Abstract
The efficacy and toxicity of oxaliplatin-based versus carboplatin/cisplatin-based doublets in patients with previously untreated nonsmall cell lung cancer (NSCLC) have been compared.
We searched published randomized controlled trials of oxaliplatin-based or carboplatin/cisplatin-based medications for NSCLC. A fixed effect model was used to analyze outcomes which were expressed as the hazard ratio for overall survival (OS) and time-to-progression (TTP), relative risk, overall response rate (ORR), disease control rate (DCR), 1-year survival, and the odds ratios for toxicity were pooled.
Eight studies involving 1047 patients were included. ORR tended to favor carboplatin/cisplatin but the effect was not significantly different compared with oxaliplatin doublets (P = 0.05). The effects of OS, TTP, DCR, and 1-year survival between the 2 regimens were comparable. Oxaliplatin doublets caused less grade 3/4 leukocytopenia and neutropenia. Grades 3 to 4 nonhematological toxicities and grades 3 to 4 hematological toxicities showed little difference between oxaliplatin doublets and carboplatin/cisplatin doublets.
Meta-analysis shows that the efficacy of oxaliplatin doublets is similar to that of other currently used platinum doublets. The lack of significant differences in the statistic analysis does not preclude genuine differences in clinical efficacy, because higher diversities between the studies covered differences between the 2 groups in each study. Oxaliplatin combined with a third-generation agent should be considered for use as alternative chemotherapy in patients who cannot tolerate conventional platinum-based regimens because the toxicity profile is much more favorable.
INTRODUCTION
Lung cancer is the most common malignancy worldwide and a leading cause of cancer-related deaths. Nonsmall cell lung cancer (NSCLC) constitutes 80% of all lung cancers1 and more than 65% of patients present with locally advanced or metastatic disease.2 Sadly, the majority of the patients treated with curative intent develop recurrence3 so that most patients therefore face the option of palliative chemotherapy and supportive care.
Cisplatin-based chemotherapy produces an improved response rate and survival benefit compared with noncisplatin regimens.4–6 New third-generation drugs including paclitaxel, docetaxel, topotecan, irinotecan, gemcitabine, and vinorelbine have been tested in Stage IV NSCLC.7–9 Randomized trials incorporating some of these agents with platinum have shown benefits over the older cisplatin-based regimens.10 However, trials using paclitaxel or gemcitabine plus carboplatin or cisplatin in both arms showed equivalent response rates.11 Currently, the carboplatin/cisplatin-based doublet regimens containing a third-generation agent are recommended as the standard first-line chemotherapy for advanced NSCLC patients with good performance status according to international guidelines.12 However, cisplatin-related toxicity, such as nephrotoxicity, severe nausea, and vomiting, has greatly hindered its use. In addition, the complexity of its administration contraindicates the use of cisplatin in a substantial proportion of patients, particularly elderly patients and those with relevant comorbidities or multiorgan metastases. Carboplatin, a cisplatin analog without significant clinical nephrotoxicity, can be used as an alternative to cisplatin. However, there is still a debate about the inferiority of carboplatin in terms of the response rate,13 progression, and overall survival (OS), derived from several meta-analyses or head-to-head comparisons.14 Furthermore, carboplatin has a relevant hematologic toxicity profile when used in equipotent dosages.15 Oxaliplatin, a third-generation platinum analog, has attracted much attention due to several potential advantages for the treatment of NSCLC compared with cisplatin and carboplatin. First, it has been proved to be associated with greater cytotoxicity and inhibition of deoxyribonucleic acid (DNA) synthesis. Second, it appears to possess a wider spectrum of activity than other platinum agents and is active against both cisplatin-sensitive and cisplatin-resistant cell lines, and also those that are cross-resistant to carboplatin.16,17 Third, oxaliplatin has a favorable toxicity profile compared with cisplatin and can be safely administered in an outpatient setting without the need for specific hydration treatment. Until now, a number of randomized clinical trials have demonstrated that oxaliplatin has promising efficacy and a favorable toxicity profile when used as the first-line treatment of advanced NSCLC in combination with either pemetrexed,18 gemcitabine,19,20 navelbine,13 paclitaxel,21 or docetaxel.22 However, most of these trials used a small cohort of patients, with inadequate statistical power to exclude potentially clinically relevant differences in efficacy.
To evaluate the efficacy and safety profile of oxaliplatin with sufficient statistical power, we have conducted a pooled analysis of all randomized controlled trials (RCTs) that have compared oxaliplatin-based doublets versus carboplatin/cisplatin-based doublets in the treatment of advanced NSCLC.
MATERIALS AND METHODS
Literature Search
In September 2014, an electronic search of the PubMed database, the Cochrane Central Register of Controlled Trials (CENTRAL) database, and the EMBASE database were performed. The detailed search strategy is described in Figure 1. Briefly, both the MeSH terms and various text words for “non-small cell lung cancer” or “Carcinoma, Non-Small-Cell Lung” were used in combination with those for “oxaliplatin.” The literature search was limited to “human studies” and “randomized control trials.” We also reviewed recent conference abstracts of the American Society of Clinical Oncology (ASCO), International Association for the Study of Lung Cancer (IASLC), and the European Society of Medical Oncology (ESMO), to identify “gray literature.” All potentially relevant studies were retrieved and their references were checked to see if there were any additional eligible studies. Furthermore, we also searched http://www.ClinicalTrials.gov for information on registered RCTs to identify trials that were registered as completed, but whose results had not yet been published. This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement issued in 2009. All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
FIGURE 1.
