Supplemental Digital Content is available in the text
Abstract
Statin intake has been reported to reduce the risk of several malignancies beyond its cholesterol-lowering effects. However, little is known regarding the survival benefit of statins for patients with colorectal cancer (CRC).
We conducted a systematic literature search of multiple databases for studies published before November 2014, which investigated associations between statin intake and CRC prognosis. Meta-analysis was performed using random-effects model. The primary outcomes of interest were all-cause mortality (ACM) and cancer-specific mortality (CSM).
Ten studies involving 76,851 patients were eligible for this meta-analysis, with 7 studies investigating prediagnosis statin use and 5 studies reporting postdiagnosis statin use. Prediagnosis statin use was associated with reduced ACM (hazard ratio [HR] 0.73, 95% confidence interval [CI] 0.61–0.88, P = 0.001) and CSM (HR 0.80, 95% CI 0.77–0.84, P < 0.001) for patients with CRC. This effect persisted when stratified by tumor site and in studies adjusted by nonsteroidal anti-inflammatory drug use. In addition, postdiagnosis statin use was associated with decreased CSM (HR 0.70, 95% CI 0.60–0.82, P < 0.001). However, we did not note reduced ACM for postdiagnosis statin use (HR 0.93, 95% CI 0.68–1.27, P = 0.639). There appeared to be an association between postdiagnosis statin use and increased ACM in KRAS-mutated CRC.
Our findings provide evidence that prediagnosis statin therapy was associated with reduced ACM and CSM in CRC patients; postdiagnosis statin therapy indicated decreased CSM. However, findings may not apply to patients with postdiagnosis statin therapy for ACM. Further studies are warranted to determine the relation between statin dose and duration on CRC survival.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of cancer death worldwide.1,2 Approximately, 1.4 million people are diagnosed with CRC and 700,000 die of CRC annually, with metastatic disease accounting for 40% to 50% of newly diagnosed patients.3 Although adjuvant chemoradiotherapy and surgical procedure are the recommended treatment for CRC and they did improve oncologic outcomes over the last decades,4–10 it remains a major bottleneck that some more effective chemopreventive agents are required to be developed to reduce the complications and mortality.
3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, also known as statins, are some of the most widely prescribed medications mainly to lower serum cholesterol.11 An increasing number of epidemiologic studies indicate that statins may serve as cancer chemopreventive agents. In addition, a meta-analysis involving >1.5 million participants aimed to evaluate the cardiovascular outcomes of statin also found that statin use was associated with 9% reduction in the risk of CRC among case–control studies.12 Besides, statins have been shown to influence the clinical outcomes through the reduction in the invasiveness or metastatic properties of CRC.13,14 Beyond the potential chemopreventive role of statins, recent studies have investigated whether taking statins before or after diagnosis can benefit prognosis for patients with CRC.15–18 Although inconsistent prognostic results exist regarding statin usage duration, disease stage, tumor site, and other medication usage such as aspirin or other nonsteroidal anti-inflammatory drug (NSAID) use status, statin remains a promising adjuvant agent for CRC. Due to inconsistent results among studies, we perform this systematic review aimed at determining whether statin use in CRC patients is associated with improved prognosis.
METHODS
Literature Search and Study Selection
Based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement,19 we conducted a systematic literature review of PubMed, Excerpta Medica dataBASE, the Cochrane Library Central Register of Controlled Trials, and the American Society of Clinical Oncology databases up till November 2014 for relevant citations, using search strategies (supplementary Table1, http://links.lww.com/MD/A288) that included exploded Medical Subject Headings terms combined with text words relating to statins and CRC prognosis. We also hand-searched the reference lists from the extracted relevant research papers, previous reviews, and meta-analyses for additional potential publications.
We considered studies eligible for inclusion if they met the following criteria: observational studies evaluated any prognostic outcomes in CRC patients comparing prediagnosis and/or postdiagnosis statin users with nonusers, and a summary statistic of hazard ratios (HRs) or relative risks (RRs) with 95% confidence intervals (CIs) could be abstracted or calculated indirectly as described by Parmar et al.20 We included studies that had a minimum length of follow-up of ≥1 year. When several studies were available for the same cohort, we retained the most recent or informative one for analysis.21,22 Studies reporting all-cause mortality (ACM) and cancer-specific mortality (CSM) were included in the main analyses. Two authors (Y.L. and L.Y.) independently performed study selection according to eligibility criteria. Institutional review board approval and patient consent were not required for this meta-analysis of observational studies.
