Abstract
Inflammation, which initiates endothelial dysfunction, vascular atherosclerosis, and oxidative stress, may negatively influence renal function and accelerate the development of end-stage renal disease (ESRD). The role of chronic osteomyelitis (COM), a chronic inflammatory disease, in the development of ESRD has not been investigated. This study explored whether patients with COM have a higher risk of ESRD than that of patients without COM.
Taiwan National Health Insurance claims from 1997 to 2010 were used to identify 24,267 newly diagnosed patients with COM and 97,068 age- and sex-matched non-COM controls for comparison. The risks of ESRD among COM patients, with adjustment for comorbidities, namely, hypertension, diabetes, coronary artery disease, congestive heart failure, and hyperlipidemia, were assessed until the end of 2010.
ESRD risk was 2.01-fold higher (95% confidence interval [CI]: 1.81–2.25) in the COM cohort than in the non-COM cohort. Regarding the joint effect of COM with comorbidity, the ESRD risk was 1.57-fold higher (95% CI: 1.23–2.00) for the COM cohort without comorbidities and increased to 2.25 (95% CI: 1.97–2.57) for the COM cohort with at least 1 comorbidity. Age-specific analysis revealed that the adjusted ESRD risk for the COM cohort increased as age decreased, with the highest hazard ratio being 17.8 (95% CI: 5.18–61.4) for patients aged 20–34 years.
This was the first study to report that COM is associated with an increased risk of ESRD, particularly among patients with comorbidities and younger patients.
INTRODUCTION
End-stage renal disease (ESRD) is becoming a major public health concern worldwide.1 ESRD can cause functional impairment and interfere with work productivity. In addition, the high cost of treatment for patients with ESRD causes a financial burden for health care systems.2 Hence, early recognition and prevention of risk factors for ESRD could diminish its social and economic cost.
Over the past decade, attention has been focused on identifying the risk factors for ESRD.3 Age, male sex, diabetes, hypertension, coronary artery disease (CAD), and congestive heart failure (CHF) are currently considered risk factors for ESRD.4,5 Inflammation has also been reported to be associated with ESRD risk.6,7 The pathophysiological explanation for the ESRD risk associated with inflammation is still unresolved but likely involves a systemic response and vascular atherosclerosis.8,9 Exploring and evaluating the ESRD risks associated with chronic inflammation-related diseases is necessary to elucidate the association between ESRD and inflammation.
Chronic osteomyelitis (COM), a lasting infection of the bones, typically evokes intense inflammation within bony structures and nearby soft tissues.10 COM is difficult to eradicate, and its therapy typically requires weeks, months, or years to complete.11 COM has been reported to increase the risk of CAD,12 dementia,13 stroke,14 depression,15 and epilepsy.16 Because CAD and stroke have many risk factors in common with ESRD,17 investigating the possible relationship between COM and ESRD is warranted. No study has connected COM with the risk of ESRD. We used a nationwide population database to assess the association of COM and risks of developing ESRD in a cohort study over a follow-up period of 14 years.
MATERIALS AND METHODS
Data Source
Data were extracted from the National Health Insurance Research Database (NHIRD) of the Taiwan National Health Insurance (NHI) program. This insurance program has provided health care for >99% of the >23 million residents of Taiwan and contracted with 97% of Taiwan's hospitals and clinics. Taiwan launched a NHI in 1995, operated by a single buyer, the government. Medical reimbursement specialists and peer review should scrutinize all insurance claims. The diagnoses were based on the International Classification of Diseases, Ninth Revision (ICD-9) codes that were judged and determined by related specialists and physicians according to the standard clinical criteria. If these hospitals or doctors made the wrong codes or diagnoses, they would be punished to pay a lot of penalty. Therefore, the diagnoses and codes used in this study should be correct and reliable.18 For this study, we used NHIR administrative data19 that contains health care data including records of inpatient claims, a registry of catastrophic illness patients, and a registry of beneficiaries. Records were linked using a scrambled, anonymous identification number for each patient to obtain a longitudinal medical history. Diagnoses are coded according to the ICD-9. This study was approved to exempt from requiring informed consent by the Institutional Review Board of China Medical University (CMU-REC-101–012).
