Abstract
We employed a population-based cohort sample to explore the risk of coronary heart disease (CHD) in relation to osteoporosis in an Asian population.
We designed a retrospective population-based cohort study from 2000 to 2010 with data obtained from Taiwan's Longitudinal Health Insurance Database. A total of 19,456 patients aged 45 years or older who had no history of CHD and had a diagnosis of osteoporosis were identified as the osteoporosis cohort. The patients in the comparison cohort were randomly selected and frequency matched according to age, sex, and year of index date at a 1:1 ratio. Both cohorts were followed from the index date until a new diagnosis of CHD was made. Baseline variables, comorbidities, and bisphosphonate and estrogen prescriptions were collected.
The overall incidence of CHD was 23.5 (per 1000 person-years) for the osteoporosis cohort and 16.7 for the comparison cohort, with a mean follow-up of 6.54 years and 6.63 years, respectively. The hazard ratio (HR) for developing CHD during follow-up was 1.30 (95% confidence interval [CI], 1.23–1.38) for the osteoporosis cohort compared with the comparison cohort after adjusting for age, sex, comorbidities, and estrogen medication. Patients with osteoporosis who received treatment with bisphosphonates or with both bisphosphonates and estrogen exhibited a significantly lower risk for CHD (adjusted HR = 0.37 and 0.23) than those who did not receive either of these 2 medications.
The results support an association between osteoporosis and CHD in Asian population.
INTRODUCTION
Osteoporosis and coronary heart disease (CHD) are 2 primary diseases that result in substantial morbidity and mortality in older people. Osteoporosis is characterized by low bone mass, structural deterioration of bone tissue, and compromised bone strength predisposing to increased susceptibility to fracture.1 The bone is a dynamic tissue that is continually being remodeled following 2 opposite and coordinated processes. Bone resorbing cells, called osteoclasts, transiently break down old bone as other bone-forming cells, known as osteoblasts, are replacing it with new tissue. Osteoblastic cells assure bone formation and mineralization by secreting bone matrix components (type I collagen and noncollagenous proteins) and play a central role in regulating bone resorption by providing essential factors such as the macrophage colony-stimulating factor and the receptor activator of nuclear factor Kappa-B ligand (RANKL) for differentiating osteoclasts.2,3 Altered osteoblastic proliferation, differentiation, secretory functions, or apoptosis rate compromise the maintenance of bone remodeling equilibrium, resulting in low bone mass or osteoporosis.
CHD, the leading cause of death worldwide, is caused by atherothrombotic vascular occlusion, which leads to progressive ischemia of the heart muscle (stable or unstable angina), acute myocardial infarction and sudden cardiac arrest.4–6 Atherosclerosis, the primary cause of CHD, is an inflammatory disease of the wall of large- and medium-sized arteries, in which immune mechanisms interact with metabolic risk factors to initiate, propagate, and activate lesions in the arterial tree.7,8 Previous studies have indicated that the initial step in CHD development is subendothelial accumulation of low-density lipoprotein (LDL) caused by endothelial dysfunction and structure alterations.5,8–11 The formation of atheromatous plaques orchestrated by a complex interplay of various cell types, such as endothelial cells, vascular smooth muscle cells, and macrophages, narrows or obstructs the coronary arteries, causing ischemia of the myocardium.5,8
Previous investigations have suggested that osteoporosis and CHD have shared risk factors, such as hypertension, diabetes, smoking, alcohol abuse, and a low level of physical activity, and that the close relationship is independent of age.12,13 Moreover, low bone mineral density (BMD) has been related to increased cardiovascular mortality and morbidity.14 Potential links underlying both diseases may be related to the calcification process that is involved in atherosclerosis and bone mineralization.13,15 Mineralization is of particular interest because numerous noncollagenous bone-related proteins mediating bone resorption have also been implicated in calcification and ossification in the vascular intima.16–18
Despite the possible inverse association between low bone density and cardiovascular events, there were few studies to address the association of osteoporosis with CHD events in Asian population and some studies in Taiwan showed no association between osteoporosis and subclinical CHD.19 The risk of CHD differs between Asian and Caucasian populations; however, most previous studies in this field have relied on Caucasian postmenopausal women and have rarely targeted an Asian population. Therefore, we used the longitudinal population-based database of the Taiwan's National Health Insurance (NHI) program to assess the association between osteoporosis and CHD in an Asian population.