Data Extraction
Two authors (Y.L. and H.Q.H.) independently extracted basic characteristics, evaluated the quality of each study, and resolved any discrepancies through a consensus discussion with a third senior author (Y.Y.). The characteristics recorded were first author, publication year, country, study name, study design, number of participants, age at baseline, tumor site, stage, statin usage duration, follow-up duration, survival analysis, and survival endpoints. We assessed the methodological quality of each study using the Newcastle–Ottawa Quality Assessment Scale,23,24 in which 3 domains including cohort selection, comparability, and outcome were evaluated with a maximum score of 9 representing the lowest risk of bias.
Statistical Analysis
We used STATA version 12.0 (StataCorp LP, College Station, TX) for meta-analysis. Survival estimates with full adjustments for known confounders of included studies were abstracted. Summary data reporting HRs or RRs with corresponding 95% CIs estimated from Cox proportional hazards models were pooled with random-effects model.25 The data regarding the association of prediagnosis and postdiagnosis statin use with survival outcomes were pooled separately. Between-study heterogeneity was evaluated by the Cochrane Q statistic (with a P < 0.10 considered statistically significant) and the I2 statistic (with an I2 exceeding 50% indicating significant heterogeneity).26 Statin usage on ACM and CSM for CRC patients was explored for primary meta-analysis. Other outcome measures such as disease-free mortality (DFM), recurrence-free mortality (RFM), or progression-free mortality (PFM) were also evaluated. To further explore the potential heterogeneity, we performed sensitivity analyses stratified by KRAS mutation status, tumor origin, aspirin, or NSAID usage. The risk of publication bias was assessed by visual inspection of a funnel plot as well as Egger test for statistical significance.27 We further determine the number of missing studies using Duval and Tweedie trim and fill method to adjust the summary HR based on all the studies including the hypothesized missing ones.28 All statistical analyses were 2-sided and P < 0.05 was chosen for significance.
RESULTS
Description of the Included Studies
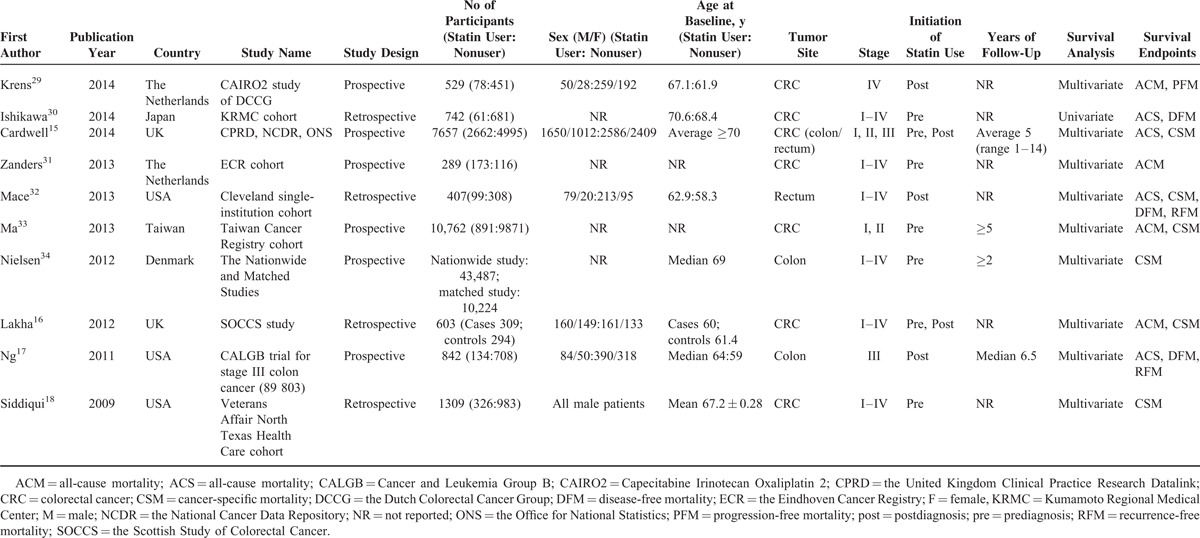
The literature search yielded a total of 532 references for eligibility. After screening the title and abstract, 66 potentially relevant studies were identified for full-text review, of which 10 met our inclusion criteria (Figure 1).15–18,29–34 The baseline characteristics of included studies15–18,29–34 were shown in Table 1. In summary, 7 studies involving 76,851 patients investigated the survival outcomes for patients of prediagnosis statin use15,16,18,30,31,33,34 and 5 studies including 10222 patients reported the prognosis impact on CRC patients of postdiagnosis statin use.15–17,29,33 The duration of follow-up ranged from 1 to 14 years. These studies were all conducted within the last 5 years (2009–2014), with 5 taking place in Europe (United Kingdom, Denmark, and The Netherlands),15,16,29,31,34 3 in North America (United States),17,18,32 and 2 in Asia (Japan and Taiwan).30,33 Several cohorts were adjusted for some conventional influential factors, including age,15–17,29,32–34 sex,15–17,33 body mass index,17,18,32 disease stage,15–17,32–34 NSAID, or metformin use.15,17,18,29,33 Three studies recruited patients with colon cancer,15,17,34 2 with rectal cancer,15,32 and 7 with both.15,16,18,29–31,33 Six studies included CRC patients with all disease stages, 1 with stage III and 1 with stage IV. The other 2 studies included stages I to III and stages II and III diseases. Assessment of methodological quality yielded an average score of 7 (range 5–8), and 8 of 10 studies had a score of ≥7 (supplementary Table 3, http://links.lww.com/MD/A288).