Study Patients
A retrospective cohort study was conducted to examine the association between COM (ICD-9 code 730.1) and ESRD (ICD-9 code 585) development. Patients aged ≥20 years with newly diagnosed COM between 1997 and 2010 were included in the study cohort. The index date was the date of COM diagnosis. Those with a history of chronic kidney disease or ESRD before the index date were excluded. The comparison cohort comprised patients who had no history of COM, chronic kidney disease, or ESRD and were frequency matched with the study cohort at a ratio of 1:4 according to age (every 5 years), sex, and index year of diagnosis. The IDC-9 codes about COM (ICD-9 code 730.1) and ESRD (ICD-9 code 585) used in this study from NHIRD in Taiwan are highly reliable, because many related studies about ESRD and COM were already published.20–23
Outcome Measures
Both cohorts were followed until a diagnosis of ESRD or until loss to follow-up, death, termination of insurance, or the end of 2010. ESRD was identified from the Registry for Catastrophic Illness Patient Database. Registration for catastrophic illness requires a diagnosis made by a physician and pathological confirmation or other supporting medical information; these documents are formally reviewed by the Bureau of NHI. Conditions diagnosed before the index date, namely, diabetes (ICD-9 code 250), hypertension (ICD-9 codes 401–405), hyperlipidemia (ICD-9 code 272), coronary heart disease (CHD) (ICD-9 codes 411.1, 411.81, 411.89, 413, 414.0, 414.8, and 414.9), CHF (ICD-9 code 428), hyperuricemia (ICD-9 code 790.61), gout (ICD-9 code 274.9), and proteinuria (ICD-9 code 791) were identified as comorbidities.
Statistical Analysis
The proportionate distributions of sociodemographic characteristics and comorbidities between the cohorts with and without COM were compared using the χ2 test. The sex-, comorbidity-, and age-specific incidence rates of ESRD per 10,000 person-years of follow-up for each cohort were calculated. Poisson regression was used to estimate the COM-to-comparison incidence rate ratio (IRR) with a 95% confidence interval (CI). To investigate the risk of developing ESRD associated with COM, Cox proportional hazard regression models were used to estimate the hazard ratios (HRs) of developing ESRD in patients with COM compared with those without COM. The Kaplan–Meier method was used with the log-rank test to compare the probability of ESRD-free events between the 2 cohorts. All statistical analyses were performed using the SAS statistical package (Version 9.2 for Windows; SAS Institute, Inc., Cary, NC). A Kaplan–Meier survival curve was plotted using R software (Version 2.14.1; R Development Core Team, Vienna, Austria). Statistical significance was set at α = 0.05.
RESULTS
We identified 24,267 patients newly diagnosed with COM between 1997 and 2010 and 97,068 patients in the non-COM comparison cohort (Table 1). The mean follow-up years in COM cohort is 5.29 ± 3.96 years and in non-COM cohort is 6.21 ± 3.88 years. In both the cohorts, more than half of the patients were ≥55 years, and 66.5% were male. Compared with the comparison cohort, patients with COM were more likely to have diabetes (28.0% vs 6.05%; P < 0.001), hypertension (30.1% vs 11.2%; P < 0.001), hyperlipidemia (6.97% vs 2.66%; P < 0.001), CHF (14.1% vs 5.09%; P < 0.001), hyperuriemia and gout (2.53% vs 0.50%; P < 0.001), and proteinuria (0.37% vs 0.11%; P < 0.001).
TABLE 1.
