Abstract
There are disparities among the results of meta-analyses under different circumstances of carotid artery stenting (CAS) versus endarterectomy (CEA) for carotid stenosis. This study aimed to assess the efficacies of CAS and CEA for carotid stenosis at 5-year intervals and worldwide.
Comparative studies simultaneously reporting CAS and CEA for carotid stenosis with at least 10 patients in each group were identified by searching PubMed and Embase in accordance with preferred reporting items for systematic reviews and meta-analyses guidelines, and by reviewing the reference lists of retrieved articles.
The studies were stratified into different subgroups according to the publication year, location in which the study was mainly performed, and randomized and nonrandomized study designs.
Thirty-five comparative studies encompassing 27,525 patients were identified. The risk ratios (RRs) of stroke/death when CAS was compared with CEA within 30 d of treatment were 1.51 (95% CI 1.32–1.74, P < 0.001) for overall, 1.50 (95% CI 1.14–1.98, P = 0.004) from 2011 to 2015, 1.61 (95% CI 1.35–1.91, P < 0.001) from 2006 to 2010, 1.59 (95% CI 1.27–1.99, P < 0.001) in North America, 1.50 (95% CI 1.24–1.81, P < 0.001) in Europe, 1.63 (95% CI 1.31–2.02, P < 0.001) for randomized, and 1.44 (95% CI 1.20–1.73, P < 0.001) for nonrandomized comparative studies. CEA decreased the risks of transient ischemic attack at 30 d (RR: 2.07, 95% CI 1.50–2.85, P < 0.001) and restenosis at 1-year (RR: 1.97, 95% CI 1.28–3.05, P = 0.002). Data from follow-up showed that the RRs of stroke/death were 0.74 (95% CI 0.55–0.99, P = 0.04) at 1 year, 1.24 (95% CI 1.04–1.46, P = 0.01) at 4 year, and 2.27 (95% CI 1.39–3.71, P = 0.001) at 10 year. This systematic review, compared with those of other meta-analyses, included all available comparative studies and analyzed them at 5-year intervals, in different continents, and under different study designs. Current evidence suggests that the efficacy of CEA is superior to CAS for freedom from stroke/death within 30 d, especially from 2006 to 2015, in North America and Europe. Meanwhile, the superiority was also observed for restenosis at 1-year, transient ischemic attack within 30 d, and stroke/death at 4- and 10-year follow-ups.
INTRODUCTION
Carotid stenosis is a major cause of ischemic stroke1 and it is estimated that ∼700,000 incidents are reported annually in the United States;2 therefore, the objective of carotid stenosis treatment is to reduce the risk of stroke or stroke-related death. Carotid endarterectomy (CEA) was introduced > 60 years ago as an effective approach to preventing stroke, and carotid artery stenting (CAS) has provided a less-invasive alternative in recent years;3 however, the results of previous meta-analyses that examined these protocols are ambiguous under different circumstances, and the therapeutic strategy of choosing between CEA and CAS is still a dilemma. Several studies have demonstrated that CAS is inferior to CEA because CAS increased the stroke or death rate within 30 d of treatment.4–6 Other studies have shown that CAS might be equivalent to CEA, especially in patients < 70 years old.7–9 In addition, the timeframes and regional discrepancies were not taken into account in previous meta-analyses.
In this meta-analysis, we systematically reviewed the current body of evidence comparing CAS with CEA in the treatment of carotid stenosis, and pooled the data for analyzing any stroke/death rate within 30 d at 5-year intervals, in different continents, and in randomized and nonrandomized comparative studies. We also pooled the data for analyzing restenosis, transient ischemic attack (TIA), and any stroke/death rates at different follow-up points.
METHODS
The systematic review and meta-analysis was performed in accordance with the standards set forth by the statement from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.10,11 As this study is a systematic review and meta-analysis, ethical approval was not required.
Data Sources and Search Results
The PubMed and Embase databases were searched from inception until February 4, 2015, restricted to studies in English and on humans. There were no restrictions on the year or the type of publication. The search strategy was amended for each database (see Table S1, Supplemental Content, which demonstrates the search strategies for PubMed and Embase databases). A hand search was also performed of all the references in the included studies for potential valuable and relevant publications.
Study Selection
The inclusion criteria were as follows: (1) comparative study simultaneously reporting CAS and CEA for carotid stenosis; (2) at least 20 patients in the study and 10 patients in each group. Based on the guidelines, reviews, case reports, clinical trial protocols, commentaries/editorials, guidelines, new techniques/devices, restenosis therapy, basic and other research, systematic reviews and/or meta-analyses were excluded. The studies reporting only CEA or CAS were also excluded. After full-text articles were assessed for eligibility, the studies from Nationwide Inpatient Sample and New York and California States data were excluded. After qualitative syntheses, studies that were the same but were reported in different years were also excluded.
Data Extraction
Two investigators (LZ and JZ) independently extracted data using a standard form. Disagreements were resolved by consensus. Data were extracted pertaining to any stroke/death, restenosis, TIA rates, and pooled for the main analysis according to the intention to treat principle.
Outcome Measurement
The studies were stratified into different subgroups according to the publication year, the location in which the study was mainly performed, and the different study designs. The primary end points were any stoke/death rates within 30 d at 5-year intervals, in different continents, and in randomized and nonrandomized comparative studies. The secondary end points were restenosis rate at 1- and 2-year follow-up, TIA rate within 30 d and 1-year follow-up, and the stroke/death rate at 1-, 2-, 3-, 4- and 10-year follow-up points.
Methodological Quality
The Cochrane Collaboration risk of bias tool was used to assess the quality of included randomized controlled trials (see Figure S1, Supplemental Content, which demonstrates the bias assessment of randomized controlled studies). The potential publication bias was tested by conducting of funnel plot, where the dotted vertical line represents the combined effect size in this outcome.
