2255. [DOI] [PubMed] [Google Scholar]
- 20.Blumentals WA, Schulman KL. Impact of oseltamivir on the incidence of secondary complications of influenza in adolescent and adult patients: results from a retrospective population-based study. Curr Med Res Opin 2007; 23:2961–2970. [DOI] [PubMed] [Google Scholar]
- 21.Sugaya N, Shinjoh M, Mitamura K, et al. Very low pandemic influenza A (H1N1) 2009 mortality associated with early neuraminidase inhibitor treatment in Japan: analysis of 1000 hospitalized children. J Infect 2011; 63:288–294. [DOI] [PubMed] [Google Scholar]
- 22.Lee IK, Liu JW, Wang L, et al. 2009 pandemic Influenza A (H1N1): clinical and laboratory characteristics in pediatric and adult patients and in patients with pulmonary involvement. Influenza Other Respir Viruses 2012; 6:e152–e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beigel H, Farrar H, Han AM, et al. Current concepts - Avian influenza A (H5N1) infection in humans. New Engl J Med 2005; 353:1374–1385. [DOI] [PubMed] [Google Scholar]
- 24.Van Kerkhove MD, Vandemaele KA, Shinde V, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med 2011; 8:e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang YM, Li WC, Huang CT, et al. Use of oseltamivir during an outbreak of influenza A in a long-term care facility in Taiwan. J Hosp Infect 2008; 68:83–87. [DOI] [PubMed] [Google Scholar]
- 26.Chien YS, Su CP, Tsai HT, et al. Predictors and outcomes of respiratory failure among hospitalized pneumonia patients with 2009 H1N1 influenza in Taiwan. J Infection 2010; 60:168–174. [DOI] [PubMed] [Google Scholar]
- 27.Aoki FY, Boivin G. Influenza virus shedding; excretion patterns and effects of antiviral treatment. J Clin Virol 2009; 44:255–261. [DOI] [PubMed] [Google Scholar]
- 28.Burch J, Corbett M, Stock C, et al. Prescription of anti-influenza drugs for healthy adults: a systematic review and meta-analysis. Lancet Infect Dis 2009; 9:537–545. [DOI] [PubMed] [Google Scholar]
- 29.Cao B, Li XW, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. New Engl J Med 2009; 361:2507–2517. [DOI] [PubMed] [Google Scholar]
- 30.Yang JR, Lin YC, Huang YP, et al. Reassortment and mutations associated with emergence and spread of oseltamivir-resistant seasonal influenza A/H1N1 viruses in 2005–2009. PloS one 2011; 6:e18177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Vries E, Stelma FF, Boucher CAB. Emergence of a multidrug-resistant pandemic influenza A (H1N1) virus. New Engl J Med 2010; 363:1381–1382. [DOI] [PubMed] [Google Scholar]
- 32.Yang JR, Huang YP, Lin YC, et al. Early findings of oseltamivir-resistant pandemic (H1N1) 2009 influenza A viruses in Taiwan. Antivir Res 2010; 88:256–262. [DOI] [PubMed] [Google Scholar]