Demographic Characteristics and Comorbidities in Cohorts With and Without Chronic Osteomyelitis
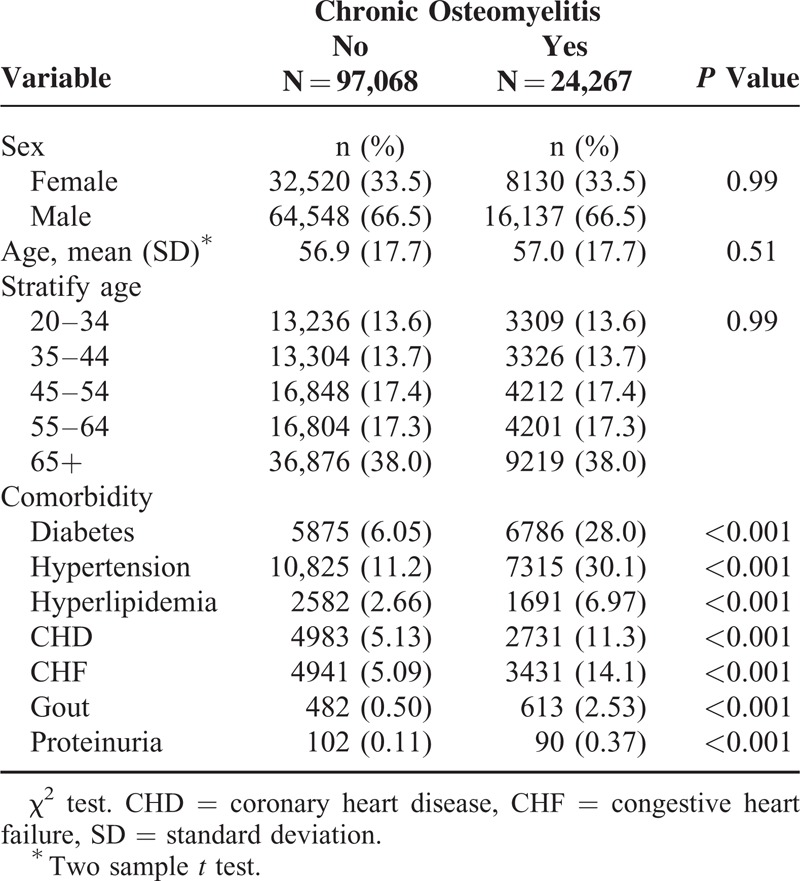
Overall, the incidence rate of ESRD was 4.55-fold higher in the COM cohort than in the non-COM cohort (59.6 vs 13.1 per 10,000 person-years), with the adjusted HR being 2.01 (95% CI: 1.81–2.25). Sex-specific analysis showed that patients with COM had a higher risk of developing ESRD compared with patients without COM for both women (adjusted HR = 1.81, 95% CI: 1.52–2.17) and men (adjusted HR = 2.03, 95% CI: 1.76–2.34). Age-specific analysis showed that the IRR was the highest in younger adults aged 20 to 34 years (IRR = 34.0, 95% CI: 28.5–40.5), with the adjusted HR being 17.8 (95% CI: 5.18–61.4). The corresponding adjusted HR decreased to 1.30 (95% CI: 1.10–1.54) for the oldest group. The adjusted HR of ESRD of the comorbidity-specific COM cohort relative to the non-COM cohort was significant for both the subgroups without comorbidity (adjusted HR = 1.57, 95% CI: 1.23–2.00) and with comorbidity (adjusted HR = 2.25, 95% CI: 1.97–2.57) (Table 2).
TABLE 2.
