Abstract
Viral encephalitis is a serious complication of hand, foot, and mouth disease (HFMD), but characteristics of cytokines response in enterovirus 71 (EV-71) and/or coxsackievirus A16 (CV-A16) associated HFMD with or without viral encephalitis remained unclear.
We performed a multigroup retrospective study and compared the serum cytokines concentrations among 16 encephalitis patients infected with EV-71 and CV-A16, 24 encephalitis patients with single EV-71 infection, 34 mild HFMD patients with EV-71 infection, 18 mild HFMD patients with CV-A16 infection, and 39 healthy control subjects.
Serum levels of interleukin (IL)-4, IL-5, IL-22, and IL-23 were significantly higher in encephalitis patients than in HFMD-alone patients when adjusting for age and sex; IL-2, tumor necrosis factor (TNF)-α, IL-4, IL-22, and IL-1β were significantly higher in HFMD-alone patients of EV-71 infection than in CV-A16 infected HFMD patients; cerebrospinal fluid level of IL-6 was lower in the EV-71/CV-A16 associated encephalitis than that in the EV-71 alone associated encephalitis patients.
Over or low expression of the cytokines cascade in HFMD patients appears to play an important role in the elicitation of the immune response to EV-71 and CV-A16. These data will be used to define a cytokine profile, which might help to recognize HFMD patients with the high risk of developing encephalitis.
INTRODUCTION
Hand, foot, and mouth disease (HFMD) is a commonly seen disease that inflicts children worldwide, characterized by mucocutaneous papulovesicular rashes on hands, feet, mouth, and buttocks lesions, and sometimes only with mouth disease (herpangina).1 The disease is generally mild and self-limited; however, in recent years, an increasing frequency of mortality associated with brainstem encephalitis in HFMD patients was reported, mostly identified in children of less than 3 years old.2 The patients rapidly develop progressive encephalitis, mostly accompanied by pulmonary edema, pulmonary hemorrhage, and cardiopulmonary collapse.2–6 In the latest large Asian-Pacific epidemic occurring in China in 2008, altogether 126 HFMD related deaths were reported, most of whom were resulted from encephalitis.7
Among the serotypes of enteroviruses that caused HFMD, human enterovirus 71 (EV-71) was most frequently associated with severe diseases. However, even given the infection of virus with same neurovirulence, the clinical sequelae differed marginally, among which host immune response was suggested to play roles.8 In the previous studies, EV-71 associated severe cases showed a marked depletion of T cells, as well as higher levels of proinflammatory cytokines and cytokines,9–11 suggesting the important role of inflammatory responses in the immune response to EV-71. Coxsackievirus A16 (CV-A16) was another enterovirus most commonly seen in HFMD patients, which rarely resulted in complicated syndromes. However, the relationship between cytokines and EV-71 and CV-A16 associated HFMD with or without encephalitis remained unclear. In the current study, by comparing multigroup of HFMD patients, we try to understand the Th1, Th2, and Th17 related cytokines responses in the clinical severity of HFMD.
METHODS
Study Population and Sample Collection
The study was designed as a retrospective study to test the serum samples that have been collected from HFMD patients during a hospital based surveillance performed in 2010. HFMD patients were recruited from children’ hospitals located in Chongqing municipality and in Shandong provinces by using a standard protocol. HFMD was diagnosed when a patient had oral ulcers and vesicular rash on the hands, feet, knees, or buttocks. Viral encephalitis was diagnosed when a patient experienced a disturbance in the level of consciousness, such as lethargy, drowsiness, or coma and had cerebrospinal fluid (CSF) pleocytosis, in accordance with the revised diagnosis criteria defined by the ministry of health, China (http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohyzs/s3586/201004/46884.htm). At acute phase of the illness, stool, serum, and CSF samples were collected from HFMD patients with encephalitis, while stool and serum samples were collected from HFMD patients without encephalitis. Healthy children were recruited from General Hospital of Beijing Military Region and used as controls, from whom the serum samples that were collected for the physical examination were obtained for tests. All samples were stored at −70 °C for batch detection of cytokine levels and EV-71/CV-A16 IgM. The study was approved by the ethic committee of the attending hospitals. We have got the written consent from the guardians of cases and controls.