FIGURE 1.
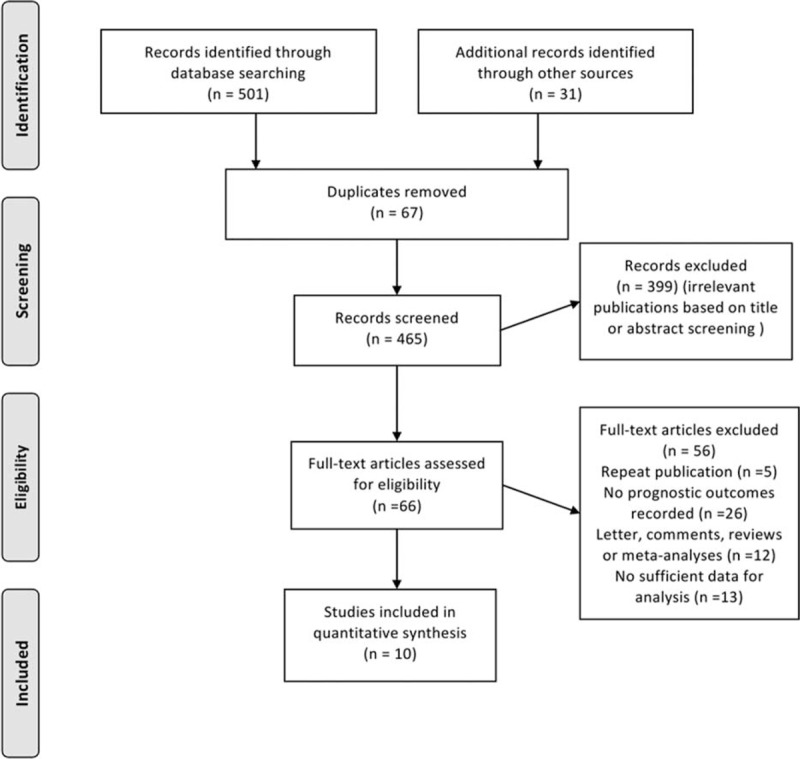
Flow diagram of study selection process investigating the effect of statin use on CRC mortality. CRC = colorectal cancer.
TABLE 1.
Baseline Characteristics of Included Studies Investigating the Survival Outcomes of Statin Use for CRC Patients
Prediagnosis Statin Use and CRC Survival
Our primary analysis regarding prediagnosis statin use and CRC survival in 7 studies estimated a pooled HR of 0.73 (95% CI 0.61–0.88, P = 0.001) for ACM and 0.80 (95% CI 0.77–0.84, P < 0.001) for CSM (Table 2), indicating 27% reduction in ACM and 20% reduction in CSM compared with statin nonusers (Figure 2A). We did not note obvious heterogeneity for ACM (I2 = 19.9%, P = 0.291) or CSM (I2 = 10.8%, P = 0.347) among the studies. CRC overall survival and CRC-specific survival benefit persisted in sensitivity analyses stratified by tumor site and NSAID adjustment (supplementary Table 2B–C, http://links.lww.com/MD/A288). For ACM, sensitivity analysis was also performed by excluding 1 study that applied univariate analysis, and the result did not alter significantly (HR 0.66, 95% CI 0.52–0.84, P = 0.001). Due to limited studies, we did not find associations between prediagnosis statin use and other outcomes such as DFM, RFM, or PFM.
TABLE 2.