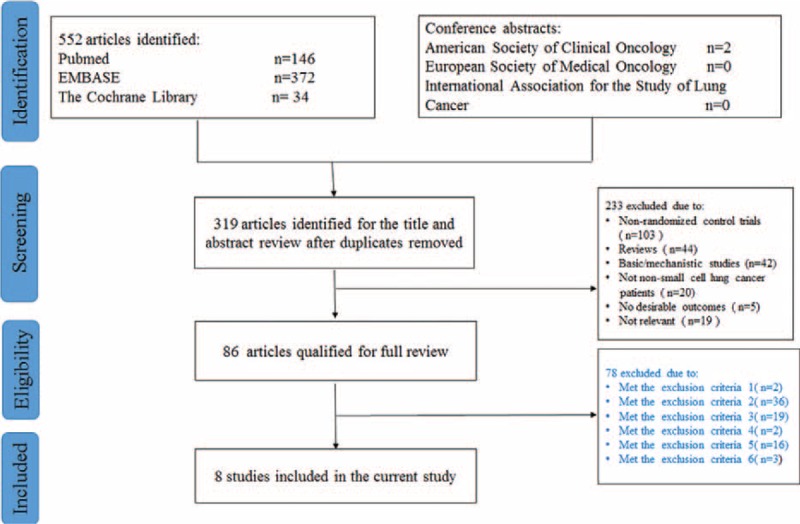
PRISMA flow chart of literature retrieval and selection.
Selection of Trials
Trials included in our study had to meet the following inclusion criteria: Participants: patients must have been cytologically or pathologically confirmed with NSCLC and in clinical stages III to IV; patients must not have previously received chemotherapy. Type of study: only clinical RCTs were deemed eligible. Types of intervention: studies that reported an oxaliplatin-based regimen and a carboplatin/cisplatin-based regimen were compared to advanced NSCLC patients in the trials. For studies using other agents as the third arm, only the 2 treatments containing oxaliplatin or cisplatin or carboplatin were included in the analysis. Type of outcome measurements: one or more of the following: overall response rate (ORR) (the sum of complete response [CR] and partial response [PR]), disease control rate (DCR) (the sum of the CR, PR, and stable disease [SD]), time-to-progression (TTP), progression-free survival (PFS), OS, 1-year survival rate, and adverse events.
Clinical trials were excluded if they did not meet the above criteria. In addition, the following types of study were also excluded. The control arm was blank or contaminated by oxaliplatin. Patients who received chemotherapy and untreated patients, or when only pretreated patients were included in the trial. Nonoriginal research (eg, review articles, case reports, and letters to the editor) or duplicated publications. The control arm used a single drug, while the experiment arm used oxaliplatin doublets. Targeted treatment, such as bevacizumab, was added to the chemotherapy. Data obtained from studies aimed at histological subtyping or pharmacokinetic analyses of NSCLC were not available.
Data Extraction
All the data were extracted by 2 independent reviewers (JY and JX) who filled in appropriate forms with disagreements being resolved by discussion with a third investigator (BWC). The following information was sought from each trial: first author, year of publication, number of the patients, percentage of male patients, performance status of patients, number of the patients eligible for response evaluation, mean age, chemotherapy regimens and doses, number and ORR, DCR, TTP, PFS, OS, 1-year survival rate, number and rate of each type of adverse events stratified by severity, and the hazard ratio (HR) for the comparison of TTP or OS of oxaliplatin doublets-treated patients with that of patients receiving carboplatin/cisplatin doublets. If the HR was not directly reported, an estimation of log HR and its variance from Kaplan–Meier curves was made according to published methodology.21 The response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) and classified as a CR, PR, SD, or progressive disease (PD). ORR was defined as CR plus PR and DCR was defined as ORR plus SD.
Quality Assessment
The methodological quality of RCTs was assessed using the 5-point Jadad scale.23 All trials were assessed by at least 2 reviewers and any disagreement was resolved by third-party consensus. In addition, the risk of bias for the included studies was also assessed.
Selection of Analysis Models
A fixed-effect model is suitable for analysis in case of homogeneity of variance between trials. We employed the Review Manager 5.0.24 software for statistical analyses in which the Peto method (an improved Mantel–Haenszel method) was used to pool odd ratios. The Peto method uses an inverse variance approach but utilizes an approximate method of estimating the log odds ratio (OR). As result, the current study met the assumption of homogeneity since the heterogeneity of overall ORR, DCR, TTP, OS, and 1-year survival values were very low between the selected publications (0–28%). Therefore, we used a fixed-effect model.
Statistical Analysis
The relative risk (RR) for ORR, DCR, and 1-year survival after treatment; the HR for OS and TTP; and the OR for the different types of toxicity was calculated using the Review Manager 5.0.24 statistical software (Cochrane Collaboration Software). The ORR, DCR, TTP, OS, and 1-year survival rate was calculated by applying an intent-to-treat analysis. Grades 3 and 4 toxicity, respectively, were analyzed by considering the number of patients evaluable for response and toxicity. RR > 1 reflected more the overall response or 1-year survival in the oxaliplatin-based arm; HR > 1 reflected more deaths or progression in the oxaliplatin-based arm, and OR > 1 indicated more toxicity in the oxaliplatin-based arm and vice versa. Publication bias was evaluated according to a funnel plot, Begg's test, and Egger's test24 by using the Review Manager 5.0.24 package. Statistical tests for heterogeneity were 1-sided; statistical tests for effect estimates were 2-sided. P values < 0.05 were regarded as being statistically significant for all included studies.
RESULTS
Characteristics of the Included Studies
The flow of study selection is demonstrated in Figure 1. Initially, 552 references were identified from PubMed, EMBASE, and the Cochrane Library; 2 relevant conference abstracts were obtained by manual searching of the ASCO, ESMO, and IASLC websites. After careful selection, a total of 8 eligible studies with 1047 patients were included in our analysis.