Incidence Rate and HR of ESRD by Sex, Age, and the Presence of Comorbidity
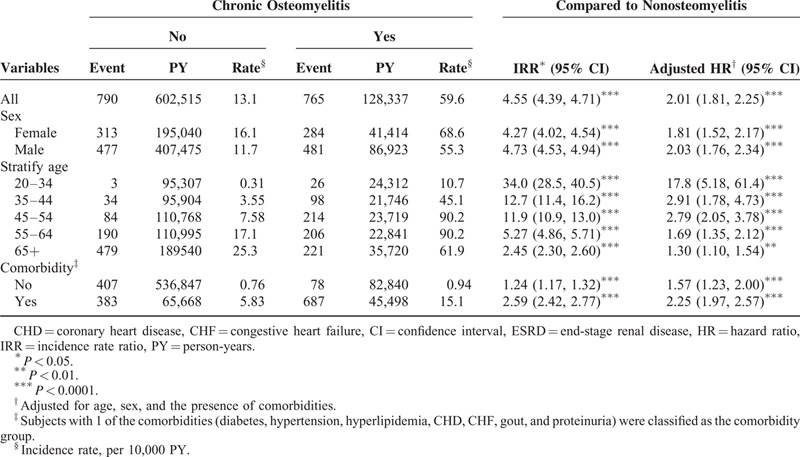
Table 3 shows the incidence rate and adjusted HR of ESRD according to the presence of individual comorbidity. A higher incidence rate of ESRD was observed in patients having any comorbidity in both the cohorts. COM patients with no comorbidity had a higher risk of developing ESRD comparing with the non-COM patients with no comorbidity (adjusted HR of diabetes = 1.53, 95% CI: 1.23–1.85; adjusted HR of hypertension = 2.06, 95% CI: 1.77–2.40; adjusted HR of hyperlipidemia = 2.02, 95% CI: 1.80–2.28; adjusted HR of CHD = 1.99, 95% CI: 1.76–2.24; adjusted HR of CHF = 2.06, 95% CI: 1.82–2.34; adjusted HR of hyperuricemia and gout = 2.04, 95% CI: 1.82–2.28; adjusted HR of proteinuria = 2.02, 95% CI: 1.81–2.25). Table 4 shows the incidence rate and adjusted HR of ESRD stratified by age categorization and the presence of comorbidity. Younger COM adults aged 20 to 34 years without comorbidities have a higher ESRD risk than non-COM adults aged 20 to 34 years without comorbidities (adjusted HR = 8.14, 95% CI: 2.04–32.6). The Kaplan–Meier survival analysis showed that patients with COM had a significantly higher rate (5.8%) of ESRD development than that of the non-COM cohort (Figure 1).
TABLE 3.
Incidence Rate and HR of ESRD by the Presence of Each Type of Comorbidity
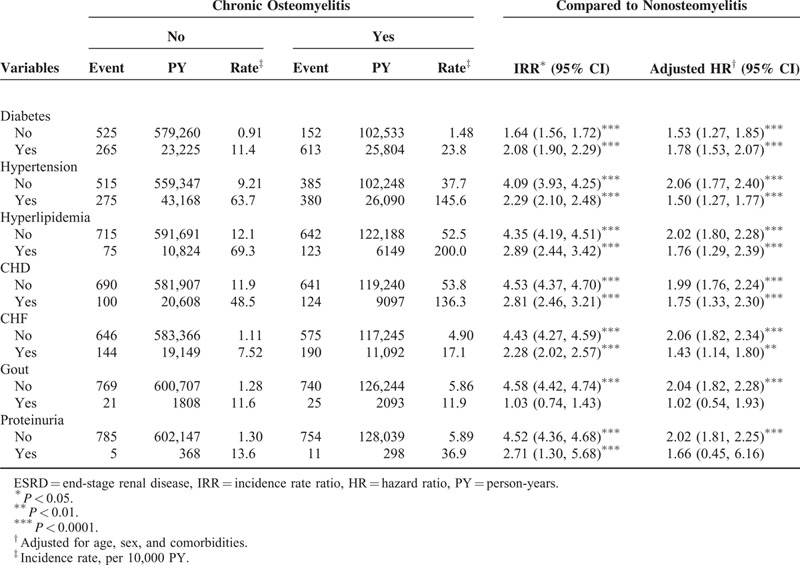
TABLE 4.