Meta-Analysis of Statin Use and Risk of ACM, CSM, DFM, RFM, and PFM
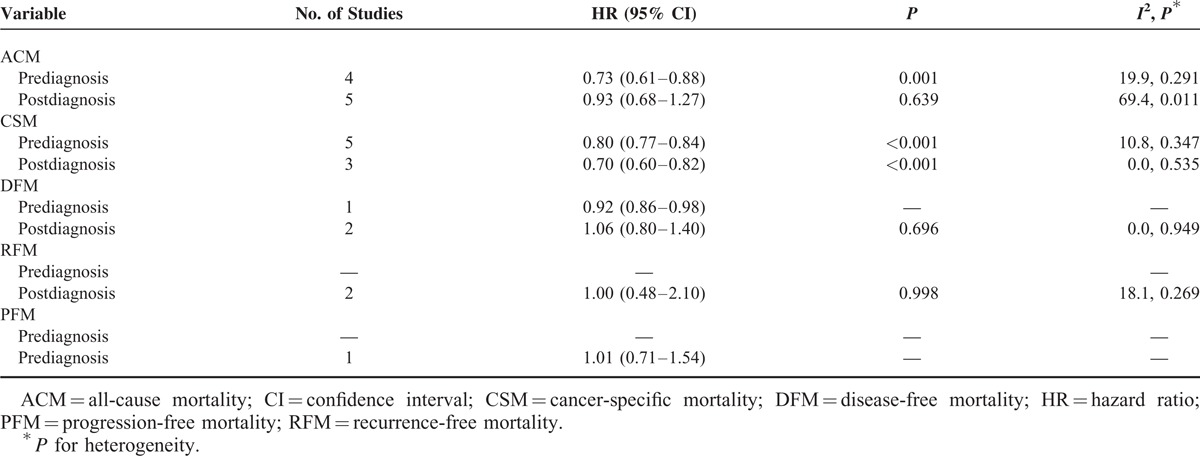
FIGURE 2.
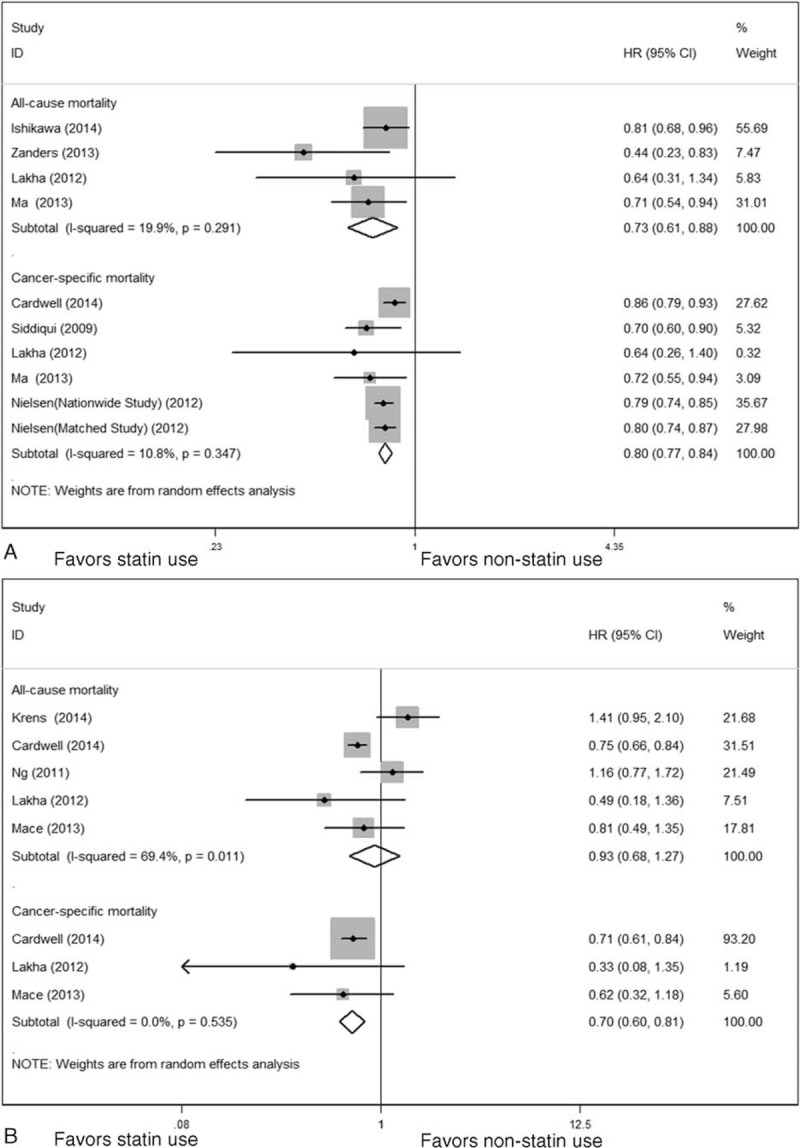
Meta-analysis of the association between statin use and CRC mortality. (A) Prediagnosis statin use and ACM and CRC-specific mortality. (B) Postdiagnosis statin use and ACM and CRC-specific mortality. ACM = all-cause mortality; CI = confidence interval; CRC = colorectal cancer; HR = hazard ratio; ID = indentity.