METHODS
Study Design
The NHI program is a universal healthcare system with coverage of over 99% of the entire population of Taiwan. The program was established in 1995 and was consolidated from 13 insurance programs covering all necessary medical expense including medication. Data for this retrospective cohort study were retrieved from Longitudinal Health Insurance Database (LHID), which consists of the claims data of 1,000,000 people randomly sampled from the 2000 registry for beneficiaries (n = 23.72 million) of the NHI program (http://www.nhi.gov.tw/english/index.aspx). The LHID consists of deidentified secondary data including outpatient and inpatient setting, and all of the aforementioned randomly sampled patients were followed to the year 2011. The International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) was used to identify health statuses. The study was approved by the Institutional Review Board of China Medical University (CMU-REC-101-012).
Sampled Patients
From 2000 to 2010, we identified patients aged 45 or more with a diagnosis of osteoporosis (ICD-9-CM codes 733.0 and 733.1) in the LHID as the osteoporosis cohort. The date of osteoporosis diagnosis was defined as the index date. We excluded patients with a diagnosis of CHD (ICD-9-CM codes 410–414) before the index date and those with incomplete age, sex, or medical record information. The look-back period is at least 4 years because the LHID is available since 1996. NHI database covers a highly representative sample of Taiwan's general population because the reimbursement policy is universal and operated by a single-buyer, the government in Taiwan. All insurance claims should be scrutinized by medical reimbursement specialists and peer review according to the standard and clinical diagnosed criteria such as dual-energy X-ray absorptiometry for osteoporosis and cardiac imaging (radionuclide myocardial perfusion imaging or coronary angiography) for CHD. Therefore, the diagnoses of osteoporosis and CHD in this study were highly reliable. The patients in the comparison cohort without a diagnosis of osteoporosis, with the same exclusion criteria, were randomly selected from LHID beneficiaries and frequency matched according to age (in 5-year bands), sex, and the year of index date at a 1:1 ratio.
Baseline Variables
We obtained baseline variables, including age, sex, comorbidities of diabetes (ICD-9-CM codes 250), hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM codes 272), chronic kidney disease (CKD) (ICD-9-CM codes 580–589), chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes 491, 492, and 496), asthma (ICD-9-CM code 493), alcohol-related illnesses (ICD-9-CM codes 291, 303, 305, 571.0, 571.1, 571.2, and 571.3), and estrogen supplement (women only) and bisphosphonate medication that were ever used.
Outcome Measurement
Both the osteoporosis and comparison cohorts were followed-up from the index date until a new diagnosis of CHD (ICD-9-CM 410–414) was made or until the patients were censored because of loss to follow-up, withdrawal from the insurance system, or the end of 2011, whichever occurred first.
Statistical Analysis
Distribution of age, sex, comorbidities, and medications were compared between the osteoporosis cohort and the comparison cohort, and were examined using the chi-square test for categorical variables and the t test for continuous variables. For estimating the cumulative incidence of CHD in osteoporosis patients and comparison patients, we performed survival analysis by using the Kaplan–Meier method, with significance based on the log-rank test. The overall and age-, sex-, and comorbidity-specific incidence densities of CHD were measured for each cohort. Univariate and multivariate Cox proportion hazard regression models were used to examine the effect of osteoporosis on the risk for CHD, shown as a hazard ratio (HR) with a 95% confidence interval (CI). The confounders including age, sex, and comorbidities of diabetes, hypertension, hyperlipidemia, CKD, COPD, asthma, and alcohol-related illnesses and estrogen supplement were adjusted in the multivariate analysis. Osteoporotic fracture was evaluated for the risk of CHD in the subgroup analysis. The effect of medications on the risk of CHD in all osteoporosis patients was analyzed. All analyses were performed using SAS statistical software for Windows (Version 9.2; SAS Institute, Inc., Cary, NC), and the significance level was set to less than .05.
RESULTS
Our study comprised a cohort of 19,456 patients with osteoporosis and a comparison cohort of 19,456 patients (Table 1). Patients were mainly aged 65 or older (45.7%) and women accounted for 80.2%. The mean age of the osteoporosis cohort was 64.0 years (standard deviation [SD] = 11.4) and that of the comparison cohort was 63.2 years (SD = 11.7). Compared with the comparison cohort, the osteoporosis cohort exhibited a significantly higher rate of comorbidities of diabetes, hypertension, hyperlipidemia, CKD, COPD, asthma and alcohol-related illness, and estrogen supplement (all P < 0.001).