Data Synthesis and Statistical Analysis
The risk ratios of any stroke/death, TIA, and restenosis were pooled across studies and analyzed using the Mantel–Haenszel statistical method to compare CAS with CEA. The amount of heterogeneity was estimated using I2 statistics, which uses values from 0 to 100% (0–24%, low heterogeneity; 25–49%, moderate heterogeneity; 50–74%, high level of heterogeneity; 75–100%, extreme heterogeneity). Random-effect meta-analysis models were chosen when heterogeneity > 50%, and fixed-effect models when heterogeneity < 50%.
All analyses were performed using the Cochrane Collaboration Review Manager (Version 5.20, Cochrane Collaboration, Copenhagen, Denmark). The probability values were two-tailed and the null hypothesis was rejected for values of P < 0.05.
RESULTS
Study Selection and Characteristics
The literature search identified 782 potentially relevant studies, as shown in the flow diagram (Figure 1). Of these, 54 full-text articles were assessed for eligibility, and 41 studies met the inclusion criteria. Six studies that were the same but were reported in different years were excluded as follows: four “Carotid Revascularization Endarterectomy versus Stenting Trial (CREST),”3,12–14 one “Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy (SPACE)”,15 and one “Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS).”16 Finally, 35 studies comprising 27,525 patients treated between January 1997 and March 2012 were included in the meta-analysis. There were 12 randomized controlled trials,17–28 three prospective controlled studies,29–31 and 20 retrospective comparative studies.32–51
FIGURE 1.
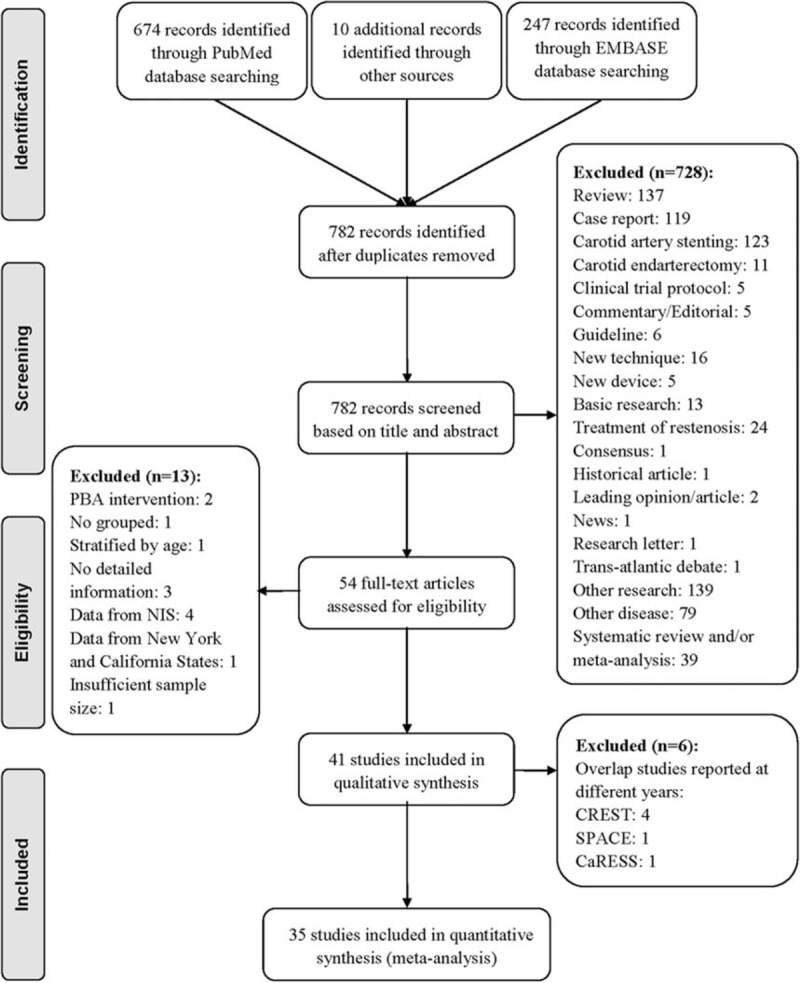
Flow diagram according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. CaRESS = carotid revascularization using endarterectomy or stenting systems, CREST = carotid revascularization endarterectomy versus stenting trial, NIS = nationwide inpatient sample, PBA = percutaneous balloon angioplasty, SPACE = stent-supported percutaneous angioplasty of the carotid artery versus endarterectomy.
The patients’ characteristics and comorbidities were summarized (see Table S2, Supplemental Content, which demonstrates the patients’ characteristics and comorbidities). The average age was 70 years, and 68.0% of patients were men. The comorbidities were hypertension (77.7%), coronary artery disease (40.4%), dyslipidemia (55.6%), diabetes mellitus (29.2%), and smoking (44.0%).
Primary End Points
The overall risk ratio of any stroke/death within 30 d of treatment was 1.51 (95% confidence interval [CI] 1.32–1.74, P < 0.001) with CAS versus CEA (Figures 2–4 A); there was low heterogeneity (I2 = 23%). The incidence of stroke/death within 30 d of treatment was 4.7% for CAS and 3.5% for CEA.
FIGURE 2.
