Abstract
Epidemiologic studies have suggested that daily fluid intake that achieves at least 2.5 L of urine output per day is protective against kidney stones. However, the precise quantitative nature of the association between fluid intake and kidney stone risk, as well as the effect of specific types of fluids on such risk, are not entirely clear.
We conducted a systematic review and dose–response meta-analysis to quantitatively assess the association between fluid intake and kidney stone risk. Based on a literature search of the PubMed, Embase, and Cochrane Library databases, 15 relevant studies (10 cohort and 5 case–control studies) were selected for inclusion in the meta-analysis with 9601 cases and 351,081 total participants.
In the dose–response meta-analysis, we found that each 500 mL increase in water intake was associated with a significantly reduced risk of kidney stone formation (relative risk (RR) = 0.93; 95% CI: 0.87, 0.98; P < 0.01). Protective associations were also found for an increasing intake of tea (RR = 0.96; 95% CI: 0.93, 0.99; P = 0.02) and alcohol (RR = 0.80, 95% CI: 0.75, 0.85; P < 0.01). A borderline reverse association were observed on coffee intake and risk of kidney stone (RR = 0.88; 95% CI: 0.76, 1.00; P = 0.05). The risk of kidney stones was not significantly related to intake of juice (RR = 1.02, 95% CI: 0.95, 1.10; P = 0.64), soda (RR = 1.03; 95% CI: 0.90, 1.17; P = 0.65), or milk (RR = 0.96; 95% CI: 0.88, 1.03; P = 0.21). Subgroup analysis and sensitivity analyses showed inconsistent results on coffee, alcohol, and milk intake.
Increased water intake is associated with a reduced risk of kidney stones; increased consumption of tea and alcohol may reduce kidney stone risk. An average daily water intake was recommended for kidney stone prevention.
INTRODUCTION
Kidney stone is a common disease that has become increasingly prevalent over the past 2 decades.1 Currently, the kidney stone prevalence rate worldwide is approximately 1.7% to 8.8%.2,3 It is more likely for men aged 60 to 69 to develop a kidney stone.4 Kidney stones can have serious clinical and economic consequences. Indeed, patients who suffer from large stones usually need surgical treatment, and in the year 2000, the cost of kidney stones was approximately $2.81 billion in the United States alone.5
Increased fluid intake may help prevent the formation of stones by diluting urine concentration, decreasing urine acidity, and by taking away excess salt.6,7 These beneficial effects, however, may be offset by the tendency of increased fluid intake to dilute stone inhibitors such as magnesium, pyrophosphate, and glycosaminoglycan.8,9 A recent meta-analysis,10 based on the results of 2 randomized trials, concluded that high water intake decreased the long-term risk of kidney stone recurrence by approximately 60%. However, pooled evidence from only 2 studies is not conclusive. Additionally, data on the association between specific beverage types and risk of stone formation are sparse.
Accordingly, this study conducted a more extensive systematic review and meta-analysis of the quantitative relationship between fluid intake and risk of kidney stones, with a particular emphasis on examining the linear or nonlinear trends of the association. This study also examined the association of specific beverage types with kidney stone risk.
MATERIALS AND METHODS
This systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (PRISMA).11 There are no ethical issues involved in our study for our data were based on published studies.
Search Strategy
Eligible studies were identified by searching the PubMed, Embase, and Cochrane Library databases for relevant studies of the association between fluid intake and risk of kidney stones. The search included studies that were published through February 15, 2015. A manual search of the references of the retrieved articles and relevant reviews was also conducted. In order to identify studies with relevant information on the exposure of interest (fluid intake), databases were searched using the following terms: “water,” “fluid∗” “liquid∗,” “beverage∗,” “tea,” “alcohol,” “wine,” “beer,” “drink∗,” “soda,” “milk,” “diet,” “coffee,” “juice∗.” For the outcome of interest (kidney stone), search terms included: “kidney stone,” “kidney calculi,” “kidney calculus,” “renal stone,” “renal calculi,” “renal calculus,” and “nephrolithiasis.” The term “Humans” was used to limit the search results. No languages were limited during the search.
Study Inclusion and Exclusion Criteria
Studies were included in the meta-analysis if they: based on a cohort, case–control, or nested case–control design; defined kidney stone cases as patients diagnosed for the first time with a kidney stone without obvious bone disease; contained exposure information on at least one of the fluids of interest (water, coffee, tea, alcohol, juice, soda, milk); and had a sufficient data information. Additionally, to be included in the dose–response analyses, relevant studies were required to contain information on quantitative serving size with at least 3 quantitative categories. We excluded cross sectional studies, grey literature, and conference abstract. Studies which reported 24-hour urine biochemical changes after fluid intake were also excluded.
Data Extraction
Two reviewers conducted data extraction independent of one another. A standardized data collection form was used which extracted the following information: first author's name, publication year, country of study, follow-up, study design, sex, age range, number of cases, the total sample size or person-years of follow-up, quantitative serving size, effect size (odd ratio, relative risk, hazard ratio) with confidence intervals (CI), and control variables. When a given study presented models that adjusted for different numbers of control variables, data were extracted from the model that adjusted for the largest number of variables. Unadjusted results were extracted only when no other results were presented. When there was no information on serving size in an article, an assumption was made that a serving size of 1 cup (or glass) was equal to 150 mL. As for alcohol, 15.2 mL of alcohol, 120 mL wine, or 240 mL beer were assumed to contain approximately 12 g alcohol (1 standard drink).12 Different periods of study (Stage I or Stage II) were regarded as 2 different studies. If multiple publications from the same data set or study were detected, we only included the publication with the longer follow-up period. Potential errors were checked by a third-party author and any divergences were resolved by discussion.