TABLE 1.
Comparison of Baseline Characteristics Between Patients With and Without Osteoporosis
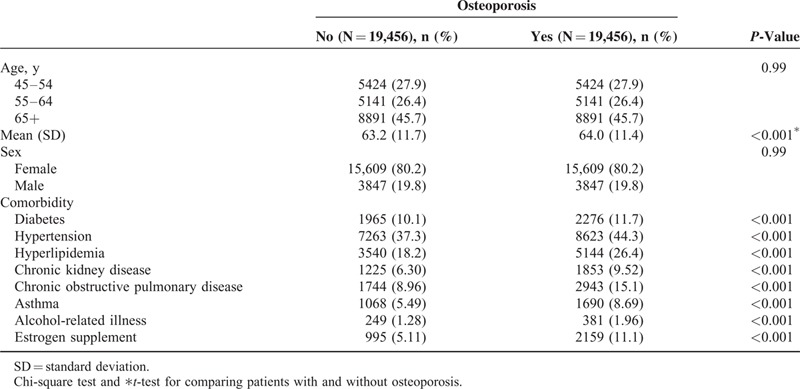
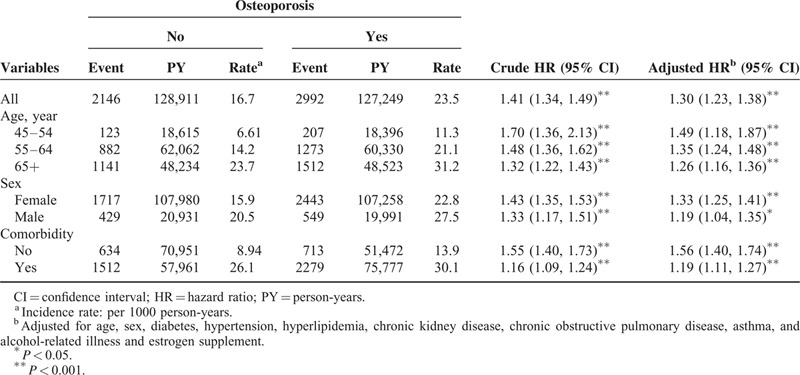
Figure 1 shows the cumulative incidence curves of CHD for patients with or without osteoporosis, with the osteoporosis cohort significantly higher than the comparison cohort (log-rank test P < 0.001). The overall incidence of CHD (per 1000 person-years) was 23.5 for the osteoporosis cohort and 16.7 for the comparison cohort, with a mean follow-up of 6.54 and 6.63 years, respectively (Table 2). After adjusting for age, sex, comorbidities, and estrogen supplement, the HR for developing CHD during the follow-up years was 1.30 (95% CI, 1.23–1.38) for the osteoporosis cohort. The incidence of CHD increased with age in both cohorts, whereas the relative risk was greatest in the youngest age-specific group (age 45–54 years) in the osteoporosis cohort compared with that in the comparison cohort (adjusted HR = 1.49; 95% CI, 1.18–1.87). The incidence rate of CHD was greater in men than in women in both cohorts. The adjusted HR of CHD was greater in the osteoporosis cohort compared with the comparison cohort for both women and men (adjusted HR = 1.33; 95% CI, 1.25–1.41 and adjusted HR = 1.19; 95% CI, 1.04–1.35, respectively). The incidence of CHD increased with comorbidities in both cohorts. For patients without comorbidities, the risk of CHD was 1.56-fold greater in the osteoporosis cohort than in the comparison cohort (95% CI, 1.40–1.74).
FIGURE 1.