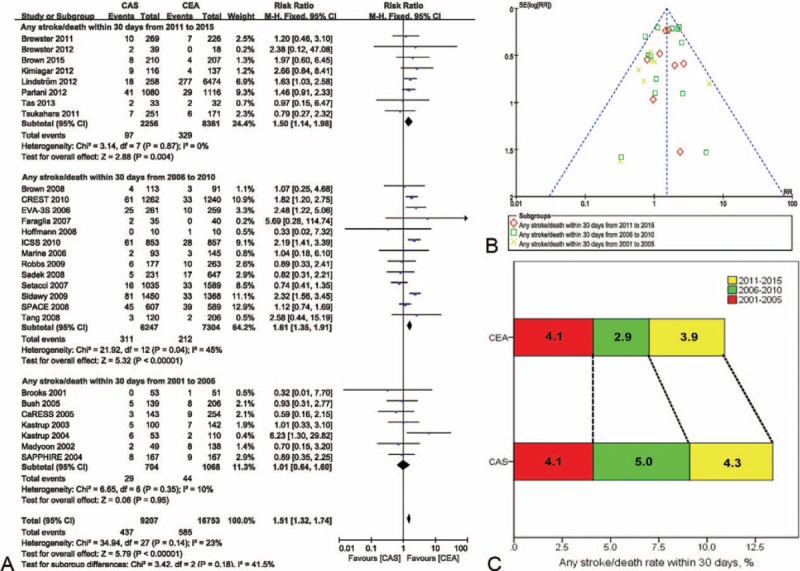
Meta-analysis of the stoke/death rate within 30 d at 5-year intervals. (A) The efficacy of CEA for freedom from stroke/death within 30 d was superior to that of CAS from 2006 to 2015. (B) The likelihood of publication bias was low. (C) The incidence rate of stroke/death within 30 d at 5-year intervals in CEA and CAS. CaRESS = carotid revascularization using endarterectomy or stenting systems, CAS = carotid artery stenting, CEA = carotid endarterectomy, CI = confidence interval (s), CREST = carotid revascularization endarterectomy versus stenting trial, EVA-3S = endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis, ICSS = international carotid stenting study, SAPPHIRE = stenting and angioplasty with protection in patients at high risk for endarterectomy, SPACE = stent-supported percutaneous angioplasty of the carotid artery versus endarterectomy.
FIGURE 4.
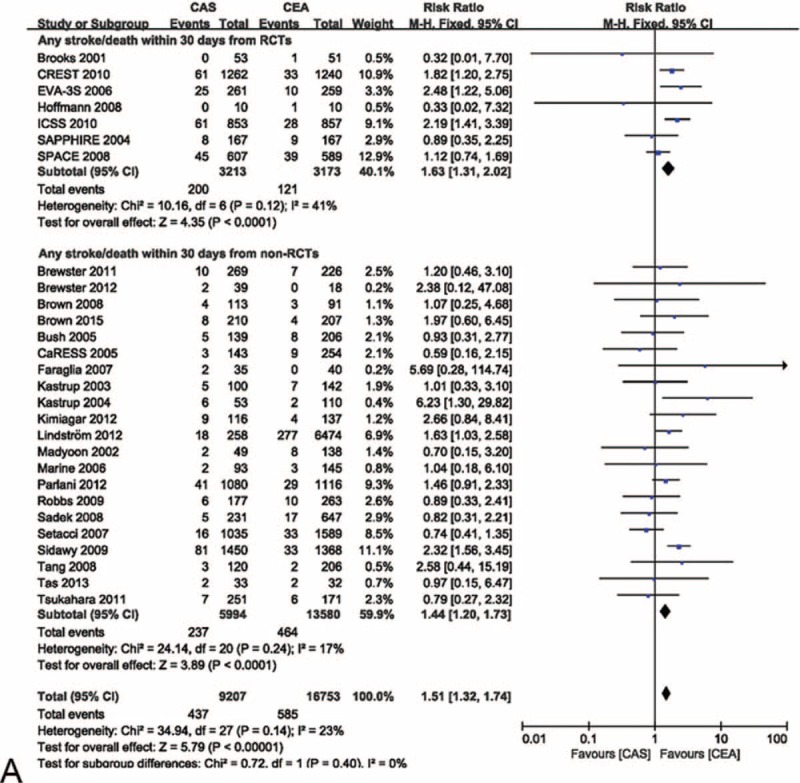
Meta-analysis of the stroke/death rate within 30 d from randomized and nonrandomized comparative studies. (A) The efficacy of CEA for freedom from stroke/death within 30 d was superior to that of CAS in randomized and nonrandomized comparative studies. (B) The likelihood of publication bias was low. CaRESS = carotid revascularization using endarterectomy or stenting systems, CAS = carotid artery stenting, CEA = carotid endarterectomy, CI = confidence interval(s), CREST = carotid revascularization endarterectomy versus stenting trial, EVA-3S = endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis, ICSS = international carotid stenting study, RCTs = randomized comparative studies, SAPPHIRE = stenting and angioplasty with protection in patients at high risk for endarterectomy, SPACE = stent-supported percutaneous angioplasty of the carotid artery versus endarterectomy.
The risk ratios of any stroke/death within 30 d were 1.50 (95% CI 1.14–1.98, P = 0.004) from 2011 to 2015, 1.61 (95% CI 1.35–1.91, P < 0.001) from 2006 to 2010, and 1.01 (95% CI 0.64–1.60, P = 0.95) from 2001 to 2005 when CAS was compared with CEA. There were low heterogeneity (I2 = 0%, 45%, and 10%, respectively) (Figure 2A). A funnel plot showed no significant evidence of asymmetry (Figure 2B). The incidence rates for CAS and CEA were 4.3% and 3.9% from 2011 to 2015, 5.0% and 2.9% from 2006 to 2010, and 4.1% for both from 2001 to 2005, respectively (Figure 2C).
The risk ratios of any stroke/death within 30 d of CAS versus CEA were 1.59 (95% CI 1.27–1.99, P < 0.001) in North America, 1.50 (95% CI 1.24–1.81, P < 0.001) in Europe, and 1.35 (95% CI 0.67–2.72, P = 0.39) in Asia. Heterogeneity was 5%, 49%, and 16%, respectively, for North America, Europe, and Asia. There was only one study from Africa,42 and the risk ratio was 0.89 (95% CI 0.33–2.41, P = 0.82) (Figure 3A). No significant evidence of asymmetry was observed in the funnel plot (Figure 3B). The incidence rates for CAS versus CEA were 4.5% versus 2.8% in North America, 5.1% versus 3.8% in Europe, 4.5% versus 3.5% in Asia, 3.4% versus 3.8% in Africa (Figure 3C).
FIGURE 3.