Postdiagnosis Statin Use and CRC Survival
Five studies provided the association between postdiagnosis statin use and CRC survival, estimating a pooled HR of 0.70 (95% CI 0.60–0.82, P < 0.001) for CSM (Table 2), indicating 30% reduction in CSM compared with statin nonusers. The survival benefits persisted when stratified by tumor site and NSAID adjustment (supplementary Table 2A, http://links.lww.com/MD/A288). However, we did not note an association between postdiagnosis statin use and ACM (HR 0.93, 95% CI 0.68–1.27, P = 0.639) with significant heterogeneity across studies (I2 = 69.4%, P = 0.011) (Figure 2B). Sensitivity analyses showed that an increased ACM was indicated for KRAS-mutated CRC (HR 1.61, 95% CI 1.07–2.43, P = 0.021) but not for KRAS wild-type CRC (HR 1.32, 95% CI 0.72–2.42, P = 0.365) (Figure 3). We did not observe reduction in ACM for postdiagnosis statin use when stratified based on tumor site and NSAID adjustment (Table 3). We did not note survival benefits for postdiagnosis statin use regarding DFM, RFM, or PFM (Table 2).
FIGURE 3.
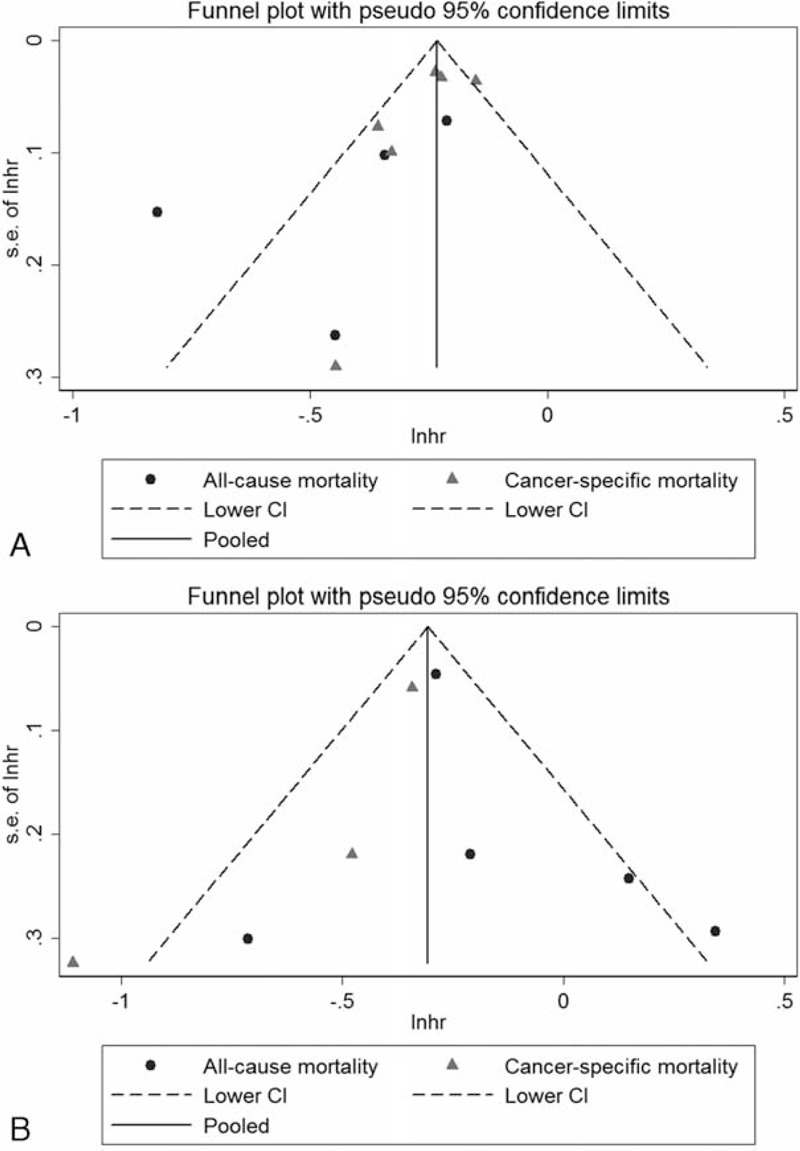
Funnel plot (with pseudo CIs) of studies investigating association between (A) prediagnosis statin use and CRC survival (B) postdiagnosis statin use and CRC survival. CI = confidence interval; CRC = colorectal cancer; Inhr = natural logarithm of hazard ratio; SE = standard error.