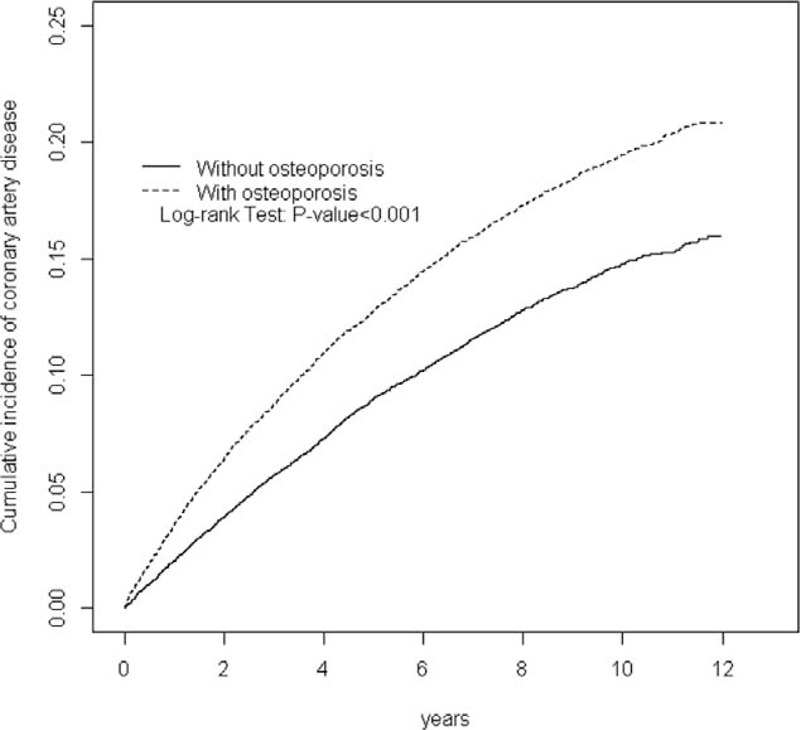
Cumulative incidence of coronary heart disease for patients with (dashed line) or without (solid line) osteoporosis.
TABLE 2.
Risk of Coronary Heart Disease in Relation to Osteoporosis and After Stratification by Age, Sex, or Comorbidity
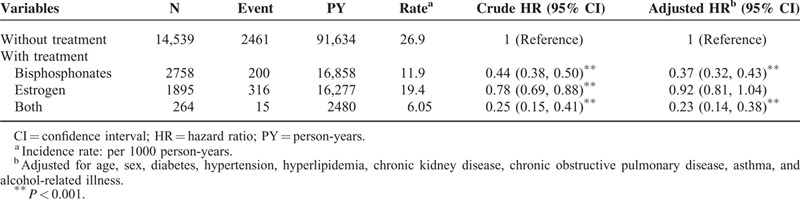
The incidences and HRs of CHD were further analyzed according to subgroup of osteoporosis (Table 3). Patients with osteoporosis-related fracture (pathologic fracture) were not significantly related to developing CHD compared with those in the comparison group, with an adjusted HR of 0.97 (95% CI, 0.75–1.25). Patients with osteoporosis who received bisphosphonates or both bisphosphonates and estrogen exhibited a significantly lower risk of CHD (adjusted HR = 0.37 and 0.23) compared with those without either of these 2 medications (Table 4).
TABLE 3.
Comparison of Risk of Coronary Heart Disease by Subgroups of the Osteoporosis
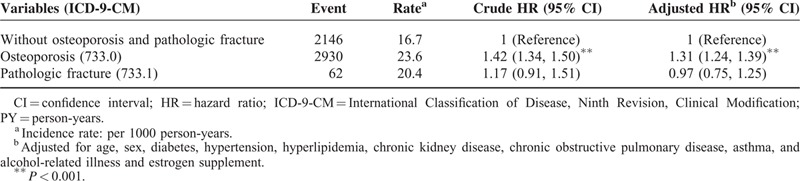
TABLE 4.
Risk of Coronary Heart Disease in Osteoporosis Patients Receiving Bisphosphonate or Estrogen
DISCUSSION
The results of this population-based cohort study suggest that patients with osteoporosis have higher risk of CHD than those without osteoporosis. Patients who have osteoporosis and have received treatment with bisphosphonates have a significantly lower risk for CHD than are those without treatment. Our findings suggest that osteoporosis is significantly associated with the risk of CHD in an Asian population.
Farhat et al14 studied the incidence of cardiovascular disease in a prospective study and found a reverse association between BMD and cardiovascular disease in black women and Caucasian men. In a selective estrogen-receptor modulator trial, osteoporosis showed a 3.9-fold increased risk of cardiovascular incidence compared with low bone mass in postmenopausal women.20 A retrospective analysis compared dual-energy X-ray absorptiometry determined BMD and angiographically determined CHD performed within a 12-month period in a population predominantly composed of Caucasian women, showing that osteoporosis is an independent risk factor for CHD.21 Moreover, osteoporosis was also reported with increased risk of morbidity and mortality caused by cardiovascular diseases.22–25 These studies have suggested a positive association between osteoporosis and the development of cardiovascular disease independent of age and other traditional cardiovascular risk factors.