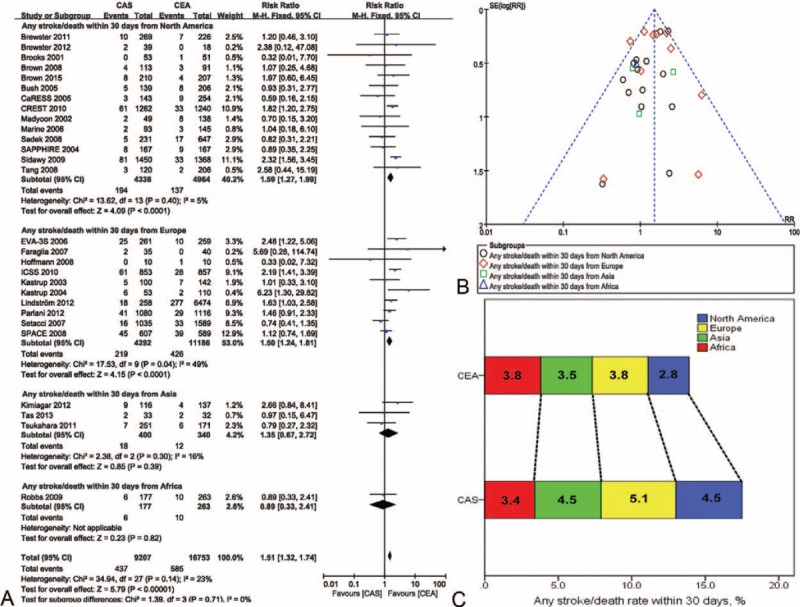
Meta-analysis of the stroke/death rate within 30 d worldwide. (A) The efficacy of CEA for freedom from stroke/death within 30 d was superior to that of CAS in North America and Europe. (B) The likelihood of publication bias was low. (C) The incidence rate of stroke/death within 30 d in different continents in CEA and CAS. CaRESS = carotid revascularization using endarterectomy or stenting systems, CAS = carotid artery stenting, CEA = carotid endarterectomy, CI = confidence interval(s), CREST = carotid revascularization endarterectomy versus stenting trial, EVA-3S = endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis, ICSS = international carotid stenting study, SAPPHIRE = stenting and angioplasty with protection in patients at high risk for endarterectomy, SPACE = stent-supported percutaneous angioplasty of the carotid artery versus endarterectomy.
The risk ratios of any stroke/death within 30 d of the randomized and nonrandomized comparative studies were 1.63 (95% CI 1.31–2.02, P < 0.001) and 1.44 (95% CI 1.20–1.73, P < 0.001), respectively. Heterogeneity was 41% and 17%, respectively, for the randomized and nonrandomized comparative studies (Figure 4 A). There was no significant evidence of asymmetry (Figure 4 B).
Secondary End Points
The risk ratios of restenosis at follow-up were 1.97 (95% CI 1.28–3.05, P = 0.002) after 1 year and 1.45 (95% CI 0.62–3.41, P = 0.39) after 2 year. Heterogeneity was 0% and 88%, respectively, after 1 and 2 year. The forest plot showed that the efficacy of CEA was superior to that of CAS at the 1-year follow-up point. The incidence rates for CAS and CEA were 7.4% and 3.6% at 1 year, and 6.6% and 5.0% at 2-year follow-up, respectively (Figure 5A). The funnel plot showed no significant evidence of asymmetry (see Figure S2A, Supplemental Content, which demonstrates the funnel plot for publication bias assessment of restenosis rate).
FIGURE 4 (Continued).
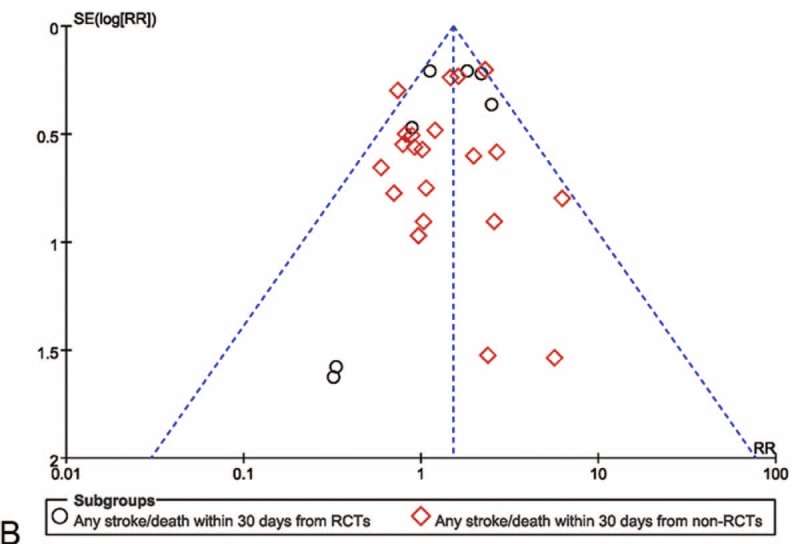
Meta-analysis of the stroke/death rate within 30 d from randomized and nonrandomized comparative studies. (A) The efficacy of CEA for freedom from stroke/death within 30 d was superior to that of CAS in randomized and nonrandomized comparative studies. (B) The likelihood of publication bias was low. CaRESS = carotid revascularization using endarterectomy or stenting systems, CAS = carotid artery stenting, CEA = carotid endarterectomy, CI = confidence interval(s), CREST = carotid revascularization endarterectomy versus stenting trial, EVA-3S = endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis, ICSS = international carotid stenting study, RCTs = randomized comparative studies, SAPPHIRE = stenting and angioplasty with protection in patients at high risk for endarterectomy, SPACE = stent-supported percutaneous angioplasty of the carotid artery versus endarterectomy.
FIGURE 5.