Determination of IgM and Specific Viral RNA for EV-71 and CV-A16
The detection for EV-71 and CV-A16 IgM in serum was performed by the Diagnostic Kit for IgM Antibody to EV-71 and CV-A16 (Beijing Wantai Biological Pharmacy Enterprise Co., Ltd, China) following the manufacture's standard protocol. The sensitivity and specificity infection were 85.1% and 97.1% for acute EV-71, 78% and 98% for CV-A16, respectively. For detection of EV-71 and CV-A16 RNA, the stool samples were diluted 1:10 in phosphate-buffered saline (PBS, pH7.2), vortexed and centrifuged. Supernatants were collected for total viral RNA extraction using the QIAamp MinElute Virus Spin Kit (Qiagen, Hilden, Germany). Then, the specific viral RNA were detected by using commercial real-time RT-PCR kit for EV-71 and CV-A16 (KingHawk Co.LTD, China), respectively, according to the manufacturer's instructions.
Determination of Cytokine Levels
The serum and CSF concentrations of interferon (IFN)-γ, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17A, IL-22, IL-23, and tumor necrosis factor (TNF) -α were measured by BioLegend LEGENDplex Custom Human 12-Plex Cytokine Panel assays (BioLegend, Inc, USA) using Luminex 200 Total System (Invitrogen, Carlsbad, CA, USA). The assays were performed according to the instructions of the manufacturers. Briefly, 25 μL serum or CSF (1:1 diluted with the assay buffer) was incubated with antibody-coated capture beads for 2 h at room temperature. After washing the beads, protein-specific biotinylated detector antibodies are added and incubated with the beads for 1 h at room temperature. Then after removal of excess biotinylated antibodies, streptavidin conjugated R-Phycoerythrin fluorescent protein (Streptavidin-RPE) is added and incubated for 30 min at room temperature. After washing of unbound Streptavidin-RPE, the beads were resuspended by the sheath fluid for 5 min. Finally, the beads are analyzed with the Luminex 200. Concentrations of these cytokines in serum and CSF were calculated from standard curves of known concentrations of recombinant human cytokines. The minimum detectable concentration was as follows: IFN-γ, 0.4 pg/mL; IL-1β, 0.6 pg/mL; IL-2, 0.3 pg/mL; IL-4, 0.2 pg/mL; IL-5, 0.7 pg/mL; IL-6, 0.3 pg/mL; IL-10, 0.2 pg/mL; IL-13, 1.4 pg/mL; IL-17A, 0.4 pg/mL; IL-22, 1.4 pg/mL; IL-23 0.8 pg/mL; TNF-α, 0.6 pg/mL.
Statistical Analysis
Cytokine levels below the limit of detection (LOD) were imputed using the formula LOD/2.12 Cytokines were analyzed as log-transformed continuous variables to achieve their right-skewed distributions. The two-sample t test or one-way ANOVA test was used to test for differences between 2 or more groups. For cytokine without right-skewed distributions after log-transform, the nonparametric Mann–Whitney U test was used to compare their differences in different groups. χ2 or Fisher exact test was used for the comparison of category variables. Logistic regression model was performed to estimate the adjusted odds ratio (OR) and 95% interval confidence (CI) of all cytokines, with effect from age and sex adjusted. All analyses were performed using the SPSS (version 11.5). A difference with P value <0.05 was considered to be significant.
RESULTS
Study Population Characteristics
A total of 92 HFMD patients and 39 normal healthy children were recruited. The sex was evenly distributed among various subgroups, while healthy controls were significantly older than those patients (P < 0.05) (Table 1) Among the 92 HFMD patients, 40 had encephalitis, comprising 24 patients with single EV-71 infection and 16 patients with an EV-71 and CV-A16 coinfection. Among the 52 patients without viral encephalitis, 34 were infected with EV-71, 18 with CV-A16, and none had EV-71 and CV-A16 coinfection. Detection for nucleic acids of other common encephalitis-related viruses, including Japanese encephalitis virus (JEV), herpes simplex virus (HSV), and human enterovirus (HEV), was all negative. All the controls were confirmed to be negative for both EV-71 and CV-A16 by RNA detection and IgM test.