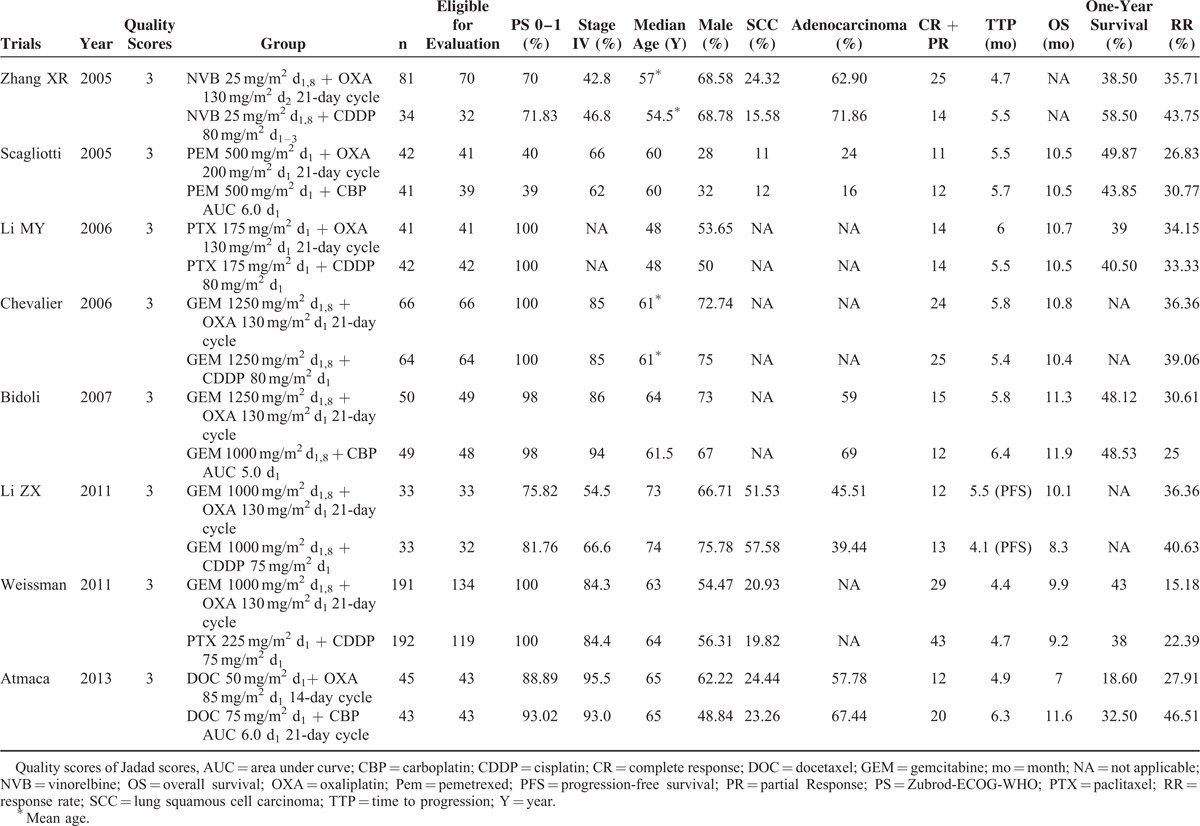
Table 1 summarizes the basic characteristics of the included studies. All the patients recruited in the 8 studies were at clinical stages III to IV of NSCLC, and all of the studies were graded with a Jadad score of at least 3. The sample sizes of the included studies ranged from 66 to 383. The median ages of the patients in the 8 studies varied from 48 to 74 years; however, the median age was well balanced between oxaliplatin (OXA) and cisplatin/carboplatin (CDDP/CBP) groups in each study. Patients’ performance status before treatment was either over 60% according to the Karnofsky performance score or <2 by the Eastern Cooperative Oncology Group performance score. Oxaliplatin-based regimens were given as first-line therapy to treat NSCLC in all studies that reported relevant information that satisfied the inclusion criteria. Four trials were gemcitabine plus oxaliplatin versus gemcitabine plus carboplatin/cisplastin. Two trials compared taxane (paclitaxel/ordocetaxel) plus carboplatin/cisplastin with taxane plus oxaliplatin. One trial compared pemetrexed plus carboplatin with pemetrexed plus oxaliplatin. Another trial compared docetaxel plus carboplatin with docetaxel plus oxaliplatin. Oxaliplatin was administered at the recommended dosage of 130 mg/m2 in 6 studies, 85 mg/m2 in 1 study, and 200 mg/m2 in another study. A typical common drug schedule is a 21-day cycle. Tumor responses and the grades of adverse events were evaluated according to the RECIST and National Cancer Institute Common Terminology Criteria (version 3.0), respectively, in all studies that specified these issues.
TABLE 1.
Characteristics of the Randomized Trials Included in the Meta-Analysis
Overall Response Rate
ORRs were reported in 8 studies,13,15,18–21,25,26 ranging from 15.2% to 46.5% with no significant difference between study heterogeneity (P = 0.76, I2 = 0%). The pooled ORRs were 26.6% and 31.2% for oxaliplatin and carboplatin/cisplastin combinations, respectively. ORRs tended to favor carboplatin/cisplatin doublets but there was no significant difference compared with the oxaliplatin doublets (RR = 0.76, 95% confidence interval [CI] = 0.58–1.00, P = 0.05, Figure 2A). Subgroup analysis revealed no significant differences in the oxaliplatin–gemcitabine doublets and taxane–oxaliplatin doublets compared with the carboplatin/cisplastin-based combinations (RR = 0.79, 95% CI = 0.56–1.13, P = 0.20; Figure 2B and RR = 0.67, 95% CI = 0.36–1.27, P = 0.22; Figure 2C).
FIGURE 2.
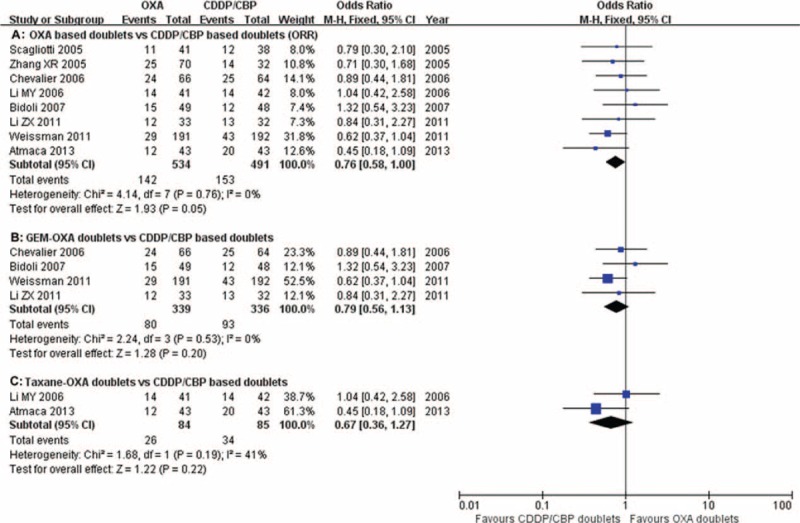
Meta-analysis of the overall response rate (ORR) among patients receiving oxaliplatin-based doublets or carboplatin/cisplatin-based doublets. The pooled RR for ORR showed that there was no significant difference between the 2 groups. Subgroup analysis revealed no significant differences between the oxaliplatin–gemcitabine doublets and taxane–oxaliplatin doublets compared with carboplatin/cisplastin-based combinations. GEM-OXA, gemcitabine/oxaliplatin regimens; RR, relative risk; Taxane-OXA, taxane/oxaliplatin regimens.