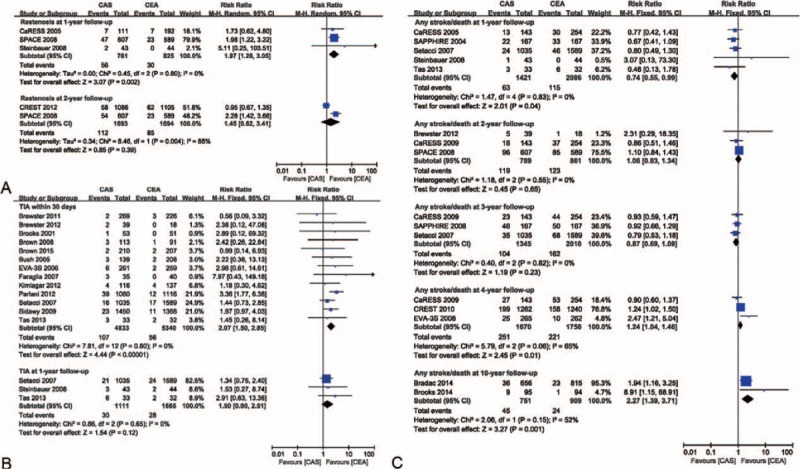
Meta-analyses of restenosis, transient ischemic attack, and stroke/death rates at different follow-up points. (A) The efficacy of CEA for freedom from restenosis was superior to that of CAS at 1-year follow-up. (B) The efficacy of CEA for freedom from transient ischemic attack was superior to that of CAS at 30 d. (C) The efficacy of CEA for freedom from stroke/death was superior to that of CAS at 4- and 10-year follow-up, but inferior to CAS at 1-year follow-up. CaRESS = carotid revascularization using endarterectomy or stenting systems, CAS = carotid artery stenting, CEA = carotid endarterectomy, CI = confidence interval(s), CREST = carotid revascularization endarterectomy versus stenting trial, EVA-3S = endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis, SAPPHIRE = stenting and angioplasty with protection in patients at high risk for endarterectomy, SPACE = stent-supported percutaneous angioplasty of the carotid artery versus endarterectomy, TIA = transient ischemic attack.
The risk ratios of TIA at 30 d and 1 year were 2.07 (95% CI 1.50–2.85, P < 0.001) and 1.50 (95% CI 0.90–2.51, P = 0.12), respectively, when CAS was compared with CEA. Heterogeneity was 0 for both. The forest plot showed that the efficacy of CEA was superior to that of CAS at 30 d. The incidence rates for CAS versus CEA were 2.2% versus 1.0% within 30 d and 2.7% versus 1.7% at 1-year follow-up, respectively (Figure 5B). No significant evidence of asymmetry was observed in the funnel plot (see Figure S2B, Supplemental Content, which demonstrates the funnel plot for publication bias assessment of TIA rate).
The risk ratios of any stroke/death were 0.74 (95% CI 0.55–0.99, P = 0.04) for 1-year, 1.06 (95% CI 0.83–1.34, P = 0.65) for 2-year, 0.87 (95% CI 0.69–1.09, P = 0.23) for 3-year, 1.24 (95% CI 1.04–1.46, P = 0.01) for 4-year, and 2.27 (95% CI 1.39–3.71, P = 0.001) for 10-year follow-up. Heterogeneity varied over the years (I2 = 0, 0, 0, 65%, and 52%, respectively). The forest plot shows that the efficacy of CEA was inferior to that of CAS at 1-year follow-up but was superior to that of CAS at 4- and 10-year follow-up examinations. The incidence rates for CAS and CEA were 4.4% and 5.5% at 1-year, 15.1% and 14.3% at 2-year, 7.7% and 8.1% at 3-year, 15.0% and 12.6% at 4-year, 6.0% and 2.6% at 10-year follow-ups, respectively (Figure 5C). There was no significant evidence of asymmetry (see Figure S2C, Supplemental Content, which demonstrates the funnel plot for publication bias assessment of stroke/death rate).
DISCUSSION
Although many meta-analyses comparing CAS with CEA for carotid stenosis have been performed, there are disparities among the results.4–9 Subgroup analyses divided by age,9 anesthesia type,52 time,53 or symptom54 were helpful to determine the best therapeutic strategy under different circumstances. To our knowledge, the present study was the first meta-analysis to take the timeframes and worldwide differences into account.
CEA was found to be superior to CAS in freedom from stroke/death within 30 d of treatment, a finding that was different from that of previous studies.55,56 The superiority was significant from 2006 to 2015 but not from 2001 to 2005 based on the 5-year interval analyses. CEA was introduced as an effective treatment option to prevent stroke in the early 1950 s, whereas CAS provided a less-invasive option until 1994.3 As with any other endovascular or surgical procedure, common sense suggests that operator skills and experience have a major impact on CAS outcomes. The procedural stroke and death rates decreased over time and differed according to the level of operator experience.57,58 It is presumed that the criteria for the initial CAS procedures were simple and uncomplicated, which might explain the lower stroke/death rates from 2001 to 2005. Meanwhile, many surgeons experienced the first 100 patients for CEA and improved the treatment effects.59 An emboli-protection device effectively reduces the stroke/death rate60,61 and was recommended with the CAS procedure.62,63 The occurrence of stroke/death within 30 d decreased from 5.0% (2006–2010) to 4.3% (2011–2015) for CAS. This improvement in the clinical outcomes of the CAS procedure was associated with the use of an emboli-protection device.64 Because these devices were used more often, it is presumed that they are related to the positive postsurgery improvements.
The occurrence of stroke/death within 30 d for CAS patients was significantly higher than that for CEA patients in North America and Europe. As discussed previously, the first successful CEA was done by DeBakey in 1953.3,65 The innovative and effective technique spread rapidly and was adopted throughout the United States, Europe, Asia, Africa, and other parts of the world. The risk of stroke/death was lower in the headstream of the technique. In fact, > 96.6% patients who underwent CEA in the present meta-analysis were from North America and Europe. Any adverse effects from the procedure were associated with operator skills as well as the operation method itself. Having more patients in need of this treatment helped to raise the proficiency level of the surgeons and decreased the adverse effects from the procedure.