TABLE 1.
Characteristics of Study Population Classified by the Clinical Phenotype
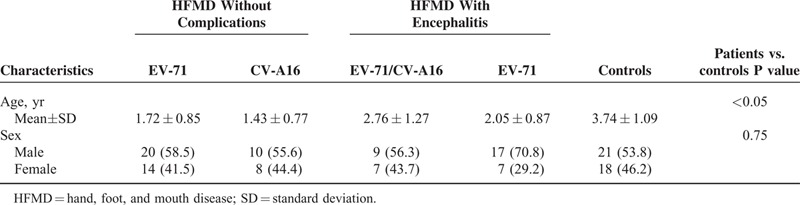
Cytokines Among HFMD Patients Regarding Their Clinical Manifestations
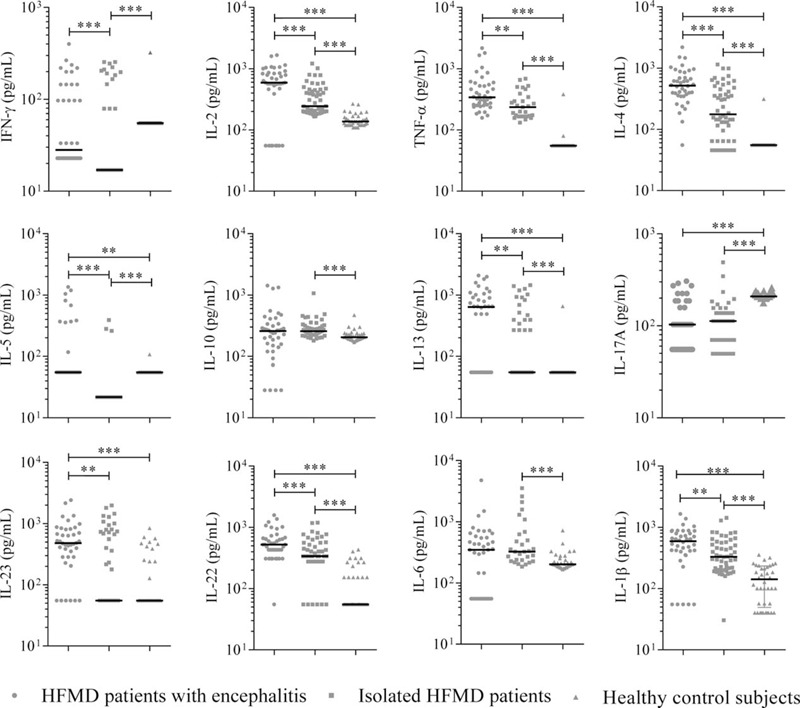
Nine of the 11 cytokines were significantly elevated among HFMD patients, in comparison to healthy controls (Figure 1). Nine of the 11 cytokines, including IFN-γ, IL-2, TNF-α, IL-4, IL-5, IL-13, IL-23, IL-22, and IL-1β, were produced with significantly higher level in encephalitis patients compared with HFMD-alone patients (P < 0.05). After adjustment for age and sex, higher levels of IL-4, IL-5, IL-22, and IL-23 remained to be significantly associated with the encephalitis (P < 0.05, Table 2). Older age conferred a protective effect on the development of HFMD associated encephalitis when adjusting for sex (OR = 0.913, 95% CI: 0.870–0.958; P < 0.001).
FIGURE 1.
Cytokines concentrations (pg/mL) in serum samples from the 3 groups of patients: HFMD patients with encephalitis (n = 40), HFMD patients without encephalitis (n = 52), and healthy controls (n = 39). Scatter plots show median within each group. The statistical significance was determined by the nonparametric Mann–Whitney U test. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. HFMD = hand, foot, and mouth disease.
TABLE 2.
The Logistic Regression Analysis on the Cytokine Concentration When Adjusting for Age and Sex
The cytokine levels of IL-2, TNF-α, IL-4, IL-5, IL-13, IL-17A, IL-22, and IL-1β were significant higher in either encephalitis patients or HFMD-alone patients compared with the healthy controls (all P < 0.05) (Figure 1).