TABLE 3.
Sensitivity Analysis of Postdiagnosis Statin Use and Risk of ACM
Publication Bias
Although funnel plots (Figure 3A–B) and Egger test did not indicate publication bias, due to the small number of studies in each survival panel, we did not definitely determine the existence of publication bias. Trim and fill analysis, however, did not change the pooled estimates of each outcome panel.
DISCUSSION
This systematic review and meta-analysis of observational studies on the impact of statin use on CRC survival underlines the strong potential of taking statins to reduce the mortality from CRC. The 7 included observational studies available so far suggest a reduction of total overall mortality by ∼27% and CRC-specific mortality by nearly 20% for patients who took statins before CRC diagnosis. This effect persists in sensitivity analyses stratified by tumor site and NSAID adjustment. In addition, meta-analyses of the identified 5 observational studies on postdiagnosis statin use suggest similar reduction of CRC-specific mortality by ∼30%, but no overall mortality reduction has been noted.
An important cumulative evidence of a possible reduction in CRC risk with statin use was noted in a meta-analysis in 2007.12 This study, involving >1.5 million participants, indicated a modest reduction in CRC risk among case–control studies (HR 0.91, 95% CI 0.87–0.96), whereas other studies concluded that statins had a slight but nonsignificant protective effect for CRC.35–38 A more updated meta-analysis including 42 studies demonstrated that after pooling results from all observational studies and randomized controlled trials (RCTs), statin use was associated with a modest reduction in the risk of CRC (RR 0.90, 95% CI 0.86–0.95), especially for lipophilic statin use. However, no significant decreased association was found in RCTs, colon cancer, or hydrophilic statin.39 Those seemingly conflicting results provide all necessities for the study of the possible biological mechanisms of statins on CRC and the impact on CRC outcomes. Furthermore, large-scale prospective studies are warranted.
There are several potential explanations for the observed association between decreased ACM or CRC-specific mortality and statin use in CRC patients. It was reported that statins might have growth inhibitory potential by inducing cell death, thereby exerting an antiproliferative effect.40 A recent study found that statins could induce apoptosis through mitochondrial effects and affected intrinsic and extrinsic pathways by upregulating Fas, the receptor for Fas ligand.41 Moreover, it was also indicated that statins could inhibit angiogenesis by reducing the production of vascular endothelial growth factor.42–44 Still, previous in vitro studies showed that statins inhibited cell-signaling pathways affecting the invasive and metastatic properties of malignancies, thus attenuating the metastatic potential of malignant cells.45
One meta-analysis has assessed the risk of cancer death among patients using statins.46 This meta-analysis, involving 22 RCTs with >80,000 participants, reported no significant association between statin use and cancer death for all cancer types, as well as for any individual cancer type including colon cancer. In colon cancer subtype, 4 studies including 27,972 participants were enrolled and no associations were found between statin use and cancer mortality. However, this meta-analysis had some limitations and should be interpreted with caution. First, some preexisting cancer patients or patients with cancer history were not excluded, which could increase in cancer mortality. Second, some important confounders, such as lifestyle factors, and clinical and pathological variables were not included for analysis. Finally, this meta-analysis, as was indicated, had publication bias; thus, the results should be interpreted in view of the above limitations.
The present analysis has several strengths. First, the exhaustive and reproducible search strategy enables us to analyze the survival of CRC patients using all the available outcome measures including ACM, cancer-specific mortality, DFM, and PFM. Although we do not search unpublished gray literature for insufficient data, the variety of cohorts cover countries from all over Europe, the United States, and Asia. Second, by combining a large sample size of >76,000 concerning the topic, we were able to provide more comprehensive synthesis of evidence for survival benefits of statin use for CRC patients both before and after diagnosis. Third, to explore the potential sources of heterogeneity and evaluate robustness of the outcome panels for ACM and CSM, we performed several sensitivity analyses according to tumor site, NSAID use, and KRAS mutation status, and the results showed consistency across subgroups.
We acknowledge that our work should be interpreted with multiple limitations. First, current number of available studies was relatively small with only 10 studies; thus, subgroup analyses could not be fully conducted and heterogeneity had not been thoroughly explored. Second, almost all studies involved applied multivariate Cox proportional hazard models adjusted for potential confounders except 1 using univariate model.30 The adjusted factors varied across studies. However, sensitivity analysis did not significantly alter the pooled results, indicating the robustness of our results. Third, duration of follow-up varied across studies, and some studies did not give detailed follow-up information, which excluded the possibility of performing subgroup analysis according to the duration of patient follow-up, although it might affect the result of patient survival.