Statistical Analysis
The term “water,” “total fluids” were regarded as water intake in this article. The relative risk (RR) was used to measure the association between beverage intake and risk of kidney stones. Odds ratios and hazard ratios were regarded to be approximately equal to the RR, since the prevalence of kidney stones in the selected studies was approximately 10% or less.13
For dose–response analyses, we fitted both linear and nonlinear models to the data (n ≥ 3). More specifically, we first estimated the RR of kidney stones per unit increment (500 mL for water, 110 mL for coffee and tea, 150 for juice, and 10 g for alcohol) of exposure within each study by generalized least-squares regression models. From these models, the regression coefficients were combined in a random-effects model, with fluid intake modeled as a continuous, linear variable.14 Then, to model possible nonlinear exposure-outcome associations, we ran models in which the exposure was modeled as a restricted cubic spline with 3 fixed knots at 10th, 50th, and 90th percentile of the exposure distribution.15 The median values or middle point of each serving size were assigned to the corresponding relative risk for each study.16 When there were open-ended categories, we assumed the length of the open ended interval to be the same as that of the adjacent interval.17 When the lowest category was not analyzed as reference category (such as),18 the method of Hamling et al19 was used to convert the results. If a study reported incomplete data, we used the method of Bekkering et al20 to evaluate the missing data. In the dose–response analyses, nonlinearity was assessed by assuming that the regression coefficient of the second spline equaled zero, which is the Wald test.15
Not all studies reported dose–response information for specific fluids. Thus, we conducted analyses which analyzed the association of specific fluid intake modeled as a dichotomous variable (intake of 1–2 servings, or about 110–240 mL vs. no intake) with kidney stone risk. If a given study did not contain dose information at the 1 to 2 serving level, the results of the category nearest to that level was used in the pooled analysis. Further sensitivity analyses were conducted to evaluate whether this influenced the results. The I2 statistics, which varied from 0% to 100%, were used to test heterogeneity, and were categorized as low (0–40%), moderate (30–60%), substantial (50–90%), and considerable (75–100%).21 A fixed-effects model was applied until slight heterogeneity (I2 < 30%) was detected; or a random-effect model was used. When the value of I2 was >75%, the combination would be terminated.21 When studies reported results separately by sex or other subtypes, the RRs were pooled using a fixed-effects model before adding the RRs to the overall meta-analysis.22 The inverse variance method was used to calculate the weight of each RR.
Egger's regression test was used to detect potential publication bias.23 Subgroup analyses were used to detect possible sources of heterogeneity and potential difference among subgroups. Sensitivity analyses were also used to detect whether results were influenced by transformation of the data, the quality of included studies, and potential confounding factors. All P-values were 2-sided.
RESULTS
Search Results and Study Characteristics
An initial literature search identified 2739 studies. After removing duplicate and unrelated studies, 2 cross-sectional studies,24,25 and 1 case–control study26 that included kidney stone cases with bone disease, 15 studies remained for the meta-analysis. A flow diagram illustrating how studies were selected is shown in Figure 1.
FIGURE 1.
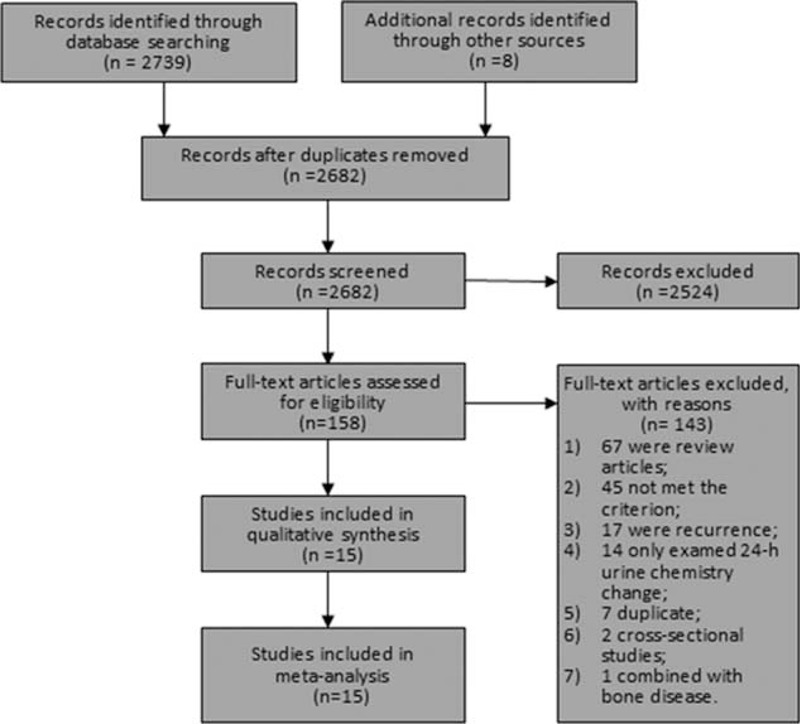
The flow diagram of study inclusion.
Among the included 15 studies, 10 were cohort studies, and 5 were case–control studies. The 15 studies combined had 9601 cases and 351,081 total participants (duplicate data from different studies were not counted). Three27–29 of the case–control studies described matching information that was utilized in matching controls to cases, while the other 2 case–control studies30,31 did not report such information. For cohort studies, the follow-up period ranged from 4 to 20 years. Nine of the studies were conducted in the United States, while 4 were conducted in China, 1 in Finland, and 1 in the United Kingdom.
Assessment of study quality was conducted according to the Newcastle Ottowa-Scale (NOS).32 The scale contains 9 items, and each item accounts for 1 point. Among the included studies, quality scores ranged from 3 to 8 points, with a mean score of 7.3 for cohort studies and mean of 4.2 for case–control studies. The main characteristics of each study are shown in Table 1 .
TABLE 1.