Disease Control Rate
Seven trials13,15,18–21,25 reported DCRs, ranging from 56.3% to 81.3%. No significant heterogeneity was detected among studies (P = 0.80, I2 = 0%). The pooled DCRs were 62.8% and 62.8% for oxaliplatin and carboplatin/cisplatin combinations, respectively. The pooled RRs for DCR revealed no differences between the oxaliplatin-based doublets and the carboplatin/cisplastin-based doublets (RR = 0.94, 95% CI = 0.71–1.24, P = 0.68; Figure 3A). Subgroup analysis revealed no significant differences in the oxaliplatin–gemcitabine doublets and taxane–oxaliplatin doublets compared with the carboplatin/cisplastin-based doublets (RR = 1.02, 95% CI = 0.72–1.43, P = 0.91; Figure 3B and RR = 0.80, 95% CI = 0.42–1.50, P = 0.48; Figure 3C).
FIGURE 3.
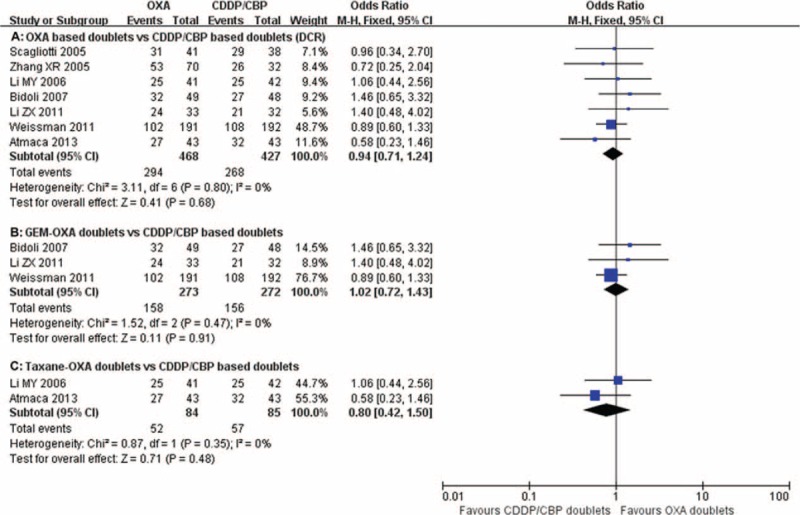
Meta-analysis of disease control rate (DCR) among patients receiving oxaliplatin doublets or carboplatin/cisplatin doublets. The pooled RR for DCR showed that there were no significant differences between the 2 groups. Subgroup analysis revealed no significant differences in the oxaliplatin–gemcitabine doublets and taxane–oxaliplatin doublets compared with carboplatin/cisplastin-based doublets. GEM-OXA, gemcitabine/oxaliplatin regimens; RR, relative risk; Taxane-OXA, taxane/oxaliplatin regimens.
Time-To-Progression or Progression-Free Survival
PFS was reported in 1 study,20 the median of which ranged from 4.1 to 5.5 months. TTP was reported in 7 studies.13,15,18,19,21,25,26 The median of TTP ranged from 4.4 to 6.0 months and 4.7 to 6.3 months in the oxaliplatin-treated and carboplatin/cisplatin-treated groups, respectively. HR data for TTP were acquired from 4 of the 5 papers.15,18,19,25 The pooled HR for TTP showed no differences between the oxaliplatin-based doublets and carboplatin/cisplatin-based doublets (HR = 0.93, 95% CI = 0.85–1.02, P = 0.14; Figure 4A). Subgroup analysis revealed no significant differences in the oxaliplatin–gemcitabine doublets compared with the carboplatin/cisplastin-based doublets (RR = 0.97, 95% CI = 0.85–1.10, P = 0.60; Figure 4B).
FIGURE 4.
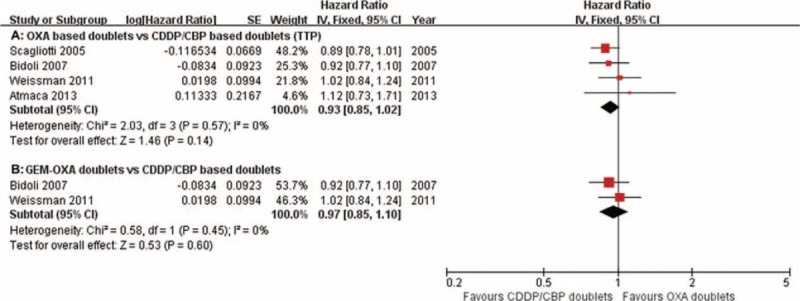
Meta-analysis of time-to-progression (TTP) among patients receiving oxaliplatin doublets or carboplatin/cisplatin doublets. The pooled HR for TTP shows that there were no significant differences between the 2 groups. Subgroup analysis revealed no significant differences in the oxaliplatin–gemcitabine doublets compared with the carboplatin/cisplastin doublets. GEM-OXA, gemcitabine/oxaliplatin regimens; HR, hazard ratio.