Comparison of Cytokines Among HFMD Patients Regarding the Infected Viruses
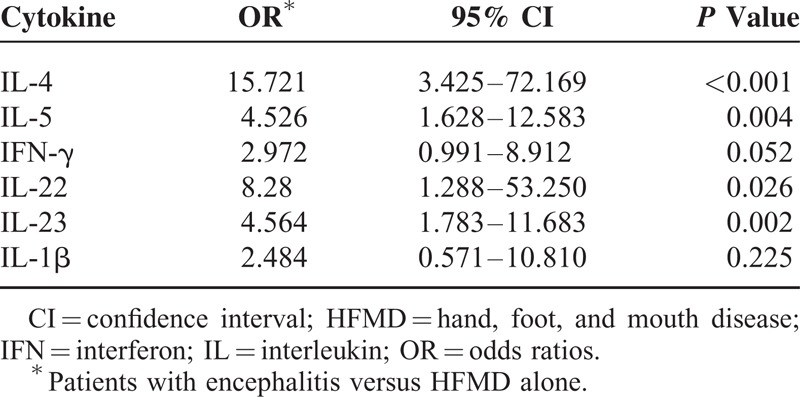
When HFMD patients without encephalitis were separately considered, 5 cytokines (IL-2, TNF-α, IL-4, IL-22, and IL-1β) were produced with significantly higher levels in patients with EV-71 infection than those with CV-A16 infection. However, significantly higher levels of IFN-γ and IL-5 were observed in the EV-71 infected patients with encephalitis (n = 24) than those without encephalitis (n = 34) (both P < 0.001) (Figure 2). The elevated IFN-γ and IL-5 were postulated to be correlated with both EV-71 infection and encephalitis development following EV-71 infection.
FIGURE 2.
Cytokines concentrations (pg/mL) in serum samples from 4 groups: EV17/CV-A16 associated viral encephalitis patients (n = 16), EV-71 associated viral encephalitis patients (n = 24), EV-71 infected mild HFMD patients (n = 18), and CV-A16 infected mild HFMD patients (n = 34). Data were analyzed by using column scatter plot. Scatter plots show median within each column. Significance determined by the nonparametric Mann–Whitney U test. HFMD = hand, foot, and mouth disease. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
The cytokines in CSF samples were only evaluated for patients with encephalitis. We found that the IL-6 level was lower in the CSF of the EV-71/CV-A16 associated encephalitis than in that of EV-71 associated encephalitis patients (P = 0.040, Figure 3). All other 10 cytokines displayed comparable levels between 2 groups (data not shown).
FIGURE 3.
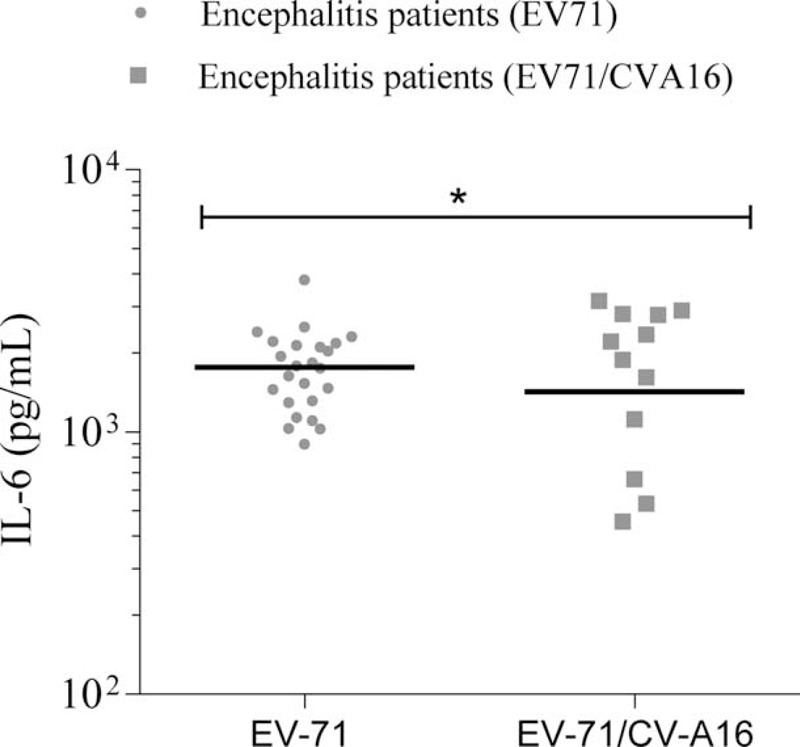
CSF concentrations of IL-6 between EV-71 with CV-A16 associated encephalitis and EV17 isolated encephalitis. Data were analyzed by using scatter plot. Scatter plot shows median within each column. Significance determined by Student t test for comparison between 2 groups. CSF = cerebrospinal fluid. ∗P < 0.05.