The incidence rate of TIA within 30 d was pronouncedly higher in CAS than in CEA. It is presumed that the complications are relevant to the procedure, in which the wire must pass through the atherosclerotic lesion with severe stenosis or total occlusion. On the other hand, the complications might be associated with the stent design. Carotid stents are now made of nitinol and available in closed-cell and open-cell designs. Although the closed-cell design might confer better plaque coverage than the open-cell design from a conceptual perspective, it still incises the plaque and causes many small emboli. The incidence rate of TIA is in accordance with the stroke/death rate within 30 d. Recent studies have demonstrated that overall survival is significantly lower in patients with postoperative TIA, which is an independent predictor of decreased survival at the 5-year follow-up.66
Restenosis is one of the main drawbacks of endovascular treatment of carotid stenosis, which would no doubt influence the therapeutic effect, especially for long lesions. Following stent deployment, inflation of the stent using a balloon catheter is mandatory. Nevertheless, neointimal hyperplasia and hemodynamic turbulence increase the possibility of in-stent restenosis.67,68 The restenosis rate in CAS is apparently higher than that in CEA at 1-year follow-up; however, the stroke/death rate in CAS is lower than that in CEA during the same time period. These conflicting results might be because of collateral compensatory circulation after CAS intervention. The advantage disappeared at 4- and 10-year follow-up examinations.
In the present study, 31 studies (95.7% patients) were from North America and Europe, whereas only three studies (2.7% patients) were from Asia and one (1.6% patients) from Africa. Scientific research has been guided by North America and Europe for many years. It should be noted that Asia, Africa, and other continents should strengthen their scientific research because their populations account for > 80% of the world population.
Study Limitations
The systematic review and meta-analysis has some limitations. First, the subgroups were stratified by the publication year, not the year of patients enrolled in. On the other hand, the subgroups were divided by the location in which the study was mainly performed, which was not rigorous to the multicenter, intercontinental studies. Second, the meta-analysis included prospective randomized controlled trials, prospective controlled studies, as well as retrospective comparative studies, which might lower the evidence level of the results. However, heterogeneity was low among the studies. Third, many confounding factors such as lesion length, stent types (closed-cell or open-cell, with or without emboli protection devices), methods of endarterectomy (conventional or eversion, with or without patch), antiplatelet therapy, and clinical manifestation of patients (symptomatic or asymptomatic) were not considered in the present study.
CONCLUSIONS
The current published body of literature suggests that the efficacy of CEA is superior to that of CAS for freedom from the stroke/death rate within 30 d of the procedures, especially that from 2006 to 2015 and in North America and Europe. The superiority of CEA over CAS was also observed for the restenosis rate at 1-year, TIA rate within 30 d, and stroke/death rate at 4- and 10-year follow-up examinations. On the contrary, the efficacy of CEA is inferior to that of CAS for the stroke/death rate at 1-year follow-up.
Footnotes
Abbreviations: CaRESS = carotid revascularization using endarterectomy or stenting systems, CAS = carotid artery stenting, CEA = carotid endarterectomy, CI = confidence interval, CREST = carotid revascularization endarterectomy versus stenting trial, EVA-3S = endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis, ICSS = international carotid stenting study, NIS = nationwide inpatient sample, PBA = percutaneous balloon angioplasty, RCTs = randomized comparative studies, RR = risk ratio, SAPPHIRE = stenting and angioplasty with protection in patients at high risk for endarterectomy, SPACE = stent-supported percutaneous angioplasty of the carotid artery versus endarterectomy, TIA = transient ischemic attack.
Lei Zhang and Zhiqing Zhao equally contributed to this study.
This study was financed by the National Natural Science Foundation of China (81330034, 81170299 and 81273522), and the Clinical Technology Key Project of China (2010gxjs063).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Petty GW, Brown RD, Jr, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke 1999; 30:2513–2516. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantese VA, Timaran CH, Chiu D, et al. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): stenting versus carotid endarterectomy for carotid disease. Stroke 2010; 41:S31–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roffi M, Mukherjee D, Clair DG. Carotid artery stenting vs. Endarterectomy. Eur Heart J 2009; 30:2693–2704. [DOI] [PubMed] [Google Scholar]
- 5.Meier P, Knapp G, Tamhane U, et al. Short term and intermediate term comparison of endarterectomy versus stenting for carotid artery stenosis: systematic review and meta-analysis of randomised controlled clinical trials. BMJ 2010; 340:c467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu ZJ, Fu WG, Guo ZY, et al. Updated systematic review and meta-analysis of randomized clinical trials comparing carotid artery stenting and carotid endarterectomy in the treatment of carotid stenosis. Ann Vasc Surg 2012; 26:576–590. [DOI] [PubMed] [Google Scholar]
- 7.Murad MH, Flynn DN, Elamin MB, et al. Endarterectomy vs stenting for carotid artery stenosis: a systematic review and meta-analysis. J Vasc Surg 2008; 48:487–493. [DOI] [PubMed] [Google Scholar]
- 8.Bonati LH, Dobson J, Algra A, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 2010; 376:1062–1073. [DOI] [PubMed] [Google Scholar]