Potential mechanisms that link osteoporosis to atherosclerosis include bone and vascular mineralization, estrogen deficiency, elevated plasma homocysteine concentrations, lipid oxidation, and ongoing inflammatory processes.13 In particular, calcium deposition and atherosclerosis have been linked to the connection between osteoporosis and cardiovascular diseases.26–28 Lower BMD is significantly associated with greater coronary and aortic calcium and calcified atherosclerosis.25,29 Biological evidence, including inflammatory cytokines, endogenous sex hormones, oxidized lipids, and vitamin D, suggest that calcified atherosclerosis is not a passive deposition, but a highly organized process that is regulated by mechanisms similar to those involved in bone mineralization.12,30 Calcified atherosclerosis is also considered to be a predicted marker of the risk for a coronary artery event.31,32 Besides, lipid accumulation has been observed in osteoporotic and aging bone. The LDL particles and LDL-associated apolipoprotein-B have been reported to have a positive association with osteoporosis.33 Osteoclast survival, activation, and differentiation are stimulated by the receptor activator of nuclear factor Kappa-B ligand (RANKL) and counteracted by osteoprotegerin (OPG).34,35 RANKL is localized at the osteoblast and stroma cell membrane, whereas OPG is secreted by osteoblasts. The oxidized LDL particles alter osteoblastic cell proliferation, migration, and apoptosis rate through oxidative stress, and therefore may contribute to alteration of bone metabolism equilibrium.2 These results elucidate the crucial role of vascular calcification in osteoporotic patient and the process of atherosclerosis.
Women with osteoporosis were associated with a greater relative risk for CHD than were those without osteoporosis in our study. Loss of estrogen protection increases the risk of atherogenesis, and an average of 10-year-earlier development of osteoporosis for menopausal women may underline the differing effects of sex hormones on the risk inequality between the sexes.36 Estrogen can prevent osteoporosis by suppressing bone resorption through RANKL and the OPG system on osteoclasts,37 and has been suggested the dominant sex steroid regulating bone resorption in aging men.38 Although estrogen exerts sex differences on bone metabolism, estrogen deficiency is documented to increase the risk of osteoporosis and is associated with greater risk of cardiovascular diseases.
Previous studies have suggested antiatherosclerotic effects of bisphosphonates, including inhibiting inflammation, intimal hyperplasia, cholesterol synthesis, and vascular calcification.39,40 Nitrogen-containing bisphosphonates can act on the cholesterol biosynthesis pathway and exert effects on lipids metabolism in postmenopausal women with osteoporosis.16 Chronic intravenous therapy with neridronate has been shown to reduce LDL-C and apolipoprotein B and to increase high density lipoprotein C.41 Alendronate significantly reduced intima-media thickness and altered the lipid profile of the carotid artery among postmenopausal women during a 1-year follow-up.42 An observational study on an Asian population also showed a lower incidence of acute myocardial infarction in bisphosphonate users in a 2-year follow-up.43 Of note, patients with osteoporotic (pathological) fractures showed a less CHD risk in our study (Table 3). The potential dual effects of bisphosphonates, which were given to more patients in osteoporotic fracture (15.2% vs. 26.6%), might have partially explained the reduced risk for CHD although the sample size is relatively small.
Certain limitations of this study should be noted. First, NHI database does not provide information on smoking, body mass index, socioeconomic status, and physical activity, which are all potential confounding factors for this study. Second, the diagnoses of osteoporosis and CHD relied on NHI records. Because the NHI database does not provide personal information about BMD and coronary artery conditions, we were unable to ascertain and assess their severity. Third, lack of information on medication compliance was inherent in this registry database study; therefore, the medication effect may be underestimated. Fourth, despite our meticulous study design in which we adequately controlled the confounding factors, data derived from a retrospective cohort study are generally limited by potential selection bias, as well as residual confounding by using other cardiovascular medications. However, even with these limitations, the present study provides valuable information regarding the association between osteoporosis and CHD risk.