DISCUSSION
There used to be studied proving that altered cellular but not humoral response might be linked to EV-71 meningoencephalitis.13 Several studies have examined cytokine and chemokine profiles in EV-71 patients with brainstem encephalitis: concentrations of IL-1β, IL-6, IL-10, IL-13, and TNF-α and IFN-γ were found to be significantly higher in EV-71 infected patients with pulmonary oedema than in those without.7,9,10 High concentrations of several chemokines in plasma, including 10 kDa-interferon-γ-induced protein, monocyte chemoattractant protein, monokine induced by IFN-γ and IL-8, have been reported in children with brainstem encephalitis and pulmonary oedema.14 In 1 study, IL-4 production was reduced in patients with meningoencephalitis, while interferon-γ production in patients with or without EV-71 meningoencephalitis was not significantly different.13 In the current study, HFMD patients with encephalitis produced an extensive immune response than patients without, EV-71 associated HFMD patients produced higher levels of cytokines than CV-A16 associated HFMD patients (IL-2, TNF-α, IL-4, IL-22, and IL-1β). Since EV-71 infection is associated with higher risk of encephalitis development, it is therefore hard to define the exact role of these cytokines in causing encephalitis. The analysis estimated simultaneously, presented significant association for younger age, EV-71 infection, IL-4, IL-5, IL-22, and IL-23. We might propose that over-presentation of other cytokines might tend to be derived from EV-71 infection, rather than the encephalitis itself. The evaluated cytokines could therefore be classified into 2 groups, the cytokines that were associated with EV-71 infection, the cytokines that were independently associated with encephalitis (IL-4, IL-5, IL-22, and IL-23) regardless of the infection types, which might further play roles in the encephalitis following EV-71 infection. Another important finding is that EV-71 and CV-A16 coinfection, despite their strong correlation with higher frequency of encephalitis, did not produce elevated cytokines in either serum or CSF; therefore, the coinfection of CV-A16 had no role in the cytokine storm produced from encephalitis.
All the evaluated cytokines in the current study were Th1, Th2, and Th17 secreted that have been involved in human innate and adaptive immunity to pathogenic infection.15,16 Under certain circumstances, the severe clinical manifestation or outcomes were induced by the stimulated host immune response, which is usually characterized by abnormal cytokine level. The complication with encephalitis is considered the second stage of the HFMD, which period is critical for taking therapeutic measures to prevent the disease progressing into the third stage, such as BE, PE, and cardiopulmonary collapse.17 Therefore, the cytokine activation accompanying EV-71 and/or CV-A16 infection could act as an important indicator, which if identified at early stage might be used to recognize patients with severe clinical outcome for further clinical treatment.
The limitation of the study should be addressed. First, we measured both CSF and serum concentration of cytokines only in patients with the encephalitis, and the association of cytokines with encephalitis risk based on the serum concentration, which might not reflect changes in the central nervous system. Furthermore, the relative small sample size might affect statistical power of the analyses, especially for the multivariate logistic regression model. Finally, the age and regional source of the patients were not matched with controls; therefore, selection bias cannot be avoided. Taken together, the current finding needs to be confirmed by further well matched studies based on larger sample sizes.