Survival
Seven studies15,18–21,25,26 reported data on the OS of oxaliplatin-treated and carboplatin/cisplatin-treated patients, with a median of 7.0 to 11.3 months and 9.2 to 11.9 months, respectively. HR data for OS were acquired from 5 of the 7 papers.15,18–20,25 The pooled HR for OS showed no difference between the oxaliplatin-based doublets and carboplatin/cisplatin-based doublets (HR = 0.98, 95% CI = 0.90–1.07, P = 0.67; Figure 5A). Subgroup analysis revealed no significant differences between the oxaliplatin–gemcitabine doublets treatment compared with the carboplatin/cisplastin-based doublets (HR = 1.01, 95% CI = 0.88–1.16, P = 0.87; Figure 5B).
FIGURE 5.
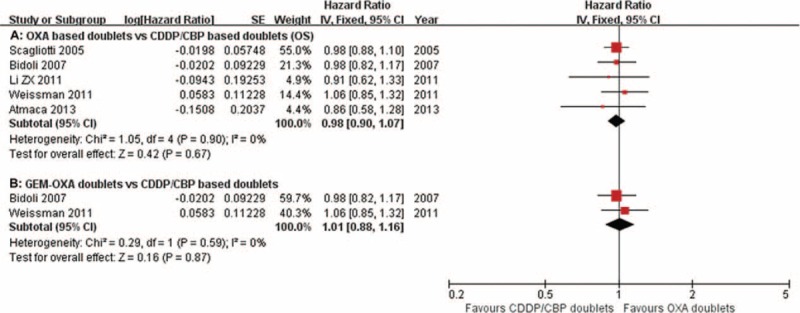
Meta-analysis of overall survival (OS) among patients receiving oxaliplatin doublets or carboplatin/cisplatin doublets. The pooled HR for OS shows that there was no significant difference between the 2 groups. Subgroup analysis revealed no significant differences in the oxaliplatin–gemcitabine doublets compared with the carboplatin/cisplastin doublets. GEM-OXA, gemcitabine/oxaliplatin regimens, HR, hazard ratio.
One-year survival rates were reported in 6 studies13,15,18,19,21,25 ranging from 18.6% to 59.4%. The pooled 1-year survival rates were 40.2% and 40.8% for oxaliplatin-based regimens and carboplatin/cisplatin-based regimens, respectively. The pooled RRs for 1-year survival also showed no difference between the oxaliplatin-based doublets and the carboplatin/cisplastin-based doublets (RR = 0.96, 95% CI = 0.72–1.27, P = 0.76; Figure 6A). Subgroup analysis failed to show any significant differences between the oxaliplatin–gemcitabine-based doublets and the taxane–oxaliplatin doublets compared with carboplatin/cisplastin doublets (RR = 1.01, 95% CI = 0.73–1.41, P = 0.95; Figure 6B and RR = 0.69, 95% CI = 0.36–1.34, P = 0.28; Figure 6C, respectively).
FIGURE 6.
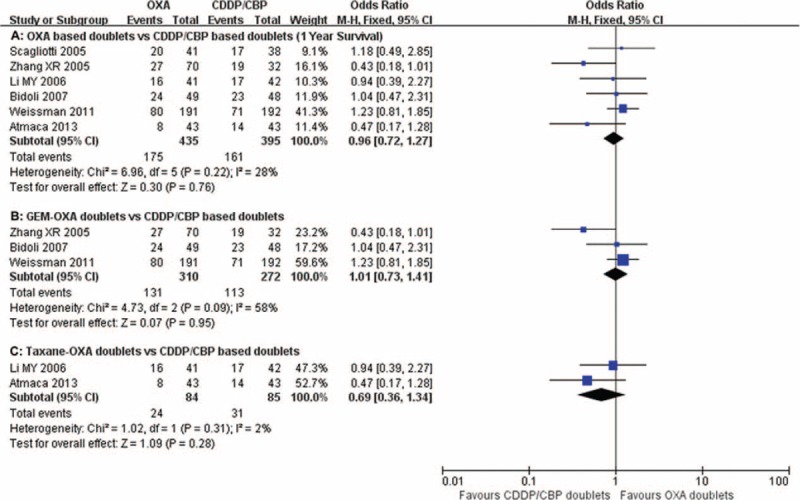
Meta-analysis of 1-year survival rates among patients receiving oxaliplatin doublets or carboplatin/cisplatin doublets. The pooled RR for 1-year survival rates shows that there were no significant differences between the 2 groups. Subgroup analysis revealed no significant differences between the oxaliplatin–gemcitabine doublets and taxane–oxaliplatin doublets compared with the carboplatin/cisplastin combinations. GEM-OXA, gemcitabine/oxaliplatin regimens; RR, relative risk; Taxane-OXA, taxane/oxaliplatin regimens.
Toxicity
The toxicity reported in the included trials, summarized according to the National Cancer Institute Common Toxicity Criteria is shown in Table 2 (only grade 3/4 toxicities are presented). Nonhematologic toxicity, such as Grades 3 and 4 nausea/vomiting, diarrhea, fatigue, and sensory neuropathy were comparable between the carboplatin/cisplatin-based group and the oxaliplatin-based group (Table 2).
TABLE 2.