Detailed Characteristic of Included Studies
Water Intake and Risk of Kidney Stone
Nine studies27,30,31,33–38 reported on the association between water intake and risk of kidney stones, with 3 of the studies33,34,38 being multiple publications of study.35 All of these studies included dose–response data. The combined RR for every 500 mL increment of water intake (linearity) a day was 0.93 (95% CI: 0.87, 0.98; P < 0.01; Figure 2). Substantial heterogeneity (I2 = 78.3%, P < 0.01) was detected among studies. Little evidence of nonlinear association was detected (P for nonlinearity test = 0.22). When using 1500 mL of daily water intake as the inference dose (RR = 1), the RRs of kidney stones for 1000 mL (approximate), 2000, 2500, and 3100 mL were 1.07 (95% CI: 1.05, 1.09), 0.92 (95% CI: 0.89, 0.95), 0.84 (95% CI: 0.78, 0.91), and 0.74 (95% CI: 0.65, 0.86), respectively.
FIGURE 2.
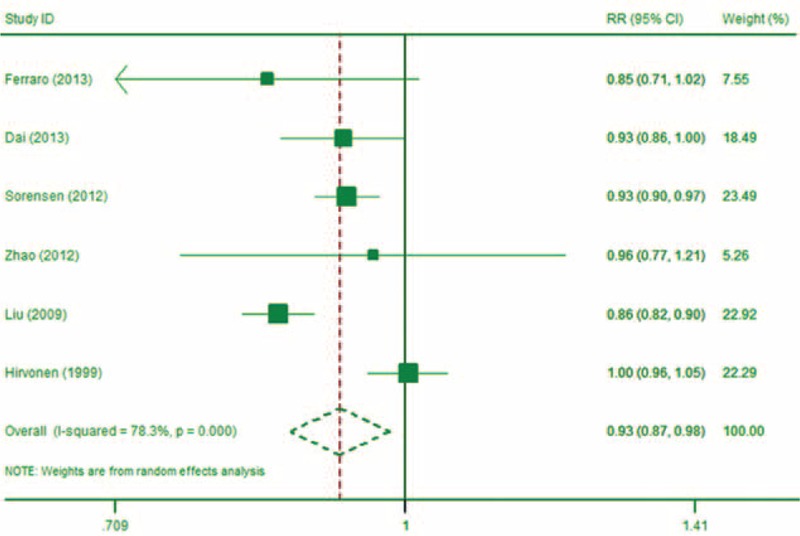
The forest plot of the linear association between water intake and risk of kidney stone (every 500 mL increment).
Coffee Consumption and Risk of Kidney Stones
Seven studies6,29,30,36,38–40 investigated the relationship between coffee consumption and risk of kidney stones (Table 1 ). Two studies6,40 were identified as multiple publications of study.38 From the remaining 5 studies, three30,38,39 reported dose–response relationship information, while two29,39 did not. Our meta-analysis showed that, compared to no coffee consumption, drinking 1 to 2 cups (about 110–240 mL) of coffee a day was associated with a RR of kidney stones of 0.88 (95% CI: 0.76, 1.00; P = 0.05; Figure 3). Moderate heterogeneity (I2 = 54.7%) was detected among studies. In dose–response meta-analysis, the RR for every 110 mL increment of coffee intake was 0.90 (95% CI: 0.87, 0.93; P < 0.01), and the Wald test showed nonsignificant of nonlinearity (P = 0.77).
FIGURE 3.
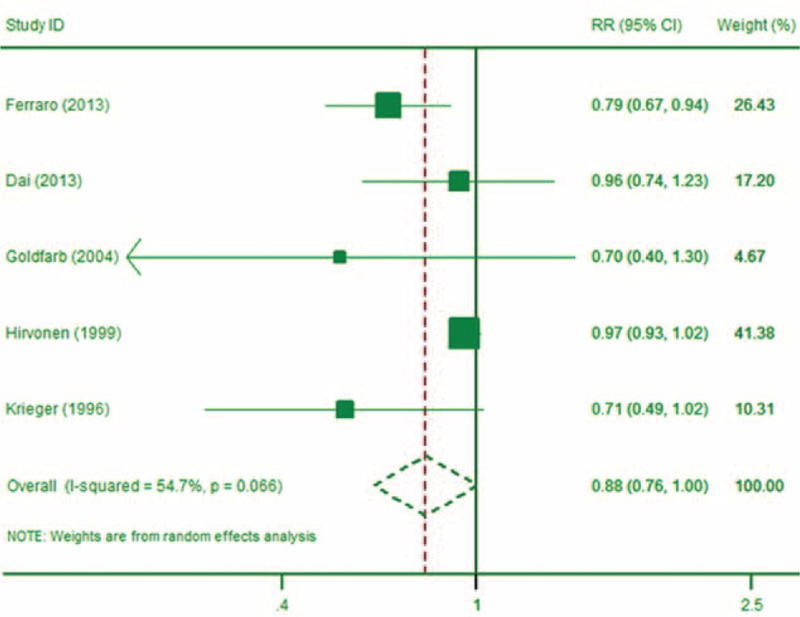
The forest plot of the association between coffee intake and risk of kidney stone (1–2 servings).
Tea Consumption and Risk of Kidney Stones
Nine studies6,28,29,30,31,36,38–40 reported data on the association between tea consumption and kidney stones. Studies6,40 were identified as the same publication of study.38 After excluding multiple publications, 3 studies reported dose–response information.30,38,39 The meta-analysis showed that, compared to no tea consumption, those who consumed 1 to 2 cups of tea per day had a RR of kidney stones of 1.06 (95% CI: 0.94, 1.20; P = 0.32; I2 = 45.5%; Figure 4). When modeled as a continuous variable, each 110 mL/day increase in tea consumption was associated with a kidney stone RR of 0.96 (95% CI: 0.93, 0.99; P = 0.02), and there was some evidence this relationship was borderline nonlinear (P = 0.07 for nonlinearity test). The protective effect of tea consumption appeared to begin at an intake level of approximately 250 mL/day. Compared to the reference dose (0 mL), those whose daily tea intake was 102, 204, 257, 525, and 825 mL, had a RR of kidney stones of 1.02 (95% CI:0.95, 1.09), 0.97 (95% CI: 0.90, 1.04), 0.91 (95% CI: 0.86, 0.99), 0.59 (95% CI: 0.39, 0.89), and 0.33 (95% CI:0.13, 0.84), respectively.