Incidence Rate and HR of ESRD by Age and the Presence of Comorbidity
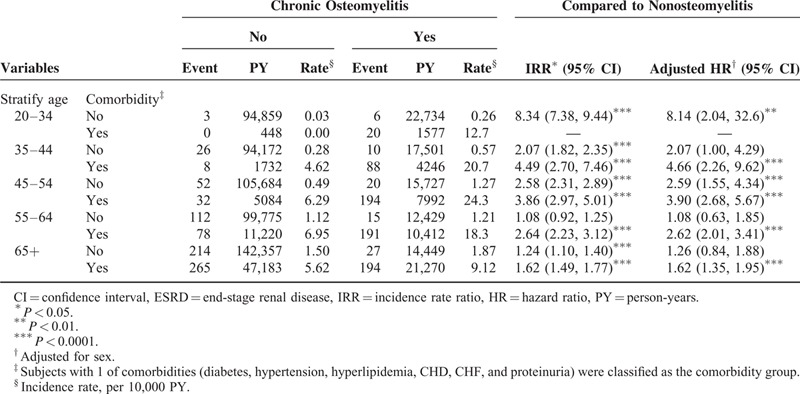
FIGURE 1.
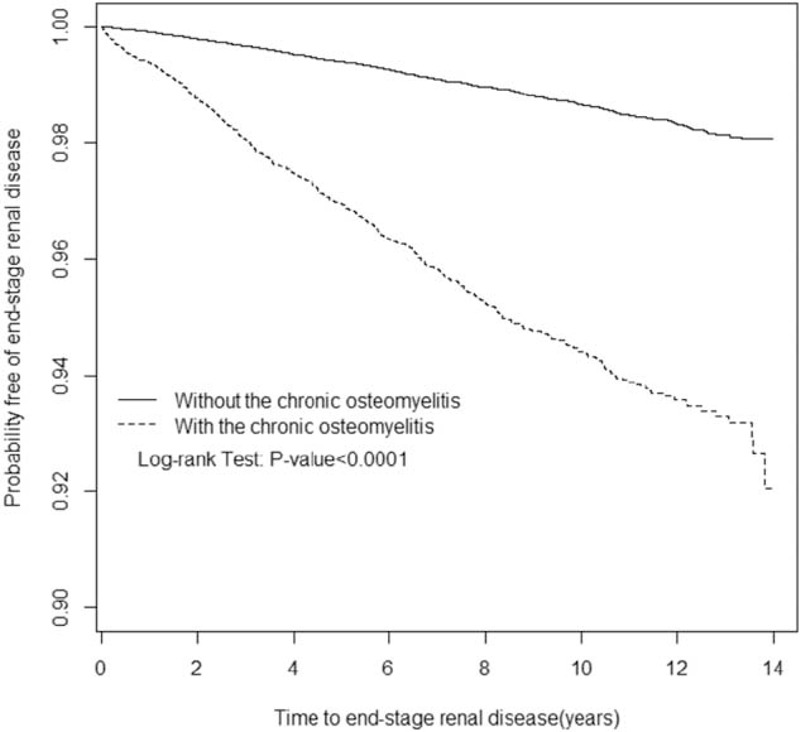
Probability free of end-stage renal disease for patients with (dashed line) or without (solid line) chronic osteomyelitis.
DISCUSSION
Previous studies have shown a link between chronic inflammatory diseases, such as hepatitis C infection,24,25 hepatitis B infection,26 stroke,27 gout,28 periodontal disease,29 and herpes zoster, and ESRD.30 Systemic inflammation is a suspected mechanism in the relationship between these diseases and an increased risk of ESRD. Using the nationally representative NHIRD to compare patients with COM and controls between 1997 and 2010, this study showed that COM, a chronic inflammatory disease, is associated with an increased risk of ESRD.
Numerous studies have shown a causal link between ESRD risk and old age,31,32 male sex,33 diabetes,33,34 hypertension,33–35 hyperlipidemia,34,36 CAD,37 and CHF.38 Our data revealed that COM patients with at least 1 of these comorbidities had an increase in ESRD risk compared with non-COM patients without comorbidities. Further analysis of the interaction between COM and individual comorbidity as well as the risk of ESRD in COM patients and matched controls with or without these comorbidities differed. Our results demonstrate that COM is potentially an independent risk factor for ESRD.