Summary of the Relative Risks of Grade 3 or Worse Toxicity Comparing Oxaliplatin-Based Doublets Versus Carboplatin/Cisplatin-Based Doublets
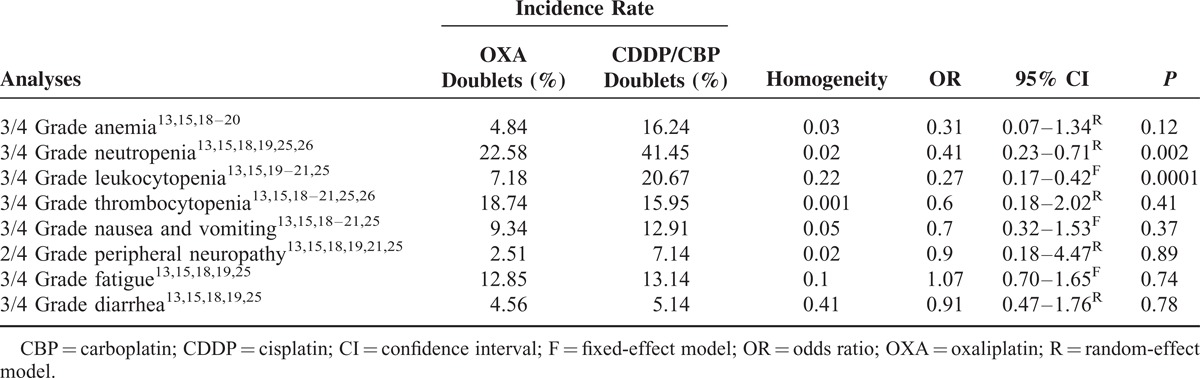
The most often reported Grades 3 and 4 adverse events were hematologic toxicity (ie, anemia, neutropenia, leukocytopenia, and thrombocytopenia). Grades 3 and 4 hematological toxicity is shown in Table 2. Grades 3 and 4 leukocytopenia (7.2% vs 20.7%, OR = 0.27, 95% CI = 0.17–0.42, P = 0.0001) and neutropenia (22.6% vs 41.5%, OR = 0.41, 95% CI = 0.23–0.71, P = 0.002) were less frequent in the oxaliplatin-based regimens than that in the carboplatin/cisplatin-based regimens. Grades 3 and 4 anemia were comparable between the carboplatin/cisplatin-based arms and the oxaliplatin-based arms. Heterogeneity was found for thrombocytopenia (I2 = 90.2%, P = 0.001), which was possibly due to the use of carboplatin or cisplatin combination in these studies. The pooled OR for thrombocytopenia showed no difference between the oxaliplatin-based doublets and the carboplatin/cisplatin-based doublets (18.7% vs 16.0%, OR = 0.60, 95% CI = 0.18–2.02, P = 0.41). Subgroup analysis according to different platinum formulations revealed that the oxaliplatin doublets caused less thrombocytopenia than the carboplatin doublets (5.2% vs 26.4%, OR = 0.15, 95% CI = 0.06–0.37, P = 0.0001) and had comparable effects to cisplatin doublets (23.2% vs 12.2%, OR = 1.56, 95% CI = 0.50–4.82, P = 0.44).
Publication Bias
After assessment by a funnel plot, Begg's test (P = 0.81), and Egger's test (P = 0.45), no publication bias was found.
DISCUSSION
Chemotherapy has been demonstrated to have a significant benefit in terms of the response rate and OS in patients with inoperable NSCLC. Currently, platinum combined with a third-generation agent regimen is regarded as the gold standard for first-line therapy for the majority of patients with NSCLC. Commonly prescribed platinum-based regimens consist of cisplatin or carboplatin combined with a third-generation drug. However, neither cisplatin nor carboplatin-based regimens have shown a clear superiority to the other treatments in terms of the response rate, PFS, and OS27,28; even though cisplatin seems to be slightly more active than carboplatin, but with a less favorable toxicity profile.29 The toxicities and the complexity of administration associated with cisplatin-based regimens limits their usefulness in a substantial proportion of patients, particularly the elderly and those in a poor condition. These toxicities may well offset the potential enhanced clinical benefits of cisplatin in the palliative setting. On the other hand, carboplatin commonly has dose-limiting myelosuppression, particularly thrombocytopenia.13 To improve the curative effect in advanced NSCLC, it is necessary to create novel treatment strategies with enhanced efficacy and/or a toxicity profile superior to that of current standard platinum-based combinations. Oxaliplatin is a third-generation platinum analog, with a similar mechanism of action to cisplatin. It is the most plausible candidate to replace cisplatin due to its apparently comparable efficacy and advantages, such as lower nephro, hematological, and gastrointestinal toxicity. Furthermore, it can be easily used in the outpatient setting and does not require specific hydration facilities.
The results15,19,22,25,26,30 from the clinical trials published to date suggest that oxaliplatin-based doublets could be potential first-line treatment options for patients with advanced NSCLC. While clinical efficacy is generally comparable to that of other currently used platinum-based regimens, the favorable toxicity profile of the oxaliplatin-based regimen makes it a desirable and appropriate alternative treatment, particularly for those patients unable to tolerate cisplatin and patients with important comorbidities. We conducted the first systematic review to address whether oxaliplatin had a comparable efficacy to cisplatin/carboplatin and to quantify any potential benefits. The toxicity profiles of oxaliplatin containing regimens were also investigated and documented.
Six of the 8 articles showed a superior efficacy of ORR in the CDDP/CBP-based group. However, statistical analysis including analysis of covariance (ANCOVA; data not shown) did not detect any significant differences between the OXA and CDDP/CBP groups. One possible reason is that the 8 selected articles were RCT studies, in which the age and clinical stages (which are the main factors affecting ORR) of patients in both the OXA and CDDP/CBP groups of each study were well balanced. For example, in Li's (2011) paper, the median age in the OXA and CDDP/CBP groups was 73 and 74 years, respectively, while in Li's (2006) paper, the median age was 48 years in both groups, which increased the intergroup heterogeneity and diminished differences between the OXA and CDDP/CBP groups.