- 9.Bonati LH, Fraedrich G. Age modifies the relative risk of stenting versus endarterectomy for symptomatic carotid stenosis—a pooled analysis of EVA-3S, SPACE and ICSS. Eur J Vasc Endovasc Surg 2011; 41:153–158. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voeks JH, Howard G, Roubin GS, et al. Age and outcomes after carotid stenting and endarterectomy: the carotid revascularization endarterectomy versus stenting trial. Stroke 2011; 42:3484–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard VJ, Lutsep HL, Mackey A, et al. Influence of sex on outcomes of stenting versus endarterectomy: a subgroup analysis of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Lancet Neurol 2011; 10:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silver FL, Mackey A, Clark WM, et al. Safety of stenting and endarterectomy by symptomatic status in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Stroke 2011; 42:675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ringleb PA, Allenberg J, Brückmann H, et al. 30 day results from the space trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet 2006; 368:1239–1247. [DOI] [PubMed] [Google Scholar]
- 16.CARESS., Steering Committee Carotid revascularization using endarterectomy or stenting systems (CaRESS): phase I clinical trial. J Endovasc Ther 2003; 10:1021–1030. [DOI] [PubMed] [Google Scholar]
- 17.Brooks WH, Jones MR, Gisler P, et al. Carotid angioplasty with stenting versus endarterectomy: 10-year randomized trial in a community hospital. JACC Cardiovasc Interv 2014; 7:163–168. [DOI] [PubMed] [Google Scholar]
- 18.Lal BK, Beach KW, Roubin GS, et al. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of crest, a randomised controlled trial. Lancet Neurol 2012; 11:755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ederle J, Dobson J, Featherstone RL, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet 2010; 375:985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinbauer MG, Pfister K, Greindl M, et al. Alert for increased long-term follow-up after carotid artery stenting: results of a prospective, randomized, single-center trial of carotid artery stenting vs carotid endarterectomy. J Vasc Surg 2008; 48:93–98. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann A, Engelter S, Taschner C, et al. Carotid artery stenting versus carotid endarterectomy−a prospective randomised controlled single-centre trial with long-term follow-up (BACASS). Schweiz Arch Neurol Psychiatr 2008; 159:84–89. [Google Scholar]
- 23.Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med 2008; 358:1572–1579. [DOI] [PubMed] [Google Scholar]
- 24.Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol 2008; 7:893–902. [DOI] [PubMed] [Google Scholar]
- 25.Mas JL, Trinquart L, Leys D, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol 2008; 7:885–892. [DOI] [PubMed] [Google Scholar]
- 26.Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med 2006; 355:1660–1671. [DOI] [PubMed] [Google Scholar]
- 27.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351:1493–1501. [DOI] [PubMed] [Google Scholar]
- 28.Brooks WH, McClure RR, Jones MR, et al. Carotid angioplasty and stenting versus carotid endarterectomy: randomized trial in a community hospital. J Am Coll Cardiol 2001; 38:1589–1595. [DOI] [PubMed] [Google Scholar]
- 29.Zarins CK, White RA, Diethrich EB, et al. Carotid revascularization using endarterectomy or stenting systems (CaRESS): 4-year outcomes. J Endovasc Ther 2009; 16:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CARESS Steering Committee Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS) phase I clinical trial: 1-year results. J Vasc Surg 2005; 42:213–219. [DOI] [PubMed] [Google Scholar]
- 31.Madyoon H, Braunstein E, Callcott F, et al. Unprotected carotid artery stenting compared to carotid endarterectomy in a community setting. J Endovasc Ther 2002; 9:803–809. [DOI] [PubMed] [Google Scholar]
- 32.Brown K, Itum DS, Preiss J, et al. Carotid artery stenting has increased risk of external carotid artery occlusion compared with carotid endarterectomy. J Vasc Surg 2015; 61:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradac O, Mohapl M, Kramar F, et al. Carotid endarterectomy and carotid artery stenting: changing paradigm during 10 years in a high-volume centre. Acta Neurochir 2014; 156:1705–1712. [DOI] [PubMed] [Google Scholar]
- 34.Taş MH, Simşek Z, Colak A, et al. Comparison of carotid artery stenting and carotid endarterectomy in patients with symptomatic carotid artery stenosis: a single center study. Adv Ther 2013; 30:845–853. [DOI] [PubMed] [Google Scholar]
- 35.Parlani G, De Rango P, Cieri E, et al. Diabetes is not a predictor of outcome for carotid revascularization with stenting as it may be for carotid endarterectomy. J Vasc Surg 2012; 55:79–89. [DOI] [PubMed] [Google Scholar]
- 36.Lindström D, Jonsson M, Formgren J, et al. Outcome after 7 years of carotid artery stenting and endarterectomy in Sweden—single centre and national results. Eur J Vasc Endovasc Surg 2012; 43:499–503. [DOI] [PubMed] [Google Scholar]
- 37.Kimiagar I, Gur AY, Auriel E, et al. Long-term follow-up of patients after carotid stenting with or without distal protective device in a single tertiary medical center. Vasc Endovascular Surg 2012; 46:536–541. [DOI] [PubMed] [Google Scholar]
- 38.Brewster LP, Beaulieu R, Kasirajan K, et al. Contralateral occlusion is not a clinically important reason for choosing carotid artery stenting for patients with significant carotid artery stenosis. J Vasc Surg 2012; 56:1291–1294. [DOI] [PubMed] [Google Scholar]
- 39.Tsukahara T, Fukuda S, Nakakuki T, et al. Indication for surgical treatment of carotid arterial stenosis in high-risk patients. Acta Neurochir Suppl 2011; 112:21–24. [DOI] [PubMed] [Google Scholar]
- 40.Brewster LP, Beaulieu R, Corriere MA, et al. Carotid revascularization outcomes comparing distal filters, flow reversal, and endarterectomy. J Vasc Surg 2011; 54:1000–1004. [DOI] [PubMed] [Google Scholar]