Fourthly, due to insufficient data reported to calculate effect estimates, we did not investigate the influence of the type of statins and their doses and duration of statin therapy on the survival of CRC patients. Therefore, further study should be conducted on the dose and duration response effects for the association between statin use and CRC survival. Fourth, the results of our analyses were derived from observational studies. Although some known potential confounders (eg, age, sex, body mass index, and disease stage) were identified and adjusted for almost all of the included studies, some other variables (eg, KRAS and BRAF mutation or microsatellite instability status) could influence our exploration of associations between statin use and CRC survival. Moreover, due to the nature of observational studies, our analysis only confirmed an association between statin use and CRC survival, and did not provide evidence for a cause–effect relationship. Another potential limitation is publication bias. Although we included meeting abstracts,31,33 we could not totally exclude the possible effect of unpublished studies on study results, which might have led to a certain degree of reporting bias. We tried to minimize this kind of bias using trim and fill methods, and the results remained unchanged. Still, our results should be treated with caution.
In summary, available evidence shows that statin therapy before diagnosis is associated with improved overall survival and CRC-specific survival; similar survival benefit regarding CRC-specific survival has been indicated for CRC patients taking statins after diagnosis. Further meta-analyses based on individual patient data are required to characterize the dose-response or duration-response associations, as well as the association in CRC patients with different molecular and pathological features to further explore the prognostic effect of statins on patients with CRC.
Footnotes
Abbreviations: ACM = all-cause mortality, CI = confidence interval, CRC = colorectal cancer, CSM = cancer-specific mortality, DFM = disease-free mortality, HR = hazard ratio, NSAID = nonsteroidal anti-inflammatory drug, PFM = progression-free mortality, RCT = randomized controlled trial, RFM = recurrence-free mortality, RR = relative risk, VEGF = vascular endothelial growth factor.
This work was supported by the Self-financing Program of the Guangxi Zhuang Autonomous Region Health Bureau (Grant No. Z2014074) and the Medicine and Health Appropriate Technology Research and Development Program of Guangxi Province (Grant No. S201413). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors alone are responsible for the content and writing of the paper.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.GLOBOCAN 2008 Web site. www.globocan.iarc.fr Accessed October 27, 2014. [Google Scholar]
- 2.Luan NN, Wu L, Gong TT, et al. Nonlinear reduction in risk for colorectal cancer by oral contraceptive use: a meta-analysis of epidemiological studies. Cancer Causes Control 2015; 26:65–78. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2011 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2014. Available at: www.cdc.gov/uscs. [Google Scholar]
- 4.Van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011; 12:575–582. [DOI] [PubMed] [Google Scholar]
- 5.Poultsides GA, Servais EL, Saltz LB, et al. Outcome of primary tumour in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol 2009; 27:3379–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanoff HK, Sargent DJ, Campbell ME, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol 2008; 26:5721–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABPC-07. J Clin Oncol 2007; 25:2198–2204. [DOI] [PubMed] [Google Scholar]
- 8.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 355:1114–1123. [DOI] [PubMed] [Google Scholar]
- 9.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 10.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18:2938–2947. [DOI] [PubMed] [Google Scholar]
- 11.Boudreau DM, Yu O, Johnson J. Statin use and cancer risk: a comprehensive review. Expert Opin Drug Saf 2010; 9:603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonovas S, Filioussi K, Flordellis CS, et al. Statins and the risk of colorectal cancer: a meta-analysis of 18 studies involving more than 1.5 million patients. J Clin Oncol 2007; 25:3462–3468. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Jung KH, Park YS, et al. Simvastatin plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) as first-line chemotherapy in metastatic colorectal patients: a multicenter phase II study. Cancer Chemother Pharmacol 2009; 64:657–663. [DOI] [PubMed] [Google Scholar]
- 14.Katz MS, Minsky BD, Saltz LB, et al. Association of statin use with a pathologic complete response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys 2005; 62:1363–1370. [DOI] [PubMed] [Google Scholar]
- 15.Cardwell CR, Hicks BM, Hughes C, et al. Statin use after colorectal cancer diagnosis and survival: a population-based cohort study. J Clin Oncol 2014; 32:3177–3183. [DOI] [PubMed] [Google Scholar]