There are several possible physiopatholoical mechanisms accounting for COM cohort that has higher ESRD risk than non-COM cohort. Chronic infection may cause infection-associated glomerulonephritis that would predispose to nephrons damage, glomerulosclerosis, and thus decline of renal function reserve. Antibiotics for treating COM may also have direct nephrotoxicity or interacting drug–drug toxic effects on renal function of COM patients. A prospective long-term follow-up of COM patients with kidney biopsy data would be necessary to help clarify the causality of COM and ESRD.
Old age has been recognized as a crucial risk factor for ESRD.39 However, in the current study, the COM subgroup of patients aged 20 to 34 years had an up to 17.8-fold increased ESRD risk compared with the non-COM subgroup of patients aged 20 to 34 years. This result is attributable to at least 2 factors. First, the competing risk between ESRD and death is higher in elderly people compared with younger patients.40 Therefore, elderly patients might have an increased risk of death from other causes before they are required to initiate dialysis. Second, elderly people have higher possibility to refuse long-term dialysis in consideration of lifespan and underlying complex comorbidities.41 Finally, we collected data in a retrospective manner and applied strict criteria to enroll patients with and those without COM. The relatively low number of ESRD events in patients without COM aged 20 to 34 years might have caused bias.
Our study has several strengths. First, this retrospective study had a follow-up length of 14 years, and a strict criteria was used to the catastrophic illness registration criteria used to identify ESRD. The long-term follow-up and strict definition of ESRD diagnosis criteria strengthened the time- and severity-dependent effects of COM on ESRD development. Second, COM and age- and sex-matched controls were selected from a dataset exceeding 22 million enrollees and encompassing >99% of the population of Taiwan. This near-total-population sample coupled with a strict case-to-control ratio of 1:4 increased the generalizability, precision, and reliability of its results. Third, an NHI monitoring and auditing system is implemented to supervise insurance claims to prevent overdiagnosis and medical resource waste. This NHI surveillance program ensures the validity of diagnosis. Finally, all recognized comorbidities and risk factors of ESRD (ie, hypertension, diabetes, hyperlipidemia, CHF, CAD, hyperuricemia and gout, and proteinuria) were considered and adjusted in this study, and the results suggest that COM is an independent risk factor for ESRD.
Several limitations of this study should be noted. First, we had no definite information on the levels of blood pressure, serum glucose, and serum lipids of the patients. Our study may thus have a confounding variability bias.
The second limitation is that the database used for our research did not provide information on lifestyle and personal health behaviors, including smoking, drinking, and obesity; these variables are known to be related to ESRD. Finally, the results of this study were obtained from insurance claims to calculate the risk of ESRD among the COM patients. Hence, patients who refused long-term dialysis or COM management may have caused us to underestimate or overestimate the effects of COM on ESRD development. This possible bias was minimized because the NHI covers >99% of Taiwan's population.
Our investigation showed that COM is an independent risk factor for ESRD. Patients with COM have a higher prevalence of conventional risk factors for ESRD. The ESRD risk of patients with COM increases if they have comorbidities (ie, hypertension, diabetes, CAD, CHF, hyperlipidemia, hyperuricemia and gout, and proteinuria). Younger patients with COM have a higher risk of ESRD. Our findings could be used to prompt clinical alerts and develop renal function screening programs for patients with COM, particularly younger patients.
Footnotes
Abbreviations: CAD = coronary artery disease, CHF = congestive heart failure, COM = chronic osteomyelitis, ESRD = end-stage renal disease, ICD = International Classification of Diseases, NHI = national Health Insurance.