Gemcitabine is one of the agents most commonly used in combination with platinum. A previous meta-analysis31 compared the efficacy of gemcitabine plus platinum combinations versus any other platinum-based regimen on survival outcomes. A subgroup analysis of 6 trials (n = 2481 patients) with a platinum-based third-generation comparator arm found a trend toward superior survival with gemcitabine-based regimens and improved PFS (HR = 0.89; 95% CI = 0.82–0.96; note the P value was not reported). Oxaliplatin/gemcitabine combination chemotherapy has been the most widely studied. The strong theoretical and biological rationales for their use arises from the observation of superposition sequence-dependent synergy between the 2 drugs.32 In a number of phase II studies in patients with metastatic NSCLC,15,30,33,34 the combination of oxaliplatin–gemcitabine demonstrated a favorable side-effect profile and an ORR that ranged from 23% to 36% with a median OS from 7.3 to 11.3 months. Our results have demonstrated that the efficacy was comparable between oxaliplatin–gemcitabine and gemcitabine plus carboplatin or cisplatin regimens, according to OS, TTP, ORR, DCR, and 1-year survival criteria. These outcomes correspond favorably with those reported in a recent meta-analysis of randomized phase II/III trials, which compared cisplatin and carboplatin.14
Taxane–platinum combinations form the mainstay of treatment for patients with advanced NSCLC in many parts of the world. In vitro data, which showed a synergistic effect between taxanes and oxaliplatin, have led to studies that combined the use of both types of drugs to treat patients with NSCLC. Two trials investigated the combination of oxaliplatin with taxanes (paclitaxel or docetaxel), and they reported encouraging results with the ORR ranging from 34.7% to 48% and a 1-year survival rate of 37% to 40% of patients.22,35 Our results have demonstrated that the efficacy was comparable between taxane–oxaliplatin doublets and taxane plus carboplatin or cisplatin regimens, according to ORR, DCR, and 1-year survival criteria.
With regard to toxicity, grades 3 and 4 nonhematological toxicities, such as diarrhea, fatigue, and vomiting/nausea and grades 3 and 4 hematological toxicities, such as thrombocytopenia and anemia, showed little difference between oxaliplatin doublets and carboplatin/cisplatin doublets. The most common adverse effect of oxaliplatin therapy has been reported to be sensory neuropathy. However, it was also associated with significantly fewer thromboembolic events and less severe neutropenia. In the present meta-analysis, we found that oxaliplatin doublets induced similar grades 3 and 4 sensory neuropathies (2.5% vs 7.1%, OR = 0.9, 95% CI = 0.18–4.47, P = 0.98) and thrombocytopenia (18.7% vs 16.0%, OR = 0.60, 95% CI = 0.18–2.02, P = 0.41) compared with carboplatin/cisplatin doublets. As to thromboembolic events, there was heterogeneity within the cisplatin/carboplatin subsets. Subgroup analysis showed that oxaliplatin doublets caused less thrombocytopenia than the carboplatin doublet (5.2% vs 26.4%, OR = 0.15, 95% CI = 0.06–0.37, P = 0.0001). We also found oxaliplatin doublets were associated with significantly less severe neutropenia (22.6% vs 41.5%, OR = 0.41, 95% CI = 0.23–0.71, P = 0.002) and leukocytopenia (7.2% vs 20.7%, OR = 0.27, 95% CI = 0.17–0.42, P = 0.0001). This is important because severe neutropenia and thromboembolic events are life-threatening toxicities and play a major role in the hospitalization and additional treatments required by patients receiving chemotherapy. Our meta-analysis indicates that oxaliplatin is particularly well tolerated in older patients and offers improved cost-effectiveness through increased patient survival rate and a significant reduction in the indirect costs of supportive care.
Although publication bias was not found according to the funnel plot, Begg's test, and Egger's test, the results need to be interpreted very cautiously because there were only 8 RCTs. It should be noted that our analysis was limited to the use of individual patient data, which in some cases was incomplete. All the outcome estimates were taken from published data, so the systematic biases and chance effects could not be minimized. Despite the limitations of our research, this meta-analysis indicated that oxaliplatin-based doublets achieved a comparable OS, TTP, 1-year survival, and response rates with favorable toxicities, compared with traditional carboplatin/cisplatin-based doublets, in patients with advanced NSCLC.
We conclude that the lack of significant differences in the statistic analysis does not preclude genuine differences in clinical efficacy, because higher diversities between the studies covered differences between the 2 groups in each study. More trials and larger sample size should be considered to continue tracing the question. Oxaliplatin combined with a third-generation drug should be considered as an efficient intervention for these patients, who cannot tolerate conventional carboplatin/cisplatin-based regimens, especially the elderly or very ill patients.
Footnotes
Abbreviations: ASCO = American Society of Clinical Oncology, CR = Complete response, DCR = Disease control rate, ESMO = European Society of Medical Oncology, HR = Hazard ratio, IASLC = International Association for the Study of Lung Cancer, NSCLC = Nonsmall cell lung cancer, OR = Odds ratios, ORR = Overall response rate, OS = Overall survival, PD = Progressive disease, PR = Partial response, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis, RCTs = Randomized controlled trials, RR = Relative risk, SD = Stable disease, TTP = Time-to-progression.