In conclusion, in a nation-wide Asian population, osteoporosis was associated with a 1.3-fold risk of incident CHD and the treatment with bisphosphonate for osteoporosis was associated with lower risk of CHD. The relative risk association with CHD is higher in women with osteoporosis. Clinicians should be aware of the close association between these 2 diseases. A CHD risk assessment allows for early identification of CHD and prompts therapeutic or preventive interventions to lower cardiovascular mobility in patients with osteoporosis.
Footnotes
Abbreviations: BMD = bone mineral density, CHD = coronary heart disease, CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, LDL = low-density lipoprotein, LHID = Longitudinal Health Insurance Database, OPG = osteoprotegerin, RANKL = receptor activator of nuclear factor Kappa-B ligand.
Authors’ contributions: Conception/design: S-JC, C-SL, and C-HK; provision of study materials: C-HK; collection and/or assembly of data: all authors; data analysis and interpretation: S-JC, C-SL, C-LL, and C-HK; manuscript writing: all authors; final approval of manuscript: all authors.
The authors have no conflicts of interest to disclose.
No commercial party having a direct or indirect interest in the subject matter of this research will confer a benefit on the authors or on any organization with which the authors are associated. This material has not previously been presented in any form.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
REFERENCES
- 1.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy, March 7–29, 2000: highlights of the conference. South Med J 2001; 94:569–573. [PubMed] [Google Scholar]
- 2.Hamel P, Abed E, Brissette L, et al. Characterization of oxidized low-density lipoprotein-induced hormesis-like effects in osteoblastic cells. Am J Physiol Cell Physiol 2008; 294:C1021–C1033. [DOI] [PubMed] [Google Scholar]
- 3.Mackie EJ. Osteoblasts: novel roles in orchestration of skeletal architecture. Int J Biochem Cell Biol 2003; 35:1301–1305. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, et al. WRITING GROUP MEMBERS Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010; 121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 5.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 2011; 17:1410–1422. [DOI] [PubMed] [Google Scholar]
- 6.Tabas I. Pulling down the plug on atherosclerosis: finding the culprit in your heart. Nat Med 2011; 17:791–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 8.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011; 145:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 1995; 15:551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon GP, Schroeder JL, Amar MJ, et al. Contribution of macromolecular structure to the retention of low-density lipoprotein at arterial branch points. Circulation 2008; 117:2919–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 2011; 12:204–212. [DOI] [PubMed] [Google Scholar]
- 12.Farhat GN, Cauley JA. The link between osteoporosis and cardiovascular disease. Clin Cases Miner Bone Metab 2008; 5:19–34. [PMC free article] [PubMed] [Google Scholar]
- 13.Anagnostis P, Karagiannis A, Kakafika AI, et al. Atherosclerosis and osteoporosis: age-dependent degenerative processes or related entities? Osteoporos Int 2009; 20:197–207. [DOI] [PubMed] [Google Scholar]
- 14.Farhat GN, Newman AB, Sutton-Tyrrell K, et al. The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporos Int 2007; 18:999–1008. [DOI] [PubMed] [Google Scholar]
- 15.den Uyl D, Nurmohamed MT, van Tuyl LH, et al. (Sub)clinical cardiovascular disease is associated with increased bone loss and fracture risk; a systematic review of the association between cardiovascular disease and osteoporosis. Arthritis Res Ther 2011; 13:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol 2001; 21:817–824. [DOI] [PubMed] [Google Scholar]
- 17.Mezquita-Raya P, de la Higuera M, García DF, et al. The contribution of serum osteoprotegerin to bone mass and vertebral fractures in postmenopausal women. Osteoporos Int 2005; 16:1368–1374. [DOI] [PubMed] [Google Scholar]
- 18.Soufi M, Schoppet M, Sattler AM, et al. Osteoprotegerin gene polymorphisms in men with coronary artery disease. J Clin Endocrinol Metab 2004; 89:3764–3768. [DOI] [PubMed] [Google Scholar]