- 16.Lakha F, Theodoratou E, Farrington SM, et al. Statin use and association with colorectal cancer survival and risk: case control study with prescription data linkage. BMC Cancer 2012; 12:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng K, Ogino S, Meyerhardt JA, et al. Relationship between statin use and colon cancer recurrence and survival: results from CALGB 89803. J Natl Cancer Inst 2011; 103:1540–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqui AA, Nazario H, Mahgoub A, et al. For patients with colorectal cancer, the long-term use of statins is associated with better clinical outcomes. Dig Dis Sci 2009; 54:1307–1311. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151:264–269. [DOI] [PubMed] [Google Scholar]
- 20.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17:2815–2834. [DOI] [PubMed] [Google Scholar]
- 21.Kuang D, Chen W, Song YZ, et al. Association between the HSPA1B ±1267A/G polymorphism and cancer risk: a meta-analysis of 14 case–control studies. Asian Pac J Cancer Prev 2014; 15:6855–6861. [DOI] [PubMed] [Google Scholar]
- 22.Guan HB, Wu L, Wu QJ, et al. Parity and pancreatic cancer risk: a dose-response meta-analysis of epidemiologic studies. PLoS One 2014; 9:e92738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle–Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603–605. [DOI] [PubMed] [Google Scholar]
- 24.Wu L, Jiang Z, Li C, et al. Prediction of heart rate variability on cardiac sudden death in heart failure patients: a systematic review. Int J Cardiol 2014; 174:857–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002; 21:589–624. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 29.Krens LL, Simkens LH, Baas JM, et al. Statin use is not associated with improved progression free survival in cetuximab treated KRAS mutant metastatic colorectal cancer patients: results from the CAIRO2 study. PLoS One 2014; 9:e112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa S, Hayashi H, Kinoshita K, et al. Statins inhibit tumour progression via an enhancer of zeste homolog 2-mediated epigenetic alteration in colorectal cancer. Int J Cancer 2014; 135:2528–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanders M, Van Herk-Sukel M, Haak H, et al. Statin use as a moderator of metformin effect on overall survival in colorectal cancer patients with diabetes. Eur J Cancer 2013; 49:S308–S309. [Google Scholar]
- 32.Mace AG, Gantt GA, Skacel M, et al. Statin therapy is associated with improved pathologic response to neoadjuvant chemoradiation in rectal cancer. Dis Colon Rectum 2013; 56:1217–1227. [DOI] [PubMed] [Google Scholar]
- 33.Ma WL, Shao YY, Hsu CH, et al. Regular statin users and colorectal cancer (CRC) prognosis. J Clin Oncol 2013; 31 15: [Google Scholar]
- 34.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2012; 367:1792–1802. [DOI] [PubMed] [Google Scholar]
- 35.Taylor ML, Wells BJ, Smolak MJ. Statins and cancer: a meta-analysis of case–control studies. Eur J Cancer Prev 2008; 17:259–268. [DOI] [PubMed] [Google Scholar]
- 36.Browning DR, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer 2007; 120:833–843. [DOI] [PubMed] [Google Scholar]
- 37.Stein EA, Corsini A, Gimpelewicz CR, et al. Fluvastatin treatment is not associated with an increased incidence of cancer. Int J Clin Pract 2006; 60:1028–1034. [DOI] [PubMed] [Google Scholar]
- 38.Bjerre LM, LeLorier J. Do statins cause cancer? A meta-analysis of large randomized clinical trials. Am J Med 2001; 110:716–723. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Tang W, Wang J, et al. Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control 2014; 25:237–249. [DOI] [PubMed] [Google Scholar]
- 40.McAnally JA, Jung M, Mo H. Farnesyl-o-acetylhydroquinone and geranyl-o-acetylhydroquinone suppress the proliferation of murine B16 melanoma cells, human prostate and colon adenocarcinoma cells, human lung carcinoma cells, and human leukemia cells. Cancer Lett 2003; 202:181–192. [DOI] [PubMed] [Google Scholar]
- 41.Sleijfer S, van der Gaast A, Planting AS, et al. The potential of statins as part of anti-cancer treatment. Eur J Cancer 2005; 41:516–522. [DOI] [PubMed] [Google Scholar]
- 42.Dimitroulakos J, Lorimer IA, Goss G. Strategies to enhance epidermal growth factor inhibition: targeting the mevalonate pathway. Clin Cancer Res 2006; 12:4426s–4431s. [DOI] [PubMed] [Google Scholar]
- 43.Dulak J, Loboda A, Jazwa A, et al. Atorvastatin affects several angiogenic mediators in human endothelial cells. Endothelium 2005; 12:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dulak J, Jozkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy. Curr Cancer Drug Targets 2005; 5:579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res 2003; 9:10–19. [PubMed] [Google Scholar]
- 46.Dale KM, Coleman CI, Henyan NN, et al. Statins and cancer risk: a meta-analysis. JAMA 2006; 295:74–80. [DOI] [PubMed] [Google Scholar]