Acknowledgments
This study was supported by China Mega-Project for Infectious Diseases Grant (2013ZX10004-202), the State Key Laboratory of Pathogen and Biosecurity (Academy of Military Medical Science, SKLPBS1442), the Youth Talent Support Program by School of Public Health, Peking University, National Natural Science Foundation of China (81102171), Shandong Province Science and Technology Development Plan (2012GHZ30031), Shandong Medical Science and Technology Development Program (2011HZ055, 2013WS0157), and Natural Science Foundation of Shandong Province (ZR2013HM052, ZR2014HP030).
Footnotes
Abbreviations: CI = confidence interval, CSF = cerebrospinal fluid, CV-A16 = coxsackievirus A16, EV71 = enterovirus 71, HFMD = hand, foot, and mouth disease, IFN = interferon, IL = interleukin, OR = odds ratios, PBS = phosphate-buffered saline, TNF = tumor necrosis factor.
The funding agencies had no role in the design and conduct of the study, collection, management, analysis, interpretation of the data, preparation, review, or approval of the manuscript. Regarding this report, the authors do not have any commercial or other association that would be considered a conflict of interest.
S.-Y.Z. and M.-Y.X. contributed equally to this article.
The authors have no conflicts of interest to disclose.
References
- 1.Chan KP, Goh KT, Chong CY, et al. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerging Infect Dis 2003; 9:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang SM, Liu CC, Tseng HW, et al. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin Infect Dis 1999; 29:184–190. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control Prevention Prevention. Deaths among children during an outbreak of hand, foot, and mouth disease: Taiwan, Republic of China, April-July 1998. MMWR Morbidity Mortality Weekly Rep 1998; 47:629–632. [PubMed] [Google Scholar]
- 4.Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 1999; 341:929–935. [DOI] [PubMed] [Google Scholar]
- 5.Liu CC, Tseng HW, Wang SM, et al. An outbreak of enterovirus 71 infection in Taiwan, 1998: epidemiologic and clinical manifestations. J Clin Virol 2000; 17:23–30. [DOI] [PubMed] [Google Scholar]
- 6.Wu JM, Wang JN, Tsai YC, et al. Cardiopulmonary manifestations of fulminant enterovirus 71 infection. Pediatrics 2002; 109:E26. [DOI] [PubMed] [Google Scholar]
- 7.Solomon T, Lewthwaite P, Perera D, et al. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10:778–790. [DOI] [PubMed] [Google Scholar]
- 8.Chang LY, Hsiung CA, Lu CY, et al. Status of cellular rather than humoral immunity is correlated with clinical outcome of enterovirus 71. Pediatr Res 2006; 60:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin TY, Hsia SH, Huang YC, et al. Proinflammatory cytokine reactions in enterovirus 71 infections of the central nervous system. Clin Infect Dis 2003; 36:269–274. [DOI] [PubMed] [Google Scholar]
- 10.Wang SM, Lei HY, Huang KJ, et al. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis 2003; 188:564–570. [DOI] [PubMed] [Google Scholar]
- 11.Wang SM, Lei HY, Yu CK, et al. Acute chemokine response in the blood and cerebrospinal fluid of children with enterovirus 71-associated brainstem encephalitis. J Infect Dis 2008; 198:1002–1006. [DOI] [PubMed] [Google Scholar]
- 12.Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hygiene 1990; 5:46–51. [Google Scholar]
- 13.Yang KD, Yang MY, Li CC, et al. Altered cellular but not humoral reactions in children with complicated enterovirus 71 infections in Taiwan. J Infect Dis 2001; 183:850–856. [DOI] [PubMed] [Google Scholar]
- 14.Lin TY, Chang LY, Huang YC, et al. Different proinflammatory reactions in fatal and non-fatal enterovirus 71 infections: implications for early recognition and therapy. Acta Paediatr 2002; 91:632–635. [DOI] [PubMed] [Google Scholar]
- 15.McGuirk P, Mills KH. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol 2002; 23:450–455. [DOI] [PubMed] [Google Scholar]
- 16.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nature reviews. Immunology 2008; 8:337–348. [DOI] [PubMed] [Google Scholar]
- 17.Lu G, Li X, Lv Y, et al. Diagnosis and treatment of critical hand-foot-mouth disease induced by enterovirus type 71 infection. Chin Pediatr Emergency Med 2008; 15:217–220. [Google Scholar]