- 19.Lin T, Liu JC, Chang LY, et al. Association between coronary artery calcification using low-dose MDCT coronary angiography and bone mineral density in middle-aged men and women. Osteoporos Int 2011; 22:627–634. [DOI] [PubMed] [Google Scholar]
- 20.Tanko LB, Christiansen C, Cox DA, et al. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res 2005; 20:1912–1920. [DOI] [PubMed] [Google Scholar]
- 21.Marcovitz PA, Tran HH, Franklin BA, et al. Usefulness of bone mineral density to predict significant coronary artery disease. Am J Cardiol 2005; 96:1059–1063. [DOI] [PubMed] [Google Scholar]
- 22.Bauer DC, Bauer DC, Palermo L, et al. Quantitative ultrasound and mortality: a prospective study. Osteoporos Int 2002; 13:606–612. [DOI] [PubMed] [Google Scholar]
- 23.Kado DM, Browner WS, Blackwell T, et al. Rate of bone loss is associated with mortality in older women: a prospective study. J Bone Miner Res 2000; 15:1974–1980. [DOI] [PubMed] [Google Scholar]
- 24.Mussolino ME, Madans JH, Gillum RF. Bone mineral density and mortality in women and men: the NHANES I epidemiologic follow-up study. Ann Epidemiol 2003; 13:692–697. [DOI] [PubMed] [Google Scholar]
- 25.Magnus JH, Broussard DL. Relationship between bone mineral density and myocardial infarction in US adults. Osteoporos Int 2005; 16:2053–2062. [DOI] [PubMed] [Google Scholar]
- 26.Doherty TM, Asotra K, Fitzpatrick LA, et al. Calcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci U S A 2003; 100:11201–11206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 2004; 24:1161–1170. [DOI] [PubMed] [Google Scholar]
- 28.Rubin MR, Silverberg SJ. Vascular calcification and osteoporosis—the nature of the nexus. J Clin Endocrinol Metab 2004; 89:4243–4245. [DOI] [PubMed] [Google Scholar]
- 29.Hyder JA, Allison MA, Wong N, et al. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol 2009; 169:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamerman D. Osteoporosis and atherosclerosis: biological linkages and the emergence of dual-purpose therapies. QJM 2005; 98:467–484. [DOI] [PubMed] [Google Scholar]
- 31.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008; 358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 32.Thompson GR, Partridge J. Coronary calcification score: the coronary-risk impact factor. Lancet 2004; 363:557–559. [DOI] [PubMed] [Google Scholar]
- 33.Adami S, Braga V, Zamboni M, et al. Relationship between lipids and bone mass in 2 cohorts of healthy women and men. Calcif Tissue Int 2004; 74:136–142. [DOI] [PubMed] [Google Scholar]
- 34.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998; 93:165–176. [DOI] [PubMed] [Google Scholar]
- 35.Silva I, Branco JC. Rank/Rankl/opg: literature review. Acta Reumatol Port 2011; 36:209–218. [PubMed] [Google Scholar]
- 36.Lambrinoudaki I, Armeni E, Georgiopoulos G, et al. Subclinical atherosclerosis in menopausal women with low to medium calculated cardiovascular risk. Int J Cardiol 2013; 164:70–76. [DOI] [PubMed] [Google Scholar]
- 37.Khosla S. Pathogenesis of age-related bone loss in humans. J Gerontol A Biol Sci Med Sci 2013; 68:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falahati-Nini A, Riggs BL, Atkinson EJ, et al. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 2000; 106:1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiore CE, Pennisi P, Pulvirenti I, et al. Bisphosphonates and atherosclerosis. J Endocrinol Invest 2009; 32:38–43.Review. [PubMed] [Google Scholar]
- 40.Strandberg TE. Alendronate, osteoporosis, and atherosclerosis. Arch Intern Med 2008; 168:2386–2387.author reply 2387. [DOI] [PubMed] [Google Scholar]
- 41.Adami S, Braga V, Guidi G, et al. Chronic intravenous aminobisphosphonate therapy increases high-density lipoprotein cholesterol and decreases low-density lipoprotein cholesterol. J Bone Miner Res 2000; 15:599–604. [DOI] [PubMed] [Google Scholar]
- 42.Ariyoshi T, Eishi K, Sakamoto I, et al. Effect of etidronic acid on arterial calcification in dialysis patients. Clin Drug Investig 2006; 26:215–222. [DOI] [PubMed] [Google Scholar]
- 43.Kang JH, Keller JJ, Lin HC. Bisphosphonates reduced the risk of acute myocardial infarction: a 2-year follow-up study. Osteoporos Int 2013; 24:271–277. [DOI] [PubMed] [Google Scholar]