FIGURE 4.
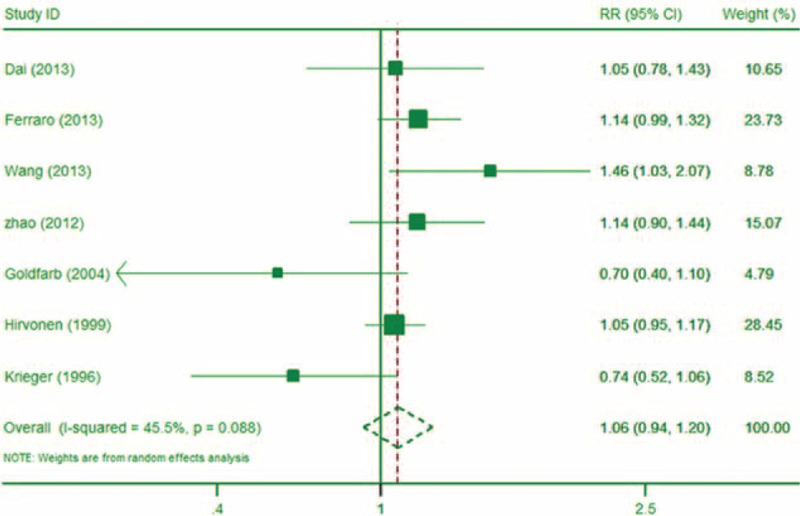
The forest plot of the association between tea intake and risk of kidney stone (1–2 servings).
Alcohol Intake and Risk of Kidney Stones
Nine studies6,18,27,29,30,36,38–40 with 7 of the studies being unique,18,27,29,30,36,38,39 reported on the association between alcohol intake and risk of kidney stones. Of these studies,18,30,36,38 reported dose–response information. Studies6,40 were identified as the same publication of study.38 The meta-analysis showed that, compared to those who did not drink alcohol, those who drank about 1 standard drink (12 g) of alcohol per day had a RR of kidney stones of 0.80 (95% CI: 0.63, 1.01; P = 0.06; I2 = 67.1%; Figure 5). When modeled as a continuous variable, each 10 g/day increase in alcohol consumption was associated with a kidney stone RR of 0.80 (95% CI: 0.75, 0.85; P < 0.01), and there was no significant evidence that this association was nonlinear (P = 0.73 for nonlinearity test).
FIGURE 5.
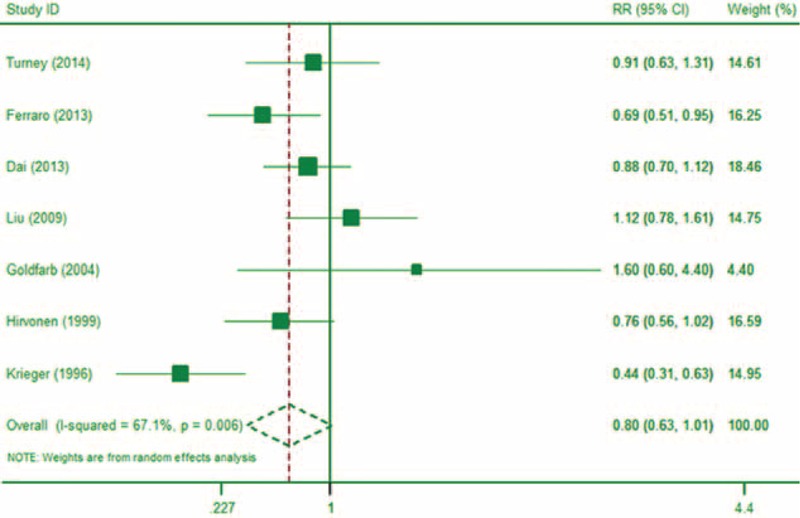
The forest plot of the association between alcohol intake and risk of kidney stone (1–2 servings).
Other Beverages and Risk of Kidney Stones
After excluding multiple publications, 3 studies30,36,38 reported on the association between juice intake and risk of kidney stones, 4 studies29,30,36,38 reported on the association between soda intake and risk of kidney stones, and 5 studies30,31,36,38,39 reported on the association between milk consumption and kidney stone risk. Limited studies reported data that could be used for dose–response analyses. The overall meta-analysis showed that, compared to those with no juice consumption, those with 1 to 2 cups per day of juice intake had a RR of kidney stones of 1.02 (95% CI: 0.95, 1.10; P = 0.64; I2 = 0.0%); compared to those with no soda consumption, those with 1 to 2 cups per day of soda intake had a RR of kidney stones of 1.03 (95% CI: 0.90, 1.17; P = 0.65; I2 = 42.6%). Additionally, compared to those with no milk consumption, those who consumed 1 to 2 cups of milk per day had a kidney stone RR of 0.96 (95% CI: 0.88, 1.03; P = 0.21; I2 = 0.0%) (see Figure 6).
FIGURE 6.