All authors have contributed significantly, and all authors are in agreement with the content of the manuscript: Conception/Design: SY-L, CH-K; Provision of study materials: CH-K; Collection and/or assembly of data: all authors; Data analysis and interpretation: all authors; Manuscript writing: all authors; Final approval of manuscript: all authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212–113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Grams ME, Chow EK, Segev DL, et al. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis 2013; 62:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zelmer J. The economic burden of end-stage renal disease in Canada. Kidney Int 2007; 72:1122–1129. [DOI] [PubMed] [Google Scholar]
- 3.Chang TI, Li S, Chen S-C, et al. Risk factors for ESRD in individuals with preserved estimated GFR with and without albuminuria: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2013; 61:S4–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int 2006; 69:375–382. [DOI] [PubMed] [Google Scholar]
- 5.Haroun MK, Jaar BG, Hoffman SC, et al. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 2003; 14:2934–2941. [DOI] [PubMed] [Google Scholar]
- 6.Huang S-T, Lin C-L, Chang Y-J, et al. Pneumococcal pneumonia infection is associated with end-stage renal disease in adult hospitalized patients. Kidney Int 2014; 86:1023–1030. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol 2009; 24:1445–1452. [DOI] [PubMed] [Google Scholar]
- 8.Vlassara H, Torreggiani M, Post JB, et al. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int 2009; 76:S3–S11. [DOI] [PubMed] [Google Scholar]
- 9.Lin SY, Lin CL, Liu JH, et al. Association between Helicobacter pylori infection and the subsequent risk of end-stage renal disease: a nationwide population-based cohort study. Int J Clin Pract 2015; 69:604–610. [DOI] [PubMed] [Google Scholar]
- 10.Uçkay I, Jugun K, Gamulin A, et al. Chronic osteomyelitis. Curr Infect Dis Rep 2012; 14:566–575. [DOI] [PubMed] [Google Scholar]
- 11.Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis 2012; 54:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiao LC, Muo CH, Chen YC, et al. Increased risk of coronary heart disease in patients with chronic osteomyelitis: a population-based study in a cohort of 23 million. Heart 2014; 100:1450–1454. [DOI] [PubMed] [Google Scholar]
- 13.Tseng CH, Huang WS, Muo CH, et al. Increased risk of dementia among chronic osteomyelitis patients. Eur J Clin Microbiol Infect Dis 2015; 34:153–159. [DOI] [PubMed] [Google Scholar]
- 14.Tseng CH, Chen JH, Muo CH, et al. Increased risk of ischaemic stroke amongst patients with chronic osteomyelitis: a population-based cohort study in Taiwan. Eur J Neurol 2015; 22:633–639. [DOI] [PubMed] [Google Scholar]
- 15.Tseng CH, Huang WS, Muo CH, et al. Increased depression risk among patients with chronic osteomyelitis. J Psychosom Res 2014; 77:535–540. [DOI] [PubMed] [Google Scholar]
- 16.Tseng CH, Huang WS, Muo CH, et al. Increased risk of epilepsy among patients diagnosed with chronic osteomyelitis. Epilepsy Res 2014; 108:1427–1434. [DOI] [PubMed] [Google Scholar]
- 17.Stack AG. Coronary artery disease and peripheral vascular disease in chronic kidney disease: an epidemiological perspective. Cardiol Clin 2005; 23:285–298. [DOI] [PubMed] [Google Scholar]
- 18.Cheng T-M. Taiwan's National Health Insurance system: high value for the dollar. Six Countries, Six Reform Models: their Healthcare Reform: Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan 2009; New Jersey: World Scientific, 171–204. [Google Scholar]
- 19.Chen Y-C, Yeh H-Y, Wu J-C, et al. Taiwan's National Health Insurance Research Database: administrative health care database as study object in bibliometrics. Scientometrics 2010; 86:365–380. [Google Scholar]
- 20.Lin S-Y, Lin C-L, Tseng C-H, et al. The association between chronic osteomyelitis and increased risk of diabetes mellitus: a population-based cohort study. Eur J Clin Microbiol Infect Dis 2014; 33:1647–1652. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao L-C, Muo C-H, Chen Y-C, et al. Increased risk of coronary heart disease in patients with chronic osteomyelitis: a population-based study in a cohort of 23 million. Heart 2014; 100:1450–1454. [DOI] [PubMed] [Google Scholar]