- 41.Sidawy AN, Zwolak RM, White RA, et al. Risk-adjusted 30-day outcomes of carotid stenting and endarterectomy: results from the svs vascular registry. J Vasc Surg 2009; 49:71–79. [DOI] [PubMed] [Google Scholar]
- 42.Robbs JV, Mulaudzi T, Paruk N, et al. Carotid intervention: stent or surgery? A prospective audit. Cardiovasc J Afr 2009; 20:336–339. [PMC free article] [PubMed] [Google Scholar]
- 43.Tang GL, Matsumura JS, Morasch MD, et al. Carotid angioplasty and stenting vs carotid endarterectomy for treatment of asymptomatic disease: single-center experience. Arch Surg 2008; 143:653–658. [DOI] [PubMed] [Google Scholar]
- 44.Sadek M, Hynecek RL, Sambol EB, et al. Carotid angioplasty and stenting, success relies on appropriate patient selection. J Vasc Surg 2008; 47:946–951. [DOI] [PubMed] [Google Scholar]
- 45.Brown KE, Fanciullo DJ, Hicks T, et al. Carotid artery stenting compared to carotid endarterectomy performed exclusively in a veteran population: one center's experience with midterm results. Ann Surg 2008; 248:110–116. [DOI] [PubMed] [Google Scholar]
- 46.Setacci C, Chisci E, de Donato G, et al. Carotid artery stenting in a single center: are six years of experience enough to achieve the standard of care? Eur J Vasc Endovasc Surg 2007; 34:655–662. [DOI] [PubMed] [Google Scholar]
- 47.Faraglia V, Palombo G, Stella N, et al. Cerebral embolization in patients undergoing protected carotid-artery stenting and carotid surgery. J Cardiovasc Surg (Torino) 2007; 48:683–688. [PubMed] [Google Scholar]
- 48.Marine LA, Rubin BG, Reddy R, et al. Treatment of asymptomatic carotid artery disease: similar early outcomes after carotid stenting for high-risk patients and endarterectomy for standard-risk patients. J Vasc Surg 2006; 43:953–958. [DOI] [PubMed] [Google Scholar]
- 49.Bush RL, Kougias P, Guerrero MA, et al. A comparison of carotid artery stenting with neuroprotection versus carotid endarterectomy under local anesthesia. Am J Surg 2005; 190:696–700. [DOI] [PubMed] [Google Scholar]
- 50.Kastrup A, Schulz JB, Raygrotzki S, et al. Comparison of angioplasty and stenting with cerebral protection versus endarterectomy for treatment of internal carotid artery stenosis in elderly patients. J Vasc Surg 2004; 40:945–951. [DOI] [PubMed] [Google Scholar]
- 51.Kastrup A, Skalej M, Krapf H, et al. Early outcome of carotid angioplasty and stenting versus carotid endarterectomy in a single academic center. Cerebrovasc Dis 2003; 15:84–89. [DOI] [PubMed] [Google Scholar]
- 52.Vaniyapong T, Chongruksut W, Rerkasem K. Local versus general anaesthesia for carotid endarterectomy. Cochrane Database Syst Rev 2013; 12:CD000126. [DOI] [PubMed] [Google Scholar]
- 53.Rantner B, Goebel G, Bonati Leo H, et al. The risk of carotid artery stenting compared with carotid endarterectomy is greatest in patients treated within 7 days of symptoms. J Vasc Surg 2013; 57:619–626. [DOI] [PubMed] [Google Scholar]
- 54.Raman G, Moorthy D, Hadar N, et al. Management strategies for asymptomatic carotid stenosisa systematic review and meta-analysis. Ann Intern Med 2013; 158:676–685. [DOI] [PubMed] [Google Scholar]
- 55.Guay J. Endovascular stenting or carotid endarterectomy for treatment of carotid stenosis: a meta-analysis. J Cardiothorac Vasc Anesth 2011; 25:1024–1029. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Shi Z, Wang Y, et al. Carotid artery stenting versus carotid endarterectomy: systematic review and meta-analysis. World J Surg 2009; 33:586–596. [DOI] [PubMed] [Google Scholar]
- 57.Theiss W, Hermanek P, Mathias K, et al. Predictors of death and stroke after carotid angioplasty and stenting: a subgroup analysis of the Pro-CAS data. Stroke 2008; 39:2325–2330. [DOI] [PubMed] [Google Scholar]
- 58.Fiehler J, Jansen O, Berger J, et al. Differences in complication rates among the centres in the SPACE study. Neuroradiology 2008; 50:1049–1053. [DOI] [PubMed] [Google Scholar]
- 59.Brothers TE. Initial experience with eversion carotid endarterectomy: absence of a learning curve for the first 100 patients. J Vasc Surg 2005; 42:429–434. [DOI] [PubMed] [Google Scholar]
- 60.Kastrup A, Gröschel K, Krapf H, et al. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke 2003; 34:813–819. [DOI] [PubMed] [Google Scholar]
- 61.Zahn R, Mark B, Niedermaier N, et al. Embolic protection devices for carotid artery stenting: better results than stenting without protection? Eur Heart J 2004; 25:1550–1558. [DOI] [PubMed] [Google Scholar]
- 62.Bates ER, Babb JD, Casey DE, Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (ACCF/SCAI/SVMB/SIR/ASITN Clinical Expert Consensus Document Committee on Carotid Stenting). J Am Coll Cardiol 2007; 49:126–170. [DOI] [PubMed] [Google Scholar]
- 63.Liapis CD, Bell PR, Mikhailidis D, et al. ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg 2009; 37:1–19. [DOI] [PubMed] [Google Scholar]
- 64.Castro-Afonso LH, Abud LG, Rolo JG, et al. Flow reversal versus filter protection: a pilot carotid artery stenting randomized trial. Circ Cardiovasc Interv 2013; 6:552–559. [DOI] [PubMed] [Google Scholar]
- 65.DeBakey ME. Successful carotid endarterectomy for cerebrovascular insufficiency. Nineteen-year follow-up. JAMA 1975; 233:1083–1085. [PubMed] [Google Scholar]
- 66.Pini R, Faggioli G, Longhi M, et al. Impact of postoperative transient ischemic attack on survival after carotid revascularization. J Vasc Surg 2014; 59:1570–1576. [DOI] [PubMed] [Google Scholar]
- 67.Brasselet C, Durand E, Addad F, et al. Effect of local heating on restenosis and in-stent neointimal hyperplasia in the atherosclerotic rabbit model: a dose-ranging study. Eur Heart J 2008; 29:402–412. [DOI] [PubMed] [Google Scholar]
- 68.May P, Arrouvel C, Revol M, et al. Detection of hemodynamic turbulence in experimental stenosis: an in vivo study in the rat carotid artery. J Vasc Res 2002; 39:21–29. [DOI] [PubMed] [Google Scholar]