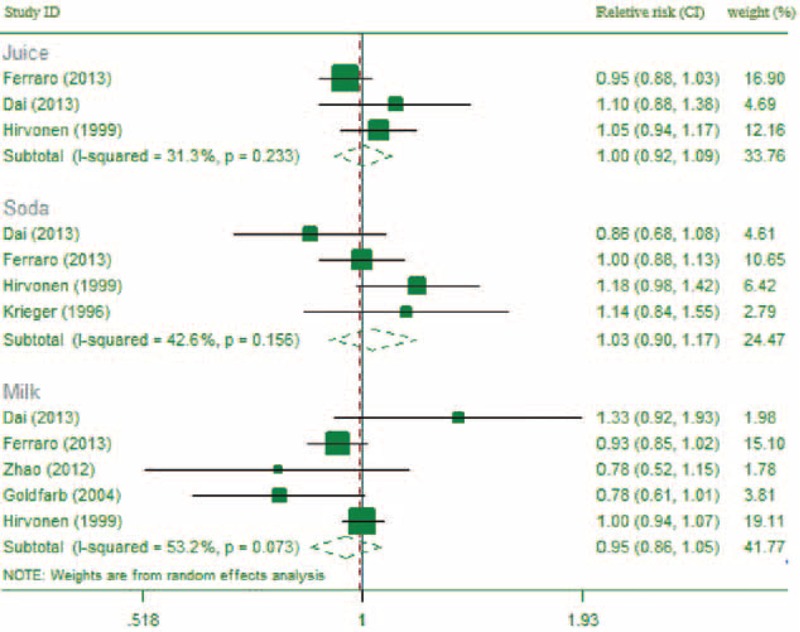
The forest plot of the association between other beverages intake and risk of kidney stone (1–2 servings).
Subgroup Analysis and Sensitivity Analysis
Subgroup analyses were conducted on country, study design, and other subtypes (if reported, such as sex). Apart from coffee and alcohol, no substantial changes of the results were found between subgroups (Table 2).
TABLE 1 (Continued).
Detailed Characteristic of Included Studies
TABLE 2.
Results of Subgroup Analysis
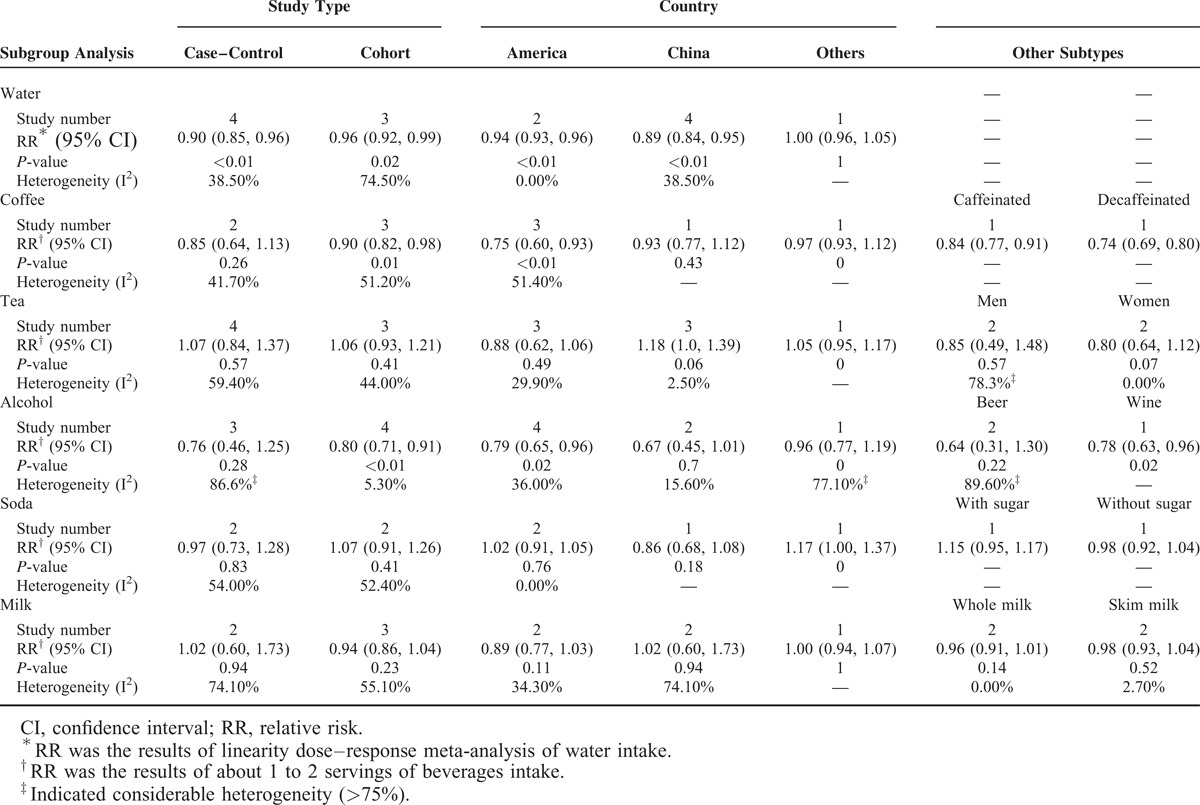
For coffee intake, the kidney stone RR associated with 1 to 2 servings of coffee per day was not significant in the subgroup of case–control studies (RR = 0.85, 95% CI: 0.64, 1.13; P = 0.26; I2 = 41.7%). A subgroup analysis showed that alcohol intake was not associated with a lower risk of kidney stones (RR = 0.76, 95% CI: 0.46, 1.25; P = 0.28; I2 = 86.6%) in case–control studies. Variation among other subgroups could not be assessed reliably due to small numbers.
As to heterogeneity of results, in most cases, the case–control studies showed a greater heterogeneity than cohort studies. Additionally, studies conducted in the United States showed mild heterogeneity, while considerably greater degrees of heterogeneity were observed in studies conducted in other countries (Finland, British).
Sensitivity analyses were conducted by omitting 1 study at a time to see whether the omission of the study influenced the overall results. We found that, for alcohol intake (about 12 g), 2 studies27,39 substantially influenced the results, as omitting either study led the results to become statistically significant (RRomit27 = 0.78, 95% CI: 0.61, 0.99; RRomit39 = 0.77, 95% CI: 0.61, 0.97). For milk intake, when omitting study,30 the results reached to statistical significant (RR = 0.90, 95% CI: 0.82, 0.98). Other fluids showed robust results.