- 22.Lin S-Y, Lin W-M, Lin C-L, et al. The relationship between secondary hyperparathyroidism and thyroid cancer in end stage renal disease: a population based cohort study. Eur J Int Med 2014; 25:276–280. [DOI] [PubMed] [Google Scholar]
- 23.Wang I-K, Lin C-L, Lin P-C, et al. Effectiveness of influenza vaccination in patients with end-stage renal disease receiving hemodialysis: a population-based study. PLOS One 2013; 8:e58317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su F-H, Su C-T, Chang S-N, et al. Association of hepatitis C virus infection with risk of ESRD: a population-based study. Am J Kidney Dis 2012; 60:553–560. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y-C, Chiou W-Y, Hung S-K, et al. Hepatitis C virus itself is a causal risk factor for chronic kidney disease beyond traditional risk factors: a 6-year nationwide cohort study across Taiwan. BMC Nephrol 2013; 14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y-C, Su Y-C, Li C-Y, et al. A nationwide cohort study suggests chronic hepatitis B virus infection increases the risk of end-stage renal disease among patients in Taiwan. Kidney Int 2015; 87:1030–1038. [DOI] [PubMed] [Google Scholar]
- 27.Hung P-H, Huang Y-T, Hsiao C-Y, et al. Young stroke patients are at high risk for subsequent end-stage renal disease: a population-based observational study. Nephrol Dialysis Transplant 2014; 29:873–878. [DOI] [PubMed] [Google Scholar]
- 28.Yu K-H, Kuo C-F, Luo S-F, et al. Risk of end-stage renal disease associated with gout: a nationwide population study. Arthritis Res Ther 2012; 14:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee C-F, Lin C-L, Lin M-C, et al. Surgical treatment for patients with periodontal disease reduces risk of end-stage renal disease: a nationwide population-based retrospective cohort study. J Periodontol 2014; 85:50–56. [DOI] [PubMed] [Google Scholar]
- 30.Lin S-Y, Liu J-H, Yeh H-C, et al. Association between herpes zoster and end stage renal disease entrance in chronic kidney disease patients: a population-based cohort study. Eur J Clin Microbiol Infect Dis 2014; 33:1809–1815. [DOI] [PubMed] [Google Scholar]
- 31.Hsu CY, Iribarren C, McCulloch CE, et al. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med Feb 2009; 169:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet 2010; 375:1296–1309. [DOI] [PubMed] [Google Scholar]
- 33.Bash LD, Astor BC, Coresh J. Risk of incident ESRD: a comprehensive look at cardiovascular risk factors and 17 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2010; 55:31–41. [DOI] [PubMed] [Google Scholar]
- 34.Levin A, Stevens PE. Early detection of CKD: the benefits, limitations and effects on prognosis. Nat Rev Nephrol 2011; 7:446–457. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds K, Gu D, Muntner P, et al. A population-based, prospective study of blood pressure and risk for end-stage renal disease in China. J Am Soc Nephrol Jun 2007; 18:1928–1935. [DOI] [PubMed] [Google Scholar]
- 36.Iseki K, Tozawa M, Ikemiya Y, et al. Relationship between dyslipidemia and the risk of developing end-stage renal disease in a screened cohort. Clin Exp Nephrol 2005; 9:46–52. [DOI] [PubMed] [Google Scholar]
- 37.Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 2006; 17:2275–2284. [DOI] [PubMed] [Google Scholar]
- 38.Stack AG, Bloembergen WE. A cross-sectional study of the prevalence and clinical correlates of congestive heart failure among incident US dialysis patients. Am J Kidney Dis 2001; 38:992–1000. [DOI] [PubMed] [Google Scholar]
- 39.Prakash S, O’Hare AM. Interaction of Aging and CKD. Sem Nephrol 2009; 29:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal R, Bunaye Z, Bekele DM, et al. Competing risk factor analysis of end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2007; 28:569–575. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman SR, Shim JK, Russ AJ. Old Age Life Extension and the Character of Medical Choice. J Gerontol Series B Psychol Sci Soc Sci 2006; 61:S175–S184. [DOI] [PMC free article] [PubMed] [Google Scholar]