This work was supported by National Natural Science Foundation of China (81272615 and 81101737), Beijing Municipal Natural Science Foundation (7092103), Beijing Municipal “215” High-Level Health Person Foundation Project (2014–3–004), Beijing Municipal “Ten, Hundred, Thousand” Person Foundation Project (2011–2013), and Capital Medical University Sciences–Clinical Research Cooperation Foundation (2011–2012).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58:71–96. [DOI] [PubMed] [Google Scholar]
- 2.Morgensztern D, Ng SH, Gao F, et al. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol 2010; 5:29–33. [DOI] [PubMed] [Google Scholar]
- 3.Carney DN, Hansen HH. Non-small-cell lung cancer—stalemate or progress? N Engl J Med 2000; 343:1261–1262. [DOI] [PubMed] [Google Scholar]
- 4.Grilli R, Oxman AD, Julian JA. Chemotherapy for advanced non-small-cell lung cancer: how much benefit is enough? J Clin Oncol 1993; 11:1866–1872. [DOI] [PubMed] [Google Scholar]
- 5.Non-small Cell Lung Cancer Collaborative Group Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. Bmj 1995; 311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 6.D’Addario G, Pintilie M, Leighl NB, et al. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol 2005; 23:2926–2936. [DOI] [PubMed] [Google Scholar]
- 7.Belani CP, Lee JS, Socinski MA, et al. Randomized phase III trial comparing cisplatin-etoposide to carboplatin-paclitaxel in advanced or metastatic non-small cell lung cancer. Ann Oncol 2005; 16:1069–1075. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Kim SY, Jung KH, et al. Randomized phase II study of gemcitabine plus cisplatin versus etoposide plus cisplatin for the treatment of locally advanced or metastatic non-small cell lung cancer: Korean Cancer Study Group experience. Lung Cancer 2006; 52:75–81. [DOI] [PubMed] [Google Scholar]
- 9.Bonomi P, Kim K, Fairclough D, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol 2000; 18:623–631. [DOI] [PubMed] [Google Scholar]
- 10.Baggstrom MQ, Stinchcombe TE, Fried DB, et al. Third-generation chemotherapy agents in the treatment of advanced non-small cell lung cancer: a meta-analysis. J Thorac Oncol 2007; 2:845–853. [DOI] [PubMed] [Google Scholar]
- 11.de Castria TB, da Silva EM, Gois AF, et al. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst Rev 2013; 8:1–56. [DOI] [PubMed] [Google Scholar]
- 12.Azzoli CG, Baker S, Jr, Temin S, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 2009; 27:6251–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang XR, Hou M, Sun JD, et al. A randomized trial comparing oxaliplatin plus vinorelbine versus cisplatin plus vinorelbine for the treatment of patients with advanced non-small-cell lung cancer. Zhonghua zhong liu za zhi 2005; 27:743–746. [PubMed] [Google Scholar]
- 14.Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Instit 2007; 99:847–857. [DOI] [PubMed] [Google Scholar]
- 15.Atmaca A, Al-Batran SE, Werner D, et al. A randomised multicentre phase II study with cisplatin/docetaxel vs oxaliplatin/docetaxel as first-line therapy in patients with advanced or metastatic non-small cell lung cancer. Br J Cancer 2013; 108:265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raymond E, Faivre S, Chaney S, et al. Cellular and molecular pharmacology of oxaliplatin. Mol Cancer Ther 2002; 1:227–235. [PubMed] [Google Scholar]
- 17.Rixe O, Ortuzar W, Alvarez M, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem Pharmacol 1996; 52:1855–1865. [DOI] [PubMed] [Google Scholar]
- 18.Scagliotti GV, Kortsik C, Dark GG, et al. Pemetrexed combined with oxaliplatin or carboplatin as first-line treatment in advanced non-small cell lung cancer: a multicenter, randomized, phase II trial. Clin Cancer Res 2005; 11 (2 Pt 1):690–696. [PubMed] [Google Scholar]
- 19.Bidoli P, Zilembo N, Cortinovis D, et al. Randomized phase II three-arm trial with three platinum-based doublets in metastatic non-small-cell lung cancer. An Italian Trials in Medical Oncology study. Ann Oncol 2007; 18:461–467. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Hou M, Wang H, et al. A randomized study of gemcitabine plus oxaliplatin versus gemcitabine plus cisplatin as the 1st line chemotherapy for advanced non-small cell lung cancer in elderly patients. Zhongguo fei ai za zhi 2011; 14:588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Huang H, Tan J, et al. A randomized clinical trial on taxol plus oxaliplatin versus taxol plus cisplatin as first-line treatment in advanced non-small cell lung cancer. Zhongguo fei ai za zhi 2006; 9:452–454. [DOI] [PubMed] [Google Scholar]
- 22.Raez LE, Santos ES, Lopes G, et al. Efficacy and safety of oxaliplatin and docetaxel in patients with locally advanced and metastatic non-small-cell lung cancer (NSCLC). Lung Cancer 2006; 53:347–353. [DOI] [PubMed] [Google Scholar]
- 23.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weissman CH, Reynolds CH, Neubauer MA, et al. A phase III randomized trial of gemcitabine-oxaliplatin versus carboplatin-paclitaxel as first-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol 2011; 6:358–364. [DOI] [PubMed] [Google Scholar]
- 26.Le Chevalier T, Thezenas S, Breton JL, et al. Randomized phase II study of gemcitabine-oxaliplatin or gemcitabine-cisplatin in chemonaive patients with advanced non-small cell lung cancer—CLEO 05. J Clin Oncol 2006; 24 (Suppl):18S. [Google Scholar]
- 27.Kelly K, Crowley J, Bunn PA, Jr, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol 2001; 19:3210–3218. [DOI] [PubMed] [Google Scholar]
- 28.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346:92–98. [DOI] [PubMed] [Google Scholar]
- 29.Kakolyris S, Ziras N, Vamvakas L, et al. Gemcitabine plus oxaliplatin combination (GEMOX regimen) in pretreated patients with advanced non-small cell lung cancer (NSCLC): a multicenter phase II study. Lung Cancer 2006; 54:347–352. [DOI] [PubMed] [Google Scholar]
- 30.Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 2002; 20:4285–4291. [DOI] [PubMed] [Google Scholar]
- 31.Raez LE, Santos ES, Webb RT, et al. A multicenter phase II study of docetaxel, oxaliplatin, and bevacizumab in first-line therapy for unresectable locally advanced or metastatic non-squamous cell histology non-small-cell lung cancer (NSCLC). Cancer Chemother Pharmacol 2013; 72:1103–1110. [DOI] [PubMed] [Google Scholar]
- 32.Faivre S, Raymond E, Woynarowski JM, et al. Supraadditive effect of 2′,2′-difluorodeoxycytidine (gemcitabine) in combination with oxaliplatin in human cancer cell lines. Cancer Chemother Pharmacol 1999; 44:117–123. [DOI] [PubMed] [Google Scholar]
- 33.Mir O, Alexandre J, Ropert S, et al. Vinorelbine and oxaliplatin in stage IV nonsmall cell lung cancer patients unfit for cisplatin: a single-center experience. Anti-Cancer Drugs 2009; 20:105–108. [DOI] [PubMed] [Google Scholar]
- 34.Radhakrishnan A, Bitran JD, Milton DT, et al. Docetaxel and oxaliplatin as first-line therapy for advanced non-small cell lung cancer: a phase II trial. J Chemother 2009; 21:439–444. [DOI] [PubMed] [Google Scholar]
- 35.Winegarden JD, Mauer AM, Otterson GA, et al. A phase II study of oxaliplatin and paclitaxel in patients with advanced non-small-cell lung cancer. Ann Oncol 2004; 15:915–920. [DOI] [PubMed] [Google Scholar]