Publication Bias
The meta-analysis results for water, tea, and alcohol intake were examined for publication bias (there were not enough studies to conduct publication bias analyses for other beverages). Egger's regression test of publication bias did not show strong evidence of publication bias (P for water = 0.92, P for tea = 0.55, P for alcohol = 0.76).
DISCUSSION
This is the first meta-analysis to have investigated the association of different types of beverages with risk of kidney stones. The meta-analysis confirmed that water intake was associated with a reduced risk of kidney stones. It also found that coffee, tea, and alcohol intake may reduce the risk of kidney stones. In contrast, juice, soda, and milk intake were not associated with kidney stone risk. Further subgroup analyses and sensitivity analyses showed unstable results for coffee and alcohol.
By increasing urine volume, increased water intake can dilute urine concentration, reduce CaOx super saturation, decrease urine acid, and remove salt.6,41 The European Association of Urology guidelines42 suggest that daily water intake should achieve at least 2.5 L of urine volume in order to prevent kidney stones. The present meta-analysis found no obvious threshold at which water intake began to be associated with reduced kidney stone risk. Instead, any increase in water intake, even at low levels of intake, was associated with reduced kidney stone risk. Thus, an average daily water intake should be recommended. The results of the present meta-analysis are partly consistent with a previous meta-analysis,10 which found that increased fluid intake (>2000 mL/day) was associated with a 61% reduction in the risk of kidney stones. Another conference abstract43 comparing the highest versus lowest level of total fluid intake, observed a 60–80% deceased risk of kidney stone in the highest level group. In our meta-analysis, greater than 2000 mL of water intake per day reduced the risk of first kidney stone occurrence risk by at least 8% compared to 1500 mL daily intake, and the highest (3100 mL) category showed a 26% reduction of kidney stone risk compared to the reference category (1500 mL). Compared to the earlier meta-analysis, our meta-analysis quantitatively evaluated the effects of different doses of water intake and thus provides more refined information.
Caffeine, which is present in both in coffee and tea, is a potential risk factor for kidney stones because it may increase the urinary calcium/creatinine ratio.44 In our study, coffee and tea intake were associated with a reduced risk of kidney stones when modeled as a continuous variable but not as a dichotomous variable. This may suggest a dose-dependent relationship between coffee (and tea) intake in prevention of stones. It is possible that some beneficial substances in coffee and tea (such as calcium)34 offset the influence of caffeine. But this result may be influenced by the inconsistency between different study designs. Further studies, especially based on cohort design, were needed.
Tea consumption appeared to show a borderline nonlinear relationship with kidney stone risk, with a reduction in risk seen mostly at intake levels above 250 mL/day. Animal experiments support this result.45,46 In rats, green tea consumption significantly decreased urinary oxalate excretion and calcium oxalate deposit formation, possibly by the antioxidative action of epigallocatechin gallate and the activity of superoxide dismutase.45,46 However, it is difficult to explain why a reduction in kidney stone risk would only occur above a certain threshold of tea consumption, though increased consumption of water might partially explain such a threshold effect.
Our meta-analysis on alcohol consumption showed inconsistent results. Although 12 g of alcohol intake showed no prevention effect on stone formation, the dose–response meta-analysis indicates alcohol intake was associated with a reduced risk of kidney stones. Our subgroup analysis also showed inconsistent results by study design and country, indicating the potential benefit effect on stone prevention. There are limited studies focusing on the mechanism by which alcohol intake could affect kidney stone risk. One possible mechanism may be the diuretic action of alcohol.47 This hypothesis may also partly explain why tea and coffee are associated with a reduced risk of kidney stones. But our results for alcohol were not particularly stable, and should therefore be interpreted with caution.
For juice intake, the overall meta-analysis showed no association between juice intake and risk of kidney stones. But the limited number of studies may results in bias. Moreover, the present study did not have sufficient data on the relation of lemon juice, cranberry juice, or pomegranate juice to kidney stone risk, though previous studies have reported that these juices are associated with a reduction in kidney stone risk.48–51 Further studies are needed.
Regarding soft drinks, the meta-analysis of Fink et al10 showed that decreased soft drink intake lowered the risk of kidney stones in patients with a high baseline consumption of soft drinks. In our meta-analysis, 110 to 240 mL of soda intake per day was not associated with stone risk. But the present study had no more data on other doses intake and the risk of stones.
In our meta-analysis, milk intake (about 1–2 servings a day) was not associated with kidney stone risk. Subgroup analyses also failed to find associations of whole or skim milk with kidney stone risk. The effect of higher doses of milk intake is still not known. But in the large cohort study of Goldfarb et al,39 intake of 5 or more cups of milk intake a day was not associated with stone risk.
We should note that confounding factors may have influenced our results.52 The most important confounder is water intake, for each type of beverage contains large amounts of water. It is difficult to control for the confounding influence of water, either by study design or by statistical adjustment. Therefore, the effects of coffee, tea, and alcohol may be overestimated while the effects of juice, soda, and milk may be underestimated. That is, coffee, tea, and alcohol may actually be less protective against kidney stone risk than our results suggest. Conversely, juice, soda, and milk intake may increase the risk of kidney stone more than is suggested by our results, since water may offset part of the risk associated with those beverages. Other variables, such as genetic factors,53 hardness of drinking water,54 concentration of beverages, body mass index,35 and geographical environment55 (such as desert) may also potentially confound the association between fluid intake and kidney stone risk. However, our sensitivity analyses showed that our results were robust, which suggests that the influence of confounders in our study was mild.
Strength and Limitations
The present study's comprehensive literature search, and strict study design, lends credibility to the study's results. Linear and nonlinear dose–response meta-analysis quantitatively evaluated the association of different levels of beverages intake on kidney stone risk, providing a more refined analysis than some prior studies. Additionally, subgroup and sensitivity analyses suggested that our study's results were robust in most cases, which strengthens the conclusions of our study.
This present study had some limitations. One limitation was that our evaluation and transformation of the raw data was fairly crude. A second limitation was that there were limited numbers of cohort studies focusing on the association between fluid intake and the risk of kidney stones. This small number of cohort studies is potentially problematic, since some research indicates that random errors induced by a small numbers of trials may cause bias in the results of meta-analyses.56,57 A third limitation was that different categories of fluid intake among studies made it difficult to pool results across studies. Indeed, some studies only reported the effect of any versus no fluid intake, or high versus no intake, while others reported 110 mL versus no intake of fluids. Although dose–response meta-analysis was used to overcome this problem, for soda and milk, there were limited studies reporting relevant data that could be used in a dose–response analysis. Furthermore, differences in how studies categorized fluid intake may have contributed to some of the heterogeneity we observed in our results, especially for juice intake. A fourth limitation was that, in our meta-analysis, most of the studies were conducted in the United States and China, and may not be generalizable to other countries. Fifth, we did not examine interactions among subgroups because the subgroup sample sizes were too small for such analyses to produce statistically reliable results.
CONCLUSIONS
Increased water are associated with a reduced risk of developing kidney stones; increased intake of coffee, tea, and alcohol showed potential benefits on stones prevention, but needs to be confirmed further. Soda and milk intake appeared to be unrelated to stone risk. Current evidence is insufficient for definitively determining the relationship between juice intake and risk of kidney stones.
Footnotes
Abbreviations: CI = confidence intervals, HR = hazard ratios, NOS = Newcastle Ottowa-Scale, ORs = odds ratios, RRs = relevant risks.
CX, CZ, and X-LW contributed equally to this work.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Pearle MS, Goldfarb DS, Assimos DG, et al. Medical management of kidney stones: AUA guideline. J Urol 2014; 192:316–324. [DOI] [PubMed] [Google Scholar]
- 2.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 2003; 63:1817–1823. [DOI] [PubMed] [Google Scholar]
- 3.Scales CD, Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol 2012; 62:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieske JC, Peña de la Vega LS, Slezak JM, et al. Renal stone epidemiology in Rochester, Minnesota: an update. Kidney Int 2006; 69:760–764. [DOI] [PubMed] [Google Scholar]
- 5.Pearle MS, Calhoun EA, Curhan GC. Urologic diseases in America project: urolithiasis. J Urol 2005; 173:848–857. [DOI] [PubMed] [Google Scholar]
- 6.Curhan GC, Walter C, Willett WC, et al. Beverage use and risk for kidney stones in women. Ann Intern Med 1998; 128:534–540. [DOI] [PubMed] [Google Scholar]
- 7.Borghi L, Meschi T, Amato F, et al. Urinary volume, water, and the recurrence in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996; 155:839–843. [PubMed] [Google Scholar]
- 8.Robertson WG. Nordin BEC. Urinary tract calculi. Metabolic Bone and Stone Disease. New York: Churchill Livingstone; 1984. 271–326. [Google Scholar]
- 9.Goldfarb S. The role of diet in the pathogenesis and therapy of nephrolithiasis. Endocrinol Metab Clin North Am 1990; 19:805–820. [PubMed] [Google Scholar]
- 10.Fink HA, Akornor JW, Garimella, et al. Diet, fluid, or supplements for secondary prevention of nephrolithiasis: a systematic review and meta-analysis of randomized trials. Eur Urol 2009; 56:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151:264–269. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. Analysis of U.S.A. Multicenter Trial Results. 2014. http://www.fda.gov/ohrms/dockets/ac/02/Slides/3857S1_05_Lipha%20-%20Mason/sld008.htm. Accessed January 2015 [Google Scholar]
- 13.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998; 280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 14.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2005; 6:40–57. [Google Scholar]
- 15.Orsini A. Multivariate dose-response meta-analysis: an update on GLST. Nordic and Baltic Users Group Meeting; Stockholm, Sweden. 2013. http://www.stata.com/meeting/nordic-and-baltic13/abst. Accessed January 2015 [Google Scholar]
- 16.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology 1993; 4:218–228. [DOI] [PubMed] [Google Scholar]
- 17.Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 2011; 343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turney BW, Appleby PN, Reynard JM, et al. Diet and risk of kidney stones in the Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Epidemiol 2014; 29:363–369. [DOI] [PubMed] [Google Scholar]
- 19.Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons form a set of estimates presented by exposure level or disease category. Stat Med 2008; 27:954–970. [DOI] [PubMed] [Google Scholar]
- 20.Bekkering GE, Harris RJ, Thomas S, et al. How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta-analysis? Am J Epidemiol 2008; 167:1017–1026. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S. eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. http://www.cochrane-handbook.org. Accessed January 2015 [Google Scholar]
- 22.Aune D, Saugstad OD, Henriksen T, et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 2014; 311:1536–1546. [DOI] [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safarinejad MR. Adult urolithiasis in a population-based study in Iran: prevalence, incidence, and associated risk factors. Urol Res 2007; 35:73–82. [DOI] [PubMed] [Google Scholar]
- 25.Soucie JM, Coates RJ, McClellan W, et al. Relation between geographic variability in kidney stones prevalence and risk factors for stones. Am J Epidemiol 1996; 143:487–495. [DOI] [PubMed] [Google Scholar]
- 26.Chen YY, Roseman JM, Devivo MJ, et al. Does fluid amount and choice influence urinary stone formation in persons with spinal cord injury? Arch Phys Med Rehabil 2002; 83:1002–1008. [DOI] [PubMed] [Google Scholar]
- 27.Liu CC, Huang SP, Wu WJ, et al. The impact of cigarette smoking, alcohol drinking and betel quid chewing on the risk of calcium urolithiasis. Ann Epidemiol 2009; 19:539–545. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Luo GT, Niu WJ, et al. Risk factors for the kidney stones: a hospital-based case-control study in a district hospital in Beijing. Beijing Da Xue Xue Bao 2013; 45:971–974. [PubMed] [Google Scholar]
- 29.Krieger JN, Kronmal RA, Coxon V, et al. Dietary and behavioral risk factors for urolithiasis: potential implications for prevention. Am J Kidney Dis 1996; 28:195–201. [DOI] [PubMed] [Google Scholar]
- 30.Dai M, Zhao A, Liu A, et al. Dietary factors and risk of kidney stone: a case-control study in southern China. J Ren Nutr 2013; 23:e21–e28. [DOI] [PubMed] [Google Scholar]
- 31.Zhao A, Dai M, Chen YJ, et al. Risk factors associated with nephrolithiasis: a case-control study in China. Asia Pac J Public Health 2012; (Epub ahead of print). PMID: 22593218. [DOI] [PubMed] [Google Scholar]
- 32.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses; 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed January 2015 [Google Scholar]
- 33.Curhan GC, Willett WC, Rimm EB, et al. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993; 328:833–838. [DOI] [PubMed] [Google Scholar]
- 34.Curhan GC, Willett WC, Knight EL, et al. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004; 164:885–891. [DOI] [PubMed] [Google Scholar]
- 35.Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol 2004; 15:3225–3232. [DOI] [PubMed] [Google Scholar]
- 36.Hirvonen T, Pietinen P, Virtanen M, et al. Nutrient intake and use of beverages and the risk of kidney stones among male smokers. Am J Epidemiol 1999; 150:187–194. [DOI] [PubMed] [Google Scholar]
- 37.Sorensen MD, Kahn AJ, Reiner AP, et al. Impact of nutritional factors on incident kidney stone formation: a report from the WHI OS. J Urol 2012; 187:1645–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferraro PM, Taylor EN, Gambaro G, et al. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol 2013; 8:1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldfarb DS, Fischer ME, Keich Y, et al. A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int 2005; 67:1053–1061. [DOI] [PubMed] [Google Scholar]
- 40.Curhan GC, Willett WC, Rimm EB, et al. Prospective study of beverage use and the risk of kidney stones. Am J Epidemiol 1996; 143:240–247. [DOI] [PubMed] [Google Scholar]
- 41.Borghi L, Guerra A, Meschi T, et al. Relationship between super saturation and calcium oxalate crystallization in normals and idiopathic calcium oxalate stone formers. Kidney Int 1999; 55:1041–1050. [DOI] [PubMed] [Google Scholar]
- 42.Türk C, Knoll T, Petrik A, et al. Guidelines on urolithiasis. Uroweb 2014; http://www.uroweb.org/guidelines/online-guidelines/http://www.uroweb.org/guidelines/online-guidelines/. Accessed January 2015. [Google Scholar]
- 43.Cheungpasitporn W, Erickson SB, Lieske JC. Treatment effect and safety of high fluid intake for the prevention of incident and recurrent kidney stones: a meta-analysis. http://tinyurl.com/llpsm2n Accessed May 21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massey LK, Sutton RA. Acute caffeine effects on urine composition and calcium kidney stone risk in calcium stone formers. J Urol 2004; 172:555–558. [DOI] [PubMed] [Google Scholar]
- 45.Itoh Y, Yasui T, Okada A, et al. Preventive effects of green tea on renal stone formation and the role of oxidative stress in nephrolithiasis. J Urol 2005; 173:271–275. [DOI] [PubMed] [Google Scholar]
- 46.Jeong BC, Kim BS, Kim JI, et al. Effects of green tea on urinary stone formation: an in vivo and in vitro study. J Endourol 2006; 20:356–361. [DOI] [PubMed] [Google Scholar]
- 47.Eggleton MG. The diuretic action of alcohol in man. J Physiol 1942; 101:172–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Odvina CV. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin J Am Soc Nephrol 2006; 1:1269–1274. [DOI] [PubMed] [Google Scholar]
- 49.Tugcu V, Kemahli E, Ozbek E, et al. Protective effect of a potent antioxidant, pomegranate juice, in the kidney of rats with nephrolithiasis induced by ethylene glycol. J Endourol 2008; 2:2723–2731. [DOI] [PubMed] [Google Scholar]
- 50.McHarg T, Rodgers A, Charlton K. Influence of cranberry juice on the urinary risk factors for calcium oxalate kidney stone formation. BJU Int 2003; 92:765–768. [DOI] [PubMed] [Google Scholar]
- 51.Touhami M, Laroubi A, Elhabazi K, et al. Lemon juice has protective activity in a rat urolithiasis model. BMC Urol 2007; 7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozkaya O, Söylemezoğlu O, Misirlioğlu M, et al. Polymorphisms in the vitamin D receptor gene and the risk of calcium nephrolithiasis in children. Eur Urol 2003; 44:150–154. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz BF, Schenkman NS, Bruce JE, et al. Calcium nephrolithiasis: effect of water hardness on urinary electrolytes. Urology 2002; 60:23–27. [DOI] [PubMed] [Google Scholar]
- 54.Xu C, Zeng XT, Liu TZ, et al. Fruits and vegetables intake and risk of bladder cancer: a PRISMA-compliant systematic review and dose-response meta-analysis of prospective cohort studies. Medicine 2015; 94:e759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masterson JH, Jourdain VJ, Collard DA, et al. Changes in urine parameters after desert exposure: assessment of stone risk in United States Marines transiently exposed to a desert environment. J Urol 2013; 189:165–170. [DOI] [PubMed] [Google Scholar]
- 56.Pogue J, Yusuf S. Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet 1998; 351:47–52. [DOI] [PubMed] [Google Scholar]
- 57.Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol 2009; 38:276–286. [DOI] [PubMed] [Google Scholar]


